Progress on Electrochemical Sensing of Pharmaceutical Drugs in Complex Biofluids
Abstract
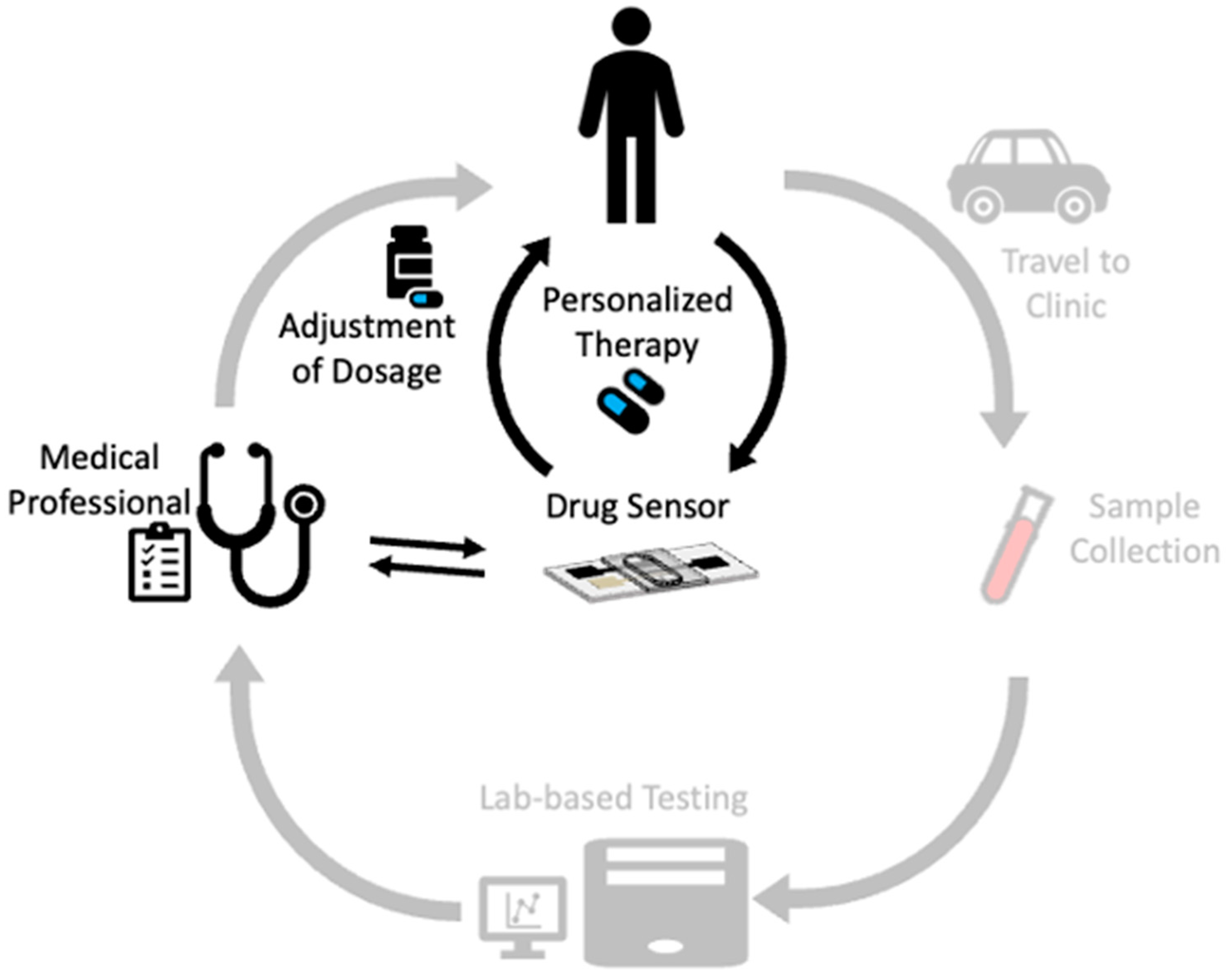
1. Introduction
2. Background
3. Advances in the Electrochemical Detection of Pharmaceuticals in Blood-Based Biofluids
| Drug and Health Condition | Electrochemical Method/Base Working Electrode/Sensing Mechanism | Complex Biofluid | Strategies to Improve Electrochemical Signal | Performance Metrics (Note that Metrics are Provided for Each of the Targets in the Order Listed in Column 1) | Ref. |
|---|---|---|---|---|---|
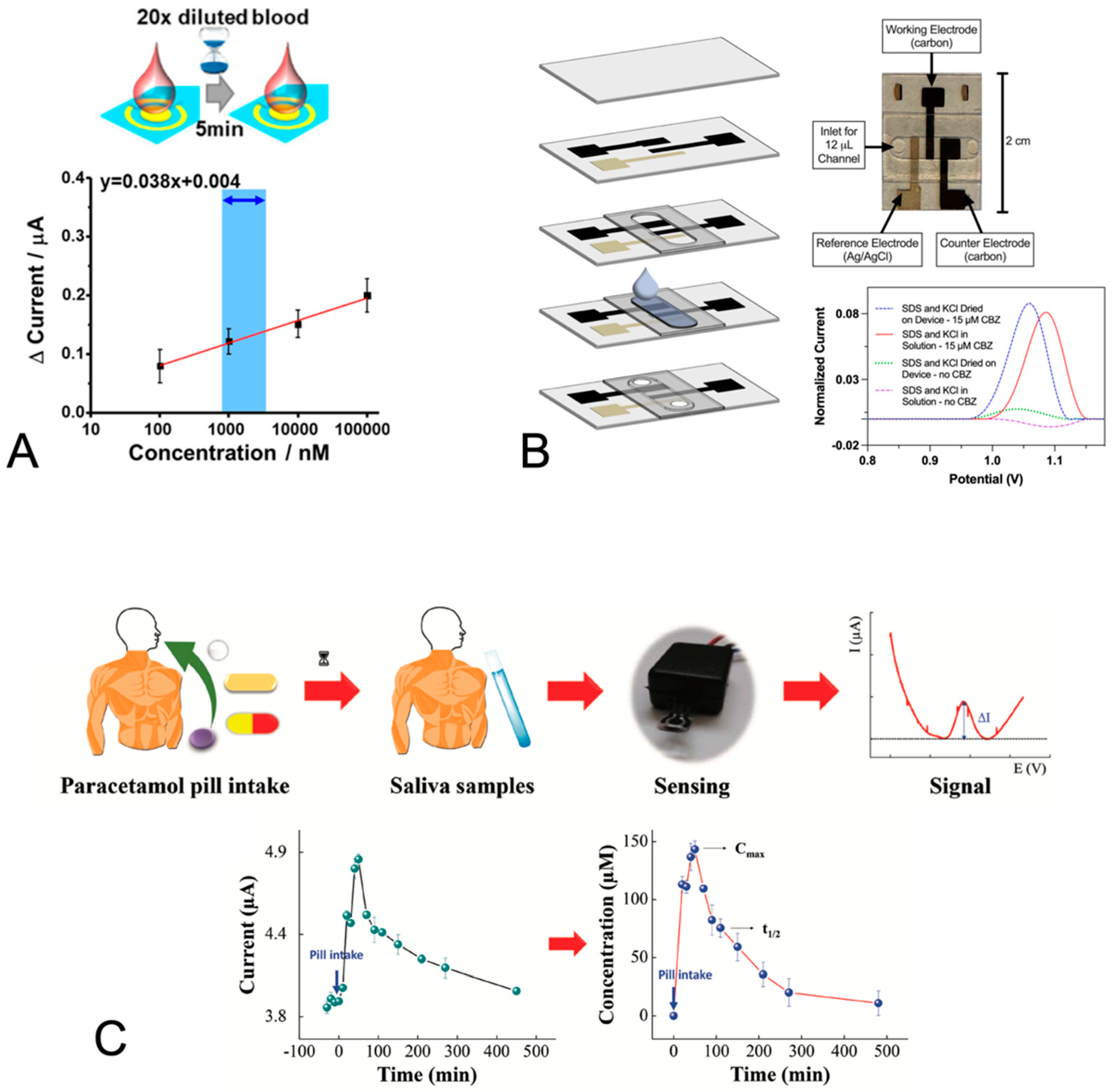
| Carbamazepine to treat epilepsy seizures | SWV; gold electrode; aptamer binding to carbamazepine reduces the distance between the methylene blue tag and the electrode and increases the current signal | Fingerstick blood-diluted 20-fold | High packing density of aptamer for increased sensitivity to the target | LOD 2.1 nM in serum for 5 min and linearly over the 17 to 51 µM therapeutic range | [61] |
| Daclatasvir, sofosbuvir, and ledipasvir for the treatment of hepatitis C | DPV; glassy carbon electrodes; oxidation of each of the drugs | Serum-diluted 200-fold | Multi-walled carbon nanotubes in an ionic liquid crystal and cobalt nanoparticles | LODs 1.9 nM, 7.3 nM, 0.28 nM; linear DRs of 0.07 to 1 µM and 5 to 50 µM, 0.3 to 8 µM and 10 to 100 µM, 0.02 to 1 µM and 3 to 100 µM; recoveries of 99.7 to 102.8% for ledipasvir | [66] |
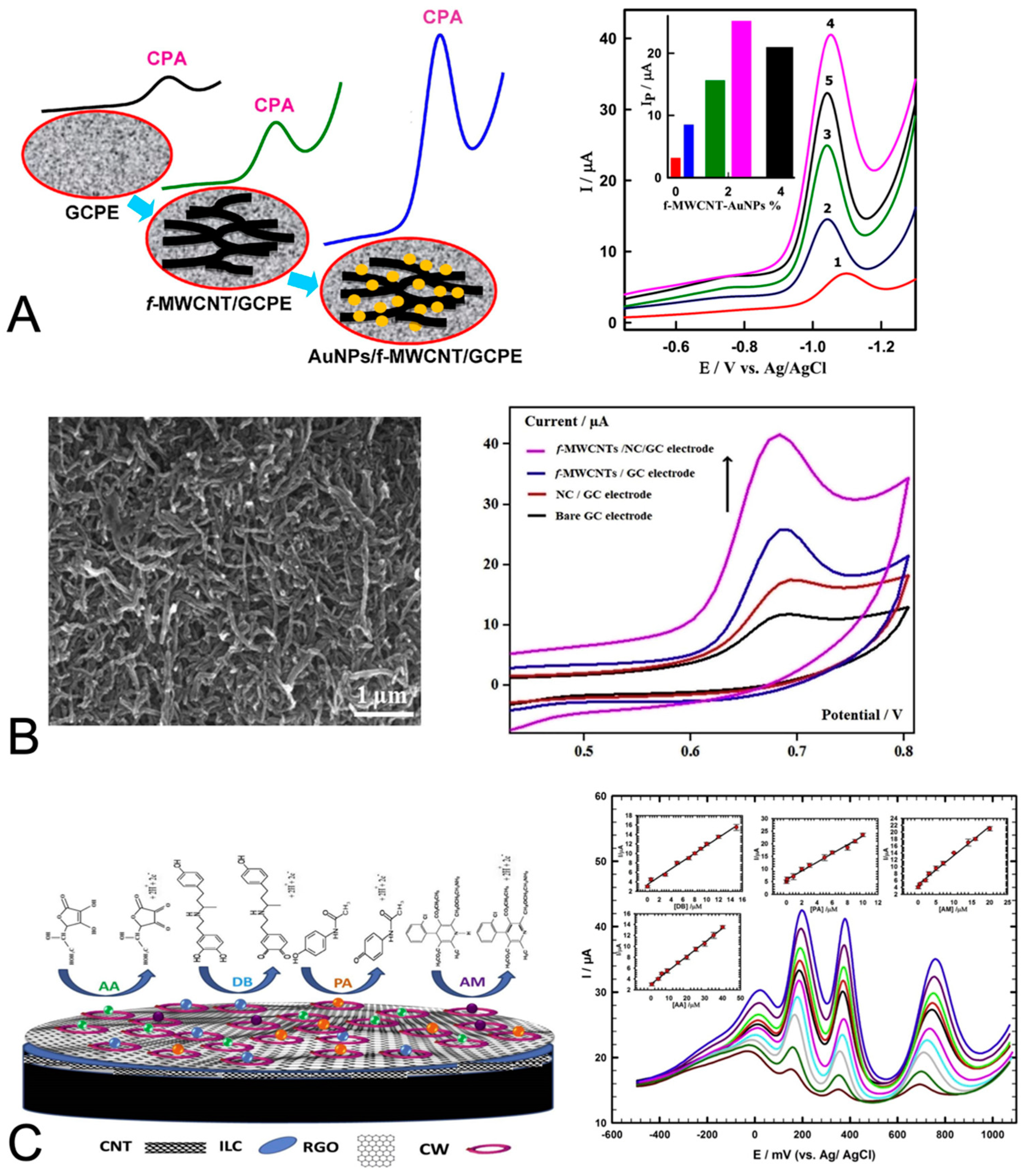
| Cyproterone acetate for the treatment of prostate cancer | SWV; glassy carbon paste electrodes; reduction of cyproterone acetate | Serum-proteins separated out with ethanol precipitation and centrifugation | Multi-walled carbon nanotubes and gold nanoparticles | LOD 17.7 nM; linear DR of 99 nM to 8.3 µM; sensitivity 117 µA/µM per cm2 | [64] |
| Regorafenib for the treatment of hepatocellular carcinoma | DPV; glassy carbon electrodes; oxidation of regorafenib | Serum-diluted 50-fold | Zirconium oxide nanoparticles and reduced graphene oxide | LOD 17 nM in buffer (not reported in serum); linear DR of 11 to 343 nM in buffer; recoveries of 97.2 to 102.6% | [40] |
| Doxorubicin and dasatinib for treatment of breast cancer | CA and SWV; carbon paste electrodes; oxidation of drugs | Serum-handling was not described | Zinc oxide nanoparticles and butyl-3-methylimidazolium tetrafluoroborate and liquid paraffin | LOD 9 nM and 0.5 µM in buffer (not reported in serum); linear DR of 0.07 to 500 µM, 9.0 nM to 0.5 µM in buffer; recoveries of 98.1 to 102.3% | [67] |
| Acetaminophen and etilefrine (with dopamine) | DPV; glassy carbon electrodes; oxidation of each of small molecule | Serum-10-fold dilution followed by another 125-fold dilution | Platinum-nickel nanoparticles and reduced graphene oxide | LOD 8.2 µM, 14.9 µM, 0.0025 µM in buffer (not reported in serum); linear DR of 4.0 to 60 µM, 4.0 to 100 µM, 0.05 to 0.5 µM in buffer; recoveries of 95 to 108% | [68] |
| Dobutamine and amlodipine for the treatment of cardiac issues and acetaminophen and ascorbic acid | DPV; glassy carbon electrodes; oxidation of each species | Serum-20-fold dilution | Composite of multi-walled carbon nanotubes, ionic liquid crystal, graphene and 18-Crown-6 enables the simultaneous detection of four species | LOD 0.50 nM, 0.14 nM, 0.09 nM, 9.2 nM; linear DR of 0.02 to 40 µM, 0.008 to 30 µM, 0.001 to 20 µM, 0.4 to 40 µM; recoveries of 97.1 to 102.7% | [62] |
| Acetaminophen (with tryptophan and caffeine) | DPV; glassy carbon electrodes; oxidation of each species | Serum-25-fold dilution | Tin sulfide and titanium dioxide on graphene oxide sheets | LOD 7.5 nM, 7.8 nM, 4.4 nM in buffer (not reported in serum); linear DR of 9.8 nM to 280 µM, 13 nM to 157 µM, 16 nM to 333 µM in buffer; recoveries of 98% and 99% for acetaminophen | [69] |
| Diclofenac sodium as an analgesic and anti-inflammatory for arthritis and other conditions | DPV and CV; glassy carbon electrodes; oxidation of diclofenac sodium | Serum-filtered and diluted 10-fold | Nanocellulose and multi-walled carbon nanotubes | LOD 0.12 µM in buffer (not reported in serum); linear DR of 0.05 to 1 µM in buffer; recoveries of 99.3 to 102.0% | [65] |
| Doxorubicin and dasatinib for the treatment of breast cancer | AdSSWV; glassy carbon electrodes; oxidation of each drug | Serum-10-fold dilution | Palladium and platinum nanoparticles with multi-walled carbon nanotubes | LOD 0.86 nM, 6.72 nM in buffer (not reported in serum); linear DR of 4.4 nM to 8.6 µM, 38 nM to 9.9 µM in buffer; recoveries of 99.1 to 100.6% | [70] |
| N-acetylcysteine for multiple indications | DPV; carbon paste electrodes; oxidation of each drug | Serum-10-fold or greater | Silica nanoparticles and boron trifluoride and 4,4′-dihydroxybiphenyl | LOD 0.33 µM in buffer (not reported in serum); linear DRs of 1.0 to 41.5 µM and 41.5 to 101.5 µM in buffer; agreed to within 1% of HPLC | [71] |
| Chloroquine to treat malaria, rheumatoid arthritis, and cancer | CV and DPV; glassy carbon electrodes; oxidation of chloroquine | Serum-5-fold dilution | Tungsten disulfide quantum dots with reduced graphene oxide | LOD 0.04 µM; linear DR of 0.5 to 82 µM | [72] |
| Olanzapine for the treatment of schizophrenia | Potentiometric measurement and carbon paste electrodes | Serum-10-fold dilution | Olanzapine- tungstophosphate | LOD 0.5 µM in buffer (not reported in serum); linear DR of 0.75 to 560 µM in buffer; recoveries of 97.8 to 101.6% | [39] |
| Epinephrine to treat allergic reactions, cardiac arrest, and hypertension | DPV; glassy carbon electrodes; oxidation of drugs | Serum-proteins separated out with ethanol precipitation and centrifugation | Zinc oxide nanoparticles and multi-walled carbon nanotubes | LOD 0.016 µM in buffer (not reported in serum); linear DR of 0.4 to 2.4 µM in buffer; recoveries of 100.4 to 101.3% | [41] |
