Capacitive, Highly Selective Zeolite-Based Ammonia Sensor for Flue Gas Applications
Abstract
1. Introduction
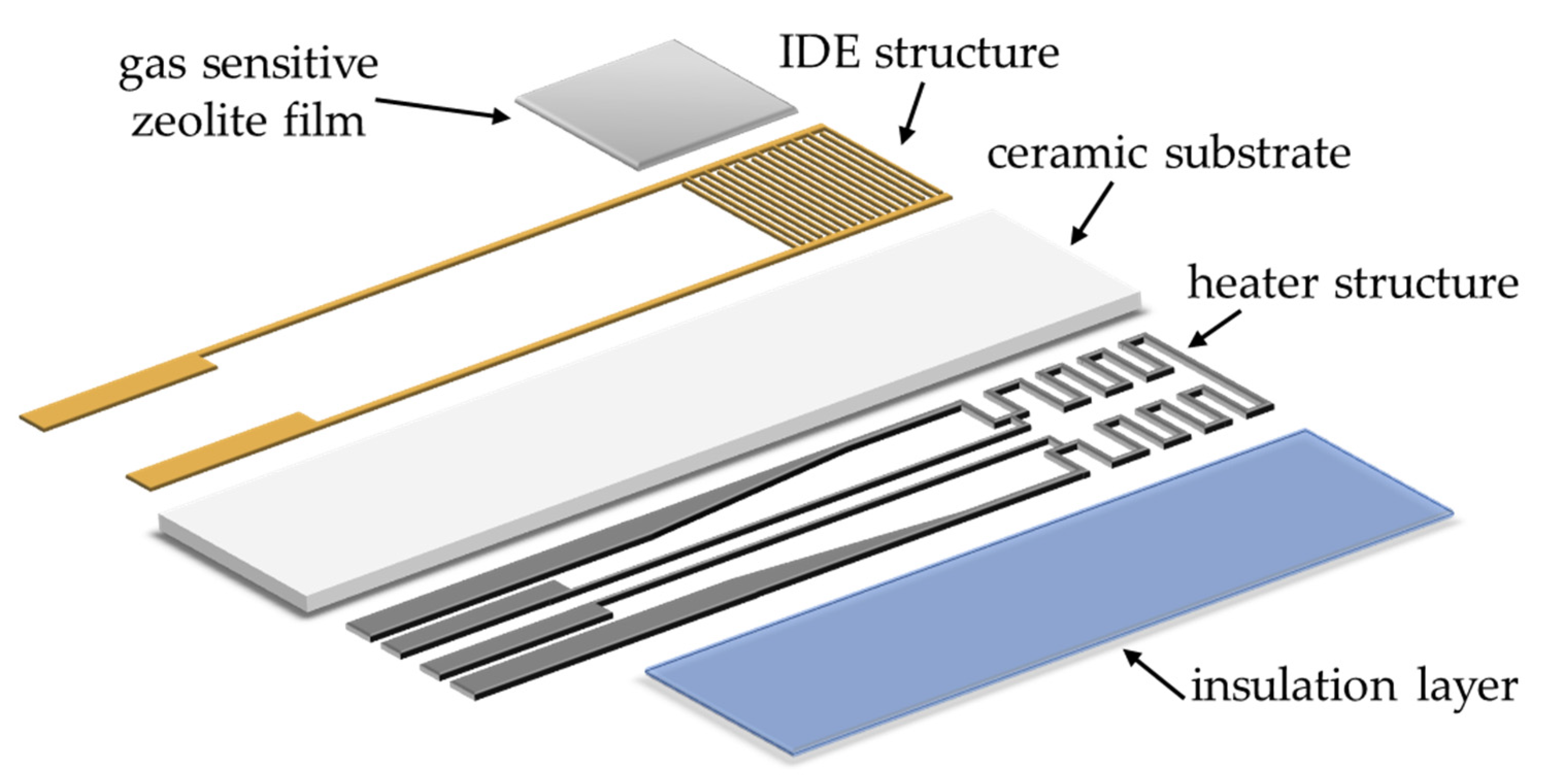
2. Structure and Operating Principle of the Sensor
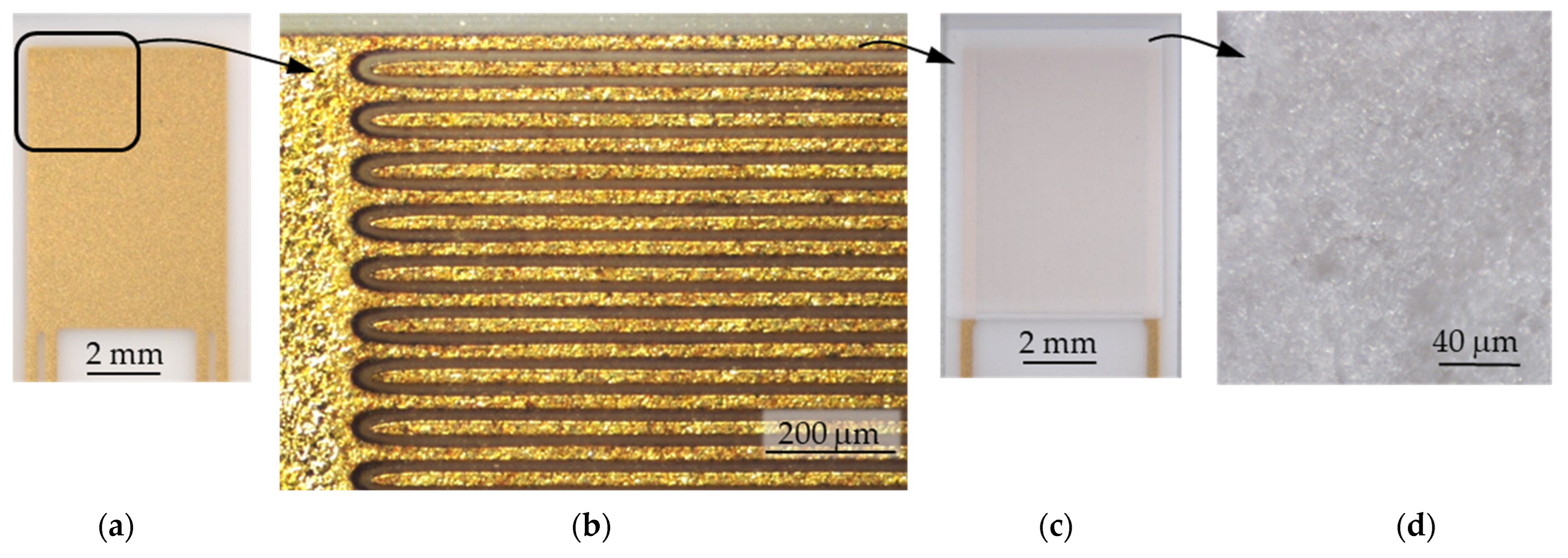
2.1. General Sensor Setup
2.2. Gas-Sensitive Zeolite Film
3. Experiments
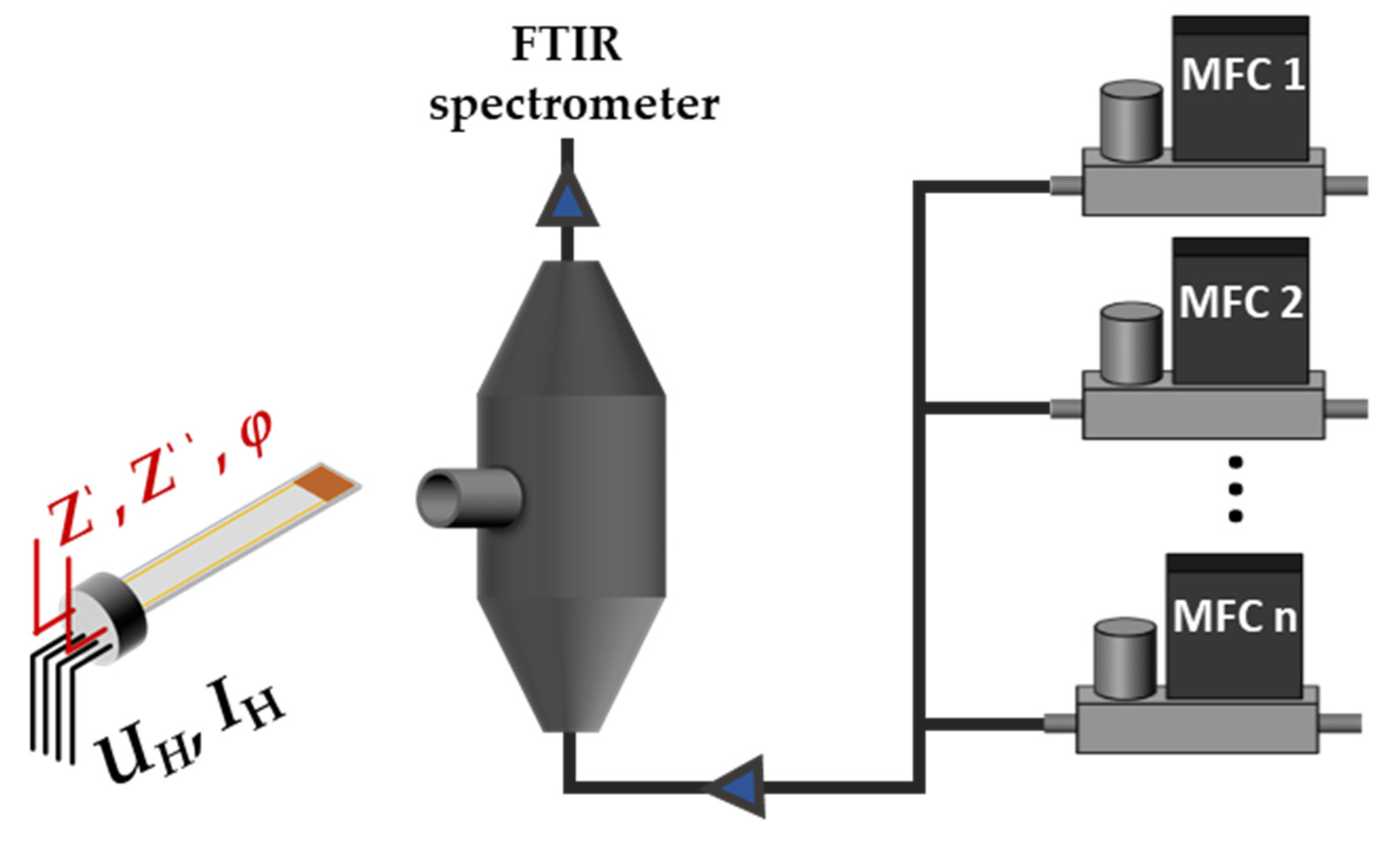
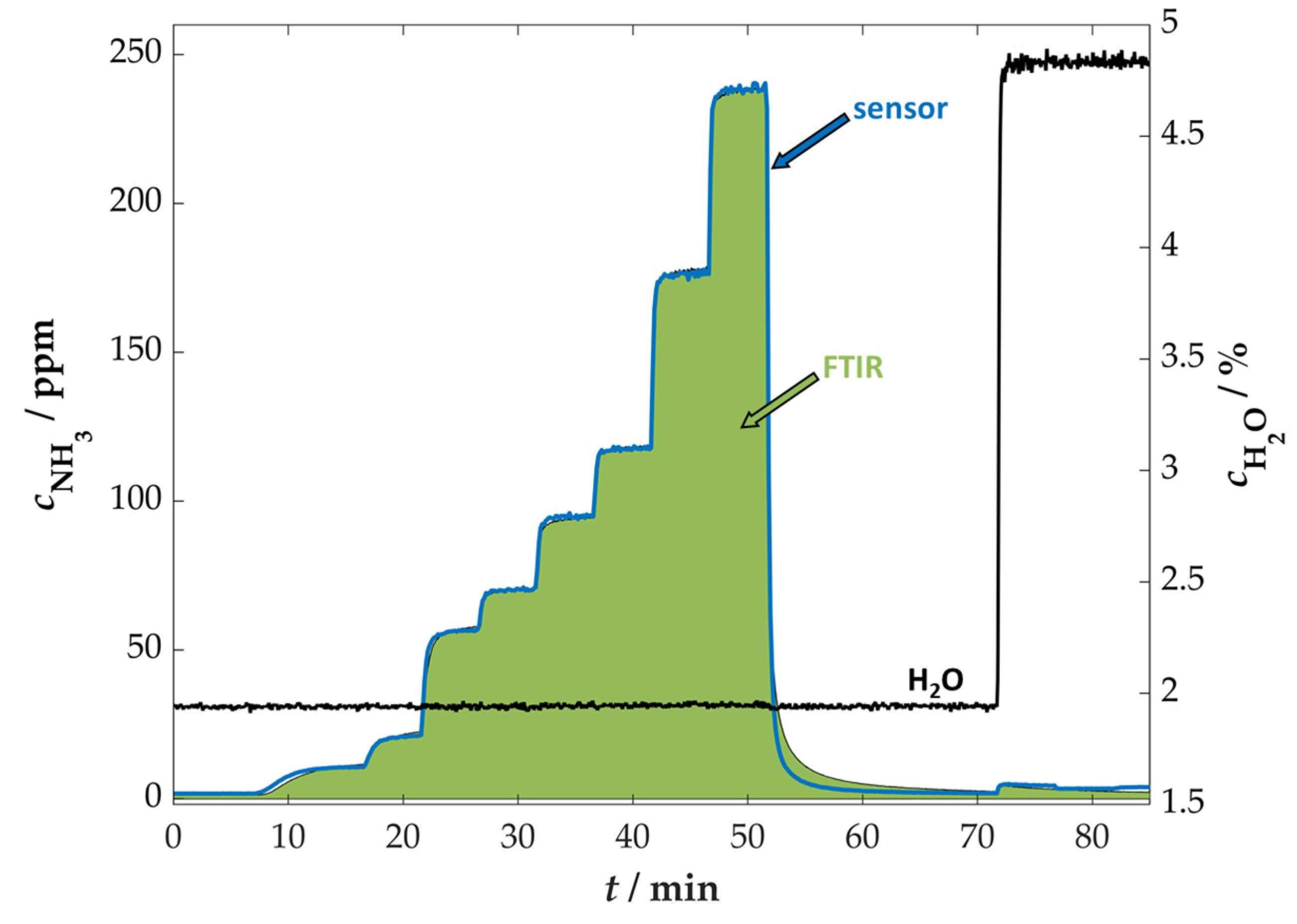
3.1. Measurement Setup
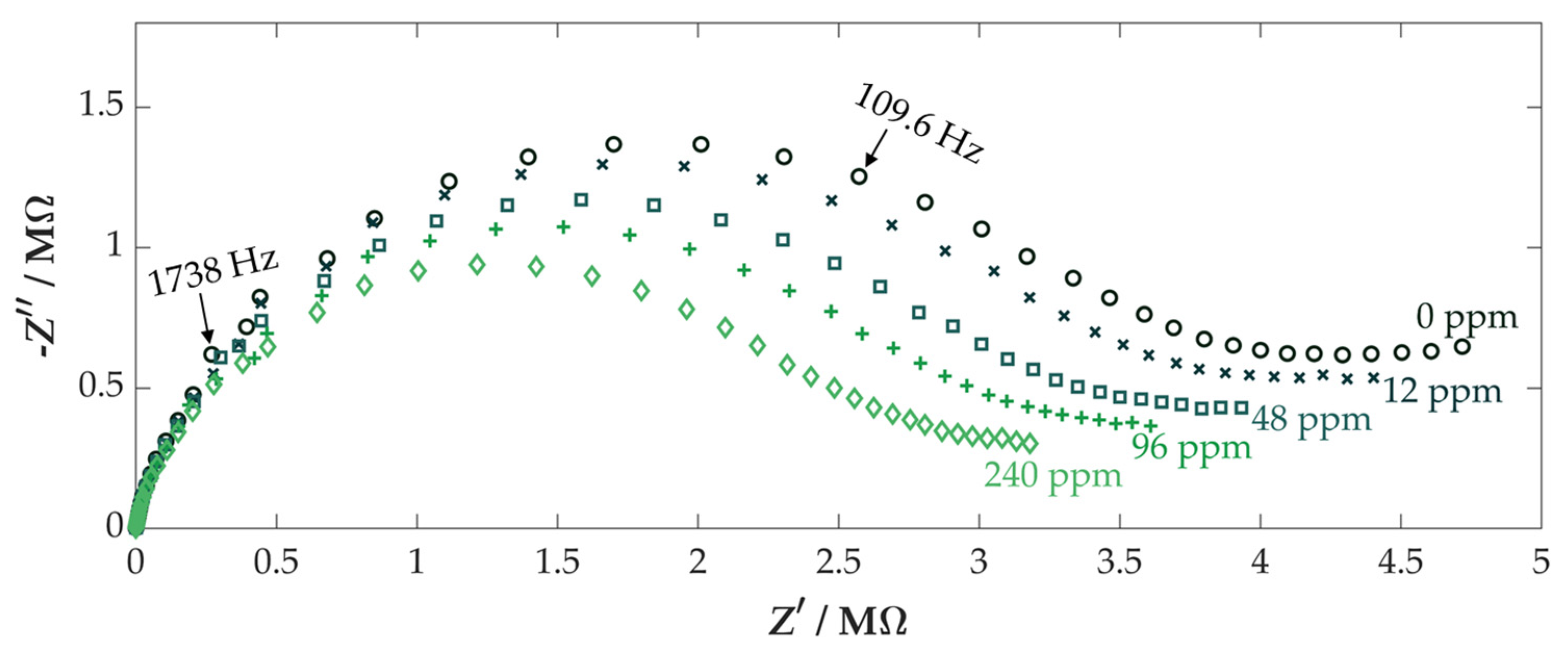
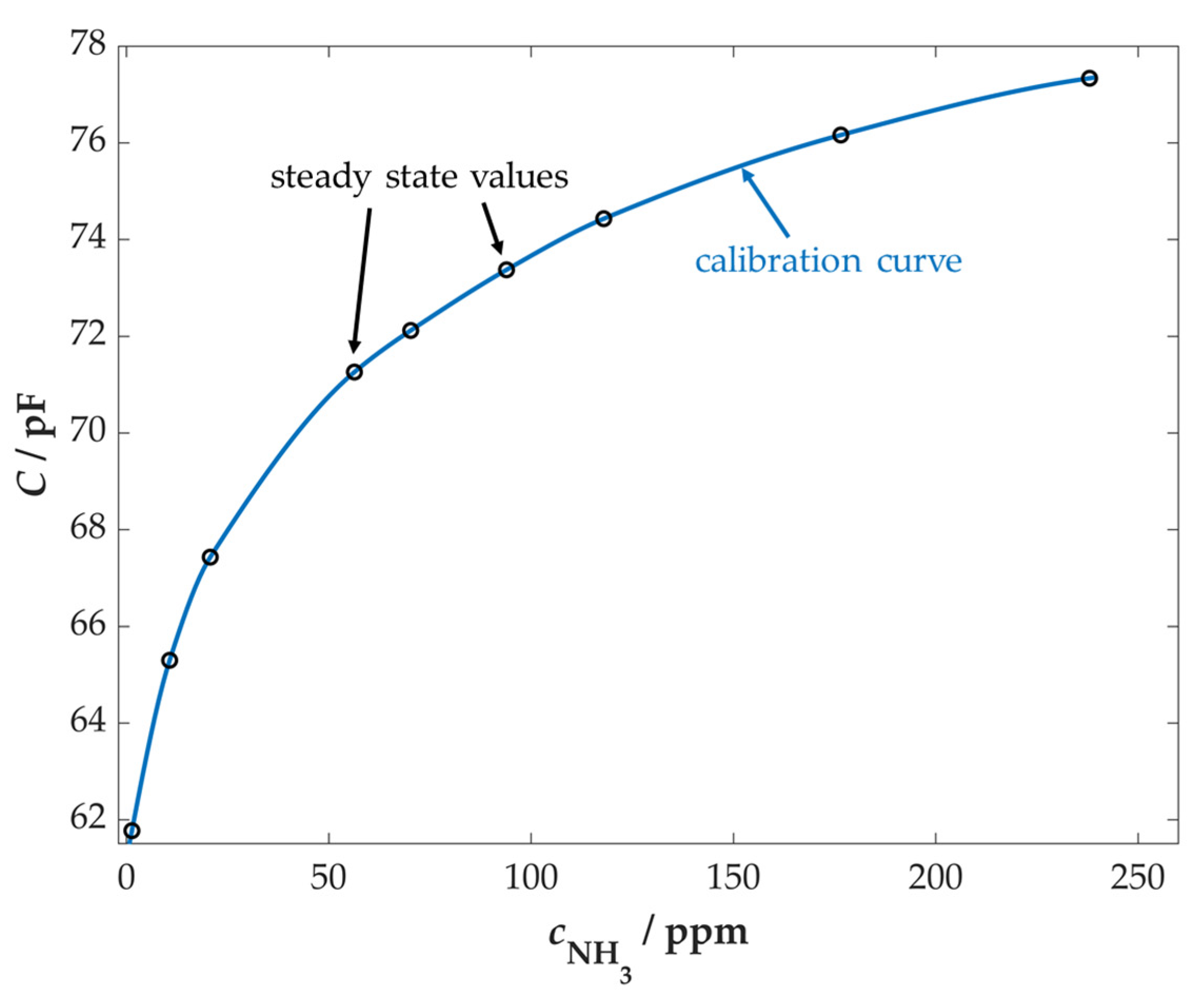
3.2. Characterization of First Sensors
3.3. Further Sensor Characterization
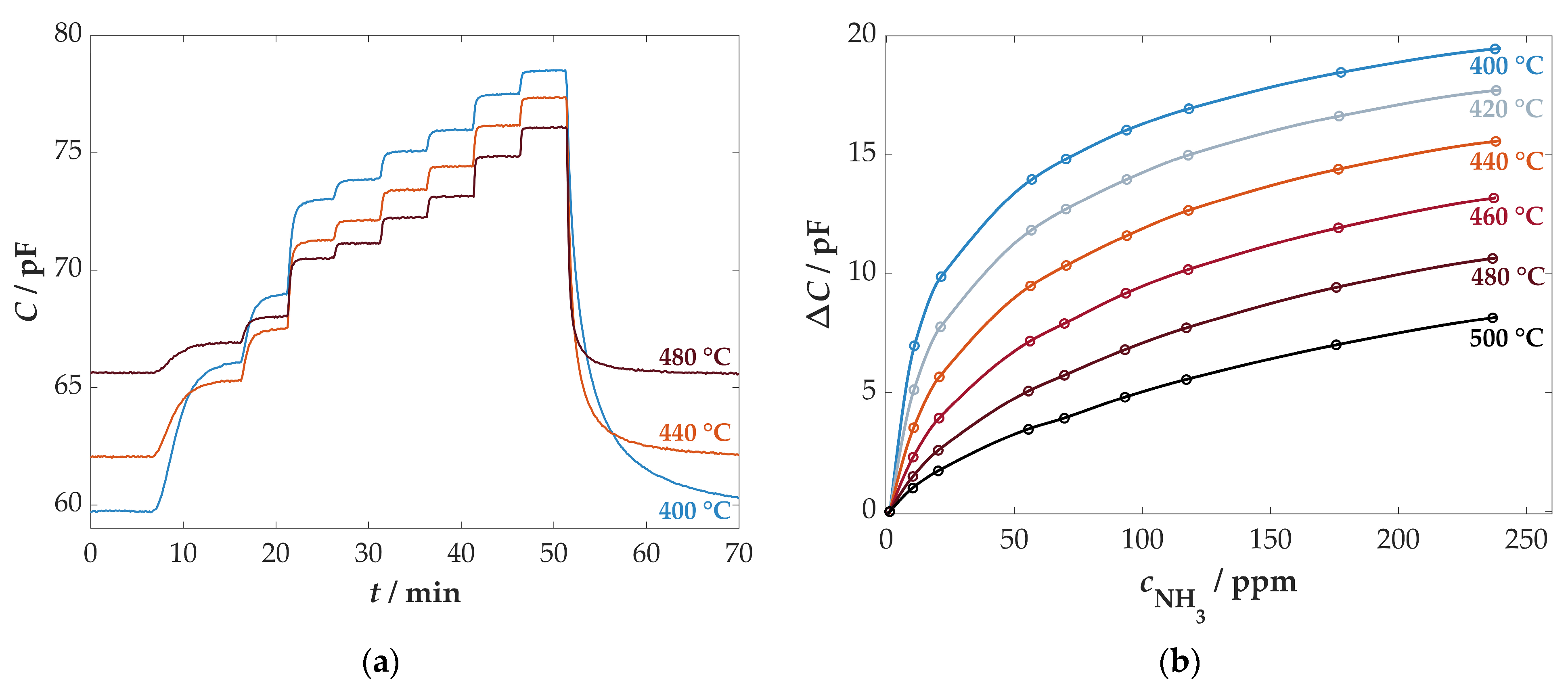
3.3.1. Influence of the Sensor Temperature
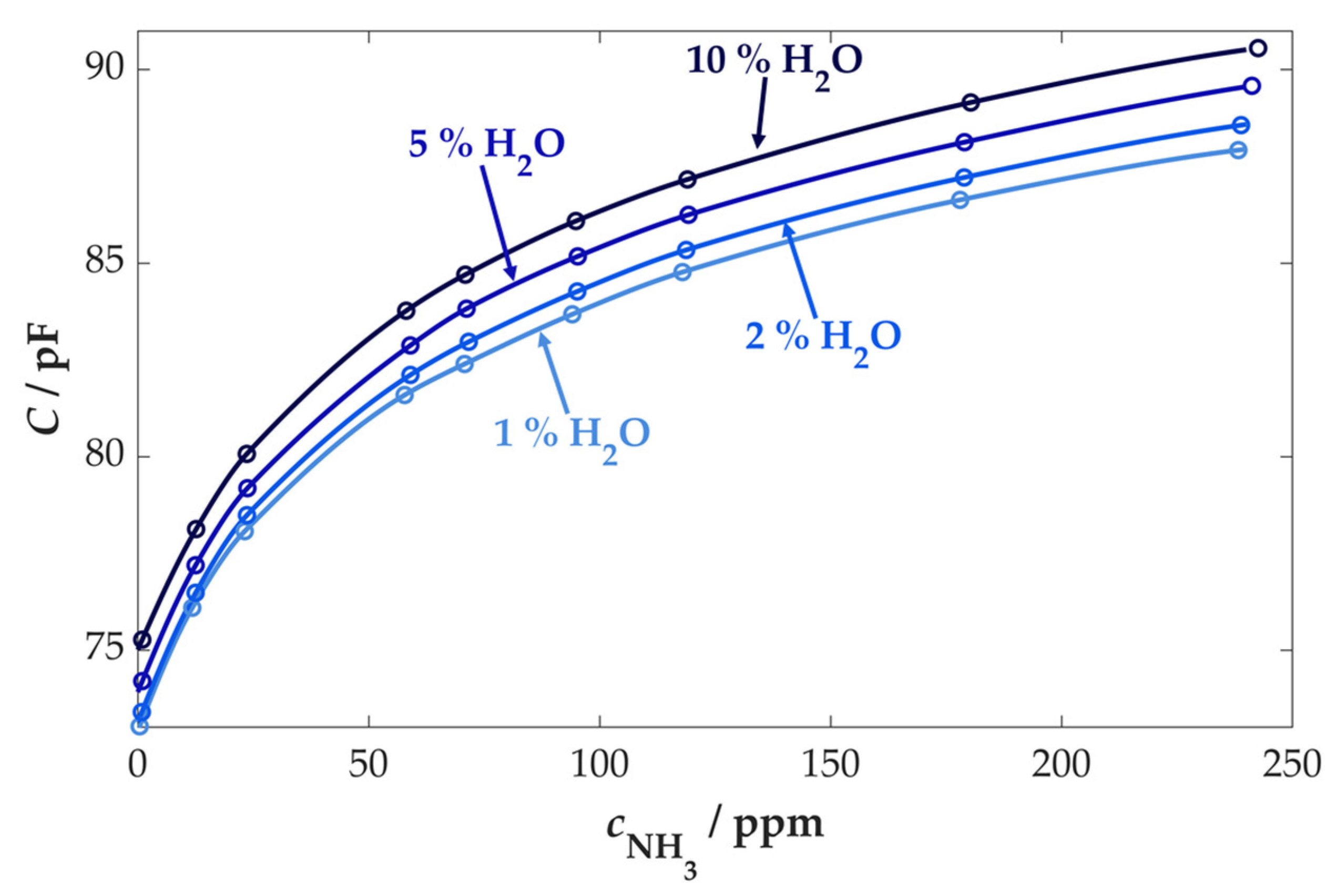
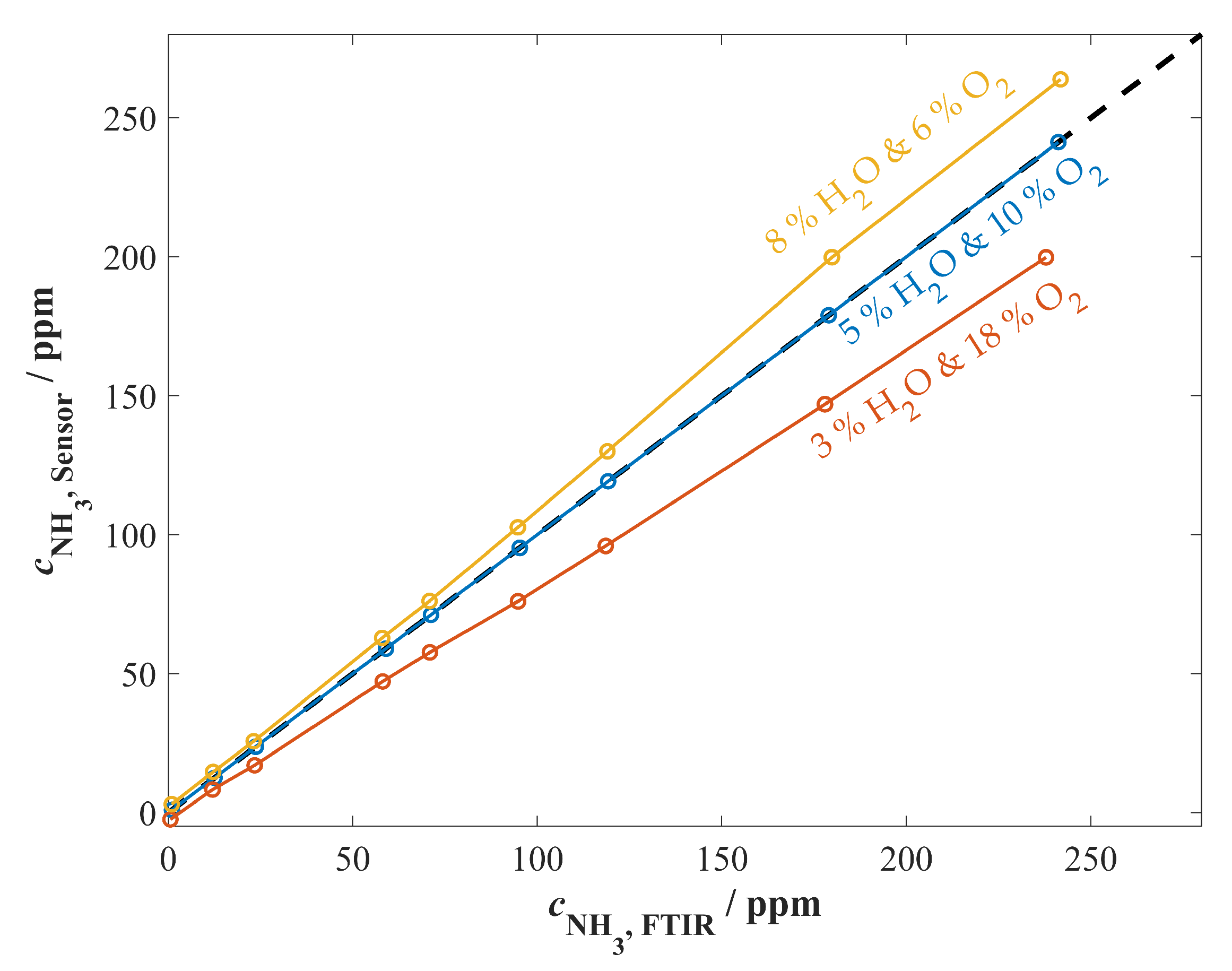
3.3.2. Cross-Sensitivity Measurements
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hasan, M.H.; Mahlia, T.M.I.; Mofijur, M.; Rizwanul Fattah, I.M.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.P.S.; Munnings, C.; Dolan, M. Ammonia as a Renewable Energy Transportation Media. ACS Sustain. Chem. Eng. 2017, 5, 10231–10239. [Google Scholar] [CrossRef]
- Alemu, M.A.; Ilbas, M. Direct ammonia fueled solid oxide fuel cells: A comprehensive review on challenges, opportunities and future outlooks. Int. J. Energy Technol. 2020, 2, 70–91. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Schar, C.M.; Onder, C.H.; Geering, H.P. Control of an SCR catalytic converter system for a mobile heavy-duty application. IEEE Trans. Control Syst. Technol. 2006, 14, 641–653. [Google Scholar] [CrossRef]
- Kröcher, O. Selective Catalytic Reduction of NOx. Catalysts 2018, 8, 459. [Google Scholar] [CrossRef]
- Chatterjee, B.; Bandyopadhyay, A. Development of Zinc Oxide Sensors for Detecting Ammonia Gas in the Ambient Air: A Critical Short Review. Environ. Qual. Manag. 2016, 26, 89–105. [Google Scholar] [CrossRef]
- Müller, V.; Pieta, H.; Schaub, J.; Ehrly, M.; Körfer, T. On-Board Monitoring to meet upcoming EU-7 emission standards—Squaring the circle between effectiveness and robust realization. Transp. Eng. 2022, 10, 100138. [Google Scholar] [CrossRef]
