A Laser-Printed Surface-Enhanced Photoluminescence Sensor for the Sub-Nanomolar Optical Detection of Mercury in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
2.2. Synthesis of the Photoluminescent Probe (d114)
2.3. Laser Fabrication of the SEPL Sensor
2.4. Characterization
2.5. Sensing Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tutov, M.V.; Sergeev, A.A.; Zadorozhny, P.A.; Bratskaya, S.Y.; Mironenko, A.Y. Dendrimeric Rhodamine Based Fluorescent Probe for Selective Detection of Au. Sens. Actuators B Chem. 2018, 273, 916–920. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Tutov, M.V.; Sergeev, A.A.; Voznesenskiy, S.S.; Bratskaya, S.Y. On/off Rhodamine Based Fluorescent Probe for Detection of Au and Pd in Aqueous Solutions. Sens. Actuators B Chem. 2017, 246, 389–394. [Google Scholar] [CrossRef]
- Tutov, M.V.; Sergeev, A.A.; Shamich, N.I.; Chepak, A.K.; Mironenko, A.Y. Synthesis and Optical Properties of Rhodamine Terminated Organosilicon Dendrimers. Dye. Pigment. 2021, 184, 108783. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Tutov, M.V.; Chepak, A.K.; Zadorozhny, P.A.; Bratskaya, S.Y. A Novel Rhodamine-Based Turn-on Probe for Fluorescent Detection of Au3+ and Colorimetric Detection of Cu2+. Tetrahedron 2019, 75, 1492–1496. [Google Scholar] [CrossRef]
- Wang, L.; Ding, H.; Ran, X.; Tang, H.; Cao, D. Recent Progress on Reaction-Based BODIPY Probes for Anion Detection. Dye. Pigment. 2020, 172, 107857. [Google Scholar] [CrossRef]
- La, M.; Hao, Y.; Wang, Z.; Han, G.-C.; Qu, L. Selective and Sensitive Detection of Cyanide Based on the Displacement Strategy Using a Water-Soluble Fluorescent Probe. J. Anal. Methods Chem. 2016, 2016, 1462013. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sedgwick, A.C.; Sun, X.; Bull, S.D.; He, X.