Electrochemical Etching-Assisted Fabrication of Quantum Tunneling Sensing Probes with Controlled Nanogap Width
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Apparatus and Characterization
2.3. Fabrication of Dual-Barrel Au-Deposited Nanoelectrodes
3. Results and Discussion
3.1. Electrochemical Etching Tunneling Electrodes Fabrication
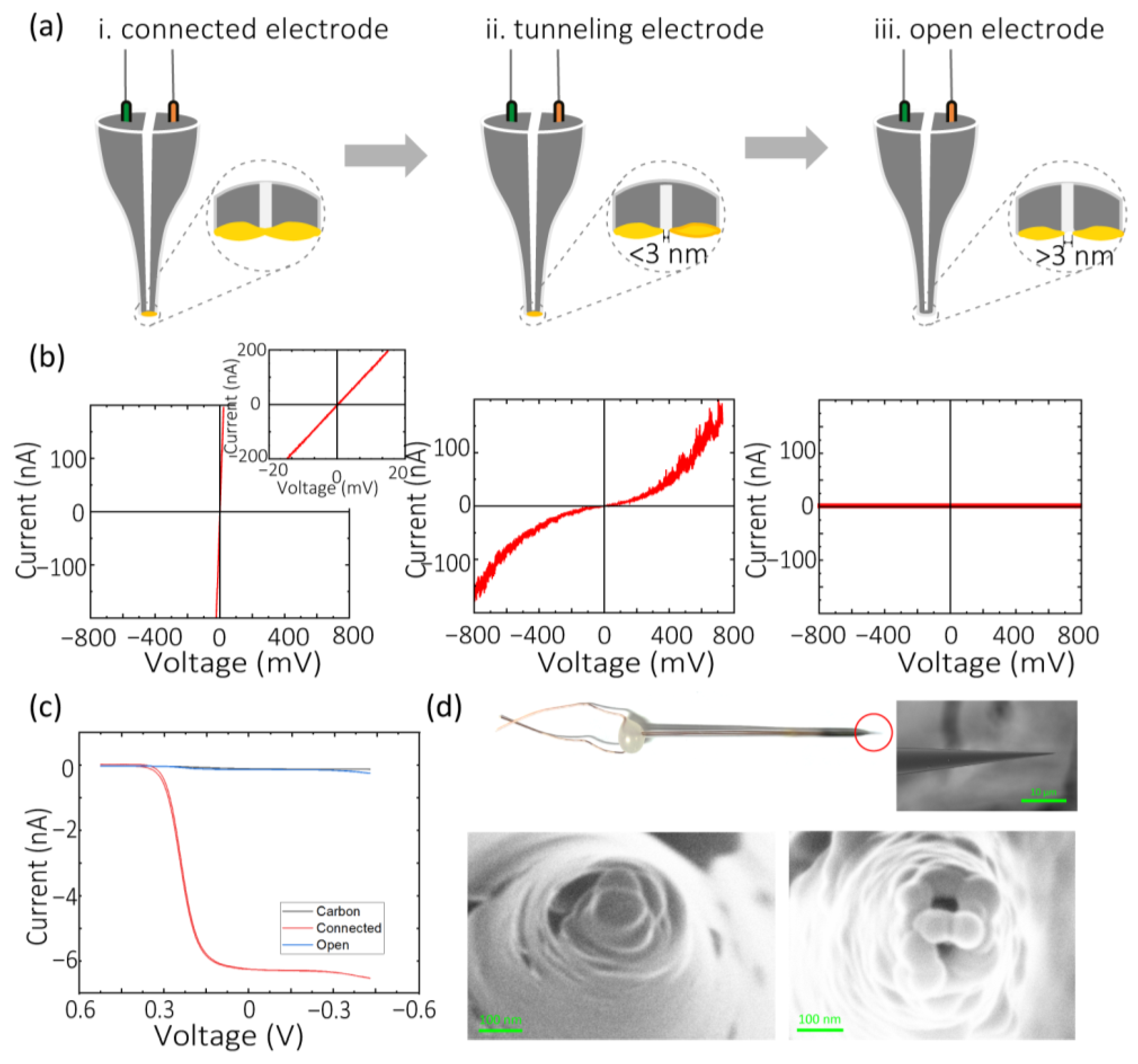
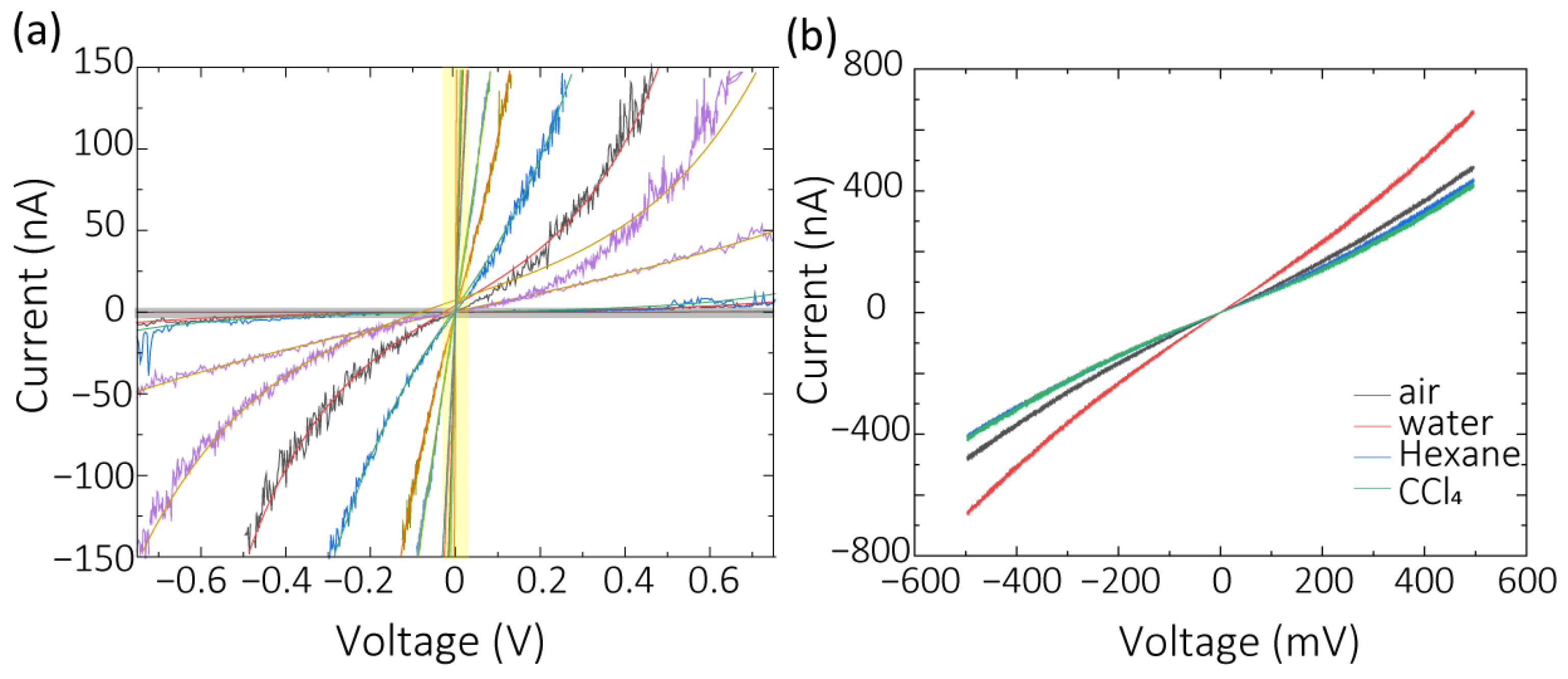
3.2. Characterization of Electrochemical Etching Electrodes
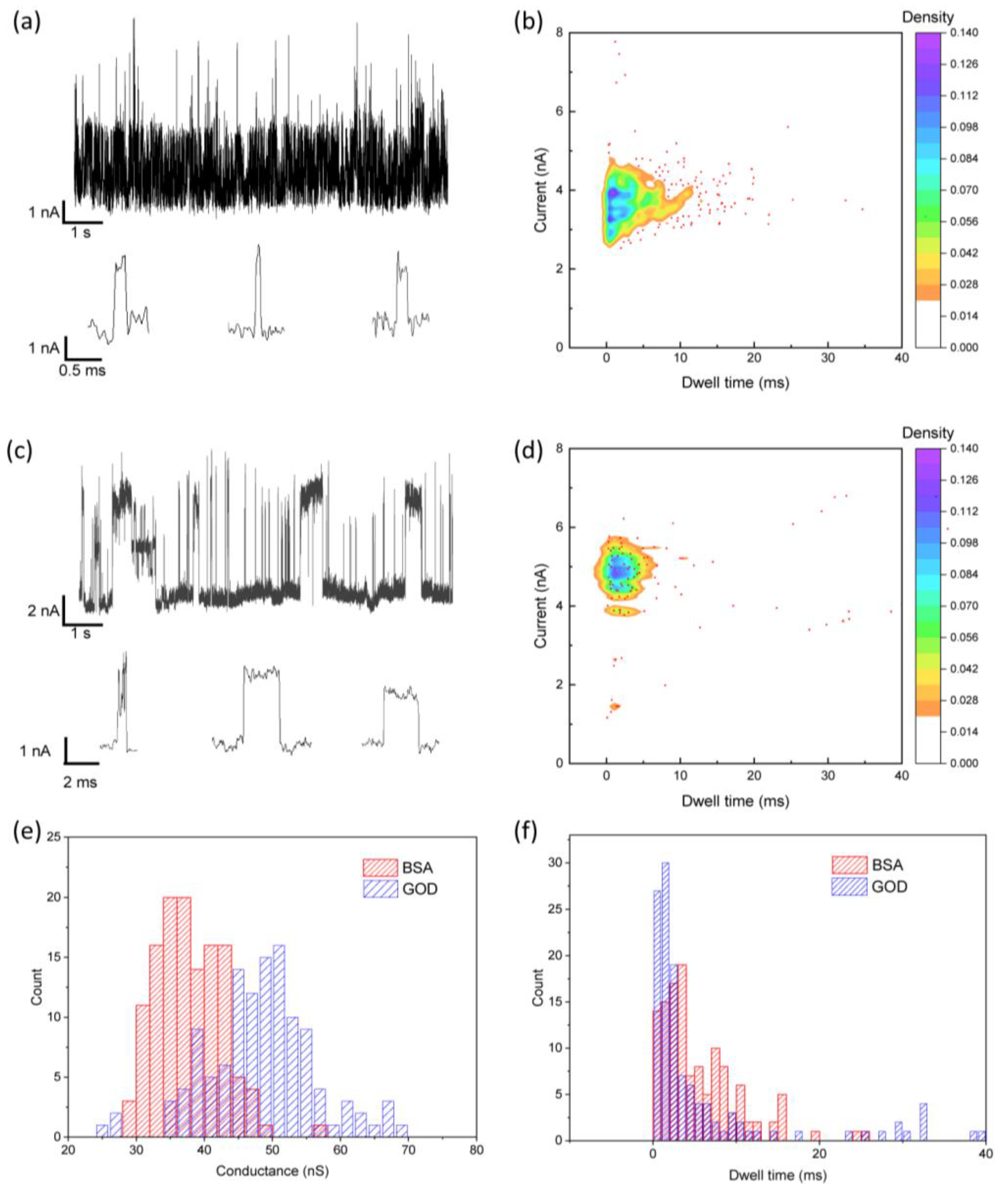
3.3. Single-Molecule Detection Using Etched-Tunneling Electrodes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, C.; Jia, C.; Guo, X. Single-Molecule Electrical Detection with Real-Time Label-Free Capability and Ultrasensitivity. Small Methods 2017, 1, 1700071. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, X.; Tao, N. Emerging Tools for Studying Single Entity Electrochemistry. Faraday Discuss. 2016, 193, 9–39. [Google Scholar] [CrossRef] [PubMed]
- Mathwig, K.; Aartsma, T.J.; Canters, G.W.; Lemay, S.G. Nanoscale Methods for Single-Molecule Electrochemistry. Annu. Rev. Anal. Chem. 2014, 7, 383–404. [Google Scholar] [CrossRef]
- Albrecht, T. Electrochemical Tunneling Sensors and Their Potential Applications. Nat. Commun. 2012, 3, 829. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Nadappuram, B.P.; Cadinu, P.; Zhao, Z.; Xue, L.; Yi, L.; Ren, R.; Wang, J.; Ivanov, A.P.; Edel, J.B. Combined Quantum Tunneling and Dielectrophoretic Trapping for Molecular Analysis at Ultra-Low Analyte Concentrations. Nat. Commun. 2021, 12, 913. [Google Scholar] [CrossRef]
