Abstract
Volatile organoamines are important industrial raw materials and chemicals. Long-term exposure to amines could be harmful to human health and even cause serious pollution. In this study, SnO2 decorated g-C3N4 material was fabricated and used as a sensor material for the detection of ethanolamine (EA). The structures, morphology, surface chemical states, and band structure were characterized, and gas sensing was studied. The results showed that SnO2 nanoparticles were dispersed on g-C3N4, and band structure was dependent on g-C3N4 doping. Notably, the interface heterojunction was conducive to electron transferring and O2 molecule adsorption; the formed reactive oxygen species enhanced the reaction between oxygen and EA, thus leading to high sensitivity to EA. This composite exhibited a high response that was 2.6 times higher than that of pure SnO2, and the detection limit reached 294 ppb. A g-C3N4/SnO2-based sensor displayed a high selectivity to EA with a fast response time (1 s) and recovery time (20 s) at low operating temperatures. In particular, this sensor exhibited a linear relationship between the response and concentration, which is required for quantitative analysis.
1. Introduction
Volatile organic amine compounds (VOACs) have a wide range of sources [1]. Natural sources mainly include animal excrement, animal and plant remains, and microbial products; non-natural sources mainly include chemical production, industrial waste discharge, and domestic waste. The harm inflicted on living organisms and the ecological environment by VOACs should not be underestimated. The excessive presence of VOACs will cause biological poisoning and environmental deterioration [2]. In China, the emissions from non-natural sources are much higher than those from natural sources [3]. It is not enough to rely on the self-regulation of the ecosystem. Therefore, it is necessary to deal with these harmful compounds artificially. Strengthening the monitoring of VOACs such as triethylamine (TEA) and ethanolamine (EA) is an important means to control their emissions and reduce their harm, so suitable detection methods and detection materials are particularly needed.
VOCs are usually detected via spectrometry, chromatography, etc., which have the advantages of low error rates and strong stability. However, their operation is complicated; they usually need to be used in combination with other instruments, and these factors restrict their portable applications. Therefore, it is necessary to develop sensing material to quickly detect VOCs on a large scale. In this regard, semiconductor metal oxide materials, due to their simple preparation and low cost, are recognized as materials with applications in chemical sensors [4,5,6,7,8]. Our previous research reported strategies for the application of ZnO and SnO2 via non-metal and metal doping, and we found that different, modified oxides can respond to different amines, such as S-ZnO for EA [9] and Cr-SnO2 for TEA [10]. Another case is CuO/SnO2: the heterojunction between the interface of CuO and SnO2 cause this composite to exhibit a high response to TEA [11]. For comparison, SnO2 is also a common sensing material [12,13,14,15]. For example, Zhang et al. prepared nanostructural SnO2 for ethanol detection and improved its performance [16]. Ma et al. prepared mesoporous SnO2 for the fast detection of formaldehyde [17]. Din et al. developed NiO/SnO2 heterojunction material to detect ethanol, and it exhibited a high performance [18]. Dai et al. prepared rod-like Au-modified ZnO nanoflowers for EA detection [19]. However, doped and oxide-modified SnO2 is only efficient in the identification of TEA and other VOCs. In order to improve its response to EA, we chose to disperse SnO2 on a matrix and construct a heterojunction by introducing non-metallic materials to this material.
Graphitic carbon nitride (g-C3N4), which possesses a two-dimensional structure and is composed of carbon and nitrogen atoms, is a promising sensitization material [20]. It is an n-type semiconductor material with many advantages, such as a high specific surface area, excellent catalytic properties, remarkable two-dimensional material properties, and a gas adsorption ability [21], so it has attracted the attention of many researchers. g-C3N4 has been used in photocatalysis [22,23], energy storage [24], gas sensing [25], and optoelectronic devices [26]. Because of its distinctive two-dimensional structure, it can be used to improve the gas sensitivity of semiconductor metal oxides for applications in gas sensors.
g-C3N4 can form heterogeneous structures with semiconductor metal oxides, which can not only cause metal oxide nanoparticles to have good dispersibility but also optimize their gas sensitivity. Cao et al. [27] synthesized a SnO2 compound material with two-dimensional g-C3N4 nanosheets via a hydrothermal route, and the compound demonstrated a high sensitivity to ethanol gas. The sensitivity to 500 ppm ethanol gas was 240 at 300 °C. Lu et al. [28] prepared a 2D/2D ZnO/g-C3N4 heterojunction material, and the results showed excellent NO2 sensing performance and a response to 7 ppm NO2 that reached 44.8. Niu et al. prepared mesoporous Co3O4 nanowires modified with g-C3N4 nanosheets, and this composite displayed a high level of response to toluene gas [29]. The high surface area and great electronic structure of two-dimensional g-C3N4 are beneficial to improving the gas sensitivity of semiconductors. These composites have enhanced response rates and selectivity compared to pure metal oxides.
