Real-Time Monitoring of H2O2 Sterilization on Individual Bacillus atrophaeus Spores by Optical Sensing with Trapping Raman Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Raman Microscope Setup
2.3. Raman Spectroscopy and Data Analysis
2.4. Scanning Electron Microscopy
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansari, I.A.; Datta, A.K. An overview of sterilization Methods for Packaging Materials Used in Aseptic Packaging Systems. Food Bioprod. Process. 2003, 81, 57–65. [Google Scholar] [CrossRef]
- Jildeh, Z.B.; Wagner, P.H.; Schöning, M.J. Sterilization of objects, products, and packaging surfaces and their characterization in different fields of industry: The status in 2020. Phys. Status Solidi A 2021, 218, 2000732. [Google Scholar] [CrossRef]
- Johnston, M.D.; Lawson, S.; Otter, J.A. Evaluation of hydrogen peroxide vapour as a method for the decontamination of surfaces contaminated with Clostridium botulinum spores. J. Microbiol. Methods 2005, 60, 403–411. [Google Scholar] [CrossRef]
- Setlow, P. Spores of Bacillus subtilis: Their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 2006, 101, 514–525. [Google Scholar] [CrossRef]
- Setlow, P. Spore Resistance Properties. Microbiol. Spectr. 2014, 2, 615–622. [Google Scholar] [CrossRef]
- Setlow, P.; Christie, G. What’s new and notable in bacterial spore killing. World J. Microbiol. Biotechnol. 2021, 37, 144. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.I.; Chung, M.S. Bacillus spores: A review of their properties and inactivation processing technologies. Food. Sci. Biotechnol. 2020, 29, 1447–1461. [Google Scholar] [CrossRef]