-P.; James, T.D. Reaction-Based Fluorescent Probes for the Detection and Imaging of Reactive Oxygen, Nitrogen, and Sulfur Species. Acc. Chem. Res. 2019, 52, 2582–2597. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Ha, J.; Cho, M.; Li, H.; Swamy, K.M.K.; Yoon, J. Recent Developments of BODIPY-Based Colorimetric and Fluorescent Probes for the Detection of Reactive Oxygen/Nitrogen Species and Cancer Diagnosis. Coord. Chem. Rev. 2021, 439, 213936. [Google Scholar] [CrossRef]
- Melnychuk, N.; Egloff, S.; Runser, A.; Reisch, A.; Klymchenko, A.S. Light-Harvesting Nanoparticle Probes for FRET-Based Detection of Oligonucleotides with Single-Molecule Sensitivity. Angew. Chemie Int. Ed. 2020, 59, 6811–6818. [Google Scholar] [CrossRef] [PubMed]
- Jurek, K.; Kabatc, J.; Kostrzewska, K.; Grabowska, M. New Fluorescence Probes for Biomolecules. Molecules 2015, 20, 13071–13079. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, R.; Samanta, S.; Qu, J.; Ohulchanskyy, T.Y. Recent Advances in Plasmon-Enhanced Luminescence for Biosensing and Bioimaging. Anal. Chim. Acta 2023, 1254, 341086. [Google Scholar] [CrossRef]
- Larson, D.R.; Zipfel, W.R.; Williams, R.M.; Clark, S.W.; Bruchez, M.P.; Wise, F.W.; Webb, W.W. Water-Soluble Quantum Dots for Multiphoton Fluorescence Imaging in vivo. Science 2003, 300, 1434–1436. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Yang, L.; Vong, C.I.; Chan, K.F.; Wu, W.K.K.; Kwong, T.N.Y.; Lo, N.W.S.; Ip, M.; Wong, S.H.; et al. Real-Time Tracking of Fluorescent Magnetic Spore–Based Microrobots for Remote Detection of C. Diff Toxins. Sci. Adv. 2019, 5, eaau9650. [Google Scholar] [CrossRef]
- Feng, X.; Liu, L.; Wang, S.; Zhu, D. Water-Soluble Fluorescent Conjugated Polymers and Their Interactions with Biomacromolecules for Sensitive Biosensors. Chem. Soc. Rev. 2010, 39, 2411. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, L.; Yang, Q.; Lv, F.; Wang, S. Water-Soluble Conjugated Polymers for Imaging, Diagnosis, and Therapy. Chem. Rev. 2012, 112, 4687–4735. [Google Scholar] [CrossRef]
