Enhanced Modification between Glucose Dehydrogenase and Mediator Using Epoxy Silane Assembly for Monitoring Glucose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
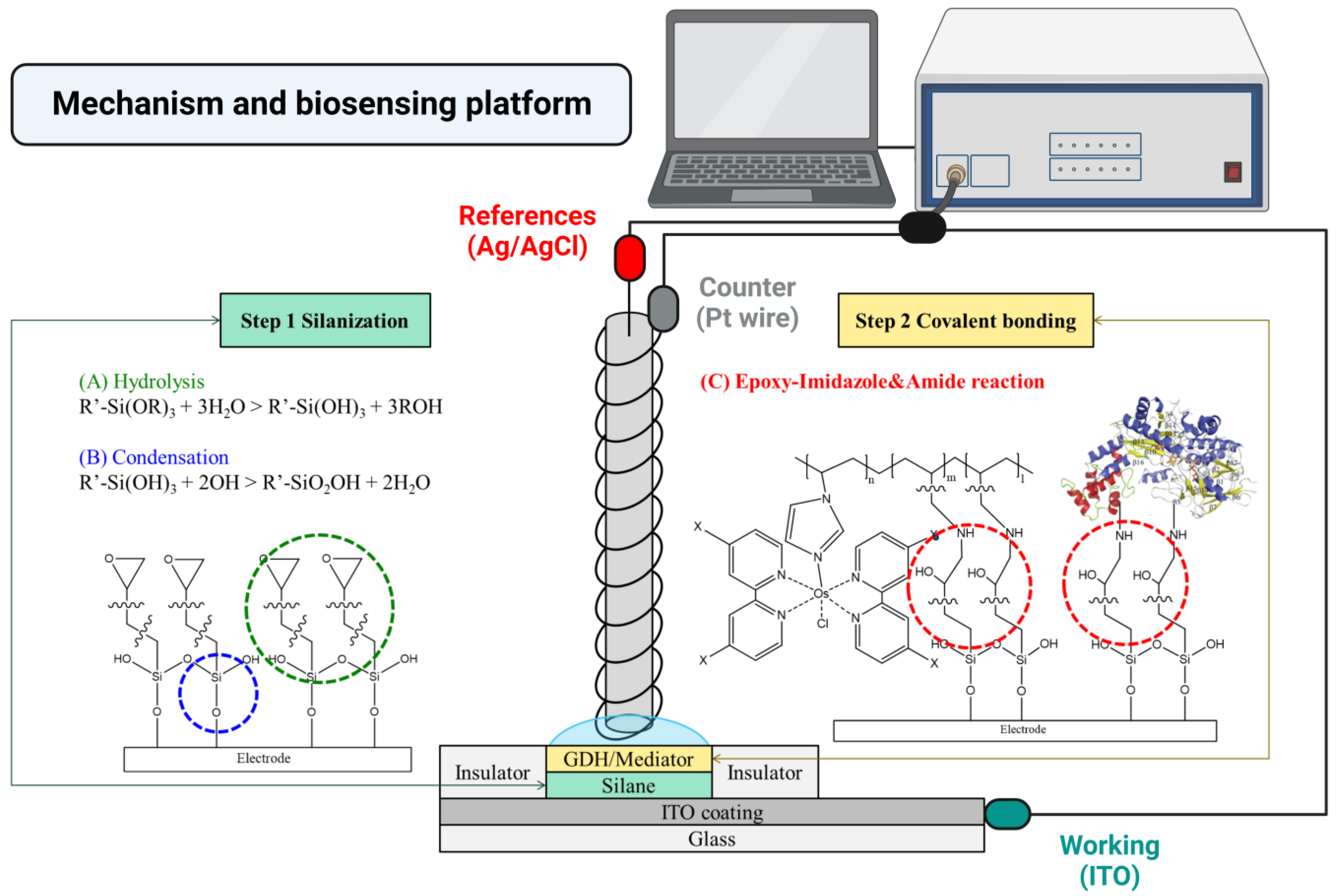
2.2. GDH/Mediator–Silane–ITO Electrode Fabrication
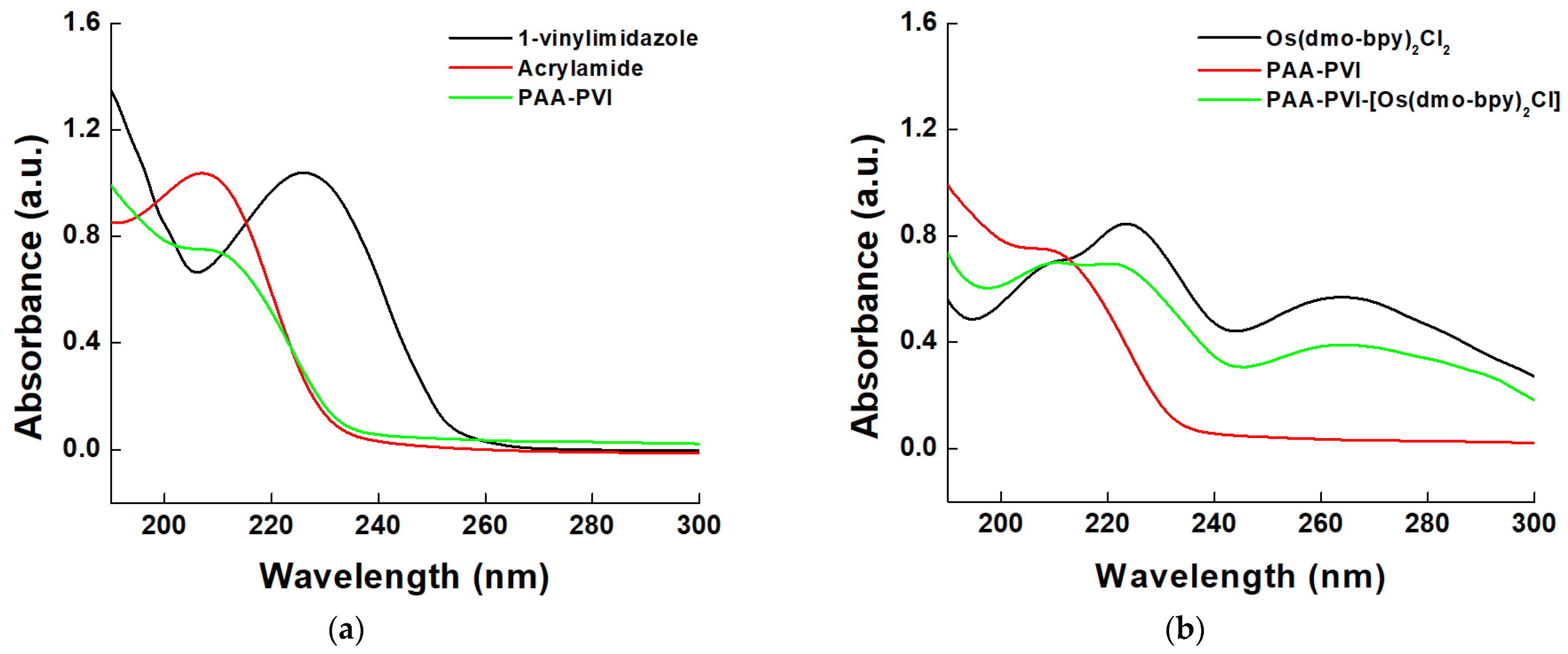
2.2.1. PAA–PVI Synthesis
2.2.2. Os(dmo–bpy)2Cl2 Synthesis
2.2.3. PAA–PVI–[Os(dmo–bpy)2Cl] Synthesis
2.2.4. GDH/Mediator–Silane–ITO Electrode Preparation
2.3. Electrochemical Analysis of GDH/Mediator–Silane–ITO Electrodes
3. Results and Discussion
3.1. Electrodes’ Characterization
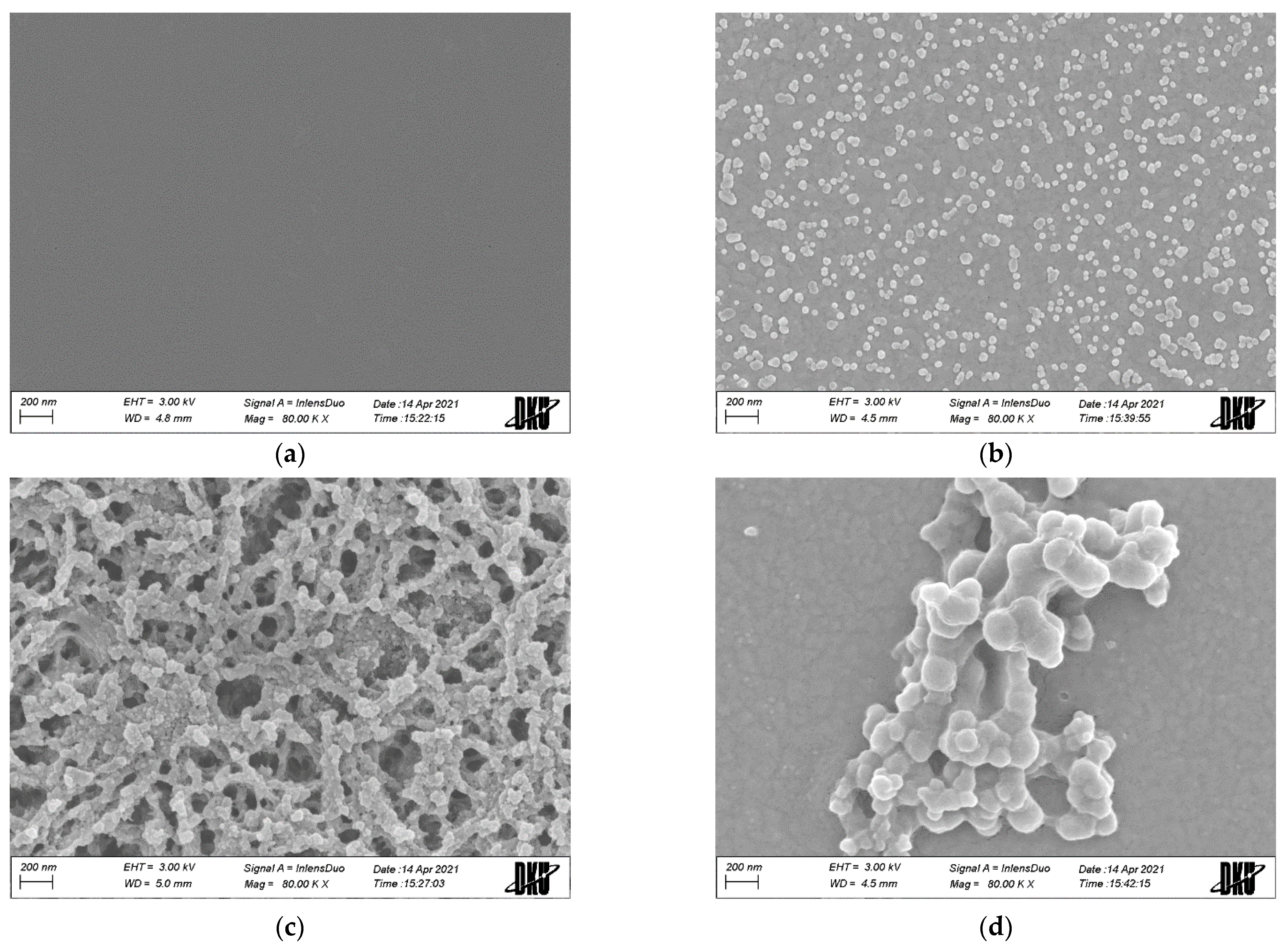
3.1.1. Morphological Properties
3.1.2. Spectroscopic Properties
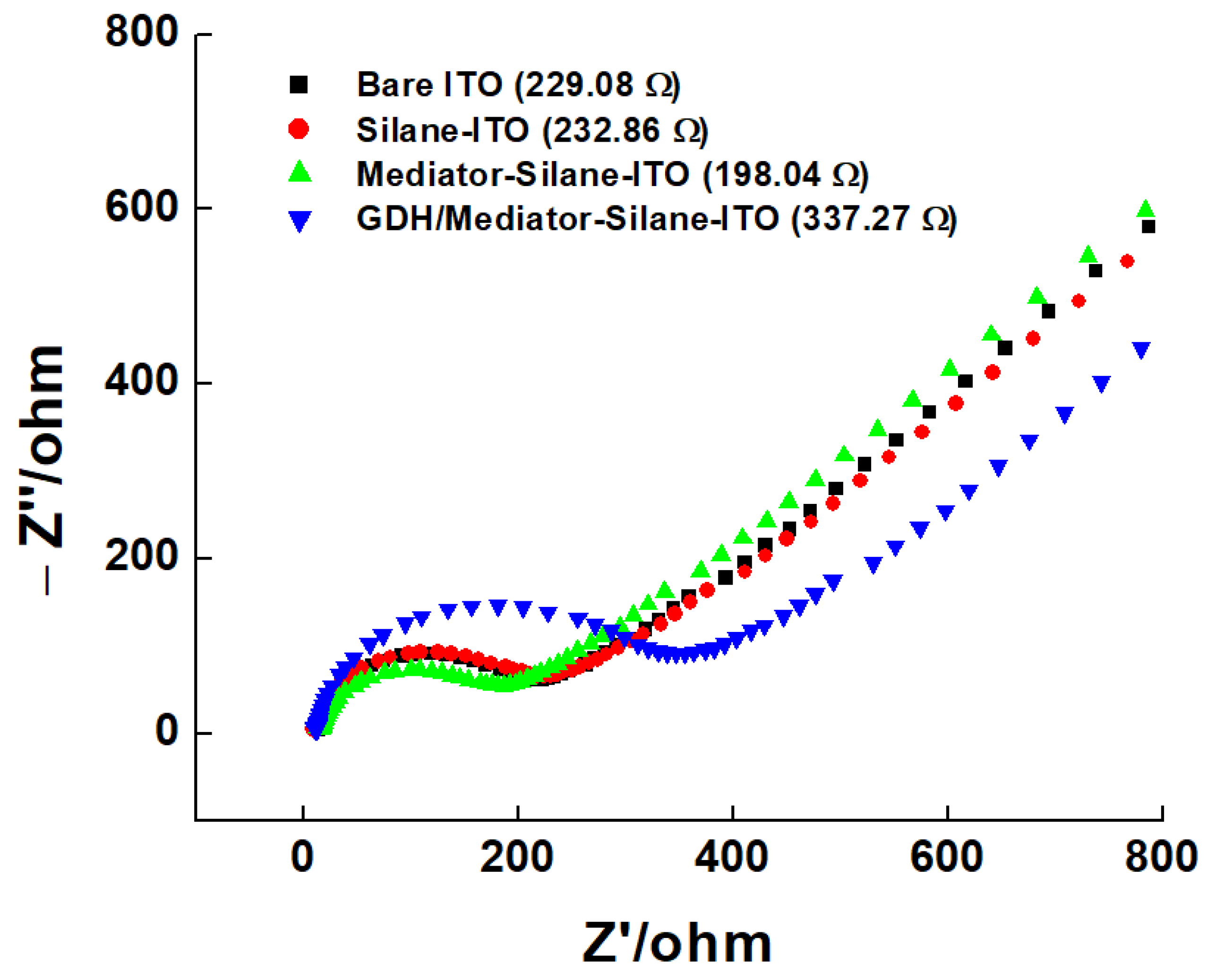
3.1.3. Electrochemical Properties
3.2. Electrodes’ Performances
3.2.1. Glucose Measurements
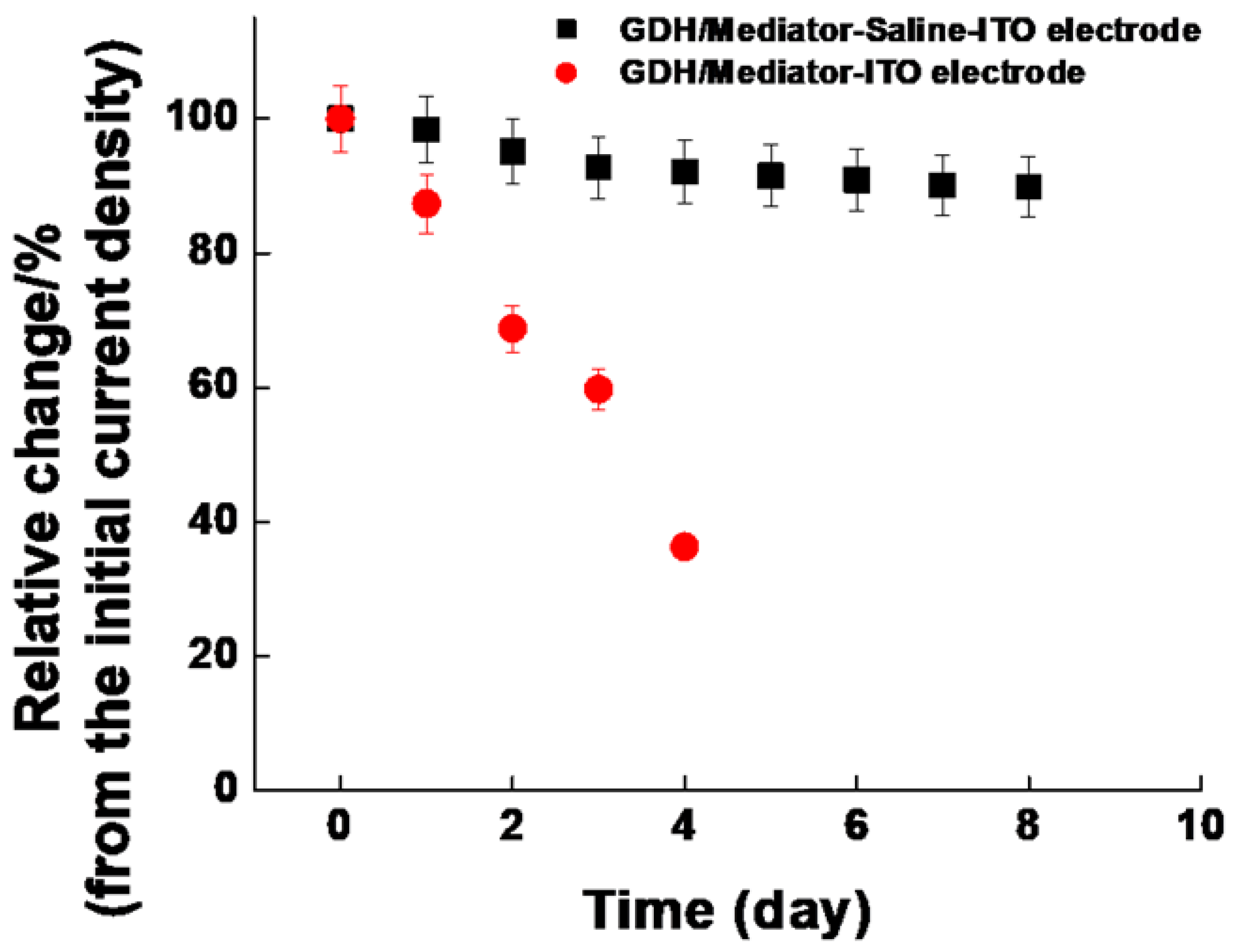
3.2.2. Sensor Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heller, A.; Feldman, B. Electrochemical Glucose Sensors and Their Applications in Diabetes Management. Chem. Rev. 2008, 108, 2482–2505. [Google Scholar] [CrossRef]
- Reach, G.; Wilson, G.S. Can Continuous Glucose Monitoring Be Used for the Treatment of Diabetes. Anal. Chem. 1992, 64, 381A–386A. [Google Scholar] [CrossRef]
- Turner, A.P.; Chen, B.; Piletsky, S.A. In Vitro Diagnostics in Diabetes: Meeting the Challenge. Clin. Chem. 1999, 45, 1596–1601. [Google Scholar] [CrossRef]
- Kobos, R.K. Enzyme-Based Electrochemical Biosensors. TrAC Trends Anal. Chem. 1987, 6, 6–9. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent Advances in Electrochemical Glucose Biosensors: A Review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Wang, J. Glucose Biosensors: 40 Years of Advances and Challenges. Electroanalysis 2001, 13, 983–988. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Horaguchi, Y.; Saito, S.; Kojima, K.; Tsugawa, W.; Ferri, S.; Sode, K. Engineering Glucose Oxidase to Minimize the Influence of Oxygen on Sensor Response. Electrochim. Acta 2014, 126, 158–161. [Google Scholar] [CrossRef]
- Tang, Z.; Louie, R.F.; Lee, J.H.; Lee, D.M.; Miller, E.E.; Kost, G.J. Oxygen Effects on Glucose Meter Measurements with Glucose Dehydrogenase- and Oxidase-Based Test Strips for Point-of-Care Testing. Crit. Care Med. 2001, 29, 1062–1070. [Google Scholar] [CrossRef]
- Okuda-Shimazaki, J.; Yoshida, H.; Sode, K. FAD Dependent Glucose Dehydrogenases—Discovery and Engineering of Representative Glucose Sensing Enzymes-. Bioelectrochemistry 2020, 132, 107414. [Google Scholar] [CrossRef]