- Setlow, B.; Setlow, P. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl. Environ. Microbiol. 1993, 59, 3418–3423. [Google Scholar] [CrossRef]
- Djouiai, B.; Thwaite, J.E.; Laws, T.R.; Commichau, F.M.; Setlow, B.; Setlow, P.; Moeller, R. Role of DNA Repair and Protective Components in Bacillus subtilis Spore Resistance to Inactivation by 400-nm-Wavelength Blue Light. Appl. Environ. Microbiol. 2018, 84, e01604-18. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G. Peroxygens and other forms of oxygen: Their use for effective cleaning, disinfection, and sterilization. In New Biocides Development. The Combined Approach of Chemistry and Microbiology; Zhu, P.C., Ed.; American Chemical Society: Washington, DC, USA, 2007; pp. 292–308. [Google Scholar]
- King, W.L.; Gould, G.W. Lysis of bacterial spores with hydrogen peroxide. J. Appl. Bacteriol. 1969, 32, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Calvisi, E.G.; Beaman, T.C.; Pankratz, H.S.; Gerhardt, P.; Marquis, R.E. Microscopic and thermal characterization of hydrogen peroxide killing and lysis of spores and protection by transition metal ions, chelators, and antioxidants. Appl. Environ. Microbiol. 1994, 60, 3192–3197. [Google Scholar] [CrossRef] [PubMed]
- Cortezzo, D.E.; Koziol-Dube, K.; Setlow, B.; Setlow, P. Treatment with oxidizing agents damages the inner membrane of spores of Bacillus subtilis and sensitizes spores to subsequent stress. J. Appl. Microbiol. 2004, 97, 838–852. [Google Scholar] [CrossRef]
- Setlow, B.; Yu, J.; Li, Y.Q.; Setlow, P. Analysis of the germination kinetics of individual Bacillus subtilis spores treated with hydrogen peroxide or sodium hypochlorite. Lett. Appl. Microbiol. 2013, 57, 259–265. [Google Scholar] [CrossRef]