- Bouhedda, F.; Fam, K.T.; Collot, M.; Autour, A.; Marzi, S.; Klymchenko, A.; Ryckelynck, M. A Dimerization-Based Fluorogenic Dye-Aptamer Module for RNA Imaging in Live Cells. Nat. Chem. Biol. 2020, 16, 69–76. [Google Scholar] [CrossRef]
- Farzan, V.M.; Kvach, M.V.; Aparin, I.O.; Kireev, D.E.; Prikazchikova, T.A.; Ustinov, A.V.; Shmanai, V.V.; Shipulin, G.A.; Korshun, V.A.; Zatsepin, T.S. Novel Homo Yin-Yang Probes Improve Sensitivity in RT-QPCR Detection of Low Copy HIV RNA. Talanta 2019, 194, 226–232. [Google Scholar] [CrossRef]
- Mironenko, A.Y.; Tutov, M.V.; Chepak, A.K.; Bratskaya, S.Y. FRET Pumping of Rhodamine-Based Probe in Light-Harvesting Nanoparticles for Highly Sensitive Detection of Cu2+. Anal. Chim. Acta 2022, 1229, 340388. [Google Scholar] [CrossRef]
- Chepak, A.; Balatskiy, D.; Tutov, M.; Mironenko, A.; Bratskaya, S. Light Harvesting Nanoprobe for Trace Detection of Hg2+ in Water. Molecules 2023, 28, 1633. [Google Scholar] [CrossRef]
- Sergeeva, K.A.; Tutov, M.V.; Zhizhchenko, A.Y.; Cherepakhin, A.B.; Leonov, A.A.; Chepak, A.K.; Mironenko, A.Y.; Sergeev, A.A. Ordered Photonic Nanojet Arrays for Luminescent Optical Sensing in Liquid and Gaseous Media. Sens. Actuators B Chem. 2023, 381, 133435. [Google Scholar] [CrossRef]
- Bratskaya, S.; Sergeeva, K.; Konovalova, M.; Modin, E.; Svirshchevskaya, E.; Sergeev, A.; Mironenko, A.; Pestov, A. Ligand-Assisted Synthesis and Cytotoxicity of ZnSe Quantum Dots Stabilized by N-(2-Carboxyethyl) chitosans. Colloids Surf. B Biointerfaces 2019, 182, 110342. [Google Scholar] [CrossRef]
- Kümmerlen, J.; Leitner, A.; Brunner, H.; Aussenegg, F.R.; Wokaun, A. Enhanced Dye Fluorescence over Silver Island Films: Analysis of the Distance Dependence. Mol. Phys. 1993, 80, 1031–1046. [Google Scholar] [CrossRef]