In this work, SnO2-decorated g-C3N4 materials were synthesized and characterized, and the band structure was regulated by changing g-C3N4. Gas-sensing performance was studied based on the response to volatile organic compounds.
2. Experimental
2.1. Chemicals and Reagents
All reagents were analytical grade and were not further purified before use. Urea was purchased from Tianjin Cameo Chemical Reagent Co., Ltd., Tianjin, China. Stannous chloride was purchased from Tianjin Guangfu Technology Development Co., Ltd., Tianjin, China. Thiourea was purchased from Tianjin Kaitong Chemical Reagent Co. Ltd., Tianjin, China. EA was purchased from Tianjin FuchenChemical Reagent Co. Ltd., Tianjin, China.
2.2. Preparation of g-C3N4
Graphitic carbon nitride (g-C3N4) was directly synthesized by pyrolysis urea in a muffle furnace. First, 20 g urea was put into an alumina crucible with a cover, then kept at 550 °C for 4 h (heating rate v = 10 °C/min). The light yellow g-C3N4 sample was collected. A certain amount of light yellow g-C3N4 powder was ultrasonically treated in deionized water for 60 min and then dried.
2.3. Synthesis of SnO2 and g-C3N4/SnO2 Materials
The procedure of material synthesis is shown in Scheme 1. In a typical synthesis of g-C3N4/SnO2-5, 16 mmol stannous chloride and 16 mmol thiourea were dissolved in 120 mL deionized water and stirred for 50 h, resulting in a solution labeled A. The theoretical content of 5 wt% g-C3N4 was stirred and mixed with 30 mL A suspension for 30 min and then kept at 140 °C for 8 h. The g-C3N4/SnO2-5 sample was obtained via centrifugation with deionized water and dried, followed by heat treatment for 2 h at 550 °C in air. According to the same procedure, pure SnO2 and SnO2 hybrid synthesized by changing g-C3N4 amount, which were labeled as g-C3N4/SnO2-x (x is the theoretical mass fraction of g-C3N4 to SnO2, i.e., 2.5% and 10%).
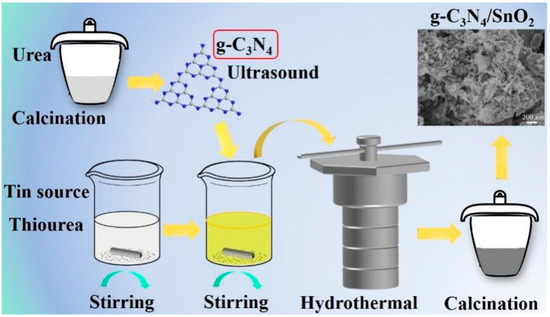
Scheme 1.
Synthetic procedure of g-C3N4 decorated SnO2 materials.
2.4. Characterization of g-C3N4/SnO2 Materials
The crystal structures of as-synthesized g-C3N4/SnO2 materials were analyzed via XRD (Shimadzu, Tokyo, Japan, XRD-6000, with high-intensity Cu Kα radiation, λ = 0.15418 nm). The morphology and microstructure were observed via SEM (Hitachi SU8010, Hitachi, Japan) and TEM (JEOL, JEM-2100F, Tokyo, Japan) at the accelerating voltage of 5 kV and 200 kV, respectively. The specific surface areas of the materials were recorded via the Bronner–Emmett–Teller (BET) method, and the pore structures were analyzed using N2 adsorption–desorption technique (MicromeriticsTristar II 3020, Atlanta, GA, USA).
2.5. Fabrication of Sensors and VOCs Detection
The gas sensors for the tests were fabricated as follows. Briefly, the SnO2-based materials are dispersed by mixing the as-synthesized SnO2 or g-C3N4/SnO2 powder with ethanol. A few drops of dispersion were coated thinly and evenly onto the surface of alumina ceramic tube with Au electrodes, Pt wires, and Ni-Cr wire as heater (Scheme 2a). Ceramic tubes coated with SnO2-based materials were dried at 80 °C for 1h and then heated at 300 °C for 2 h. Finally, the element should be welded on the pedestal and then inserted in the test circuit (Scheme 2b).
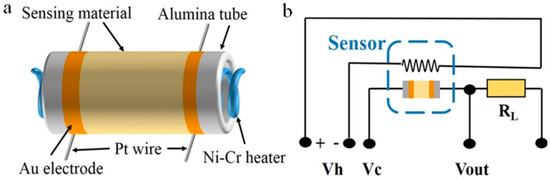
Scheme 2.
The construction of gas sensor (a) and electric circuit of test device (b).
The gas sensing properties were measured using WS-30A static test system (Winsen Electronics Co. Ltd., Zhengzhou, China). The sensitivity (S) of sensor is defined as Ra/Rg, where Ra and Rg denote the resistance in air and target gas, respectively. The environment’s humidity is about 35%.