- Arreola, J.; Keusgen, M.; Wagner, T.; Schöning, M.J. Combined calorimetric gas- and spore-based biosensor array for online monitoring and sterility assurance of gaseous hydrogen peroxide in aseptic filling machines. Biosens. Bioelectron. 2019, 143, 111628. [Google Scholar] [CrossRef]
- Oberländer, J.; Mayer, M.; Greeff, A.; Keusgen, M.; Schöning, M.J. Spore-based biosensor to monitor the microbicidal efficacy of gaseous hydrogen peroxide sterilization processes. Biosens. Bioelectron. 2018, 104, 87–94. [Google Scholar] [CrossRef]
- Deng, X.; Shi, J.; Kong, M.G. Physical Mechanisms of Inactivation of Bacillus subtilis Spores Using Cold Atmospheric Plasmas. IEEE Trans. Plasma Sci. 2006, 34, 1310–1316. [Google Scholar] [CrossRef]
- Baeumner, A.J.; Jones, C.; Wong, C.Y.; Price, A. A generic sandwich-type biosensor with nanomolar detection limits. Anal. Bioanal. Chem. 2004, 378, 1587–1593. [Google Scholar] [CrossRef]
- Tehri, N.; Kumar, N.; Raghu, H.V.; Vashishth, A. Biomarkers of bacterial spore germination. Ann. Microbiol. 2018, 68, 513–523. [Google Scholar] [CrossRef]
- Campbell, G.A.; Mutharasan, R. Piezoelectric-excited millimeter-sized cantilever (PEMC) sensors detect Bacillus anthracis at 300spores/mL. Biosens. Bioelectron. 2006, 21, 1684–1692. [Google Scholar] [CrossRef]
- Wang, D.B.; Cui, M.M.; Li, M.; Zhang, X.E. Biosensors for the Detection of Bacillus anthracis. Acc. Chem. Res. 2021, 54, 4451–4461. [Google Scholar] [CrossRef]
- Zhou, Y.; Yu, B.; Levon, K. Potentiometric sensor for dipicolinic acid. Biosens. Bioelectron. 2005, 20, 1851–1855. [Google Scholar] [CrossRef] [PubMed]