- Igarashi, S.; Ohtera, T.; Yoshida, H.; Witarto, A.B.; Sode, K. Construction and Characterization of Mutant Water-Soluble PQQ Glucose Dehydrogenases with Altered Km Values—Site-Directed Mutagenesis Studies on the Putative Active Site. Biochem. Biophys. Res. Commun. 1999, 264, 820–824. [Google Scholar] [CrossRef] [PubMed]
- Haque, A.J.; Nandhakumar, P.; Yang, H. Specific and Rapid Glucose Detection Using NAD-dependent Glucose Dehydrogenase, Diaphorase, and Osmium Complex. Electroanalysis 2019, 31, 876–882. [Google Scholar] [CrossRef]
- Yamaoka, H.; Yamashita, Y.; Ferri, S.; Sode, K. Site Directed Mutagenesis Studies of FAD-Dependent Glucose Dehydrogenase Catalytic Subunit of Burkholderia Cepacia. Biotechnol. Lett. 2008, 30, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Milton, R.D.; Giroud, F.; Thumser, A.E.; Minteer, S.D.; Slade, R.C.T. Glucose Oxidase Progressively Lowers Bilirubin Oxidase Bioelectrocatalytic Cathode Performance in Single-Compartment Glucose/Oxygen Biological Fuel Cells. Electrochim. Acta 2014, 140, 59–64. [Google Scholar] [CrossRef]
- Özgeriş, F.B.; Yeltekin, A.Ç.; Ucar, A.; Çağlar, Ö.; Parlak, V.; Arslan, M.E.; Türkez, H.; Atamanalp, M.; Alak, G. Toxic Releases and Exposure Assessment: A Multi-Endpoint Approach in Fish for Ferrocene Toxicity. Process Saf. Environ. Prot. 2023, 169, 636–645. [Google Scholar] [CrossRef]
- Loew, N.; Tsugawa, W.; Nagae, D.; Kojima, K.; Sode, K. Mediator Preference of Two Different FAD-Dependent Glucose Dehydrogenases Employed in Disposable Enzyme Glucose Sensors. Sensors 2017, 17, 2636. [Google Scholar] [CrossRef]
- Okurita, M.; Suzuki, N.; Loew, N.; Yoshida, H.; Tsugawa, W.; Mori, K.; Kojima, K.; Klonoff, D.C.; Sode, K. Engineered Fungus Derived FAD-Dependent Glucose Dehydrogenase with Acquired Ability to Utilize Hexaammineruthenium(III) as an Electron Acceptor. Bioelectrochemistry 2018, 123, 62–69. [Google Scholar] [CrossRef]
- Ishida, K.; Orihara, K.; Muguruma, H.; Iwasa, H.; Hiratsuka, A.; Tsuji, K.; Kishimoto, T. Comparison of Direct and Mediated Electron Transfer in Electrodes with Novel Fungal Flavin Adenine Dinucleotide Glucose Dehydrogenase. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2018, 34, 783–787. [Google Scholar] [CrossRef]
- Nakabayashi, Y.; Omayu, A.; Yagi, S.; Nakamura, K.; Motonaka, J. Evaluation of Osmium(II) Complexes as Electron Transfer Mediators Accessible for Amperometric Glucose Sensors. Anal. Sci. 2001, 17, 945–950. [Google Scholar] [CrossRef]