3. Results and Discussion
Figure 1a illustrates the synthetic route of SnO2 nanoparticles anchored on the g-C3N4 (g-C3N4/SnO2) by simple hydrolysis at room temperature, hydrothermal, and heat treatment. Thiourea was used as the accelerant and stabilizer, and the yellow precursor was obtained by hydrolysis, dehydration, and partial oxidation of stannum ion. This is a slow process in which only a small amount of Sn2+ is oxidized to tin dioxide. In the stirring process, thioureas (CH4N2S), which produce amino (−NH2), imino(−NH), and mercaptan (−SH), react with HCl generated via hydrolysis of tin tetrachloride (SnCl4), which accelerates the continuous hydrolysis process. Moreover, the coupling of Sn4+ with sulfhydryl groups plays a stabilizing role, contributing to the formation of stable nanoscale particles. During the hydrothermal process, g-C3N4 is agglomerated on the surface, forming a sheet structure of g-C3N4 coated with tin dioxide precursor nanoparticles. Finally, the g-C3N4 composite coated with SnO2 nanoparticles was prepared via drying and calcination.
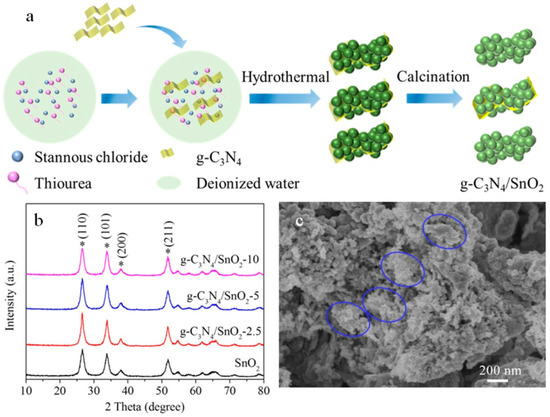
Figure 1.
(a) Schematic diagram of the formation process; (b) XRD patterns of as-synthesized g-C3N4/SnO2 (* denotes SnO2 characteristic peaks); (c) SEM image of g-C3N4/SnO2-5 material.
The phase structure and crystal structure were characterized via XRD. Figure S1 shows (100) and (002) planes corresponding to g-C3N4 (JCPDS, No. 87-1526) at 13.0° and 27.6°, respectively. Figure 1b shows the XRD patterns of the pure SnO2 nanoparticles and g-C3N4/SnO2 composites. The positions of 26.6°, 33.9°, 37.9°, and 51.8°, where the diffraction peaks are located, corresponding to (110), (101), (200), and (211) plants of the tetragonal rutile structure SnO2 (JCPDS, No. 41-1445), respectively. However, no other peaks of impurity, including g-C3N4 diffraction peaks, were detected in the XRD patterns, which may be due to the trace amounts of g-C3N4 in the g-C3N4/SnO2 composite materials, which did not reach the detection limit of the XRD. It is also possible that the diffraction peak of g-C3N4 near 27.6° and that of SnO2 at 26.6°overlaps. There is no obvious diffraction peak shift in the diffraction pattern, indicating that g-C3N4 and SnO2 do not exist independently, which means that n-n heterojunctions form on the contact interface between the two materials. Figure 1c shows the morphology of the g-C3N4/SnO2-5 composite. SnO2 nanoparticles are dispersed on the nanosheets of g-C3N4 and form a supported particle group (shown with a blue oval). The SEM images of g-C3N4, SnO2, and other g-C3N4/SnO2 show layer, nanoparticles aggregation, and nanosheets-supported particle group (Figure S2).
In order to analyze the chemical composition and state of g-C3N4/SnO2-5 material, XPS characterization was performed. Figure S3 shows the full XPS spectrum of g-C3N4/SnO2-5 material, in which the peaks are related to C, N, O, and Sn. It indicates the successful synthesis of g-C3N4/SnO2 composite material, which indirectly proves the existence of heterojunction between g-C3N4 and SnO2.
Figure 2a shows the high-resolution spectrum of C 1s, and the binding energies of 284.5, 285.1, 286.2, and 286.9 eV belong to the C-C bond of sp2, C-N, C-NH2, and C=NH in the triazine ring, respectively [30]. Figure 2b shows the spectrum of N 1s, and the binding energies of 399.7, 401.7, 402.00 eV and 402.65 eV belong to sp2 hybrid nitrogen (C-N=C), tertiary nitrogen (N-(C)3), N-O and C-N-H structure, respectively [31]. It indicates the existence of g-C3N4 in the g-C3N4/SnO2-5 composite. Figure 2c is the spectrum of O 1s, and the peaks appear at 530.95 and 531.8 eV. The peak at 530.95 eV is attributed to the lattice oxygen of SnO2, and the peak at 531.8 eV is attributed to defective oxygen. In Figure 2d, the peaks of 495.5 eV and 487.15 eV correspond to Sn 3d3/2 and Sn 3d5/2, indicating that Sn exists in the form of Sn4+. The results show that the material is composed of g-C3N4 and SnO2.