- Di Ventra, M.; Taniguchi, M. Decoding DNA, RNA and Peptides with Quantum Tunneling. Nat. Nanotech 2016, 11, 117–126. [Google Scholar] [CrossRef]
- Xin, N.; Guan, J.; Zhou, C.; Chen, X.; Gu, C.; Li, Y.; Ratner, M.A.; Nitzan, A.; Stoddart, J.F.; Guo, X. Concepts in the Design and Engineering of Single-Molecule Electronic Devices. Nat. Rev. Phys. 2019, 1, 211–230. [Google Scholar] [CrossRef]
- Simmons, J.G. Generalized Formula for the Electric Tunnel Effect between Similar Electrodes Separated by a Thin Insulating Film. J. Appl. Phys. 1963, 34, 1793–1803. [Google Scholar] [CrossRef]
- Xu, B.; Tao, N.J. Measurement of Single-Molecule Resistance by Repeated Formation of Molecular Junctions. Science 2003, 301, 1221–1223. [Google Scholar] [CrossRef]
- Li, X.-M.; Wang, Y.-H.; Seng, J.-W.; Zheng, J.-F.; Cao, R.; Shao, Y.; Chen, J.-Z.; Li, J.-F.; Zhou, X.-S.; Mao, B.-W. Z-Piezo Pulse-Modulated STM Break Junction: Toward Single-Molecule Rectifiers with Dissimilar Metal Electrodes. ACS Appl. Mater. Interfaces 2021, 13, 8656–8663. [Google Scholar] [CrossRef]
- Tsutsui, M.; Shoji, K.; Taniguchi, M.; Kawai, T. Formation and Self-Breaking Mechanism of Stable Atom-Sized Junctions. Nano Lett. 2008, 8, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Xiang, D.; Jeong, H.; Lee, T.; Mayer, D. Mechanically Controllable Break Junctions for Molecular Electronics. Adv. Mater. 2013, 25, 4845–4867. [Google Scholar] [CrossRef]
- Vrouwe, S.A.G.; Van Der Giessen, E.; Van Der Molen, S.J.; Dulic, D.; Trouwborst, M.L.; Van Wees, B.J. Mechanics of Lithographically Defined Break Junctions. Phys. Rev. B 2005, 71, 035313. [Google Scholar] [CrossRef]