- Zakeeruddin, S.M.; Fraser, D.M.; Nazeeruddin, M.-K.; Grätzel, M. Towards Mediator Design: Characterization of Tris-(4,4′-Substituted-2,2′-Bipyridine) Complexes of Iron(II), Ruthenium(II) and Osmium(II) as Mediators for Glucose Oxidase of Aspergillus Niger and Other Redox Proteins. J. Electroanal. Chem. 1992, 337, 253–283. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Zakeeruddin, S.M.; Kalyanasundaram, K. Enhanced Intensities of the Ligand-to-Metal Charge-Transfer Transitions in Ruthenium(III) and Osmium(III) Complexes of Substituted Bipyridines. J. Phys. Chem. 1993, 97, 9607–9612. [Google Scholar] [CrossRef]
- Kim, H.-H.; Mano, N.; Zhang, Y.; Heller, A. A Miniature Membrane-Less Biofuel Cell Operating under Physiological Conditions at 0.5 V. J. Electrochem. Soc. 2003, 150, A209. [Google Scholar] [CrossRef]
- Jeon, W.-Y.; Lee, C.-J.; Kim, H.-H.; Choi, Y.-B.; Lee, B.-H.; Jo, H.-J.; Jeon, S.-Y. Glucose Detection via Ru-Mediated Catalytic Reaction of Glucose Dehydrogenase. Adv. Mater. Lett. 2018, 9, 220–224. [Google Scholar] [CrossRef]
- Beck, R.W.; Bergenstal, R.M.; Riddlesworth, T.D.; Kollman, C.; Li, Z.; Brown, A.S.; Close, K.L. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care 2019, 42, 400–405. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 7. Diabetes Technology: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S97–S112. [Google Scholar] [CrossRef]
- Lucarelli, F.; Ricci, F.; Caprio, F.; Valgimigli, F.; Scuffi, C.; Moscone, D.; Palleschi, G. GlucoMen Day Continuous Glucose Monitoring System: A Screening for Enzymatic and Electrochemical Interferents. J. Diabetes Sci. Technol. 2012, 6, 1172–1181. [Google Scholar] [CrossRef]
- Wisniewski, N.; Moussy, F.; Reichert, W.M. Characterization of Implantable Biosensor Membrane Biofouling. Fresenius’ J. Anal. Chem. 2000, 366, 611–621. [Google Scholar] [CrossRef]
- Moon, B.-U.; Koster, S.; Wientjes, K.J.C.; Kwapiszewski, R.M.; Schoonen, A.J.M.; Westerink, B.H.C.; Verpoorte, E. An Enzymatic Microreactor Based on Chaotic Micromixing for Enhanced Amperometric Detection in a Continuous Glucose Monitoring Application. Anal. Chem. 2010, 82, 6756–6763. [Google Scholar] [CrossRef]
- Klonoff, D.C. Continuous Glucose Monitoring. Diabetes Care 2005, 28, 1231–1239. [Google Scholar] [CrossRef]