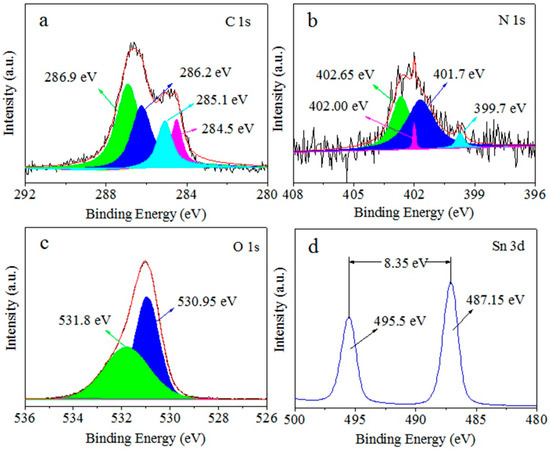
Figure 2.
XPS spectra of the C 1s (a), N 1s (b), O 1s (c), and Sn 3d (d) of as-synthesized g-C3N4/SnO2-5 nanomaterial.
Figure 3a shows that SnO2 and g-C3N4/SnO2-5 materials possess characteristics of type IV isotherms, and the specific surface areas of SnO2 and g-C3N4/SnO2-5 are 24.5 and 25.7 m2 g−1, respectively. When the relative pressure P/P0 is between 0.4 and 1.0, the adsorption capacity rises rapidly, showing a H3-type hysteresis loop, indicating that a narrow-slit aggregate structure is formed between g-C3N4 and SnO2 nanoparticles, and there are abundant mesoporous structures. Figure 3b shows the pore size distribution of SnO2 and g-C3N4/SnO2-5. It can be seen from the figure that both materials have smaller pore size distributions, and the average pore sizes of SnO2 and g-C3N4/SnO2-5 are 8.2 nm and 10.8 nm, respectively. The increased specific surface area is beneficial to improve the gas sensing property.
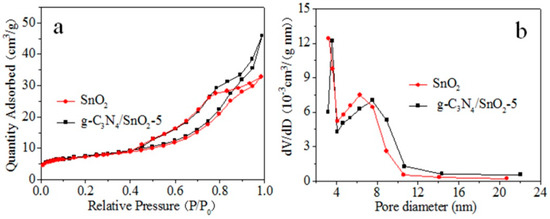
Figure 3.
N2 adsorption–desorption isotherms (a) and pore size distribution (b) of as-synthesized g-C3N4/SnO2−5.
The gas-sensing properties of these materials in the atmosphere were investigated. The working temperature has a great influence on the reaction kinetics of gas molecules and oxygen on the surface of the material, and it also has an effect on the gas-sensing behavior of the material. The optimal working temperature of SnO2 and g-C3N4/SnO2 materials for detecting ethanolamine (EA) was explored. Figure 4a shows the response of the sensor to 100 ppm EA gas in the test range of 200–280 °C. The optimal operating temperature is 240 °C for the four samples obtained by changing the g-C3N4 content. The response to EA increases with increasing temperature when the temperature is less than 240 °C. The possible reason for this is that when the optimal working temperature is lower than 240 °C, the activity of adsorbed oxygen on the surface of SnO2-based materials gradually increases with the increase in temperature, and the sensitivity to EA shows an upward trend. When the optimal working temperature is higher than 240 °C, the oxygen molecules, which are adsorbed on the surface of SnO2-based materials, are rapidly desorbed before reaction with EA molecules as temperature increases, resulting in a decrease in the sensitivity to EA. It can also be seen that the sensitivity to EA increases first and then decreases with the increase in g-C3N4 content in SnO2-based materials at the optimum working temperature. The g-C3N4/SnO2-5 shows the best gas sensitivity. The responses of pure SnO2, g-C3N4/SnO2-2.5, g-C3N4/SnO2-5, and g-C3N4/SnO2-10 to 100 ppm EA are 16.5, 18.7, 23.3, and 22, respectively. The optimal amount of g-C3N4 doped in SnO2 is conducive to better dispersion of the composite. The formation of heterojunctions and the increase in defects, which are between the 2D sheet g-C3N4 and SnO2 nanoparticles, provide more active sites for gas adsorption and reaction, and active sites improve the gas sensitivity. However, when the content of g-C3N4 in the composite exceeds a certain threshold (5 wt%), g-C3N4 nanosheets can be connected to form a micro-bridge on the surface. The micro-bridge may result in the reduction of the resistance of the g-C3N4/SnO2 composite, finally leading to a decrease in the performance of gas sensing.