- Dostovalov, A.; Bronnikov, K.; Korolkov, V.; Babin, S.; Mitsai, E.; Mironenko, A.; Tutov, M.; Zhang, D.; Sugioka, K.; Maksimovic, J.; et al. Hierarchical Anti-Reflective Laser-Induced Periodic Surface Structures (LIPSSs) on Amorphous Si Films for Sensing Applications. Nanoscale 2020, 12, 13431–13441. [Google Scholar] [CrossRef] [PubMed]
- Borodaenko, Y.; Gurbatov, S.; Tutov, M.; Zhizhchenko, A.; Kulinich, S.A.; Kuchmizhak, A.; Mironenko, A. Direct Femtosecond Laser Fabrication of Chemically Functionalized Ultra-Black Textures on Silicon for Sensing Applications. Nanomaterials 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Mironenko, A.Y.; Tutov, M.V.; Sergeev, A.A.; Mitsai, E.V.; Ustinov, A.Y.; Zhizhchenko, A.Y.; Linklater, D.P.; Bratskaya, S.Y.; Juodkazis, S.; Kuchmizhak, A.A. Ultratrace Nitroaromatic Vapor Detection via Surface-Enhanced Fluorescence on Carbazole-Terminated Black Silicon. ACS Sens. 2019, 4, 2879–2884. [Google Scholar] [CrossRef]
- Sergeeva, K.A.; Tutov, M.V.; Voznesenskiy, S.S.; Shamich, N.I.; Mironenko, A.Y.; Sergeev, A.A. Highly-Sensitive Fluorescent Detection of Chemical Compounds via Photonic Nanojet Excitation. Sens. Actuators B Chem. 2020, 305, 127354. [Google Scholar] [CrossRef]
- Borodaenko, Y.; Khairullina, E.; Levshakova, A.; Shmalko, A.; Tumkin, I.; Gurbatov, S.; Mironenko, A.; Mitsai, E.; Modin, E.; Gurevich, E.L.; et al. Noble-Metal Nanoparticle-Embedded Silicon Nanogratings via Single-Step Laser-Induced Periodic Surface Structuring. Nanomaterials 2023, 13, 1300. [Google Scholar] [CrossRef]
- Taylor, A.B.; Zijlstra, P. Single-Molecule Plasmon Sensing: Current Status and Future Prospects. ACS Sens. 2017, 2, 1103–1122. [Google Scholar] [CrossRef]