| Azithromycin for the treatment of bacterial infections | DPV; glassy carbon electrodes; oxidation of azithromycin | Plasma-filtered using 0.45 µm filter and diluted 10-fold | Molecularly imprinted polymer | LOD 0.85 nM in buffer (not reported in serum); linear DR of 13 nM to 67 µM in buffer; recovery of 102.4% | [73] |
| Epirubicin and methotrexate for breast cancer treatment | DPV; glassy carbon electrodes; oxidation of each drug | Serum-filtered using 0.45 µm filter and diluted 5-fold | Zinc oxide nanoflowers doped with cerium | LOD 2.3 nM, 6.3 nM in buffer (not reported in serum); linear DR of 0.01 to 600 µM, 0.01 to 500 µM in buffer; recoveries of 98.0 to 102.3% | [74] |
| Rifampicin to treat bacterial infections | DPV; glassy carbon electrodes; oxidation of drugs | Serum-indicated dilution of 3-fold | Titanium dioxide nanoparticles on reduced graphene oxide | LOD 0.03 µM in buffer (not reported in serum); linear DR of 0.01 to 0.1 nM in buffer; recoveries of 95 to 100% | [75] |
| Levofloxacin for treating bacterial infections | Potentiometric measurement; carbon paste electrodes | Serum-diluted 25-fold | PVC coating | LOD 10 µM in buffer (not reported in serum); linear DR of 10−2 to 10−4 M in buffer; recoveries of 95.6 to 98.7% for CPE | [76] |
| Mefenamic acid, a non-steroidal anti-inflammatory drug | CV and DPV; carbon paste electrodes; oxidation of mefenamic acid | Serum-handling not described | Copper vanadium oxide nanostructures (Cu5V2O10) | LOD 2.3 nM in buffer (not reported in serum); linear DR of 0.01 to 470 µM in buffer; recoveries of 98.3 to 110% | [77] |
| Mefenamic acid, a non-steroidal anti-inflammatory drug | CV and DPV; carbon paste electrodes; oxidation of mefenamic acid | Serum-handling not described | Terbium titanate nanostructures (Tb2Ti2O7) | LOD 2.4 nM in buffer (not reported in serum); linear DR of 0.01 to 400 µM in buffer; recoveries of 92.0 to 107% | [78] |
4. Advances in the Electrochemical Detection of Pharmaceuticals in Alternative Fluids
4.1. Detection of Analyte Drugs in Saliva
4.2. Detection of Analyte Drugs in Sweat
4.3. Detection of Analyte Drugs in Interstitial Fluid
4.4. Detection of Analyte Drugs in Urine
| Drug and Health Condition | Electrochemical Method/Base Working Electrode/Sensing Mechanism | Complex Biofluid | Strategies to Improve Electrochemical Signal | Performance Metrics (Note that Metrics are Provided for Each of the Targets in the Order Listed in Column 1) | Ref. |
|---|---|---|---|---|---|
| Interferon gamma for treating cancer and infections | Amperometry; screen-printed carbon electrodes treated with p-ABA diazonium salt to immobilize capture Ab; reduction of benzoquinone from an enzymatic reaction of label HRP, hydroquinone, and H2O2 | Saliva collected with a Salivette and then extracted using centrifugation | Optimization of parameters including the capture Ab concentration and the concentrations of detected Ab and enzymes | LOD 1.6 pg/mL in buffer; linear DR of 2.5 to 2000 pg/mL in buffer; in saliva, measurements agreed with ELISA to within 3% | [97] |
| Carbamazepine to treat epilepsy seizures | SWV; stencil-printed carbon electrodes; carbamazepine oxidation | Saliva—pooled, commercially purchased | Sodium dodecyl sulfate in solution | LOD 1 µM; average QR of 0.85 µM from 0 to 15 µM | [79] |
| SWV; stencil-printed carbon electrodes; carbamazepine oxidation | Saliva—pooled, commercially purchased | Sodium dodecyl sulfate film on electrodes | LOD 1 µM; average QR of 1.6 µM from 0 to 15 µM for field-use format sensor | [80] | |
| Acetaminophen/ paracetamol as an analgesic | DPV; oxygen-terminated boron-doped diamond electrode; oxidation of acetaminophen | Saliva and sweat—saliva was processed by centrifugation before use | Hydrogen- terminated boron-doped diamond electrode with a Nafion layer | LOD 1 µM; strong correlation in saliva (R2 = 0.92) and sweat (R2 = 0.95) with LC-MS/MS | [82] |
| Acetaminophen/ paracetamol to manage pain | DPV; screen-printed carbon electrodes; oxidation of acetaminophen | Saliva, unprocessed | Electrochemical pretreatment consisting of cyclic voltammetry of 0.5 M sulfuric acid increased the electrode conductivity and signal | LOD 14.5 µM; linear DR 25 to 150 µM | [81] |
| Benzodiazepine for the treatment of depression, anxiety, and insomnia | DPV; laser-scribed graphene electrodes functionalized with Ab capture; oxidation current change with Ab-Ag binding | Saliva collected with a swab and extracted by centrifugation | Optimization of capture of the Ab concentration and blocking agent treatment | LOD 9.7 ng/mL in buffer; DR of 1.0 pg/mL to 500 ng/mL in buffer; simultaneous detection with amphetamine and cocaine in saliva | [98] |
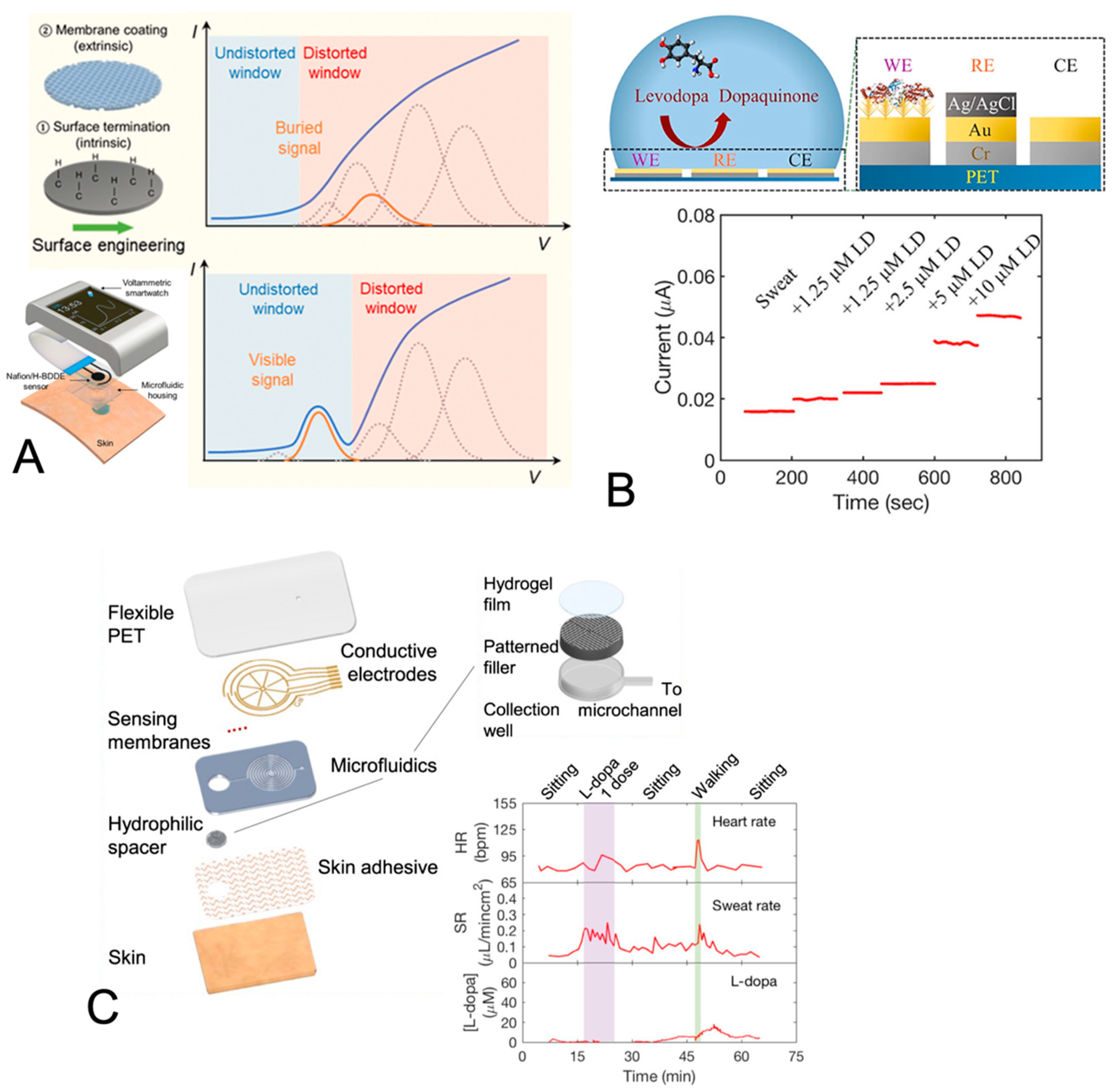
| Levodopa for the treatment of Parkinson’s disease | Amperometry; gold-coated electrodes with tyrosinase; tyrosinase-catalyzed oxidation of levodopa | Simulated sweat using iontophoretic stimulation or exercise | Gold nanodendrite structures on gold electrodes and Nafion film | LOD 1 µM; DR of 0 to 20 µM; sensitivity of 17 nA/µM | [83] |
| Amperometry; gold-coated electrodes with tyrosinase; tyrosinase-catalyzed oxidation of levodopa | Sweat generated at rest | Gold nanodendrite structures on the gold electrodes and the Nafion-TBAB film | LOD 3 µM in buffer; linear DR of 0 to 50 µM in buffer; in situ sweat analysis | [84] | |
| Levodopa for treatment of Parkinson’s disease | Amperometry; screen-printed carbon electrodes coated with crosslinked tyrosinase; dopaquinone reduction | Fingertip sweat using a touch sensor | Note that an advantage to their method is its robustness to fouling of the electrode via unintended quinone polymerization reactions | LOD 300 nM in buffer; linear DR of 1 to 30 µM in buffer; in situ sweat analysis | [85] |
| Acetaminophen/ paracetamol to manage pain and paroxetine as an antidepressant | DPV; screen-printed carbon electrodes | Artificial sweat and human sweat combined with artificial sweat in equal parts | Pretreatment consisting of cyclic voltammetry of 0.5 M sulfuric acid | LOD 0.25 µM, 0.49 µM in artificial sweat; recoveries of 106% and 112% in artificial sweat | [86] |