- Orbay, S.; Kocaturk, O.; Sanyal, R.; Sanyal, A. Molecularly Imprinted Polymer-Coated Inorganic Nanoparticles: Fabrication and Biomedical Applications. Micromachines 2022, 13, 1464. [Google Scholar] [CrossRef]
- Labib, M.; Zamay, A.S.; Kolovskaya, O.S.; Reshetneva, I.T.; Zamay, G.S.; Kibbee, R.J.; Sattar, S.A.; Zamay, T.N.; Berezovski, M.V. Aptamer-based viability impedimetric sensor for bacteria. Anal. Chem. 2012, 84, 8966–8969. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, X.; Liu, T.; Zhang, Z.; Kong, J.; Yu, H.; Yan, J.; Luan, D.; Zhao, Y.; Bian, X. Reusable and universal impedimetric sensing platform for the rapid and sensitive detection of pathogenic bacteria based on bacteria-imprinted polythiophene film. Analyst 2022, 147, 4433–4441. [Google Scholar] [CrossRef]
- Kirchner, P.; Li, B.; Spelthahn, H.; Henkel, H.; Schneider, A.; Friedrich, P.; Kolstad, J.; Keusgen, M.; Schöning, M.J. Thin-film calorimetric H2O2 gas sensor for the validation of germicidal effectivity in aseptic filling processes. Sens. Actuators B Chem. 2011, 154, 257–263. [Google Scholar] [CrossRef]
- Kirchner, P.; Oberländer, J.; Friedrich, P.; Berger, J.; Rysstad, G.; Keusgen, M.; Schöning, M.J. Realisation of a calorimetric gas sensor on polyimide foil for applications in aseptic food industry. Sens. Actuators B Chem. 2012, 170, 60–66. [Google Scholar] [CrossRef]
- Stöckel, S.; Schumacher, W.; Meisel, S.; Elschner, M.; Rösch, P.; Popp, J. Raman spectroscopy-compatible inactivation method for pathogenic endospores. Appl. Environ. Microbiol. 2010, 76, 2895–2907. [Google Scholar] [CrossRef]
- Chan, J.W. Recent advances in laser tweezers Raman spectroscopy (LTRS) for label-free analysis of single cells. J. Biophotonics 2013, 6, 36–48. [Google Scholar] [CrossRef]