- Pan, R.; Yang, Y.; Wang, Y.; Li, S.; Liu, Z.; Su, Y.; Quan, B.; Li, Y.; Gu, C.; Li, J. Nanocracking and Metallization Doubly Defined Large-Scale 3D Plasmonic Sub-10 Nm-Gap Arrays as Extremely Sensitive SERS Substrates. Nanoscale 2018, 10, 3171–3180. [Google Scholar] [CrossRef] [PubMed]
- Missault, N.; Schwarzacher, W. Bridging the Nanogap: Au Electrodeposition at Parallel Electrodes with NM-Scale Spacing. J. Electrochem. Soc. 2021, 168, 082511. [Google Scholar] [CrossRef]
- Naitoh, Y.; Tani, Y.; Koyama, E.; Nakamura, T.; Sumiya, T.; Ogawa, T.; Misawa, G.; Shima, H.; Sugawara, K.; Suga, H.; et al. Single-Molecular Bridging in Static Metal Nanogap Electrodes Using Migrations of Metal Atoms. J. Phys. Chem. C 2020, 124, 14007–14015. [Google Scholar] [CrossRef]
- Manheller, M.; Trellenkamp, S.; Waser, R.; Karthäuser, S. Reliable Fabrication of 3 Nm Gaps between Nanoelectrodes by Electron-Beam Lithography. Nanotechnology 2012, 23, 125302. [Google Scholar] [CrossRef]
- Li, H.; Wani, I.H.; Hayat, A.; Jafri, S.H.M.; Leifer, K. Fabrication of Reproducible Sub-5 Nm Nanogaps by a Focused Ion Beam and Observation of Fowler-Nordheim Tunneling. Appl. Phys. Lett. 2015, 107, 103108. [Google Scholar] [CrossRef]
- Nagase, T.; Gamo, K.; Kubota, T.; Mashiko, S. Direct Fabrication of Nano-Gap Electrodes by Focused Ion Beam Etching. Thin Solid. Film. 2006, 499, 279–284. [Google Scholar] [CrossRef]
- Sun, L.F.; Chin, S.N.; Marx, E.; Curtis, K.S.; Greenham, N.C.; Ford, C.J.B. Shadow-Evaporated Nanometre-Sized Gaps and Their Use in Electrical Studies of Nanocrystals. Nanotechnology 2005, 16, 631–634. [Google Scholar] [CrossRef]