| Levodopa for treatment of Parkinson’s disease | SWV and CA; carbon paste electrodes with tyrosinase in microneedles; oxidation of levodopa | Artificial interstitial fluid | Nafion | LOD 0.5 µM in artificial ISF; linear DR of 0.5 to 3 µM in artificial ISF | [88] |
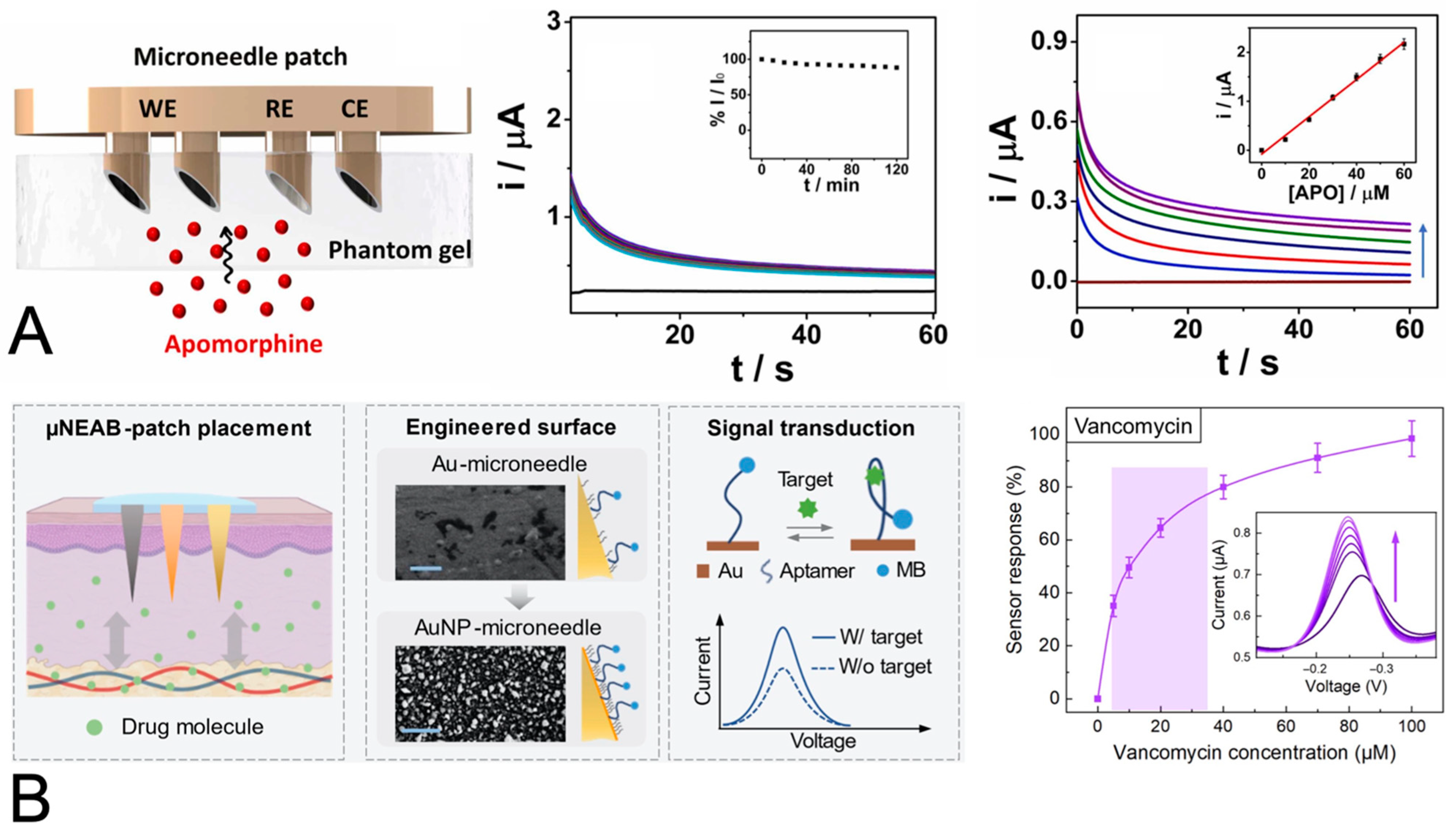
| Apomorphine for treatment of Parkinson’s disease | SWV and CA; carbon paste electrodes in microneedles; apomorphine oxidation | Artificial interstitial fluid containing protein interferents | 2% rhodium nanoparticles and 1% Nafion film for stability against protein interferents | LOD 0.6 µM/0.75 µM (SWA/CA) in buffer; linear DR of 10 to 60 µM in the skin mimic model; sensitivity of 3.8 nA/µM in the skin mimic model | [89] |
| Tobramycin and vancomycin for bacterial infections | SWV; gold coating of an acupuncture needle; the binding of aptamer to the antibiotic reduces the distance between the methylene blue tag and the electrode and increases the current signal | Interstitial fluid— in vivo on rat | Gold nanoparticle coating enhances the signal compared to evaporated gold film | Response curves from 0 to 100 µM for each in artificial ISF; correlation between blood and ISF tobramycin levels in mice | [90] |
| Cyproterone acetate for the treatment of prostate cancer | SWV; glassy carbon paste electrodes; reduction of cyproterone acetate | Urine—diluted with buffer | Multi-walled carbon nanotubes and gold nanoparticles | LOD 17.9 nM; linear DR of 99 nM to 5.0 µM; sensitivity 173 µA/µM per cm2 | [64] |
| Diclofenac sodium as an analgesic and anti-inflammatory for arthritis and other conditions | DPV; glassy carbon electrodes; oxidation of diclofenac sodium | Urine—diluted 4-fold | Nanocellulose and multi-walled carbon nanotubes | LOD 0.12 µM in buffer (not reported in urine); linear DR of 0.05 to 1 µM in buffer; recoveries of 98.0 to 104.0% | [65] |
| Doxorubicin and dasatinib for the treatment of breast cancer | AdSSWV; glassy carbon electrodes; oxidation of each drug | Urine—10-fold dilution | Palladium and platinum nanoparticles with multi-walled carbon nanotubes | LOD 0.86 nM, 6.72 nM in buffer (not reported in urine); linear DR of 4.4 nM to 8.6 µM, 38 nM to 9.9 µM in buffer; recoveries of 98.8 to 99.5% | [70] |
| Diclofenac sodium as an analgesic and anti-inflammatory for arthritis and other conditions | DPV; screen-printed carbon electrodes; oxidation of diclofenac sodium | Urine—centrifuged to remove solids | Platinum nanoflowers with reduced graphene oxide facilitated additional analyte on the electrode and improved electron transfer | LOD 40 nM in buffer (not reported in urine); linear DR 0.1 to 100 µM in buffer; recoveries of 84 to 105% | [92] |
| Azithromycin for the treatment of bacterial infections | DPV; glassy carbon electrodes; oxidation of azithromycin | Urine and tears— filtered using an 0.45 µm filter and diluted 10-fold | Molecularly imprinted polymer | LOD 0.85 nM in buffer (not reported in urine); linear DR of 13 nM to 67 µM in buffer; recoveries of 98.0 to 106.3% | [73] |
| Epirubicin and methotrexate for breast cancer treatment | DPV; glassy carbon electrodes; oxidation of each drug | Urine—filtered using 0.45 µm filter and diluted 5-fold | Zinc oxide nanoflowers doped with cerium | LOD 2.3 nM, 6.3 nM in buffer (not reported in urine); linear DR of 0.01 to 600 µM, 0.01 to 500 µM in buffer; recoveries of over 97.1 to 102.6% | [74] |
| Levofloxacin for treating bacterial infections | Potentiometric measurement; carbon paste electrodes | Urine—diluted 25-fold | PVC coating | LOD 10 µM in buffer (not reported in urine); linear DR of 10−2 to 10−4 M in buffer; recoveries of 94.5 to 98.4% for CPE | [76] |
| Triamterene as a diuretic | CV, CA, SWV; boron-doped diamond electrodes; reduction of triamterene | Pooled and individual urine | Note that the electrode choice provides resistance to biofouling, stability, and a relatively large potential window | LOD 7.80 nM pooled and 20.8 nM individual urine | [91] |
| Nalbuphine as an analgesic | Potentiometric measurement; screen-printed carbon electrodes | Urine—diluted 10-fold | Composite of polyaniline with multi-walled carbon nanotubes and PVC with molecularly imprinted polymer beads | LOD 11 µM in buffer (not reported in urine); linear DR of 43 to 3300 µM in buffer; recoveries of 91.0 to 101.5% | [94] |
| Methotrexate for cancer treatment | DPV; screen-printed graphite electrodes; oxidation of methotrexate and folic acid | Urine—centrifuged, supernatant filtered using 0.45 µm filter and diluted at least 2.5-fold | Composite of iron oxide and polypyrrole and palladium | LOD 7.0 nM in buffer (not reported in urine); linear DR of 0.03 to 100 µM in buffer; recoveries of 97.8 to 103.1% | [93] |
| Sulfanilamide for the treatment of bacterial infections | CV and DPV; 3D printed carbon black-PLA electrodes; oxidation of sulfanilamide | Artificial urine—diluted 10-fold | Electrode pretreatment of NaOH solution at 1.4 V and −1 V for 200 s each | LOD 12 nM in buffer; linear DR of 1 to 39 µM in buffer; recoveries of 99.1 to 101.9% in synthetic urine | [99] |
| Trimethoprim for the treatment of bacterial infections | DPV; carbon paste electrodes with iron oxide and multi-walled carbon nanotubes; oxidation of trimethoprim | Urine—centrifuged and supernatant analyzed | Layered structure consisting of base electrode material and reduced graphene oxide and molecularly imprinted polymer with iron oxide and multi-walled carbon nanotubes | LOD 1.2 nM in buffer (not reported in urine); linear DRs of 0.004 to 0.08 µM and 0.08 to 500 µM in buffer; recoveries of 95.0 to 110.0% | [95] |
| Aminophylline for the treatment of bronchial asthma | DPV; glassy carbon electrodes; oxidation of aminophylline | Urine—filtered and diluted | Molecularly imprinted polymer and graphene oxide | LOD 2.1 pM in buffer (not reported in urine); linear DR of 37 pM to 1 mM in buffer; recoveries of 98.2 to 99.6% | [96] |