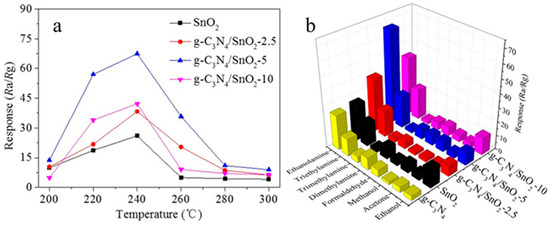
Figure 4.
(a) The response to 100 ppm EA at different temperatures; (b) selectivity to EA on exposure to various volatile gases.
Selectivity is also one of the important parameters to measure the gas-sensing performance of materials. At the optimum operating temperature of 240 °C, the sensing properties of SnO2-based materials with different g-C3N4 contents to 100 ppm of volatile organic compounds are tested. The results indicate good selectivity to EA (Figure 4b), which may be due to the fact that the bond energy of the C-N bond in the EA molecule is 307 kJ mol−1, indicating EA has strong reducibility compared with other volatile organic compounds. Therefore, as-prepared material exhibits better selectivity to EA. In addition, selectivity is dependent on the type of oxides and structure of composites. This is the reason that ZnO/g-C3N4 has no response to EA and only to DMA [21].
Figure 5a shows the transient response–recovery characteristics of SnO2 and g-C3N4/SnO2 to EA in the concentration range of 1 to 100 ppm at 240 °C. The results show that materials have a fast response and recovery ability for EA detection in a wide concentration range, and the response increases with increasing concentration. Notably, these materials exhibit an obvious response to 1–10 ppm of low-concentration EA (Figure 5b). Figure 6a displays a good linear relationship between response and concentration of EA. The detection limit of the g-C3N4/SnO2-5 sensor to EA is 294 ppb. To explore the stability of the g-C3N4/SnO2-5 sensor, the responses to low-concentration EA were recorded within nine days (Figure 6b), and the response value to 100 ppm EA at interval of 2 days reaches around 50 after 5 days, indicating g-C3N4/SnO2-5 sensor has better stability to EA.
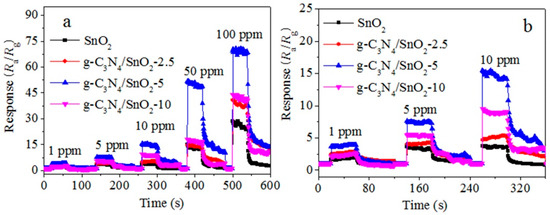
Figure 5.
Transient response–recovery profiles of composite material at 240 °C after exposure to (a) different concentration EA and (b) low concentration (1–5 ppm) EA.
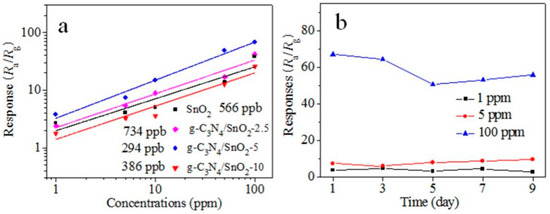
Figure 6.
(a) Linear relationships between response and concentration of EA; (b) stability of material within nine days.
The effect of band structure changes on sensing performance has been analyzed by calculating the band gap energy of as-synthesized g-C3N4/SnO2 from UV-vis results (Figure S4 and Figure 7a,c), and the linear extrapolation formula is referred to as our previous work [21]. The band gap energy is 2.92 and 2.86 eV for g-C3N4 and SnO2, and 2.81 eV for g-C3N4/SnO2-5 (Figure 7b,d). The results show that less energy can drive electrons to transfer from the valence to the conduction band, and the increased free electrons are helpful in improving the response to EA.
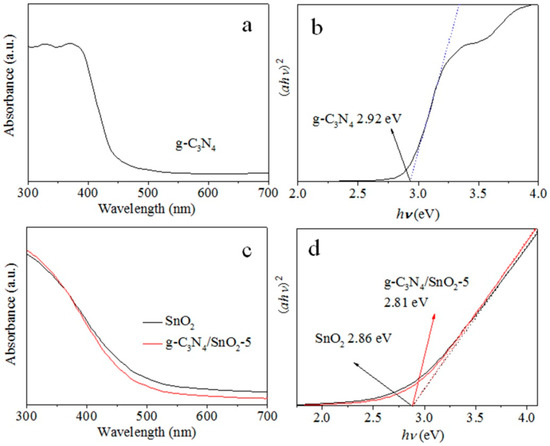
Figure 7.
UV-vis DRS (a,c) and the band gap energy (b,d) of g-C3N4, SnO2, and g-C3N4/SnO2-5 materials (The dashed line is tangent extension line).