- Jiang, R.; Li, B.; Fang, C.; Wang, J. Metal/Semiconductor Hybrid Nanostructures for Plasmon-Enhanced Applications. Adv. Mater. 2014, 26, 5274–5309. [Google Scholar] [CrossRef]
- Fusco, Z.; Rahmani, M.; Tran-Phu, T.; Ricci, C.; Kiy, A.; Kluth, P.; Della Gaspera, E.; Motta, N.; Neshev, D.; Tricoli, A. Photonic Fractal Metamaterials: A Metal–Semiconductor Platform with Enhanced Volatile-Compound Sensing Performance. Adv. Mater. 2020, 32, 2002471. [Google Scholar] [CrossRef]
- Borodaenko, Y.; Syubaev, S.; Khairullina, E.; Tumkin, I.; Gurbatov, S.; Mironenko, A.; Mitsai, E.; Zhizhchenko, A.; Modin, E.; Gurevich, E.L.; et al. On-Demand Plasmon Nanoparticle-Embedded Laser-Induced Periodic Surface Structures (LIPSSs) on Silicon for Optical Nanosensing. Adv. Opt. Mater. 2022, 10, 2201094. [Google Scholar] [CrossRef]
- Gurbatov, S.O.; Modin, E.; Puzikov, V.; Tonkaev, P.; Storozhenko, D.; Sergeev, A.; Mintcheva, N.; Yamaguchi, S.; Tarasenka, N.N.; Chuvilin, A.; et al. Black Au-Decorated TiO2 Produced via Laser Ablation in Liquid. ACS Appl. Mater. Interfaces 2021, 13, 6522–6531. [Google Scholar] [CrossRef]
- Zuev, D.A.; Makarov, S.V.; Mukhin, I.S.; Milichko, V.A.; Starikov, S.V.; Morozov, I.A.; Shishkin, I.I.; Krasnok, A.E.; Belov, P.A. Fabrication of Hybrid Nanostructures via Nanoscale Laser-Induced Reshaping for Advanced Light Manipulation. Adv. Mater. 2016, 28, 3087–3093. [Google Scholar] [CrossRef]
- Borodaenko, Y.; Syubaev, S.; Gurbatov, S.; Zhizhchenko, A.; Porfirev, A.; Khonina, S.; Mitsai, E.; Gerasimenko, A.V.; Shevlyagin, A.; Modin, E.; et al. Deep Subwavelength Laser-Induced Periodic Surface Structures on Silicon as a Novel Multifunctional Biosensing Platform. ACS Appl. Mater. Interfaces 2021, 13, 54551–54560. [Google Scholar] [CrossRef]
- Bonse, J.; Gräf, S. Maxwell Meets Marangoni—A Review of Theories on Laser-Induced Periodic Surface Structures. Laser Photon. Rev. 2020, 14, 2000215. [Google Scholar] [CrossRef]