- Feldman, B.; Brazg, R.; Schwartz, S.; Weinstein, R. A Continuous Glucose Sensor Based on Wired EnzymeTM Technology—Results from a 3-Day Trial in Patients with Type 1 Diabetes. Diabetes Technol. Ther. 2003, 5, 769–779. [Google Scholar] [CrossRef]
- Tamada, J.A.; Garg, S.; Jovanovic, L.; Pitzer, K.R.; Fermi, S.; Potts, R.O. The Cygnus Research Team Noninvasive Glucose Monitoring: Comprehensive Clinical Results. JAMA 1999, 282, 1839. [Google Scholar] [CrossRef] [PubMed]
- Sreenan, S.; Andersen, M.; Thorsted, B.L.; Wolden, M.L.; Evans, M. Increased Risk of Severe Hypoglycemic Events with Increasing Frequency of Non-Severe Hypoglycemic Events in Patients with Type 1 and Type 2 Diabetes. Diabetes Ther. 2014, 5, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Acciaroli, G.; Vettoretti, M.; Facchinetti, A.; Sparacino, G. Calibration of Minimally Invasive Continuous Glucose Monitoring Sensors: State-of-The-Art and Current Perspectives. Biosensors 2018, 8, 24. [Google Scholar] [CrossRef]
- Yamamoto, K.; Zeng, H.; Shen, Y.; Ahmed, M.; Kato, T. Evaluation of an Amperometric Glucose Biosensor Based on a Ruthenium Complex Mediator of Low Redox Potential. Talanta 2005, 66, 1175–1180. [Google Scholar] [CrossRef]
- Gerard, M. Application of Conducting Polymers to Biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Wilson, N.G.; McCreedy, T.; Greenway, G.M. In-Situ Immobilisation of Glucose Oxidase on a Novel Microporous Silica Support. Analyst 2000, 125, 237–239. [Google Scholar] [CrossRef]
- Liu, B.; Hu, R.; Deng, J. Fabrication of an Amperometric Biosensor Based on the Immobilization of Glucose Oxidase in a Modified Molecular Sieve Matrix. Analyst 1997, 122, 821–826. [Google Scholar] [CrossRef]
- DeSantis, G.; Jones, J.B. Chemical Modification of Enzymes for Enhanced Functionality. Curr. Opin. Biotechnol. 1999, 10, 324–330. [Google Scholar] [CrossRef]
- Polizzi, K.M.; Bommarius, A.S.; Broering, J.M.; Chaparro-Riggers, J.F. Stability of Biocatalysts. Curr. Opin. Chem. Biol. 2007, 11, 220–225. [Google Scholar] [CrossRef]
- Qiu, J.-D.; Zhou, W.-M.; Guo, J.; Wang, R.; Liang, R.-P. Amperometric Sensor Based on Ferrocene-Modified Multiwalled Carbon Nanotube Nanocomposites as Electron Mediator for the Determination of Glucose. Anal. Biochem. 2009, 385, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Togo, M.; Takamura, A.; Asai, T.; Kaji, H.; Nishizawa, M. An Enzyme-Based Microfluidic Biofuel Cell Using Vitamin K3-Mediated Glucose Oxidation. Electrochim. Acta 2007, 52, 4669–4674. [Google Scholar] [CrossRef]