- Dubois, V.; Bleiker, S.J.; Stemme, G.; Niklaus, F. Scalable Manufacturing of Nanogaps. Adv. Mater. 2018, 30, 1801124. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, W.; Zhu, D. Nanogap Electrodes. Adv. Mater. 2010, 22, 286–300. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Tang, L. Sub-5 Nm Nanogap Electrodes towards Single-Molecular Biosensing. Biosens. Bioelectron. 2022, 213, 114486. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Li, H.B.; Domulevicz, L.; Hihath, J. An On-Chip Break Junction System for Combined Single-Molecule Conductance and Raman Spectroscopies. Adv. Funct. Mater. 2020, 30, 2000615. [Google Scholar] [CrossRef]
- Zhang, S.; Guo, C.; Ni, L.; Hans, K.M.; Zhang, W.; Peng, S.; Zhao, Z.; Guhr, D.C.; Qi, Z.; Liu, H.; et al. In-Situ Control of on-Chip Angstrom Gaps, Atomic Switches, and Molecular Junctions by Light Irradiation. Nano Today 2021, 39, 101226. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Yin, K.; Zhang, S.; Zhao, Z.; Tan, M.; Xu, X.; Zhao, Z.; Wang, M.; Xu, B.; et al. In Situ Adjustable Nanogaps and In-Plane Break Junctions. Small Methods 2023, 7, 2201427. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Kazuma, E.; Yokota, Y.; Kim, Y. Fabrication of Sharp Gold Tips by Three-Electrode Electrochemical Etching with High Controllability and Reproducibility. J. Phys. Chem. C 2018, 122, 16950–16955. [Google Scholar] [CrossRef]
- Wang, X.; Cui, Y.; Ren, B. Fabrication of Au tips for tip-enhanced Raman spectroscopy. Chem. J. Chin. Univ.-Chin. 2007, 28, 522–525. [Google Scholar]