- Boger, T.; Rose, D.; He, S.; Joshi, A. Developments for future EU7 regulations and the path to zero impact emissions—A catalyst substrate and filter supplier’s perspective. Transp. Eng. 2022, 10, 100129. [Google Scholar] [CrossRef]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Nelles, M.; Deprie, K.; Jalalipour, H. The role of biogenic wastes and residues in a climate-neutral society: Carbon source, bioenergy and negative emissions. Waste Manag. Res. 2023, 41, 741–743. [Google Scholar] [CrossRef]
- Lauer, M.; Dotzauer, M.; Millinger, M.; Oehmichen, K.; Jordan, M.; Kalcher, J.; Majer, S.; Thraen, D. The Crucial Role of Bioenergy in a Climate-Neutral Energy System in Germany. Chem. Eng. Technol. 2023, 46, 501–510. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Williams, A.; Jones, J.M.; Ma, L.; Pourkashanian, M. Pollutants from the combustion of solid biomass fuels. Prog. Energy Combust. Sci. 2012, 38, 113–137. [Google Scholar] [CrossRef]
- Tomlin, A.S. Air Quality and Climate Impacts of Biomass Use as an Energy Source: A Review. Energy Fuels 2021, 35, 14213–14240. [Google Scholar] [CrossRef]
- König, M.; Eisinger, K.; Hartmann, I.; Müller, M. Combined removal of particulate matter and nitrogen oxides from the exhaust gas of small-scale biomass combustion. Biomass Convers. Biorefinery 2019, 9, 201–212. [Google Scholar] [CrossRef]
- Zhu, M.; Lai, J.-K.; Tumuluri, U.; Ford, M.E.; Wu, Z.; Wachs, I.E. Reaction Pathways and Kinetics for Selective Catalytic Reduction (SCR) of Acidic NOx Emissions from Power Plants with NH3. ACS Catal. 2017, 7, 8358–8361. [Google Scholar] [CrossRef]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Hofmann, L.; Rusch, K.; Fischer, S.; Lemire, B. Onboard Emissions Monitoring on a HD Truck with an SCR System Using NOx Sensors; SAE International: Pittsburgh, PA, USA, 2004; Volume 113, pp. 559–572. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; van den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Malins, C.; Doyle, A.; MacCraith, B.D.; Kvasnik, F.; Landl, M.; Simon, P.; Kalvoda, L.; Lukas, R.; Pufler, K.; Babusík, I. Personal ammonia sensor for industrial environments. J. Environ. Monit. 1999, 1, 417–422. [Google Scholar] [CrossRef]
- Karunagaran, B.; Uthirakumar, P.; Chung, S.J.; Velumani, S.; Suh, E.-K. TiO2 thin film gas sensor for monitoring ammonia. Mater. Charact. 2007, 58, 680–684. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Wu, X.-H.; Su, Q.; Li, Y.-F.; Zhou, Z.-L. Ammonia-sensing characteristics of Pt and SiO2 doped SnO2 materials. Solid-State Electron. 2001, 45, 347–350. [Google Scholar] [CrossRef]