- An, N.; Zhou, C.H.; Zhuang, X.Y.; Tong, D.S.; Yu, W.H. Immobilization of Enzymes on Clay Minerals for Biocatalysts and Biosensors. Appl. Clay Sci. 2015, 114, 283–296. [Google Scholar] [CrossRef]
- De Lumley-Woodyear, T.; Rocca, P.; Lindsay, J.; Dror, Y.; Freeman, A.; Heller, A. Polyacrylamide-Based Redox Polymer for Connecting Redox Centers of Enzymes to Electrodes. Anal. Chem. 1995, 67, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.-Y.; Choi, Y.-B.; Kim, H.-H. Ultrasonic Synthesis and Characterization of Poly(Acrylamide)-Co-Poly(Vinylimidazole)@MWCNTs Composite for Use as an Electrochemical Material. Ultrason. Sonochem. 2018, 43, 73–79. [Google Scholar] [CrossRef]
- Csoeregi, E.; Schmidtke, D.W.; Heller, A. Design and Optimization of a Selective Subcutaneously Implantable Glucose Electrode Based on “Wired” Glucose Oxidase. Anal. Chem. 1995, 67, 1240–1244. [Google Scholar] [CrossRef]
- Gregg, B.A.; Heller, A. Cross-Linked Redox Gels Containing Glucose Oxidase for Amperometric Biosensor Applications. Anal. Chem. 1990, 62, 258–263. [Google Scholar] [CrossRef]
- Mano, N.; Heller, A. Detection of Glucose at 2 FM Concentration. Anal. Chem. 2005, 77, 729–732. [Google Scholar] [CrossRef]
- Olejnik, A.; Karczewski, J.; Dołęga, A.; Siuzdak, K.; Grochowska, K. Novel Approach to Interference Analysis of Glucose Sensing Materials Coated with Nafion. Bioelectrochemistry 2020, 135, 107575. [Google Scholar] [CrossRef]
- Pruna, R.; Palacio, F.; Martínez, M.; Blázquez, O.; Hernández, S.; Garrido, B.; López, M. Organosilane-Functionalization of Nanostructured Indium Tin Oxide Films. Interface Focus 2016, 6, 20160056. [Google Scholar] [CrossRef]
- Kamra, T.; Chaudhary, S.; Xu, C.; Johansson, N.; Montelius, L.; Schnadt, J.; Ye, L. Covalent Immobilization of Molecularly Imprinted Polymer Nanoparticles Using an Epoxy Silane. J. Colloid Interface Sci. 2015, 445, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Kidchob, T.; Yoko, T. Hybrid Organic-Inorganic Sol-Gel Materials Based on Epoxy-Amine Systems. J. Sol-Gel Sci. Technol. 2005, 35, 225–235. [Google Scholar] [CrossRef]
- Balaji, J.; Sethuraman, M.G. Corrosion Protection of Copper with 3-Glycidoxypropyltrimethoxysilane-Based Sol–Gel Coating through 3-Amino-5-Mercapto-1,2,4-Triazole Doping. Res. Chem. Intermed. 2016, 42, 1315–1328. [Google Scholar] [CrossRef]