The sensing mechanism of n-type semiconductor materials conforms to the space charge layer model. When the sensor is moved from air to VOCs gases, the electron depletion layer formed on the surface of g-C3N4/SnO2-5 changes, which can explain the gas sensitivity of g-C3N4/SnO2-5 [30]. Therefore, the effect of different environmental conditions on the depletion layer width of g-C3N4/SnO2-5 material is studied to explain the resistance variation of g-C3N4/SnO2-5 sensor in air and EA. Figure 8 illustrates the formation of the electron depletion layer of the g-C3N4/SnO2-5 sensor in air and EA gas. When g-C3N4/SnO2-5 sensing material is in the air, oxygen molecules are adsorbed on the surface of g-C3N4/SnO2-5 sensing material to form surface adsorbed oxygen (Figure 8), which will capture free electrons from the conduction band of g-C3N4/SnO2-5 sensing material to form surface adsorbed oxygen ions (Oα−). There is a strong interaction between gas and surface [32]. A wide electron depletion layer is generated on the surface of g-C3N4/SnO2-5, which causes a decrease in the carrier concentration and an increase in the resistance of the gas sensor. The formulas are as follows:
O2 (gas) = O2 (ads)
O2 (ads) + αe− = Oα− (ads)
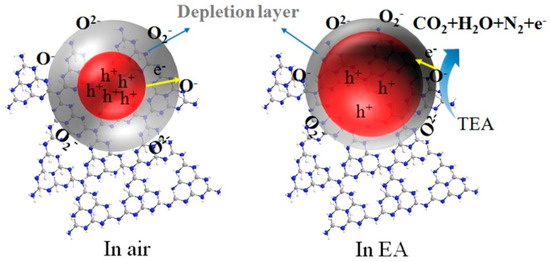
Figure 8.
Schematic diagram of EA gas sensing mechanism of g−C3N4/SnO2 material.
When the gas sensor is exposed to EA gas, it will react with chemisorbed oxygen (Figure 8). The reaction is represented as follows [23]:
N(CH2CH3)3 (gas) + Oα− (ads) = CO2 + H2O + N2 + αe−
The captured electrons are released back into the conduction band of g-C3N4/SnO2-5, and the thickness of the electron depletion layer of g-C3N4/SnO2-5 material decreases, which results in an increase in the carrier concentration and decrease in the resistance of the g-C3N4/SnO2-5 sensor. When g-C3N4/SnO2-5 is exposed to air again, the chemisorption oxygen is produced, and the electron depletion layer becomes wide. Compared with pure SnO2, g-C3N4/SnO2-5 exhibits superior gas sensitivity to EA. The possible reason is that g-C3N4 acts as a matrix to disperse SnO2 nanoparticles and prevent aggregation of SnO2 nanoparticles, which facilitates the adsorption and diffusion of oxygen molecules and EA molecules, and thus enhances the reaction of EA molecules with adsorbed oxygen ions, which are dependent on types of nano or microparticles and structure of sensing materials. In addition, the n-n heterojunction formed between the g-C3N4 and SnO2 interface. When EA molecules cross through this interface, the electrical properties change at the heterojunction. The electrons transfer from the conduction band of g-C3N4 to the conduction band of SnO2, and the electrons and holes separate and finally a high potential barrier is created. The heterostructure between g-C3N4 and SnO2 may inhibit the recombination of electron–hole pairs and promote the rapid transfer of electrons from EA to the surface of g-C3N4/SnO2. As a result, the electrical conductivity of the heterojunction increases, which results in a high response.
To obtain theoretical evidence of energy band structure and conduction, model construction and calculation are conducted, and the details are put in Supporting Information according to similar methods [30,33,34]. Figure 9a–c show the structure models of g-C3N4, SnO2, and g-C3N4/SnO2 materials, and the calculated energy band structures are shown in Figure 9d–f. The band gaps of the pure g-C3N4 and SnO2 are marked in Figure 9d–f as 1.26 and 1.00 eV, respectively. It can also be seen that the top of the valence band (VBT) and the bottom of the conduction band (CBB) of g-C3N4 are centered at the Γ point and the A point, respectively (Figure 9d), while the VBT and CBB of SnO2 are located at the S and X point (Figure 9e), which imply that g-C3N4 is an indirect band gap semiconductor and SnO2 was a direct band gap semiconductor. [30] For the g-C3N4/SnO2 material, it is clear that the energy band curves are more fine and close after the heterojunction is formed between SnO2 and g-C3N4 (Figure 9f), leading to the electrons being reconstructed, and the band gap is closed to 0.0 eV (Figure 9f), indicating that the conductivity of g-C3N4/SnO2 material has been improved significantly.