- Pijolat, C.; Pupier, C.; Sauvan, M.; Tournier, G.; Lalauze, R. Gas detection for automotive pollution control. Sens. Actuators B Chem. 1999, 59, 195–202. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent Advances in Ammonia Gas Sensors Based on Carbon Nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef]
- Tang, Q.B.; Guo, Y.J.; Tang, Y.L.; Long, G.D.; Wang, J.L.; Li, D.J.; Zu, X.T.; Ma, J.Y.; Wang, L.; Torun, H.; et al. Highly sensitive and selective Love mode surface acoustic wave ammonia sensor based on graphene oxides operated at room temperature. J. Mater. Sci. 2019, 54, 11925–11935. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Ghosh, A.; Zhang, C.; Shi, S.Q.; Zhang, H. High-Temperature Gas Sensors for Harsh Environment Applications: A Review. Clean—Soil Air Water 2019, 47, 1800491. [Google Scholar] [CrossRef]
- Rodríguez-González, L.; Rodríguez-Castellon, E.; Jimenez-Lopez, A.; Simon, U. Correlation of TPD and impedance measurements on the desorption of NH3 from zeolite H-ZSM-5. Solid State Ion. 2008, 179, 1968–1973. [Google Scholar] [CrossRef]
- Schönauer, D.; Nieder, T.; Wiesner, K.; Fleischer, M.; Moos, R. Investigation of the electrode effects in mixed potential type ammonia exhaust gas sensors. Solid State Ion. 2011, 192, 38–41. [Google Scholar] [CrossRef]
- Zhang, W.; Qi, S.; Pantaleo, G.; Liotta, L.F. WO3–V2O5 Active Oxides for NOx SCR by NH3: Preparation Methods, Catalysts’ Composition, and Deactivation Mechanism—A Review. Catalysts 2019, 9, 527. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Yuan, Y.; Wang, B.; Huang, J.; Xia, F.; Zhang, H.; Xiao, J. Effects of sintering temperature on sensing properties of V2O5-WO3-TiO2 electrode for potentiometric ammonia sensor. Sens. Actuators B Chem. 2017, 241, 268–275. [Google Scholar] [CrossRef]
- Miura, N.; Sato, T.; Anggraini, S.A.; Ikeda, H.; Zhuiykov, S. A review of mixed-potential type zirconia-based gas sensors. Ionics 2014, 20, 901–925. [Google Scholar] [CrossRef]
- Da Wang, Y.; Yao, S.; Shost, M.; Yoo, J.-H.; Cabush, D.; Racine, D.; Cloudt, R.; Willems, F. Ammonia Sensor for Closed-Loop SCR Control. SAE Int. J. Passeng. Cars—Electron. Electr. Syst. 2009, 1, 323–333. [Google Scholar] [CrossRef]
- Franke, M.E.; Simon, U. Solvate-supported proton transport in zeolites. Chemphyschem 2004, 5, 465–472. [Google Scholar] [CrossRef]