- Hossain, M.M.; Morshed, J.; Tsujimura, S. Designing a Cross-Linked Redox Network for a Mediated Enzyme-Based Electrode. Chem. Commun. 2021, 57, 6999–7002. [Google Scholar] [CrossRef]
- Zafar, M.N.; Wang, X.; Sygmund, C.; Ludwig, R.; Leech, D.; Gorton, L. Electron-Transfer Studies with a New Flavin Adenine Dinucleotide Dependent Glucose Dehydrogenase and Osmium Polymers of Different Redox Potentials. Anal. Chem. 2012, 84, 334–341. [Google Scholar] [CrossRef]
- Ohara, T.J.; Rajagopalan, R.; Heller, A. “Wired” Enzyme Electrodes for Amperometric Determination of Glucose or Lactate in the Presence of Interfering Substances. Anal. Chem. 1994, 66, 2451–2457. [Google Scholar] [CrossRef]
- Streeter, I.; Wildgoose, G.G.; Shao, L.; Compton, R.G. Cyclic Voltammetry on Electrode Surfaces Covered with Porous Layers: An Analysis of Electron Transfer Kinetics at Single-Walled Carbon Nanotube Modified Electrodes. Sens. Actuators B Chem. 2008, 133, 462–466. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Ansari, A.A.; Sumana, G.; Malhotra, B.D. Zinc Oxide-Chitosan Nanobiocomposite for Urea Sensor. Appl. Phys. Lett. 2008, 93, 163903. [Google Scholar] [CrossRef]
- Van Benschoten, J.J.; Lewis, J.Y.; Heineman, W.R.; Roston, D.A.; Kissinger, P.T. Cyclic Voltammetry Experiment. J. Chem. Educ. 1983, 60, 772. [Google Scholar] [CrossRef]
- Pekel, N.; Güven, O. Investigation of Complex Formation between Poly(N-Vinyl Imidazole) and Various Metal Ions Using the Molar Ratio Method. Colloid Polym. Sci. 1999, 277, 570–573. [Google Scholar] [CrossRef]
- Verma, A.; Chaudhary, P.; Tripathi, R.K.; Yadav, B.C. The Functionalization of Polyacrylamide with MoS2 Nanoflakes for Use in Transient Photodetectors. Sustain. Energy Fuels 2021, 5, 1394–1405. [Google Scholar] [CrossRef]
- Pekel, N.; Güven, O. Spectroscopic and Thermal Studies of Poly[( N -Vinylimidazole)-Co-(Maleic Acid)] Hydrogel and Its Quaternized Form. Polym. Int. 2008, 57, 637–643. [Google Scholar] [CrossRef]
- Paris, J.P.; Brandt, W.W. Charge Transfer Luminescence of a Ruthenium(II) Chelate. J. Am. Chem. Soc. 1959, 81, 5001–5002. [Google Scholar] [CrossRef]
- Baldo, M.A.; Daniele, S.; Mazzocchin, G.A. Cyclic Voltammetry at Mercury Microelectrodes. Effects of Mercury Thickness and Scan Rate. Electrochim. Acta 1996, 41, 811–818. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, A.; Hou, T.; Li, F. A Sensitive and Versatile “Signal-on” Electrochemical Aptasensor Based on a Triple-Helix Molecular Switch. Analyst 2014, 139, 6272–6278. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Zhao, J.; Tang, S.; Shen, W.; Lee, H.K. Logarithmic Data Processing Can Be Used Justifiably in the Plotting of a Calibration Curve. Anal. Chem. 2021, 93, 12156–12161. [Google Scholar] [CrossRef]