- Redding, B.; Schwab, M.; Pan, Y.L. Raman Spectroscopy of Optically Trapped Single Biological Micro-Particles. Sensors 2015, 15, 19021–19046. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.; Block, S.M. Optical trapping of metallic Rayleigh particles. Opt. Lett. 1994, 19, 930–932. [Google Scholar] [CrossRef]
- Chang, W.-T.; Lin, H.-L.; Chen, H.-C.; Wu, Y.-M.; Chen, W.-J.; Lee, Y.-T.; Liau, I. Real-time molecular assessment on oxidative injury of single cells using Raman spectroscopy. J. Raman Spectrosc. 2009, 40, 1194–1199. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.; Huang, Y.; Feng, S.; Zheng, Z.; Liu, X.; Liu, M. Label-free detection of hydrogen peroxide-induced oxidative stress in human retinal pigment epithelium cells via laser tweezers Raman spectroscopy. Biomed. Opt. Express. 2019, 10, 500–513. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, S.S.; Li, Y.Q. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 2006, 78, 6936–6941. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kong, L.; Wang, G.; Setlow, P.; Li, Y.Q. Monitoring the wet-heat inactivation dynamics of single spores of Bacillus species by using Raman tweezers, differential interference contrast microscopy, and nucleic acid dye fluorescence microscopy. Appl. Environ. Microbiol. 2011, 77, 4754–4769. [Google Scholar] [CrossRef]
- Sarshar, M.; Wong, W.T.; Anvari, B. Comparative study of methods to calibrate the stiffness of a single-beam gradient-force optical tweezers over various laser trapping powers. J. Biomed. Opt. 2014, 19, 115001. [Google Scholar] [CrossRef]