- Broadhead, E.J.; Tibbetts, K.M. Fabrication of Gold–Silicon Nanostructured Surfaces with Reactive Laser Ablation in Liquid. Langmuir 2020, 36, 10120–10129. [Google Scholar] [CrossRef]
- Ran, P.; Jiang, L.; Li, X.; Li, B.; Zuo, P.; Lu, Y. Femtosecond Photon-Mediated Plasma Enhances Photosynthesis of Plasmonic Nanostructures and Their SERS Applications. Small 2019, 15, 1804899. [Google Scholar] [CrossRef]
- Li, C.; Hu, J.; Jiang, L.; Xu, C.; Li, X.; Gao, Y.; Qu, L. Shaped Femtosecond Laser Induced Photoreduction for Highly Controllable Au Nanoparticles Based on Localized Field Enhancement and Their SERS Applications. Nanophotonics 2020, 9, 691–702. [Google Scholar] [CrossRef]
- Garcia-Lechuga, M.; Puerto, D.; Fuentes-Edfuf, Y.; Solis, J.; Siegel, J. Ultrafast Moving-Spot Microscopy: Birth and Growth of Laser-Induced Periodic Surface Structures. ACS Photonics 2016, 3, 1961–1967. [Google Scholar] [CrossRef]
- Zhang, D.; Ranjan, B.; Tanaka, T.; Sugioka, K. Underwater Persistent Bubble-Assisted Femtosecond Laser Ablation for Hierarchical Micro/Nanostructuring. Int. J. Extrem. Manuf. 2020, 2, 015001. [Google Scholar] [CrossRef]
- Zhang, D.; Sugioka, K. Hierarchical Microstructures with High Spatial Frequency Laser Induced Periodic Surface Structures Possessing Different Orientations Created by Femtosecond Laser Ablation of Silicon in Liquids. Opto-Electron. Adv. 2019, 2, 19000201–19000218. [Google Scholar] [CrossRef]
- Allahyari, E.; Nivas, J.J.; Skoulas, E.; Bruzzese, R.; Tsibidis, G.D.; Stratakis, E.; Amoruso, S. On the Formation and Features of the Supra-Wavelength Grooves Generated during Femtosecond Laser Surface Structuring of Silicon. Appl. Surf. Sci. 2020, 528, 146607. [Google Scholar] [CrossRef]