| Ketoconazole for the treatment of fungal infections | DPV, CV, CA; carbon paste electrodes; oxidation of ketoconazole | Urine—centrifuged, filtered, and diluted | Metal–organic framework composed of cerium and 1,3,5 benzene tricarboxylic acid and ionic liquid | LOD 0.04 µM in buffer; linear DR of 0.1 to 110 µM in buffer; recoveries of 96.7 to 102.0% | [100] |
5. Summary and Ongoing Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanavio, B.; Krol, S. On the slow diffusion of point-of-care systems in therapeutic drug monitoring. Front. Bioeng. Biotechnol. 2015, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Sardini, E.; Serpelloni, M.; Tonello, S. Printed Electrochemical Biosensors: Opportunities and Metrological Challenges. Biosensors 2020, 10, 166. [Google Scholar] [CrossRef]
- Zabihollahpoor, A.; Rahimnejad, M.; Najafpour-Darzi, G.; Moghadamnia, A.A. Recent advances in electroanalytical methods for the therapeutic monitoring of antiepileptic drugs: A comprehensive review. J. Pharm. Biomed. Anal. 2020, 188, 113394. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D.; Ong, J.J.; Goyanes, A.; Orlu, M.; Gaisford, S.; Elbadawi, M.; Basit, A.W. Electrochemical biosensors: A nexus for precision medicine. Drug Discov. Today 2021, 26, 69–79. [Google Scholar] [CrossRef]
- Mobed, A.; Shirafkan, M.; Charsouei, S.; Sadeghzadeh, J.; Ahmadalipour, A. Biosensors technology for anti-epileptic drugs. Clin. Chim. Acta 2022, 533, 175–182. [Google Scholar] [CrossRef]
- Ozbek, O.; Berkel, C.; Isildak, O. Applications of Potentiometric Sensors for the Determination of Drug Molecules in Biological Samples. Crit. Rev. Anal. Chem. 2022, 52, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Siwal, S.S.; Chauhan, G.; Saini, A.K.; Kumari, A.; Thakur, V.K. Recent advances in electrochemical-based sensors amplified with carbon-based nanomaterials (CNMs) for sensing pharmaceutical and food pollutants. Chemosphere 2022, 304, 135182. [Google Scholar] [CrossRef]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping healthcare with wearable biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef]
- Caldevilla, R.; Morais, S.L.; Cruz, A.; Delerue-Matos, C.; Moreira, F.; Pacheco, J.G.; Santos, M.; Barroso, M.F. Electrochemical Chemically Based Sensors and Emerging Enzymatic Biosensors for Antidepressant Drug Detection: A Review. Int. J. Mol. Sci. 2023, 24, 8480. [Google Scholar] [CrossRef]
- Ensom, M.H.H.; Davis, G.A.; Cropp, C.D.; Ensom, R.J. Clinical pharmacokinetics in the 21st century—Does the evidence support definitive outcomes? Clin. Pharmacokinet. 1998, 34, 265–279. [Google Scholar] [CrossRef]
- Seiden, L.G.; Connor, G.S. The importance of drug titration in the management of patients with epilepsy. Epilepsy Behav. 2022, 128, 108517. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M.; Bicchi, M.M. Antiseizure Medications for Adults with Epilepsy A Review. J. Am. Med. Assoc. 2022, 327, 1269–1281. [Google Scholar] [CrossRef]
- Perucca, E.; Meador, K.J. Adverse effects of antiepileptic drugs. Acta Neurol. Scand. 2005, 112, 30–35. [Google Scholar] [CrossRef]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef] [PubMed]
- St. Louis, E.K.; Minimizing, A.E.D. Adverse Effects: Improving Quality of Life in the Interictal State in Epilepsy Care. Curr. Neuropharmacol. 2009, 7, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Mahrer-Imhof, R.; Jaggi, S.; Bonomo, A.; Hediger, H.; Eggenschwiler, P.; Kramer, G.; Oberholzer, E. Quality of life in adult patients with epilepsy and their family members. Seizure Eur. J. Epilepsy 2013, 22, 128–135. [Google Scholar] [CrossRef]
- Kubota, H.; Awaya, Y. Assessment of health-related quality of life and influencing factors using QOLIE-31 in Japanese patients with epilepsy. Epilepsy Behav. 2010, 18, 381–387. [Google Scholar] [CrossRef]
- Perucca, E. Overtreatment in epilepsy: Adverse consequences and mechanisms. Epilepsy Res. 2002, 52, 25–33. [Google Scholar] [CrossRef]
- Landmark, C.J.; Johannessen, S.I.; Patsalos, P.N. Therapeutic drug monitoring of antiepileptic drugs: Current status and future prospects. Expert Opin. Drug Metab. Toxicol. 2020, 16, 227–238. [Google Scholar] [CrossRef]
- Zucker, I.; Prendergast, B.J. Sex differences in pharmacokinetics predict adverse drug reactions in women. Biol. Sex Differ. 2020, 11, 32. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex Differences in Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar]
- Adab, N.; Chadwick, D.W. Management of women with epilepsy during pregnancy. Obstet. Gynaecol. 2006, 8, 20–25. [Google Scholar] [CrossRef]
- Patel, S.I.; Pennell, P.B. Management of epilepsy during pregnancy: An update. Ther. Adv. Neurol. Disord. 2016, 9, 118–129. [Google Scholar] [CrossRef]
- Thomas, S.V. Management of epilepsy and pregnancy. J. Postgrad. Med. 2006, 52, 57–64. [Google Scholar]
- Schoretsanitis, G.; Paulzen, M.; Unterecker, S.; Schwarz, M.; Conca, A.; Zernig, G.; Gründer, G.; Haen, E.; Baumann, P.; Bergemann, N.; et al. TDM in psychiatry and neurology: A comprehensive summary of the consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology, update 2017, a tool for clinicians. World J. Biol. Psychiatry 2018, 19, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Grundmann, M.; Kacirova, I.; Urinovska, R. Therapeutic monitoring of psychoactive drugs–Antidepressants: A review. Biomed. Pap. 2015, 159, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.A.; Norris, R.; Paterson, D.L.; Martin, J.H. Therapeutic drug monitoring of antimicrobials. Br. J. Clin. Pharmacol. 2012, 73, 27–36. [Google Scholar] [PubMed]
- Mallon, P.W.G.; Ray, J.; Cooper, D.A. Effect of therapeutic drug monitoring on outcome in antiretroviral experienced HIV-infected individuals. J. Clin. Virol. 2003, 26, 223–227. [Google Scholar] [CrossRef]