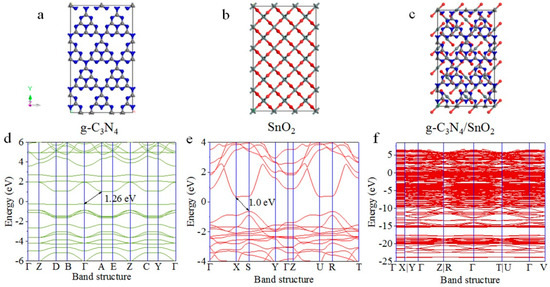
Figure 9.
The crystal structure of (a) g−C3N4, (b) SnO2, and (c) g−C3N4/SnO2. The calculated energy band structure of (d) g−C3N4, (e) SnO2, and (f) g−C3N4/SnO2.
To further explore the change in conductivity of g-C3N4/SnO2 material, the total density of states (TDOS) and partial density of states (PDOS) of g-C3N4, SnO2, and g-C3N4/SnO2 were calculated, and the results are summarized in Figure 10. The main contribution of the valence band edge is C2p and N2p orbitals, and the conduction band edge is C2p and N2p orbitals for pure g-C3N4 (Figure 10a). The top of the SnO2 valence band is Sn5s, 5p orbitals, and the bottom of the conduction band is Sn5p,4d and O2p orbitals (Figure 10b). For comparison, the DOS peaks shift to the left, and the peak at −15 eV increases. Notably, a new peak occurs near the Fermi level after SnO2 modification (Figure 10c,d), showing that there is a strong orbital hybridization between SnO2 and g-C3N4, and there is significant electron exchange between them.
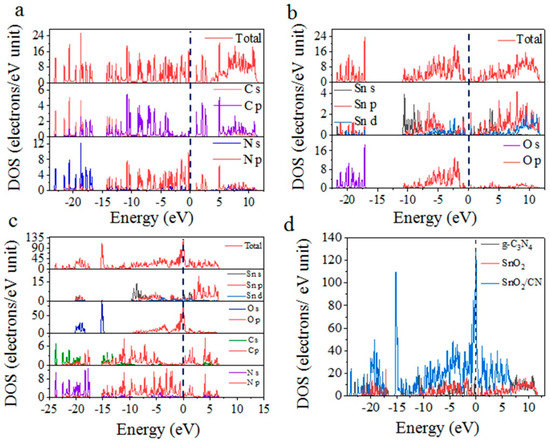
Figure 10.
TDOS and PDOS of (a) g−C3N4, (b) SnO2, and (c) g−C3N4/SnO2. (d) TDOS comparison of three materials.
4. Conclusions
g-C3N4/SnO2 material was synthesized via a hydrothermal and pyrolysis route. Structural and morphology characterizations show that g-C3N4 acts as a matrix of SnO2 to prevent the particles from agglomeration. As-obtained g-C3N4/SnO2 exhibited higher response and selectivity to EA compared with pure SnO2. The response to 100 ppm EA reaches ca. 68 at 240 °C, and it is superior to that of SnO2. Enhanced sensing performance is attributed to the morphology, energy band, and structure of composites consisting of nanoparticles anchored on g-C3N4 layers, and the heterojunctions formed between SnO2 and g-C3N4 interface resulting in fast electron transfer and increasing interstitial lattice defect sites, which can adsorb more O2 molecules and provide more reactive oxygen species to react with EA molecules. This research provides a strategy to prepare new composite material for the effective detection of EA and other related organoamines released from tobacco and chemical industries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemosensors11050296/s1, Figure S1: XRD patterns of g-C3N4; Figure S2: SEM images of as-synthesized materials (a) g-C3N4,(b) SnO2, (c) g-C3N4/SnO2-2.5, and (d) g-C3N4/SnO2-10; Figure S3: XPS spectra of the full range spectrum of g-C3N4/SnO2-5 material; Figure S4: (a) UV–Vis absorption spectra of g-C3N4, SnO2, and g-C3N4/SnO2 and (b) bandgap energy of g-C3N4, SnO2, and g-C3N4/SnO2.
Author Contributions
J.L.: Experimental work, data curation, and writing—original draft. K.X.: Data analysis and draft revision. Y.W.: Data analysis and draft revision. R.Z.: Supervision, conceptualization, and writing—review and editing. Y.S.: data curation and draft review and revision. J.D.: Experimental design, supervision, conceptualization, and writing—revision and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the National Natural Science Foundation of China (No. 51572185), the Key R&D program of Shanxi Province (International Cooperation, No. 201903D421079), and the National Natural Science Foundation of Shanxi (No. 20210302123173).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests.