- Kay, K.E.; Batista, L.M.F.; Tibbetts, K.M.; Ferri, J.K. Stability of Uncapped Gold Nanoparticles Produced via Laser Reduction in Liquid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129860. [Google Scholar] [CrossRef]
- Frias Batista, L.M.; Kunzler, K.; John, M.G.; Clark, B.; Bullock, A.; Ferri, J.; Gupton, B.F.; Tibbetts, K.M. Laser Synthesis of Uncapped Palladium Nanocatalysts. Appl. Surf. Sci. 2021, 557, 149811. [Google Scholar] [CrossRef]
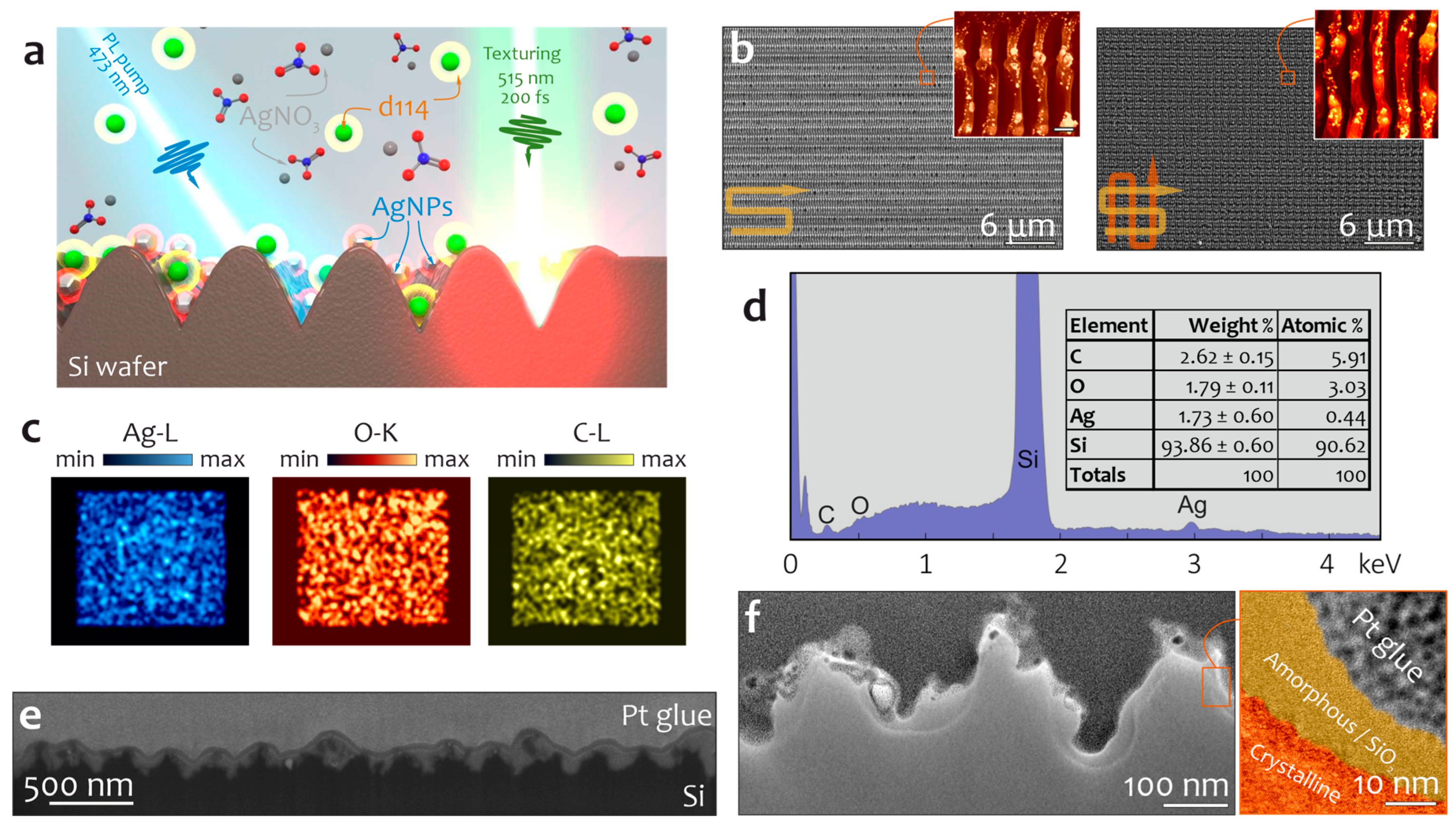
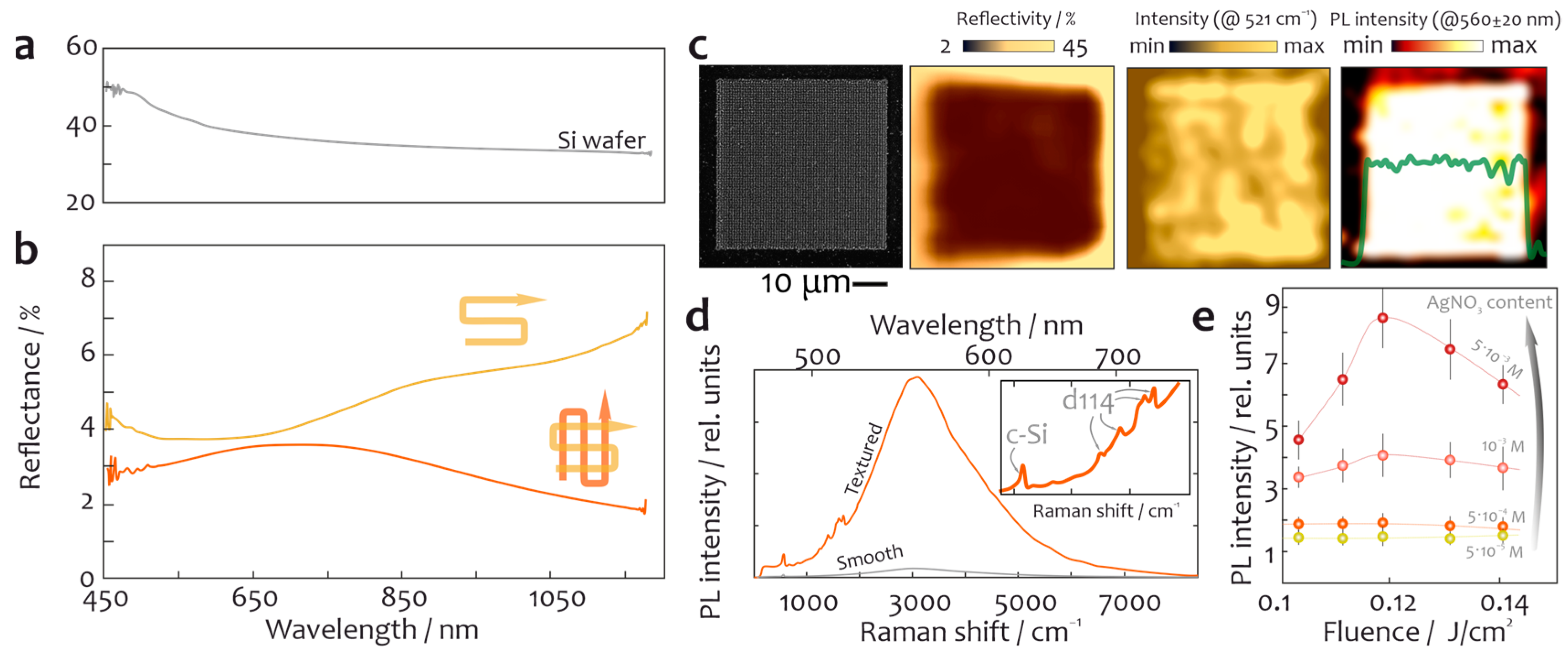
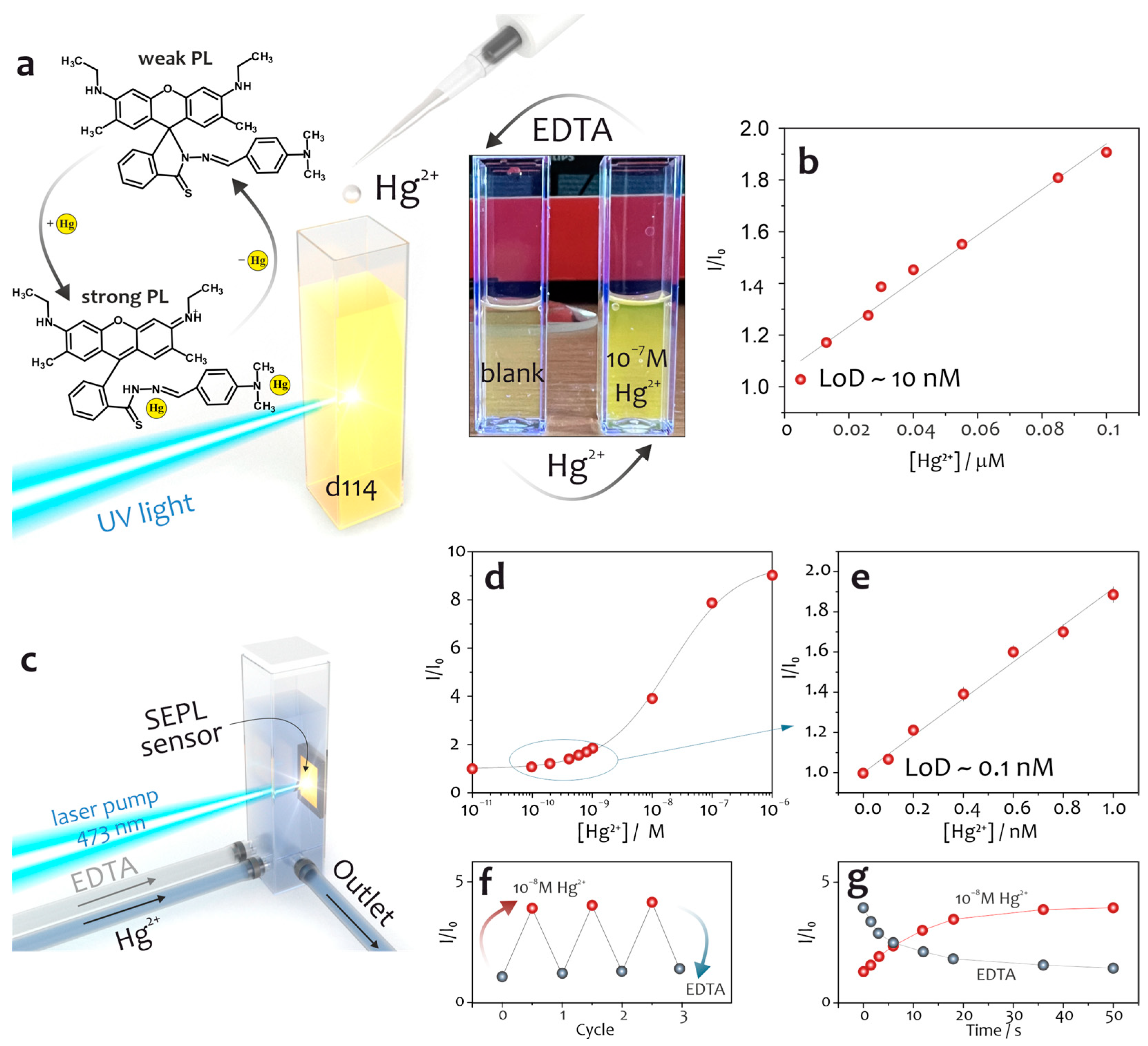
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borodaenko, Y.; Gurbatov, S.; Modin, E.; Chepak, A.; Tutov, M.; Mironenko, A.; Kuchmizhak, A. A Laser-Printed Surface-Enhanced Photoluminescence Sensor for the Sub-Nanomolar Optical Detection of Mercury in Water. Chemosensors 2023, 11, 307. https://doi.org/10.3390/chemosensors11050307
Borodaenko Y, Gurbatov S, Modin E, Chepak A, Tutov M, Mironenko A, Kuchmizhak A. A Laser-Printed Surface-Enhanced Photoluminescence Sensor for the Sub-Nanomolar Optical Detection of Mercury in Water. Chemosensors. 2023; 11(5):307. https://doi.org/10.3390/chemosensors11050307
Chicago/Turabian StyleBorodaenko, Yulia, Stanislav Gurbatov, Evgeny Modin, Aleksandr Chepak, Mikhail Tutov, Aleksandr Mironenko, and Aleksandr Kuchmizhak. 2023. "A Laser-Printed Surface-Enhanced Photoluminescence Sensor for the Sub-Nanomolar Optical Detection of Mercury in Water" Chemosensors 11, no. 5: 307. https://doi.org/10.3390/chemosensors11050307
APA StyleBorodaenko, Y., Gurbatov, S., Modin, E., Chepak, A., Tutov, M., Mironenko, A., & Kuchmizhak, A. (2023). A Laser-Printed Surface-Enhanced Photoluminescence Sensor for the Sub-Nanomolar Optical Detection of Mercury in Water. Chemosensors, 11(5), 307. https://doi.org/10.3390/chemosensors11050307