- Huang, S.S.; Chen, D.; Pelczar, P.L.; Vepachedu, V.R.; Setlow, P.; Li, Y.Q. Levels of Ca2+-dipicolinic acid in individual bacillus spores determined using microfluidic Raman tweezers. J. Bacteriol. 2007, 189, 4681–4687. [Google Scholar] [CrossRef]
- Hashimoto, T.; Frieben, W.R.; Conti, S.F. Germination of single bacterial spores. J. Bacteriol. 1969, 98, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kong, L.; Wang, G.; Setlow, P.; Li, Y.-Q. Combination of Raman tweezers and quantitative differential interference contrast microscopy for measurement of dynamics and heterogeneity during the germination of individual bacterial spores. J. Biomed. Opt. 2010, 15, 056010. [Google Scholar] [CrossRef]
- Stringer, S.C.; Webb, M.D.; George, S.M.; Pin, C.; Peck, M.W. Heterogeneity of times required for germination and outgrowth from single spores of nonproteolytic Clostridium botulinum. Appl. Environ. Microbiol. 2005, 71, 4998–5003. [Google Scholar] [CrossRef]
- Blázquez-Castro, A. Optical Tweezers: Phototoxicity and Thermal Stress in Cells and Biomolecules. Micromachines 2019, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Houk, K.N.; Foote, C.S.; Fukuto, J.M.; Ignarro, L.J.; Squadrito, G.L.; Davies, K.J. Free radical biology and medicine: It’s a gas, man. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R491–R511. [Google Scholar] [CrossRef] [PubMed]
- Neuman, K.C.; Chadd, E.H.; Liou, G.F.; Bergman, K.; Block, S.M. Characterization of photodamage to Escherichia coli in optical traps. Biophys. J. 1999, 77, 2856–2863. [Google Scholar] [CrossRef]
- Westberg, M.; Bregnhøj, M.; Blázquez-Castro, A.; Breitenbach, T.; Etzerodt, M.; Ogilby, P.R. Control of singlet oxygen production in experiments performed on single mammalian cells. J. Photochem. Photobiol. A 2016, 321, 297–308. [Google Scholar] [CrossRef]