- Moos, R.; Müller, R.; Plog, C.; Knezevic, A.; Leye, H.; Irion, E.; Braun, T.; Marquardt, K.-J.; Binder, K. Selective ammonia exhaust gas sensor for automotive applications. Sens. Actuators B Chem. 2002, 83, 181–189. [Google Scholar] [CrossRef]
- Simons, T.; Simon, U. Zeolites as nanoporous, gas-sensitive materials for in situ monitoring of DeNOx-SCR. Beilstein J. Nanotechnol. 2012, 3, 667–673. [Google Scholar] [CrossRef]
- Smit, B.; Maesen, T.L.M. Molecular simulations of zeolites: Adsorption, diffusion, and shape selectivity. Chem. Rev. 2008, 108, 4125–4184. [Google Scholar] [CrossRef]
- Ozin, G.A.; Kuperman, A.; Stein, A. Advanced Zeolite, Materials Science. Angew. Chem. Int. Ed. Engl. 1989, 28, 359–376. [Google Scholar] [CrossRef]
- Helfrecht, B.A.; Semino, R.; Pireddu, G.; Auerbach, S.M.; Ceriotti, M. A new kind of atlas of zeolite building blocks. J. Chem. Phys. 2019, 151, 154112. [Google Scholar] [CrossRef]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Bingre, R.; Louis, B.; Nguyen, P. An Overview on Zeolite Shaping Technology and Solutions to Overcome Diffusion Limitations. Catalysts 2018, 8, 163. [Google Scholar] [CrossRef]
- Devadas, M.; Kröcher, O.; Wokaun, A. Catalytic investigation of Fe-ZSM5 in the selective catalytic reduction of NOx with NH3. React. Kinet. Catal. Lett. 2005, 86, 347–354. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Long, Y. Zeolite-based Materials for Gas Sensors. Sensors 2006, 6, 1751–1764. [Google Scholar] [CrossRef]
- Sahner, K.; Hagen, G.; Schönauer, D.; Reis, S.; Moos, R. Zeolites—Versatile materials for gas sensors. Solid State Ion. 2008, 179, 2416–2423. [Google Scholar] [CrossRef]
- Kurzweil, P.; Maunz, W.; Plog, C. Impedance of zeolite-based gas sensors. Sens. Actuators B Chem. 1995, 25, 653–656. [Google Scholar] [CrossRef]
- Marr, I.; Reiss, S.; Hagen, G.; Moos, R. Planar zeolite film-based potentiometric gas sensors manufactured by a combined thick-film and electroplating technique. Sensors 2011, 11, 7736–7748. [Google Scholar] [CrossRef] [PubMed]
- Reiß, S.; Hagen, G.; Moos, R. Zeolite-based Impedimetric Gas Sensor Device in Low-cost Technology for Hydrocarbon Gas Detection. Sensors 2008, 8, 7904–7916. [Google Scholar] [CrossRef]