- Pargas, C.D.; Elhessy, A.H.; Abouei, M.; Gesheff, M.G.; Conway, J.D. Tobramycin Blood Levels after Local Antibiotic Treatment of Bone and Soft Tissue Infection. Antibiotics 2022, 11, 336. [Google Scholar] [CrossRef]
- Boyle, A.; Ondo, W. Role of Apomorphine in the Treatment of Parkinson’s Disease. CNS Drugs 2015, 29, 83–89. [Google Scholar] [CrossRef]
- Oertel, W.H.; Quinn, N.P. Parkinson’s disease: Drug therapy. Bailliere’s Clin. Neurol. 1997, 6, 89–108. [Google Scholar]
- Almalki, R.S.; Eweis, H.; Kamal, F.; Kutbi, D. Methotrexate Toxicity: Molecular Mechanisms and Management. J. Pharm. Res. Int. 2021, 33, 204–217. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Edison, T.; Velmurugan, B.K.; Jacob, J.A.; Karuppusamy, I. Toxicity of Doxorubicin (Dox) to different experimental organ systems. Life Sci. 2018, 200, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Brunner, A.M.; Costa, D.B.; Heist, R.S.; Garcia, E.; Lindeman, N.I.; Sholl, L.M.; Oxnard, G.R.; Johnson, B.E.; Hammerman, P.S. Treatment-Related Toxicities in a Phase II Trial of Dasatinib in Patients with Squamous Cell Carcinoma of the Lung. J. Thorac. Oncol. 2013, 8, 1434–1437. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.M.; Lima, I.S.F.; Baracos, V.E.; Bies, R.R.; McCargar, L.J.; Reiman, T.; Mackey, J.R.; Kuzma, M.; Damaraju, V.L.; Sawyer, M.B. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother. Pharmacol. 2011, 67, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Giordano, N.; Nardi, P.; Santacroce, C.; Geraci, S.; Gennari, C. Acute hepatitis induced by cyproterone acetate. Ann. Pharmacother. 2001, 35, 1053–1055. [Google Scholar] [CrossRef]
- Sastre, J.; Argiles, G.; Benavides, M.; Feliu, J.; Garcia-Alfonso, P.; Garcia-Carbonero, R.; Grávalos, C.; Guillén-Ponce, C.; Martínez-Villacampa, M.; Pericay, C. Clinical management of regorafenib in the treatment of patients with advanced colorectal cancer. Clin. Transl. Oncol. 2014, 16, 942–953. [Google Scholar] [CrossRef]
- Burgess, S.; Partovi, N.; Yoshida, E.M.; Erb, S.R.; Azalgara, V.M.; Hussaini, T. Drug Interactions with Direct-Acting Antivirals for Hepatitis C: Implications for HIV and Transplant Patients. Ann. Pharmacother. 2015, 49, 674–687. [Google Scholar] [CrossRef]
- Rouhani, M.; Soleymanpour, A. A new selective carbon paste electrode for potentiometric analysis of olanzapine. Measurement 2019, 140, 472–478. [Google Scholar]
- Venu, M.; Venkateswarlu, S.; Reddy, Y.V.M.; Seshadri Reddy, A.; Gupta, V.K.; Yoon, M.; Madhavi, G. Highly Sensitive Electrochemical Sensor for Anticancer Drug by a Zirconia Nanoparticle-Decorated Reduced Graphene Oxide Nanocomposite. ACS Omega 2018, 3, 14597–14605. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Madhusudana Reddy, T.; Venu Gopal, T.; Venkataprasad, G.; Kotakadi, V.S.; Palakollu, V.N.; Karpoormath, R. A simple sonochemical assisted synthesis of nanocomposite (ZnO/MWCNTs) for electrochemical sensing of Epinephrine in human serum and pharmaceutical formulation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124038. [Google Scholar] [CrossRef]
- Jarrar, Q.B.; Hakim, M.N.; Zakaria, Z.A.; Cheema, M.S.; Moshawih, S. Renal ultrastructural alterations induced by various preparations of mefenamic acid. Ultrastruct. Pathol. 2020, 44, 130–140. [Google Scholar] [CrossRef]
- Aslan, M.; Kirimlioglu, E.; Afsar, E.; Ceker, T.; Yilmaz, C. Increased PUFA levels in kidney epithelial cells in the course of diclofenac toxicity. Toxicol. Vitr. 2020, 66, 104836. [Google Scholar] [CrossRef]
- Moling, O.; Cairon, E.; Rimenti, G.; Rizza, F.; Pristera, R.; Mian, P. Severe hepatotoxicity after therapeutic doses of acetaminophen. Clin. Ther. 2006, 28, 755–760. [Google Scholar] [CrossRef]
- Mazer, M.; Perrone, J. Acetaminophen-Induced Nephrotoxicity: Pathophysiology, Clinical Manifestations, and Management. J. Med. Toxicol. 2008, 4, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Nicolle, E.; Devillier, P.; Delanoy, B.; Durand, C.; Bessard, G. Therapeutic monitoring of nalbuphine: Transplacental transfer and estimated pharmacokinetics in the neonate. Eur. J. Clin. Pharmacol. 1996, 49, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Starakis, I.; Lekkou, A.; Blikas, A.; Labropoulou-Karatza, C. Drug-induced cardiotoxicity due to aminophylline treatment: A case report. Curr. Ther. Res. Clin. Exp. 2003, 64, 367–374. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kiang, T.K.L.; Ranamukhaarachchi, S.A.; Ensom, M.H.H. Revolutionizing Therapeutic Drug Monitoring with the Use of Interstitial Fluid and Microneedles Technology. Pharmaceutics 2017, 9, 43. [Google Scholar] [CrossRef]
- Chiu, M.L.; Lawi, W.; Snyder, S.T.; Wong, P.K.; Liao, J.C.; Gau, V. Matrix Effects—A Challenge Toward Automation of Molecular Analysis. JALA Charlottesv. Va. 2010, 15, 233–242. [Google Scholar] [CrossRef]
- Kaczor-Urbanowicz, K.E.; Carreras-Presas, C.M.; Aro, K.; Tu, M.; Garcia-Godoy, F.; Wong, D.T.W. Saliva diagnostics—Current views and directions. Exp. Biol. Med. 2017, 242, 459–472. [Google Scholar] [CrossRef]
- de Castro, M.D.L. Sweat as a clinical sample: What is done and what should be done. Bioanalysis 2016, 8, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J.; Cheng, Y.; Chen, J.; Zhao, H.; Ren, X. Urine Analysis has a Very Broad Prospect in the Future. Front. Anal. Sci. 2022, 1, 812301. [Google Scholar] [CrossRef]
- Patsalos, P.N.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs by Use of Saliva. Ther. Drug Monit. 2013, 35, 4–29. [Google Scholar] [PubMed]
- Patsalos, P.N.; Spencer, E.P.; Berry, D.J. Therapeutic Drug Monitoring of Antiepileptic Drugs in Epilepsy: A 2018 Update. Ther. Drug Monit. 2018, 40, 526–548. [Google Scholar] [CrossRef]
- Vasudev, A.; Tripathi, K.D.; Puri, V. Correlation of serum and salivary carbamazepine concentration in epileptic patients: Implications for therapeutic drug monitoring. Neurol. India. 2002, 50, 60–62. [Google Scholar]