- Blázquez-Castro, A.; Westberg, M.; Bregnhøj, M.; Breitenbach, T.; Mogensen, D.J.; Etzerodt, M.; Ogilby, P.R. Light-initiated oxidative stress. In Oxidative Stress; Sies, H., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 363–388. [Google Scholar] [CrossRef]
- Oberländer, J.; Kirchner, P.; Boyen, H.-G.; Schöning, M.J. Detection of hydrogen peroxide vapor by use of manganese(IV) oxide as catalyst for calorimetric gas sensors. Phys. Status Solidi A 2014, 211, 1372–1376. [Google Scholar] [CrossRef]
- McDonnell, G. The Use of Hydrogen Peroxide for Disinfection and Sterilization Applications. In PATAI’S Chemistry of Functional Groups; Rappoport, Z., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 1–34. [Google Scholar] [CrossRef]
- Malyshev, D.; Dahlberg, T.; Wiklund, K.; Andersson, P.O.; Henriksson, S.; Andersson, M. Mode of Action of Disinfection Chemicals on the Bacterial Spore Structure and Their Raman Spectra. Anal. Chem. 2021, 93, 3146–3153. [Google Scholar] [CrossRef]
- Malyshev, D.; Öberg, R.; Dahlberg, T.; Wiklund, K.; Landström, L.; Andersson, P.O.; Andersson, M. Laser induced degradation of bacterial spores during micro-Raman spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 265, 120381. [Google Scholar] [CrossRef]
- McDonnell, G.; Russell, A.D. Antiseptics and disinfectants: Activity, action, and resistance. Clin. Microbiol. Rev. 1999, 12, 147–179. [Google Scholar] [CrossRef]
- Malyshev, D.; Robinson, N.F.; Öberg, R.; Dahlberg, T.; Andersson, M. Reactive oxygen species generated by infrared laser light in optical tweezers inhibits the germination of bacterial spores. J. Biophotonics 2022, 15, 118668. [Google Scholar] [CrossRef]