- Dietrich, M.; Rauch, D.; Simon, U.; Porch, A.; Moos, R. Ammonia storage studies on H-ZSM-5 zeolites by microwave cavity perturbation: Correlation of dielectric properties with ammonia storage. J. Sens. Sens. Syst. 2015, 4, 263–269. [Google Scholar] [CrossRef]
- Rauch, D.; Kubinski, D.; Cavataio, G.; Upadhyay, D.; Moos, R. Ammonia Loading Detection of Zeolite SCR Catalysts using a Radio Frequency based Method. SAE Int. J. Engines 2015, 8, 1126–1135. [Google Scholar] [CrossRef]
- Franke, M.E.; Simon, U.; Moos, R.; Knezevic, A.; Müller, R.; Plog, C. Development and working principle of an ammonia gas sensor based on a refined model for solvate supported proton transport in zeolites. Phys. Chem. Chem. Phys. 2003, 5, 5195–5198. [Google Scholar] [CrossRef]
- Yoo, Y.; Choi, B.-D. Readout Circuits for Capacitive Sensors. Micromachines 2021, 12, 960. [Google Scholar] [CrossRef]
- König, M.; Müller, M.; Hartmann, I. Emission reduction process for the energetic use of biogenic residues. IOP Conf. Ser. Earth Environ. Sci. 2021, 642, 012006. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, G.; Shao, Y.; Riffat, S.B. Experimental investigation on flue gas emissions of a domestic biomass boiler under normal and idle combustion conditions. Int. J. Low-Carbon Technol. 2010, 5, 88–95. [Google Scholar] [CrossRef]
- Ritter, T.; Wiegärtner, S.; Hagen, G.; Moos, R. Simulation of a thermoelectric gas sensor that determines hydrocarbon concentrations in exhausts and the light-off temperature of catalyst materials. J. Sens. Sens. Syst. 2017, 6, 395–405. [Google Scholar] [CrossRef]
- Hagen, G.; Herrmann, J.; Zhang, X.; Kohler, H.; Hartmann, I.; Moos, R. Application of a Robust Thermoelectric Gas Sensor in Firewood Combustion Exhausts. Sensors 2023, 23, 2930. [Google Scholar] [CrossRef]
- Wiegärtner, S.; Hagen, G.; Kita, J.; Reitmeier, W.; Hien, M.; Grass, P.; Moos, R. Thermoelectric hydrocarbon sensor in thick-film technology for on-board-diagnostics of a diesel oxidation catalyst. Sens. Actuators B Chem. 2015, 214, 234–240. [Google Scholar] [CrossRef]
- Herrmann, J.; Hagen, G.; Kita, J.; Noack, F.; Bleicker, D.; Moos, R. Multi-gas sensor to detect simultaneously nitrogen oxides and oxygen. J. Sens. Sens. Syst. 2020, 9, 327–335. [Google Scholar] [CrossRef]
- Wöhrl, T.; Herrmann, J.; Kita, J.; Moos, R.; Hagen, G. Methods to investigate the temperature distribution of heated ceramic gas sensors for high-temperature applications. J. Sens. Sens. Syst. 2023, 12, 205–214. [Google Scholar] [CrossRef]