- Ren, B.; Picardi, G.; Pettinger, B. Preparation of Gold Tips Suitable for Tip-Enhanced Raman Spectroscopy and Light Emission by Electrochemical Etching. Rev. Sci. Instrum. 2004, 75, 837–841. [Google Scholar] [CrossRef]
- Takami, T.; Kitamura, R.; Hiramoto, T.; Oki, S.; Yoshioka, K.; Aoyama, Y. Automatic Shutdown System of Alternating Current Electrochemical Etching for the Preparation of a Platinum/Iridium Tip for Scanning Tunneling Microscopy and the Investigation of the Byproduct of Platinum Chloride Particles. Jpn. J. Appl. Phys. 2019, 58, SIIC05. [Google Scholar] [CrossRef]
- Tahmasebipour, G.; Hojjat, Y.; Ahmadi, V.; Abdullah, A. Optimization of STM/FIM Nanotip Aspect Ratio Based on the Taguchi Method. Int. J. Adv. Manuf. Technol. 2009, 44, 80–90. [Google Scholar] [CrossRef]
- Mao, B.; Ren, B.; Cai, X.; Xiong, L. Electrochemical Oscillatory Behavior Under a Scanning Electrochemical Microscopic Configuration. J. Electroanal. Chem. 1995, 394, 155–160. [Google Scholar] [CrossRef]
- Penner, R.M.; Heben, M.J.; Lewis, N.S. Preparation and Electrochemical Characterization of Conical and Hemispherical Ultramicroelectrodes. Anal. Chem. 1989, 61, 1630–1636. [Google Scholar] [CrossRef]
- Hugelmann, M.; Schindler, W. Tunnel Barrier Height Oscillations at the Solid/Liquid Interface. Surf. Sci. 2003, 541, L643–L648. [Google Scholar] [CrossRef]
- Prokopuk, N.; Son, K.-A.; Waltz, C. Electron Tunneling through Fluid Solvents. J. Phys. Chem. C 2007, 111, 6533–6537. [Google Scholar] [CrossRef]
- Taniguchi, M. Single-Molecule Analysis Methods Using Nanogap Electrodes and Their Application to DNA Sequencing Technologies. Bull. Chem. Soc. Jpn. 2017, 90, 1189–1210. [Google Scholar] [CrossRef]