- Tsiropoulos, I.; Kristensen, O.; Klitgaard, N.A. Saliva and serum concentration of lamotrigine in patients with epilepsy. Ther. Drug Monit. 2000, 22, 517–521. [Google Scholar] [CrossRef]
- Meearelli, O.; Voti, P.L.; Pro, S.; Romolo, F.S.; Rotolo, M.; Pulitano, P.; Accornero, N.; Vanacore, N. Saliva and serum levetiracetam concentrations in patients with epilepsy. Ther. Drug Monit. 2007, 29, 313–318. [Google Scholar] [CrossRef]
- Li, R.R.; Sheng, X.Y.; Ma, L.Y.; Yao, H.X.; Cai, L.X.; Chen, C.Y.; Zhu, S.N.; Zhou, Y.; Wu, Y.; Cui, Y.M. Saliva and Plasma Monohydroxycarbamazepine Concentrations in Pediatric Patients with Epilepsy. Ther. Drug Monit. 2016, 38, 365–370. [Google Scholar] [CrossRef]
- Chiappin, S.; Antonelli, G.; Gatti, R.; De Palo, E.F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef]
- Diao, X.; Huestis, M.A. Approaches, Challenges, and Advances in Metabolism of New Synthetic Cannabinoids and Identification of Optimal Urinary Marker Metabolites. Clin. Pharmacol. Therapeutics. 2017, 101, 239–253. [Google Scholar] [CrossRef]
- Chung, S.; Singh, N.K.; Gribkoff, V.K.; Hall, D.A. Electrochemical Carbamazepine Aptasensor for Therapeutic Drug Monitoring at the Point of Care. ACS Omega 2022, 7, 39097–39106. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Galal, A.; Ahmed, Y.M.; El-Ads, E.H. Design strategy and preparation of a conductive layered electrochemical sensor for simultaneous determination of ascorbic acid, dobutamine, acetaminophen and amlodipine. Sens. Actuators B Chem. 2019, 297, 126648. [Google Scholar] [CrossRef]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ibrahim, H.; Almandil, N.; Kawde, A.-N. Gold nanoparticles/f-MWCNT nanocomposites modified glassy carbon paste electrode as a novel voltammetric sensor for the determination of cyproterone acetate in pharmaceutical and human body fluids. Sens. Actuators B Chem. 2018, 274, 123–132. [Google Scholar] [CrossRef]
- Shalauddin, M.; Akhter, S.; Basirun, W.J.; Bagheri, S.; Anuar, N.S.; Johan, M.R. Hybrid nanocellulose/f-MWCNTs nanocomposite for the electrochemical sensing of diclofenac sodium in pharmaceutical drugs and biological fluids. Electrochim. Acta 2019, 304, 323–333. [Google Scholar] [CrossRef]
- Atta, N.F.; Galal, A.; Ahmed, Y.M. Electrochemical Method for the Determination of Three New Anti-Hepatitis C Drugs: Application in Human Blood Serum. J. Electrochem. Soc. 2018, 165, B442. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.R.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous determination of doxorubicin and dasatinib as two breast anticancer drugs uses an amplified sensor with ionic liquid and ZnO nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Veera Manohara Reddy, Y.; Sravani, B.; Maseed, H.; Łuczak, T.; Osińska, M.; SubramanyamSarma, L.; Srikanth, V.V.S.S.; Madhavi, G. Ultrafine Pt–Ni bimetallic nanoparticles anchored on reduced graphene oxide nanocomposites for boosting electrochemical detection of dopamine in biological samples. New J. Chem. 2018, 42, 16891–16901. [Google Scholar] [CrossRef]
- Murugan, E.; Kumar, K. Fabrication of SnS/TiO2@GO Composite Coated Glassy Carbon Electrode for Concomitant Determination of Paracetamol, Tryptophan, and Caffeine in Pharmaceutical Formulations. Anal. Chem. 2019, 91, 5667–5676. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Li, Y.; Shen, Y.; Huang, Y. Mesoporous Pd@Pt core–shell nanoparticles supported on multi-walled carbon nanotubes as a sensing platform: Application in simultaneous electrochemical detection of anticancer drugs doxorubicin and dasatinib. Anal. Methods 2019, 11, 443–453. [Google Scholar] [CrossRef]
- Farahani, K.Z.; Benvidi, A.; Rezaeinasab, M.; Abbasi, S.; Abdollahi-Alibeik, M.; Rezaeipoor-Anari, A.; Zarchi, M.A.K.; Abadi, S.S.A.D.M. Potentiality of PARAFAC approaches for simultaneous determination of N-acetylcysteine and acetaminophen based on the second-order data obtained from differential pulse voltammetry. Talanta 2019, 192, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Tiwari, P.; Mall, V.K.; Srivastava, S.K.; Prakash, R. Voltammetric determination of the antimalarial drug chloroquine using a glassy carbon electrode modified with reduced graphene oxide on WS2 quantum dots. Microchim. Acta. 2019, 186, 415. [Google Scholar] [CrossRef] [PubMed]
- Stoian, I.-A.; Iacob, B.-C.; Dudaș, C.-L.; Barbu-Tudoran, L.; Bogdan, D.; Marian, I.O.; Bodoki, E.; Oprean, R. Biomimetic electrochemical sensor for the highly selective detection of azithromycin in biological samples. Biosens. Bioelectron. 2020, 155, 112098. [Google Scholar] [CrossRef] [PubMed]
- Jandaghi, N.; Jahani, S.; Foroughi, M.M.; Kazemipour, M.; Ansari, M. Cerium-doped flower-shaped ZnO nano-crystallites as a sensing component for simultaneous electrochemical determination of epirubicin and methotrexate. Microchim. Acta 2019, 187, 24. [Google Scholar] [CrossRef]
- Veera Manohara Reddy, Y.; Sravani, B.; Łuczak, T.; Mallikarjuna, K.; Madhavi, G. An ultra-sensitive rifampicin electrochemical sensor based on titanium nanoparticles (TiO2) anchored reduced graphene oxide modified glassy carbon electrode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125533. [Google Scholar] [CrossRef]
- Abdel-Haleem, F.M.; Gamal, E.; Rizk, M.S.; Madbouly, A.; El Nashar, R.M.; Anis, B.; Elnabawy, H.M.; Khalil, A.S.G.; Barhoum, A. Molecularly Imprinted Electrochemical Sensor-Based Fe2O3@MWCNTs for Ivabradine Drug Determination in Pharmaceutical Formulation, Serum, and Urine Samples. Front. Bioeng. Biotechnol. 2021, 9, 648704. [Google Scholar] [CrossRef]
- Monsef, R.; Salavati-Niasari, M. Hydrothermal architecture of Cu5V2O10 nanostructures as new electro-sensing catalysts for voltammetric quantification of mefenamic acid in pharmaceuticals and biological samples. Biosens. Bioelectron. 2021, 178, 113017. [Google Scholar] [CrossRef]
- Valian, M.; Khoobi, A.; Salavati-Niasari, M. Synthesis, characterization and electrochemical sensors application of Tb2Ti2O7 nanoparticle modified carbon paste electrode for the sensing of mefenamic acid drug in biological samples and pharmaceutical industry wastewater. Talanta 2022, 247, 123593. [Google Scholar] [CrossRef]