- Plog, C.; Maunz, W.; Kurzweil, P.; Obermeier, E.; Scheibe, C. Combustion gas sensitivity of zeolite layers on thin-film capacitors. Sens. Actuators B Chem. 1995, 25, 403–406. [Google Scholar] [CrossRef]
- Mamishev, A.; Sundara-Rajan, K.; Yang, F.; Du, Y.; Zahn, M. Interdigital sensors and transducers. Proc. IEEE 2004, 92, 808–845. [Google Scholar] [CrossRef]
- Franke, M.E.; Simon, U. Characteristics of Proton Hopping in Zeolite H-ZSM5. Phys. Status Solidi (B) 2000, 218, 287–290. [Google Scholar] [CrossRef]
- Simon, U.; Sanders, D.; Jockel, J.; Heppel, C.; Brinz, T. Design strategies for multielectrode arrays applicable for high-throughput impedance spectroscopy on novel gas sensor materials. J. Comb. Chem. 2002, 4, 511–515. [Google Scholar] [CrossRef]
- Sharma, R.K.; Chan, P.C.; Tang, Z.; Yan, G.; Hsing, I.-M.; Sin, J.K. Investigation of stability and reliability of tin oxide thin-film for integrated micro-machined gas sensor devices. Sens. Actuators B Chem. 2001, 81, 9–16. [Google Scholar] [CrossRef]
- Liang, T.-X.; Sun, W.Z.; Wang, L.-D.; Wang, Y.H.; Li, H.-D. Effect of surface energies on screen printing resolution. IEEE Trans. Compon. Packag. Manuf. Technol. Part B 1996, 19, 423–426. [Google Scholar] [CrossRef]
- Bancroft, R.C. A Survey of Laser Trimming. Microelectron. Int. 1984, 2, 18–24. [Google Scholar] [CrossRef]
- Wagner, R.H. Functional Laser Trimming: An Overview. Laser Process. Semicond. Hybrids 1986, 611, 8–18. [Google Scholar] [CrossRef]
- Vankoningsveld, H.; Jansen, J.; Vanbekkum, H. The monoclinic framework structure of zeolite H-ZSM-5. Comparison with the orthorhombic framework of as-synthesized ZSM-5. Zeolites 1990, 10, 235–242. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H.; Meier, W.M. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar] [CrossRef]
- Chen, P.; Schönebaum, S.; Simons, T.; Rauch, D.; Dietrich, M.; Moos, R.; Simon, U. Correlating the Integral Sensing Properties of Zeolites with Molecular Processes by Combining Broadband Impedance and DRIFT Spectroscopy—A New Approach for Bridging the Scales. Sensors 2015, 15, 28915–28941. [Google Scholar] [CrossRef]
- Di Iorio, J.R.; Bates, S.A.; Verma, A.A.; Delgass, W.N.; Ribeiro, F.H.; Miller, J.T.; Gounder, R. The Dynamic Nature of Brønsted Acid Sites in Cu–Zeolites During NOx Selective Catalytic Reduction: Quantification by Gas-Phase Ammonia Titration. Top. Catal. 2015, 58, 424–434. [Google Scholar] [CrossRef]