- Takamatsu, S.; Takano, H.; Binh-Khiem, N.; Takahata, T.; Iwase, E.; Matsumoto, K.; Shimoyama, I. Liquid-Phase Packaging of a Glucose Oxidase Solution with Parylene Direct Encapsulation and an Ultraviolet Curing Adhesive Cover for Glucose Sensors. Sensors 2010, 10, 5888–5898. [Google Scholar] [CrossRef]
- EMA ICH Q2(R2) Validation of Analytical Procedures—Scientific Guideline. Available online: https://www.ema.europa.eu/en/ich-q2r2-validation-analytical-procedures-scientific-guideline (accessed on 2 July 2023).
- Lee, I.; Probst, D.; Klonoff, D.; Sode, K. Continuous Glucose Monitoring Systems—Current Status and Future Perspectives of the Flagship Technologies in Biosensor Research-. Biosens. Bioelectron. 2021, 181, 113054. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; De Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable Salivary Uric Acid Mouthguard Biosensor with Integrated Wireless Electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef]
- Mäkilä, E.; Kirveskari, P. A Study of Ascorbic Acid in Human Saliva. Arch. Oral Biol. 1969, 14, 1285–1292. [Google Scholar] [CrossRef]
- Piechotta, G.; Albers, J.; Hintsche, R. Novel Micromachined Silicon Sensor for Continuous Glucose Monitoring. Biosens. Bioelectron. 2005, 21, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Chinnadayyala, S.R.; Park, K.D.; Cho, S. Editors’ Choice—Review—In Vivo and In Vitro Microneedle Based Enzymatic and Non-Enzymatic Continuous Glucose Monitoring Biosensors. ECS J. Solid State Sci. Technol. 2018, 7, Q3159–Q3171. [Google Scholar] [CrossRef]
- Invernale, M.A.; Tang, B.C.; York, R.L.; Le, L.; Hou, D.Y.; Anderson, D.G. Microneedle Electrodes Toward an Amperometric Glucose-Sensing Smart Patch. Adv. Healthc. Mater. 2014, 3, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Ramírez, G.; Li, Y.-C.; Kim, J.; Jia, W.; Bandodkar, A.J.; Nuñez-Flores, R.; Miller, P.R.; Wu, S.-Y.; Narayan, R.; Windmiller, J.R.; et al. Microneedle-Based Self-Powered Glucose Sensor. Electrochem. Commun. 2014, 47, 58–62. [Google Scholar] [CrossRef]