- Wentland, L.; Downs, C.; Fu, E. Comparison of signal enhancement strategies for carbamazepine detection in undiluted human saliva using an electrochemical sensor with stencil-printed carbon electrodes. Anal. Methods 2022, 14, 3103–3114. [Google Scholar] [CrossRef]
- Wentland, L.; Cook, J.M.; Minzlaff, J.; Ramsey, S.A.; Johnston, M.L.; Fu, E. Field-use device for the electrochemical quantification of carbamazepine levels in a background of human saliva. J. Appl. Electrochem. 2023, 53, 523–534. [Google Scholar] [CrossRef]
- Gomes, N.O.; Raymundo-Pereira, P.A. On-Site Therapeutic Drug Monitoring of Paracetamol Analgesic in Non-Invasively Collected Saliva for Personalized Medicine. Small 2023, 19, e2206753. [Google Scholar] [CrossRef]
- Lin, S.Y.; Yu, W.Z.; Wang, B.; Zhao, Y.C.; En, K.; Zhu, J.L.; Cheng, X.; Zhou, C.; Lin, H.; Wang, Z.; et al. Noninvasive wearable electroactive pharmaceutical monitoring for personalized therapeutics. Proc. Natl. Acad. Sci. USA 2020, 117, 19017–19025. [Google Scholar]
- Tai, L.C.; Liaw, T.S.; Lin, Y.J.; Nyein, H.Y.Y.; Bariya, M.; Ji, W.B.; Hettick, M.; Zhao, C.; Zhao, J.; Hou, L.; et al. Wearable Sweat Band for Noninvasive Levodopa Monitoring. Nano Lett. 2019, 19, 6346–6351. [Google Scholar] [CrossRef] [PubMed]
- Nyein, H.Y.Y.; Bariya, M.; Tran, B.; Ahn, C.H.; Brown, B.J.; Ji, W.B.; Davis, N.; Javey, A. A wearable patch for continuous analysis of thermoregulatory sweat at rest. Nat. Commun. 2021, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.M.; Teymourian, H.; De la Paz, E.; Sempionatto, J.R.; Mahato, K.; Sonsa-ard, T.; Huang, N.; Longardner, K.; Litvan, I.; Wang, J. Non-Invasive Sweat-Based Tracking of L-Dopa Pharmacokinetic Profiles Following an Oral Tablet Administration. Angew. Chem. Int. Ed. 2021, 60, 19074–19078. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N., Jr. Wearable glove-embedded sensors for therapeutic drug monitoring in sweat for personalized medicine. Chem. Eng. J. 2022, 435, 135047. [Google Scholar] [CrossRef]
- Windmiller, J.R.; Zhou, N.; Chuang, M.-C.; Valdés-Ramírez, G.; Santhosh, P.; Miller, P.R.; Narayan, R.; Wang, J. Microneedle array-based carbon paste amperometric sensors and biosensors. Analyst 2011, 136, 1846–1851. [Google Scholar] [CrossRef]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable Electrochemical Microneedle Sensor for Continuous Monitoring of Levodopa: Toward Parkinson Management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Goud, K.Y.; Mahato, K.; Teymourian, H.; Longardner, K.; Litvan, I.; Wang, J. Wearable electrochemical microneedle sensing platform for real-time continuous interstitial fluid monitoring of apomorphine: Toward Parkinson management. Sens. Actuators B Chem. 2022, 354, 131234. [Google Scholar] [CrossRef]
- Lin, S.; Cheng, X.; Zhu, J.; Wang, B.; Jelinek, D.; Zhao, Y.; Wu, T.-Y.; Horrillo, A.; Tan, J.; Yeung, J.; et al. Wearable microneedle-based electrochemical aptamer biosensing for precision dosing of drugs with narrow therapeutic windows. Sci. Adv. 2022, 8, eabq4539. [Google Scholar] [CrossRef]
- Ishii, K.; Ogata, G.; Einaga, Y. Electrochemical detection of triamterene in human urine using boron-doped diamond electrodes. Biosens. Bioelectron. 2022, 217, 114666. [Google Scholar] [CrossRef] [PubMed]
- Kimuam, K.; Rodthongkum, N.; Ngamrojanavanich, N.; Chailapakul, O.; Ruecha, N. Single step preparation of platinum nanoflowers/reduced graphene oxide electrode as a novel platform for diclofenac sensor. Microchem. J. 2020, 155, 104744. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Shahsavari, S.; Nejad, F.G. Simultaneous and selective electrochemical sensing of methotrexate and folic acid in biological fluids and pharmaceutical samples using Fe3O4/ppy/Pd nanocomposite modified screen printed graphite electrode. Chemosphere 2022, 291, 132736. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.S.M.; Kamel, A.H.; Fathy, M.A. A novel screen-printed potentiometric electrode with carbon nanotubes/polyaniline transducer and molecularly imprinted polymer for the determination of nalbuphine in pharmaceuticals and biological fluids. Anal. Chim. Acta 2022, 1227, 340239. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, R.; Zheng, L.; Cao, Q. An Electrochemical Sensor for Trimethoprim Based on a Magnetic Molecularly Imprinted Carbon Paste Electrode. Chemosensors 2023, 11, 339. [Google Scholar] [CrossRef]
- Saher, A.; Bahgat, A.; Molouk, A.; Mortada, W.; Khalifa, M. MIP/GO/GCE Sensor for the Determination of Aminophylline in Pharmaceutical Ingredients and Urine Samples. Anal. Bioanal. Chem. Research. 2023, 10, 435–443. [Google Scholar]
- Sánchez-Tirado, E.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical immunosensor for the determination of the cytokine interferon gamma (IFN-γ) in saliva. Talanta 2020, 211, 120761. [Google Scholar] [CrossRef]
- Beduk, D.; Beduk, T.; de Oliveira Filho, J.I.; Ait Lahcen, A.; Aldemir, E.; Guler Celik, E.; Salama, K.N.; Timur, S. Smart Multiplex Point-of-Care Platform for Simultaneous Drug Monitoring. ACS Appl. Mater. Interfaces 2023, 15, 37247–37258. [Google Scholar] [CrossRef]
- Lisboa, T.P.; Alves, G.F.; de Faria, L.V.; de Souza, C.C.; Matos, M.A.C.; Matos, R.C. 3D-printed electrode an affordable sensor for sulfanilamide monitoring in breast milk, synthetic urine, and pharmaceutical formulation samples. Talanta 2022, 247, 123610. [Google Scholar] [CrossRef]
- Tajik, S.; Sharifi, F.; Aflatoonian, B.; Di Bartolomeo, A. A New Electrochemical Sensor for the Detection of Ketoconazole Using Carbon Paste Electrode Modified with Sheaf-like Ce-BTC MOF Nanostructure and Ionic Liquid. Nanomaterials 2023, 13, 523. [Google Scholar] [CrossRef]
- Tachi, T.; Kaji, N.; Tokeshi, M.; Baba, Y. Simultaneous Separation, Metering, and Dilution of Plasma from Human Whole Blood in a Microfluidic System. Anal. Chem. 2009, 81, 3194–3198. [Google Scholar] [CrossRef] [PubMed]