- Kustov, A.L.; Egeblad, K.; Kustova, M.; Hansen, T.W.; Christensen, C.H. Mesoporous Fe-containing ZSM-5 zeolite single crystal catalysts for selective catalytic reduction of nitric oxide by ammonia. Top. Catal. 2007, 45, 159–163. [Google Scholar] [CrossRef]
- Jiang, T.; Göltl, F.; Bulo, R.E.; Sautet, P. Effect of Temperature on the Adsorption of Short Alkanes in the Zeolite SSZ-13—Adapting Adsorption Isotherms to Microporous Materials. ACS Catal. 2014, 4, 2351–2358. [Google Scholar] [CrossRef]
- Schmohl, A.; Miklos, A.; Hess, P. Effects of adsorption-desorption processes on the response time and accuracy of photoacoustic detection of ammonia. Appl. Opt. 2001, 40, 2571–2578. [Google Scholar] [CrossRef]
- Vaittinen, O.; Metsälä, M.; Persijn, S.; Vainio, M.; Halonen, L. Adsorption of ammonia on treated stainless steel and polymer surfaces. Appl. Phys. B 2014, 115, 185–196. [Google Scholar] [CrossRef]
- Hagen, G.; Schulz, A.; Knörr, M.; Moos, R. Four-Wire Impedance Spectroscopy on Planar Zeolite/Chromium Oxide Based Hydrocarbon Gas Sensors. Sensors 2007, 7, 2681–2692. [Google Scholar] [CrossRef]
- Chen, P.; Rizzotto, V.; Xie, K.; Simon, U. Tracking mobile active sites and intermediates in NH3-SCR over zeolite catalysts by impedance-based in situ spectroscopy. React. Chem. Eng. 2019, 4, 986–994. [Google Scholar] [CrossRef]
- Franke, M.E.; Sierka, M.; Simon, U.; Sauer, J. Translational proton motion in zeolite H-ZSM-5. Energy barriers and jump rates from DFT calculations. Phys. Chem. Chem. Phys. 2002, 4, 5207–5216. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Vermesse, J.; Vidal, D.; Malbrunot, P. Gas Adsorption on Zeolites at High Pressure. Langmuir 1996, 12, 4190–4196. [Google Scholar] [CrossRef]
- Mofarahi, M.; Gholipour, F. Gas adsorption separation of CO2/CH4 system using zeolite 5A. Microporous Mesoporous Mater. 2014, 200, 1–10. [Google Scholar] [CrossRef]
- Hayhurst, D.T. Gas adsorption by some natural zeolites. Chem. Eng. Commun. 1980, 4, 729–735. [Google Scholar] [CrossRef]
- Ohlin, L.; Bazin, P.; Thibault-Starzyk, F.; Hedlund, J.; Grahn, M. Adsorption of CO2, CH4, and H2O in Zeolite ZSM-5 Studied Using In Situ ATR-FTIR Spectroscopy. J. Phys. Chem. C 2013, 117, 16972–16982. [Google Scholar] [CrossRef]