- Hwa, K.-Y.; Subramani, B.; Chang, P.-W.; Chien, M.; Huang, J.-T. Transdermal Microneedle Array-Based Sensor for Real Time Continuous Glucose Monitoring. Int. J. Electrochem. Sci. 2015, 10, 2455–2466. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Valdés-Ramírez, G.; Zhou, N.; Zhou, M.; Miller, P.R.; Jin, C.; Brozik, S.M.; Polsky, R.; Katz, E.; Narayan, R.; et al. Bicomponent Microneedle Array Biosensor for Minimally-Invasive Glutamate Monitoring. Electroanalysis 2011, 23, 2302–2309. [Google Scholar] [CrossRef]

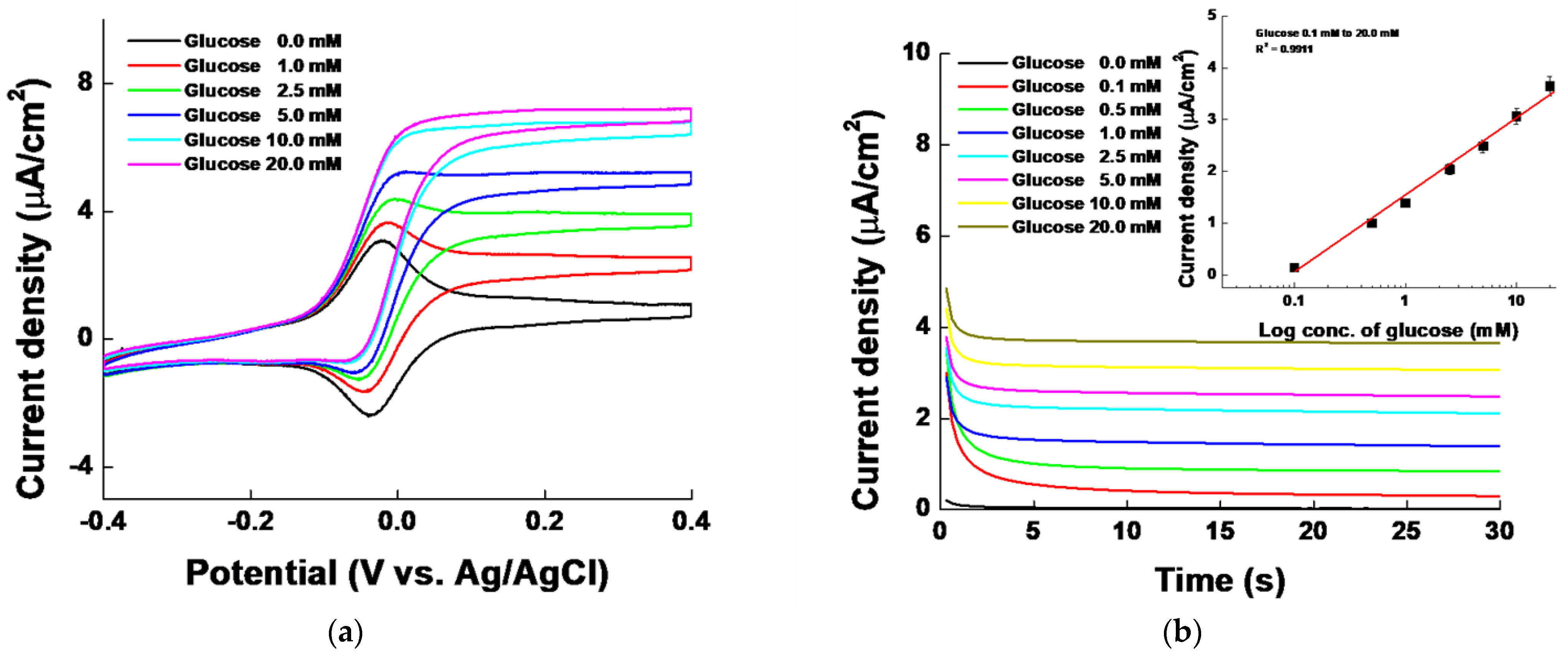

| Test Number | BKG Signal | Net. Signal | ICH Q2(R2) Guideline | 0.1–1 mM net. Calibration Curve |
|---|---|---|---|---|
| 1 | −7.31 × 10−9 | 2.52 × 10−2 | LOD = 3.3 s/m | Y = 1.5454X |
| 2 | −6.98 × 10−9 | 2.47 × 10−2 | LOQ = 10 s/m | R2 = 0.9777 |
| 3 | −7.02 × 10−9 | 2.48 × 10−2 | Standard deviation (s) = 0.001175 μA/cm2 | LOQ = 0.007604 mM |
| 4 | −6.96 × 10−9 | 2.46 × 10−2 | Sensitivity (m) = 1.5454 μA/cm2·mM | LOD = 0.002509 mM |
| 5 | −7.84 × 10−9 | 2.77 × 10−2 |
| Reports | Electrode Type | Dynamic Range | LOD | Sensitivity | Continuous Glucose Monitoring |
|---|---|---|---|---|---|
| [74] | GOX/PEDOT/Carbon | 36–432 mg/dL | N/A | N/A | 24% lost for 7 d |
| [75] | GOX/TIF/Carbon | 90–450 mg/dL | N/A | N/A | 25% lost for 60 h |
| [76] | GOX/MPA/Au | 50–400 mg/dL | N/A | N/A | 20% lost for 7 d |
| [77] | GOX/PPD | 18–252 mg/dL | 21 µM | 0.353 µA/mM | 3% lost for 8 h |
| Our study | GDH/Mediator–Silane–ITO | 1.8–360.3 mg/dL (0.1–20.0 mM) | 2.509 μM | 1.5454 μA/cm2·mM | 10.11% lost for 8 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, T.-W.; Jeon, W.-Y.; Choi, Y.-B. Enhanced Modification between Glucose Dehydrogenase and Mediator Using Epoxy Silane Assembly for Monitoring Glucose. Chemosensors 2023, 11, 485. https://doi.org/10.3390/chemosensors11090485
Seo T-W, Jeon W-Y, Choi Y-B. Enhanced Modification between Glucose Dehydrogenase and Mediator Using Epoxy Silane Assembly for Monitoring Glucose. Chemosensors. 2023; 11(9):485. https://doi.org/10.3390/chemosensors11090485
Chicago/Turabian StyleSeo, Tae-Won, Won-Yong Jeon, and Young-Bong Choi. 2023. "Enhanced Modification between Glucose Dehydrogenase and Mediator Using Epoxy Silane Assembly for Monitoring Glucose" Chemosensors 11, no. 9: 485. https://doi.org/10.3390/chemosensors11090485
APA StyleSeo, T.-W., Jeon, W.-Y., & Choi, Y.-B. (2023). Enhanced Modification between Glucose Dehydrogenase and Mediator Using Epoxy Silane Assembly for Monitoring Glucose. Chemosensors, 11(9), 485. https://doi.org/10.3390/chemosensors11090485