- Helton, K.L.; Nelson, K.E.; Fu, E.; Yager, P. Conditioning saliva for use in a microfluidic biosensor. Lab. Chip. 2008, 8, 1847–1851. [Google Scholar] [CrossRef] [PubMed]
- Kava, A.A.; Beardsley, C.; Hofstetter, J.; Henry, C.S. Disposable glassy carbon stencil printed electrodes for trace detection of cadmium and lead. Anal. Chim. Acta 2020, 1103, 58–66. [Google Scholar] [CrossRef]
- Chen, Z.; Patel, R.; Berry, J.; Keyes, C.; Satterfield, C.; Simmons, C.; Neeson, A.; Cao, X.; Wu, Q. Development of Screen-Printable Nafion Dispersion for Electrochemical Sensor. Appl. Sci. 2022, 12, 6533. [Google Scholar] [CrossRef]
| Class | Example(s) from the Studies Reviewed | Motivation for Drug Monitoring |
|---|---|---|
| Drugs to suppress seizures in the treatment of epilepsy | Carbamazepine | Highly variable pharmacokinetics; strong interactions with other common drugs; and/or high toxicity [13,14] |
| Drugs to treat bacterial or fungal infections | Tobramycin; vancomycin; levofloxacin; rifampicin; azithromycin; sulfanilamide; trimethoprim; ketoconazole | Potential for kidney injury for tobramycin [29] |
| Drugs to treat Parkinson’s disease | Apomorphine; levodopa | Side effects of nausea for apomorphine [30] and decreased efficacy and increased motor disturbances with use for levodopa [31] |
| Drugs to treat depression | Paroxetine; benzodiazepines | Highly variable pharmacokinetics with a longer time for clearance with aging and renal/hepatic damage for paroxetine [26] |
| Drugs to treat cancer | Methotrexate; doxorubicin; dasatinib; epirubicin; cyproterone acetate; regorafenib; interferon gamma | Pulmonary and hepatotoxicity for methotrexate [32]; cardiotoxicity for doxorubicin [33]; adverse effects include dyspnea, fatigue, nausea for dasatinib [34]; hepatotoxicity for epirubicin [35]; hepatotoxicity for cyproterone acetate [36]; adverse effects include dyspnea, fatigue, nausea for regorafenib [37] |
| Drugs to treat hepatitis C viral infection | Daclatasvir; sofosbuvir; ledipasvir | Potential adverse drug–drug interactions for transplant and HIV patients [38] |
| Drugs to treat psychiatric disorders | Olanzapine | Side effects of overdose such as nausea, slurred speech, vomiting, damage to the aorta resulting in bleeding or death [39] |
| Drugs to treat cardiac conditions | Etilefrine; epinephrine | Overdosing on etilefrine can cause heart failure, hypertension, and erectile dysfunction [40]; epinephrine has interactions with other common compounds [41] |
| Anti-inflammatory and analgesic | Mefenamic acid; diclofenac sodium | Potential for renal toxicity [42,43] |
| Analgesic | Acetaminophen/ paracetamol; nalbuphine | Hepatotoxicity [44] and nephrotoxicity [45] for acetaminophen; potential for TDM in neonates for nalbuphine [46] |
| Drugs for the treatment of bronchial asthma | Aminophylline | Potential for drug-induced cardiotoxicity [47] |
| Complex Biofluid | Major Components | Advantages | Disadvantages |
|---|---|---|---|
| Blood | Ions, proteins, glucose, amino acids, lipids, hormones, erythrocytes, leukocytes, platelets [49] | Gold standard; uniform across individuals; small fingerstick volumes (20 µL) are compatible with point-of-care collection | Invasive and painful; larger venipuncture volumes require a phlebotomist, which is inconvenient and limited to low-frequency collection |
| Serum | Ions, proteins, glucose, amino acids, lipids, hormones [49] | ||
| Saliva | Ions, small molecules, proteins, mucins, hormones, blood-derived compounds, food debris, uric acid [59] | Noninvasive; moderate (1 mL) volume; easy to sample; frequent donation possible and on demand; could be compatible with continuous wearable device | Properties variable across individuals; variability throughout the day for individuals including pH; possible food contamination |
| Sweat | Ions, small molecules, proteins, pyruvate, lactate urea, antigens, antibodies, ethanol [51] | Noninvasive; compatible with continuous wearable devices | Low secretion rate (10 to 100 nL/min per cm2) volume unless stimulated; variability in the rate secreted; possible contamination from cosmetics or the environment |
| Urine | Inorganic salts, urea, uric acid, proteins, enzymes, nucleic acids, vitamins, hormones, amino acids, mesothelin, beta-microglobulin, antibiotics, urokinase, mycomycin [52] | Noninvasive; large (many mL) volume; easy to sample; there may be a longer time window available for drug detection compared to other biological matrices such as saliva or blood [60] | Sampling is not always possible ‘on demand’; contamination potential if the collection is not conducted carefully [60] |
| Interstitial fluid | Amino acids, carbohydrates, fatty acids [48] | Noninvasive; compatible with continuous wearable devices | Very small (nL) volume unless suction used |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, E.; Khederlou, K.; Lefevre, N.; Ramsey, S.A.; Johnston, M.L.; Wentland, L. Progress on Electrochemical Sensing of Pharmaceutical Drugs in Complex Biofluids. Chemosensors 2023, 11, 467. https://doi.org/10.3390/chemosensors11080467
Fu E, Khederlou K, Lefevre N, Ramsey SA, Johnston ML, Wentland L. Progress on Electrochemical Sensing of Pharmaceutical Drugs in Complex Biofluids. Chemosensors. 2023; 11(8):467. https://doi.org/10.3390/chemosensors11080467
Chicago/Turabian StyleFu, Elain, Khadijeh Khederlou, Noël Lefevre, Stephen A. Ramsey, Matthew L. Johnston, and Lael Wentland. 2023. "Progress on Electrochemical Sensing of Pharmaceutical Drugs in Complex Biofluids" Chemosensors 11, no. 8: 467. https://doi.org/10.3390/chemosensors11080467
APA StyleFu, E., Khederlou, K., Lefevre, N., Ramsey, S. A., Johnston, M. L., & Wentland, L. (2023). Progress on Electrochemical Sensing of Pharmaceutical Drugs in Complex Biofluids. Chemosensors, 11(8), 467. https://doi.org/10.3390/chemosensors11080467