- Kohler, H.; Ojha, B.; Illyaskutty, N.; Hartmann, I.; Thiel, C.; Eisinger, K.; Dambacher, M. In situ high-temperature gas sensors: Continuous monitoring of the combustion quality of different wood combustion systems and optimization of combustion process. J. Sens. Sens. Syst. 2018, 7, 161–167. [Google Scholar] [CrossRef]
- Zhang, X.; Ojha, B.; Bichlmaier, H.; Hartmann, I.; Kohler, H. Extensive Gaseous Emissions Reduction of Firewood-Fueled Low Power Fireplaces by a Gas Sensor Based Advanced Combustion Airflow Control System and Catalytic Post-Oxidation. Sensors 2023, 23, 4679. [Google Scholar] [CrossRef] [PubMed]
- Hunger, B.; Heuchel, M.; Matysik, S.; Beck, K.; Einicke, W.D. Adsorption of water on ZSM-5 zeolites. Thermochim. Acta 1995, 269–270, 599–611. [Google Scholar] [CrossRef]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef]
- Rodríguez-González, L.; Hermes, F.; Bertmer, M.; Rodríguez-Castellón, E.; Jiménez-López, A.; Simon, U. The acid properties of H-ZSM-5 as studied by NH3-TPD and 27Al-MAS-NMR spectroscopy. Appl. Catal. A Gen. 2007, 328, 174–182. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K. Combustion, Chemistry, and Carbon Neutrality. Chem. Rev. 2023, 123, 5139–5219. [Google Scholar] [CrossRef]
- Simon, I.; Bârsan, N.; Bauer, M.; Weimar, U. Micromachined metal oxide gas sensors: Opportunities to improve sensor performance. Sens. Actuators B Chem. 2001, 73, 1–26. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wöhrl, T.; Kita, J.; Moos, R.; Hagen, G. Capacitive, Highly Selective Zeolite-Based Ammonia Sensor for Flue Gas Applications. Chemosensors 2023, 11, 413. https://doi.org/10.3390/chemosensors11070413
Wöhrl T, Kita J, Moos R, Hagen G. Capacitive, Highly Selective Zeolite-Based Ammonia Sensor for Flue Gas Applications. Chemosensors. 2023; 11(7):413. https://doi.org/10.3390/chemosensors11070413
Chicago/Turabian StyleWöhrl, Thomas, Jaroslaw Kita, Ralf Moos, and Gunter Hagen. 2023. "Capacitive, Highly Selective Zeolite-Based Ammonia Sensor for Flue Gas Applications" Chemosensors 11, no. 7: 413. https://doi.org/10.3390/chemosensors11070413
APA StyleWöhrl, T., Kita, J., Moos, R., & Hagen, G. (2023). Capacitive, Highly Selective Zeolite-Based Ammonia Sensor for Flue Gas Applications. Chemosensors, 11(7), 413. https://doi.org/10.3390/chemosensors11070413