- Zwolak, M.; Di Ventra, M. Electronic Signature of DNA Nucleotides via Transverse Transport. Nano Lett. 2005, 5, 421–424. [Google Scholar] [CrossRef]
- Ron, I.; Sepunaru, L.; Itzhakov, S.; Belenkova, T.; Friedman, N.; Pecht, I.; Sheves, M.; Cahen, D. Proteins as Electronic Materials: Electron Transport through Solid-State Protein Monolayer Junctions. J. Am. Chem. Soc. 2010, 132, 4131–4140. [Google Scholar] [CrossRef]
- Ron, I.; Pecht, I.; Sheves, M.; Cahen, D. Proteins as Solid-State Electronic Conductors. Acc. Chem. Res. 2010, 43, 945–953. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, B.; He, Q.; Jiang, T.; Zeng, B.; Kuang, C.; Liu, X.; Tang, L. Electrochemical Etching-Assisted Fabrication of Quantum Tunneling Sensing Probes with Controlled Nanogap Width. Chemosensors 2023, 11, 480. https://doi.org/10.3390/chemosensors11090480
Shao B, He Q, Jiang T, Zeng B, Kuang C, Liu X, Tang L. Electrochemical Etching-Assisted Fabrication of Quantum Tunneling Sensing Probes with Controlled Nanogap Width. Chemosensors. 2023; 11(9):480. https://doi.org/10.3390/chemosensors11090480
Chicago/Turabian StyleShao, Bangrui, Qiuxiang He, Tao Jiang, Biaofeng Zeng, Cuifang Kuang, Xu Liu, and Longhua Tang. 2023. "Electrochemical Etching-Assisted Fabrication of Quantum Tunneling Sensing Probes with Controlled Nanogap Width" Chemosensors 11, no. 9: 480. https://doi.org/10.3390/chemosensors11090480
APA StyleShao, B., He, Q., Jiang, T., Zeng, B., Kuang, C., Liu, X., & Tang, L. (2023). Electrochemical Etching-Assisted Fabrication of Quantum Tunneling Sensing Probes with Controlled Nanogap Width. Chemosensors, 11(9), 480. https://doi.org/10.3390/chemosensors11090480