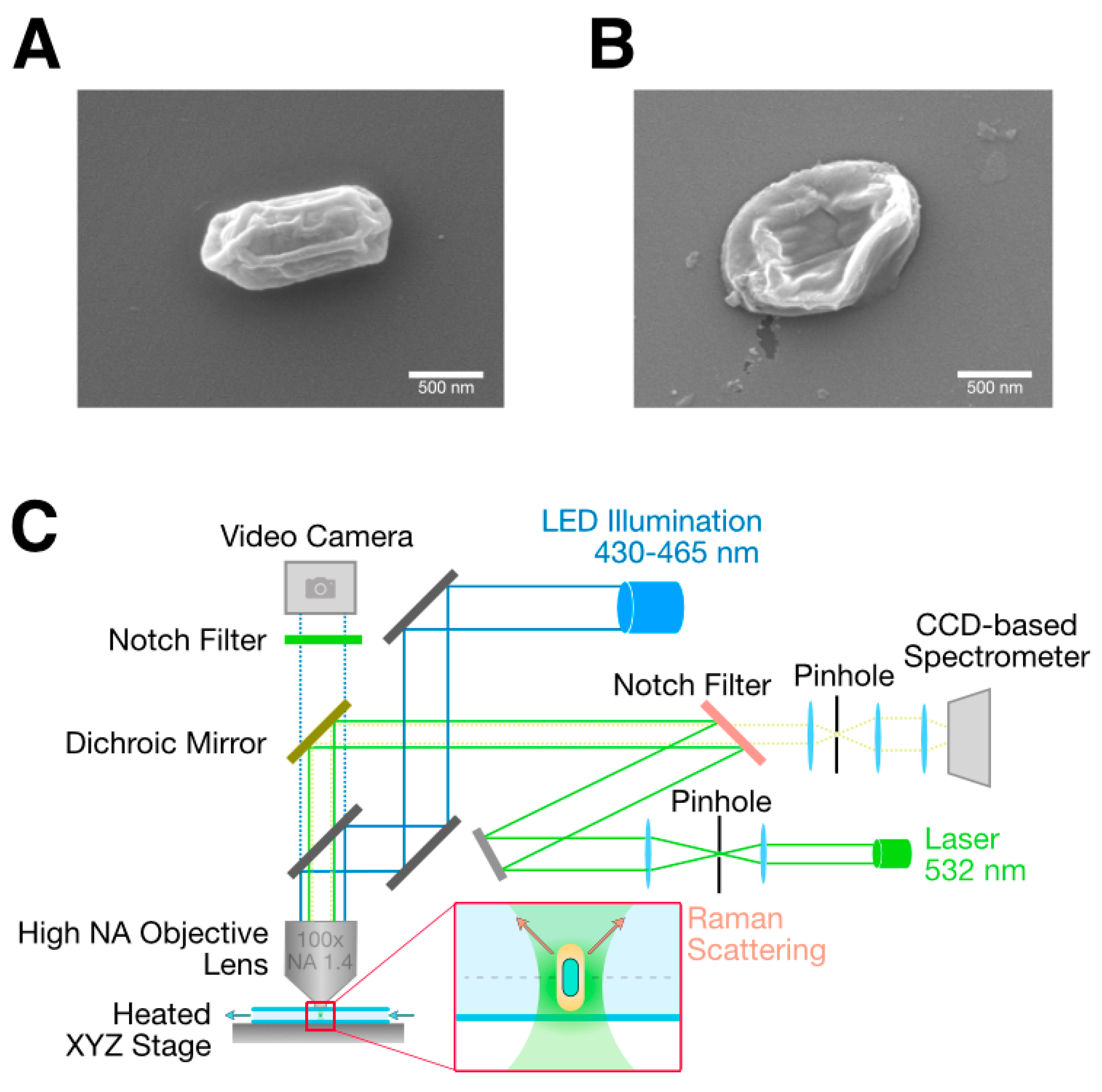
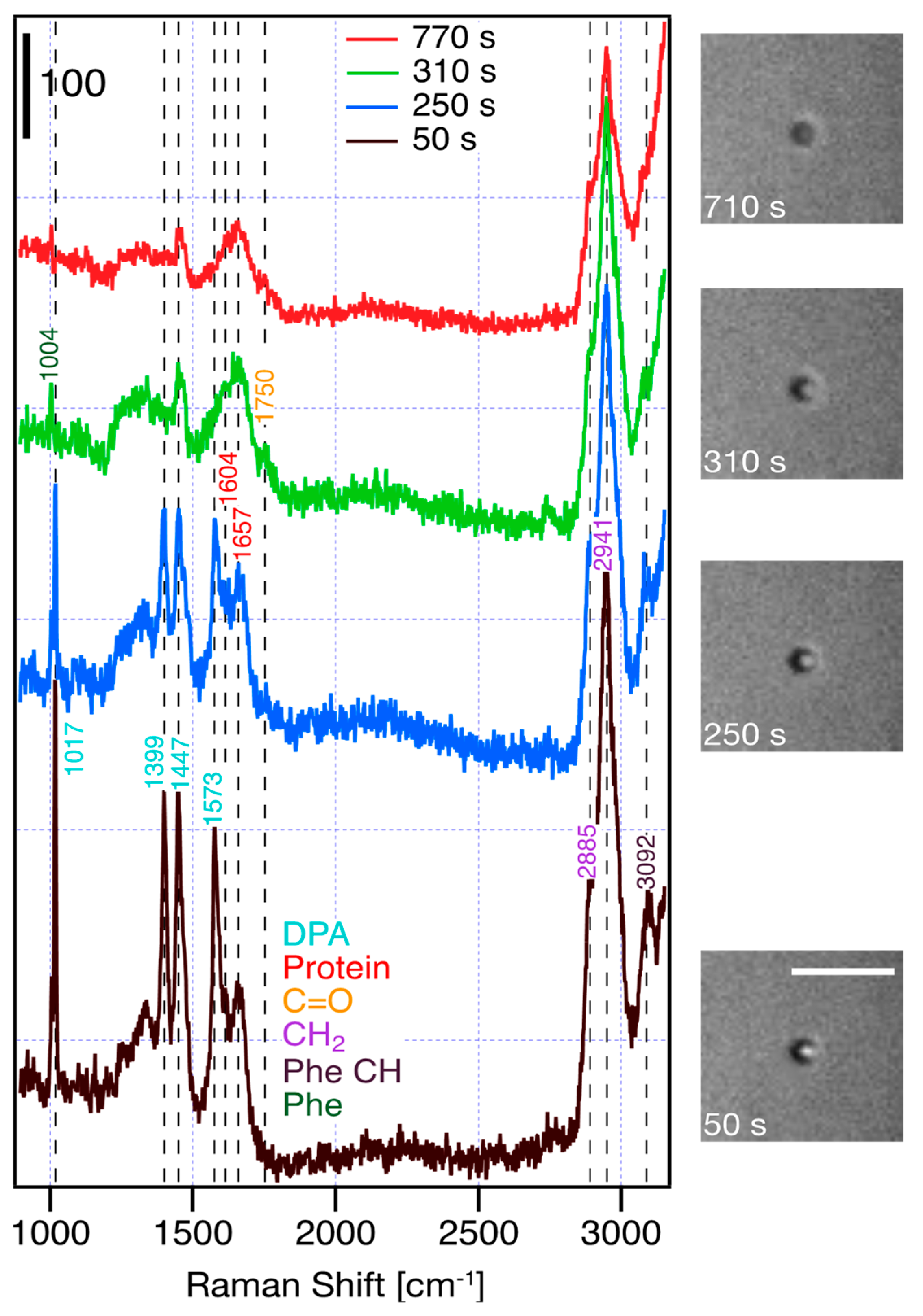
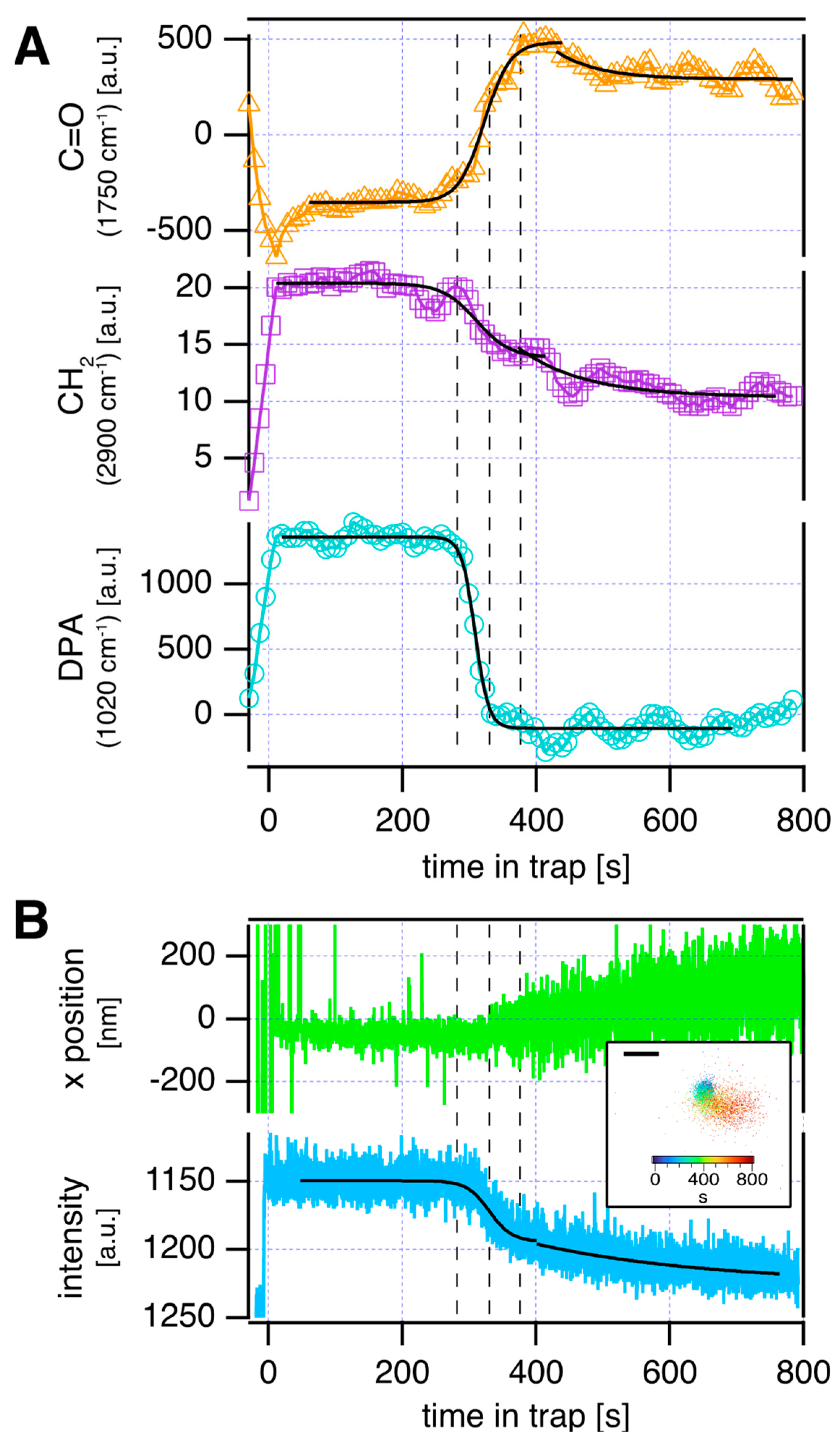


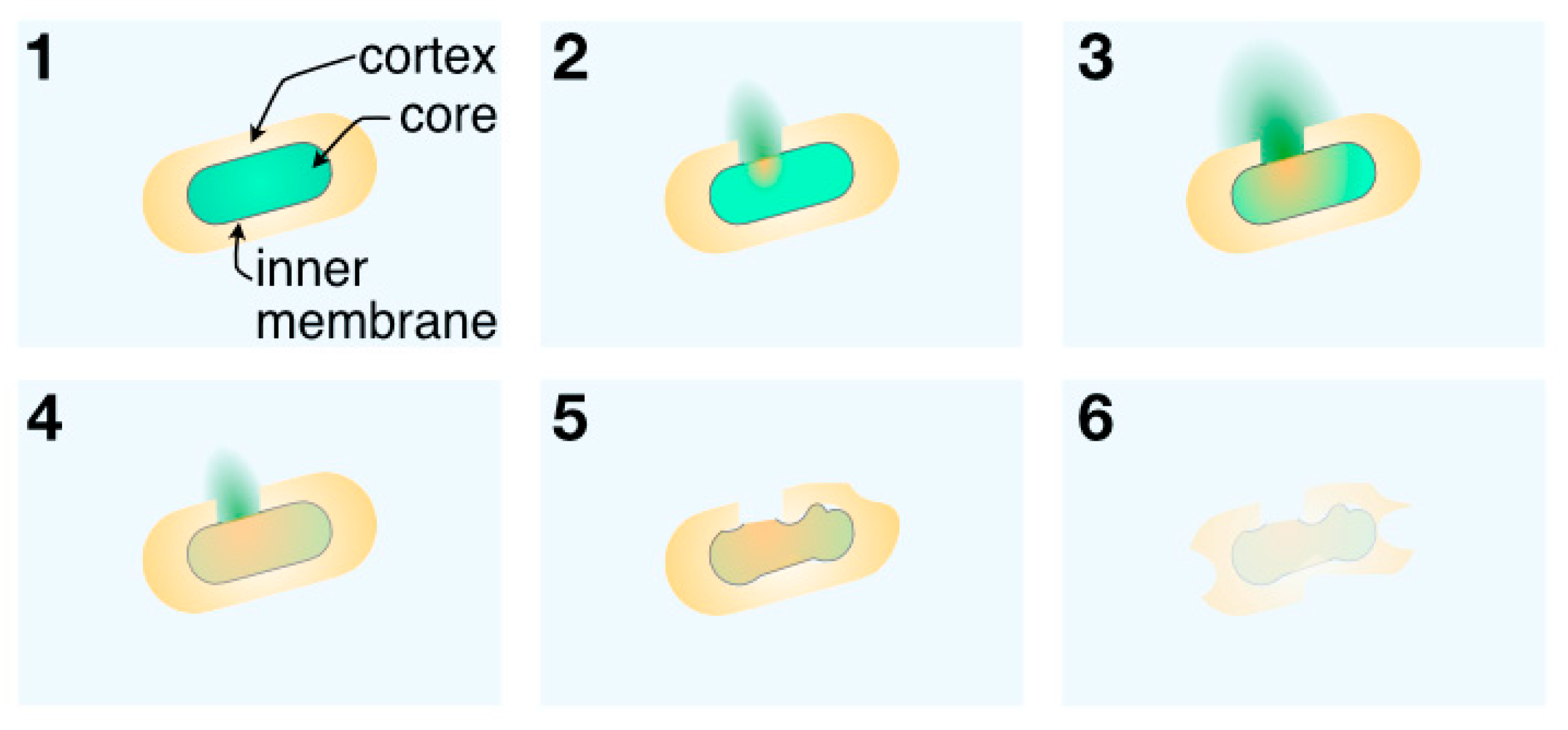
| Raman Shift [cm−1] | Assignment |
|---|---|
| 1004 | Phe (ring breathing) |
| 1017 | DPA ring breathing |
| 1399 | DPA COO− stretch (symmetric) |
| 1447 | DPA C-C ring stretch |
| 1573 | DPA COO− stretch (asymmetric) |
| 1604 | Protein (Tyr, Trp, Phe) |
| 1657 | amide I |
| 1750 | C=O |
| 2885 | CH2 |
| 2941 | CH2 |
| 3092 | Phenyl CH |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertz, M.; Molinnus, D.; Schöning, M.J.; Homma, T. Real-Time Monitoring of H2O2 Sterilization on Individual Bacillus atrophaeus Spores by Optical Sensing with Trapping Raman Spectroscopy. Chemosensors 2023, 11, 445. https://doi.org/10.3390/chemosensors11080445
Bertz M, Molinnus D, Schöning MJ, Homma T. Real-Time Monitoring of H2O2 Sterilization on Individual Bacillus atrophaeus Spores by Optical Sensing with Trapping Raman Spectroscopy. Chemosensors. 2023; 11(8):445. https://doi.org/10.3390/chemosensors11080445
Chicago/Turabian StyleBertz, Morten, Denise Molinnus, Michael J. Schöning, and Takayuki Homma. 2023. "Real-Time Monitoring of H2O2 Sterilization on Individual Bacillus atrophaeus Spores by Optical Sensing with Trapping Raman Spectroscopy" Chemosensors 11, no. 8: 445. https://doi.org/10.3390/chemosensors11080445
APA StyleBertz, M., Molinnus, D., Schöning, M. J., & Homma, T. (2023). Real-Time Monitoring of H2O2 Sterilization on Individual Bacillus atrophaeus Spores by Optical Sensing with Trapping Raman Spectroscopy. Chemosensors, 11(8), 445. https://doi.org/10.3390/chemosensors11080445