References
- Lv, D.; Lu, S.; Tan, X.; Shao, M.; Wang, L. Source profiles, emission factors and associated contributions to secondary pollution of volatile organic compounds (VOCs) emitted from a local petroleum refinery in Shandong. Environ. Pollut. 2021, 3, 116589. [Google Scholar] [CrossRef] [PubMed]
- Hung, F.H.; Tsang, K.F.; Wu, C.K.; Liu, Y.; Wan, W.H. An Adaptive Indoor Air Quality Control Scheme for Minimizing Volatile Organic Compounds Density. IEEE Access 2020, 8, 22357–22365. [Google Scholar] [CrossRef]
- Qin, J.; Wang, X.; Yang, Y.; Qin, Y.; Shi, S.; Xu, P.; Chen, R.; Zhou, X.; Tan, J.; Wang, X. Source apportionment of VOCs in a typical medium-sized city in North China Plain and implications on control policy. J. Environ. Sci. 2021, 107, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Han, E.; Meng, F.; Zuo, K. Detection and identification of volatile organic compounds based on temperature-modulated ZnO sensors. IEEE Trans. Instrum. Meas. 2020, 69, 4533–4544. [Google Scholar] [CrossRef]
- Wang, X.; Chen, F.; Yang, M.; Guo, L.; Xie, N.; Kou, X.; Song, Y.; Wang, Q.; Sun, Y.; Lu, G. Dispersed WO3 nanoparticles with porous nanostructure for ultrafast toluene sensing. Sens. Actuators B Chem. 2019, 289, 195–206. [Google Scholar] [CrossRef]
- Meng, F.; Zheng, H.; Chang, Y.; Zhao, Y.; Li, M.; Wang, C.; Sun, Y.; Liu, J. One-stepsynthesis of Au/SnO2/RGO nanocomposites and their VOC sensing properties. IEEE Trans. Nanotechnol. 2018, 17, 212–219. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, L.; Xu, H.; Xing, Y.; Chen, J.; Bie, L. Ultrathin CeO2 nanosheets as bifunctional sensing materials for humidity and formaldehyde detection. Rare Met. 2021, 40, 1614–1621. [Google Scholar] [CrossRef]
- Bhat, P.; Kumar, S.N. Evaluation of IDE-based flexible thin film ZnO sensor for VOC sensing in a custom designed gas chamber at room temperature. J. Mater. Sci.-Mater. Electron. 2022, 33, 1529–1541. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, W.; Gao, N.; Zhao, R.; Ahmed, M.; Li, J.; Du, J.; Asefa, T. Highly sensitive and selective gas-phase ethanolamine sensor by doping sulfur into nanostructured ZnO. Sens. Acutators B Chem. 2019, 296, 126633. [Google Scholar] [CrossRef]
- Zhang, K.; Xie, K.; Ahmed, M.; Chai, Z.; Zhao, R.; Li, J.; Du, J. Cr-doped SnO2 microrod adhering nanoparticles for enhanced triethylamine sensing performance. Mater. Lett. 2022, 312, 131684. [Google Scholar] [CrossRef]
- Shang, Y.; Shi, W.; Zhao, R.; Ahmed, M.; Li, J.; Du, J. Simple self-assembly of 3D laminated CuO/SnO2 hybrid for the detection of triethylamine. Chin. Chem. Lett. 2020, 31, 2055–2058. [Google Scholar] [CrossRef]
- Du, J.; Xie, Y.; Yao, H.; Zhao, R.; Li, J. Size-dependent gas sensing and selectivity of SnO2 quantum dots toward volatile compounds. Appl. Surf. Sci. 2015, 346, 256–262. [Google Scholar] [CrossRef]
- Acharyya, S.; Jana, B.; Nag, S.; Saha, G.; Guha, P.K. Single resistive sensor for selective detection of multiple VOCs employing SnO2 hollow spheres and machine learning algorithm: A proof of concept. Sens. Acutators B Chem. 2020, 321, 128484. [Google Scholar] [CrossRef]
- Meng, F.; Ji, H.; Yuan, Z.; Chen, Y.; Gao, H. Dynamic Measurement and Recognition Methods of SnO2 Sensor to VOCs under Zigzag-rectangular Wave Temperature Modulation. IEEE Sens. J. 2021, 21, 10915–10922. [Google Scholar] [CrossRef]
- Du, J.; He, S.; Zhao, R.; Chen, S.; Guo, T.; Wang, H. Facile self-assembly of SnO2 nanospheres for volatile amines gas sensing. Mater. Lett. 2017, 186, 318–321. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, Z.; Zhou, T.; Fei, T.; Wang, R.; Zhang, T. Improvement of gas sensing performance for tin dioxide sensor through construction of nanostructures. J. Colloid Interface Sci. 2019, 557, 673–682. [Google Scholar] [CrossRef]
- Ma, X.; Gao, R.; Zhang, T.; Sun, X.; Li, T.; Gao, S.; Zhang, X.; Xu, Y.; Cheng, X.; Huo, L. Mesoporous SnO2 nanospheres sensor for fast detection of HCHO and its application in safety detection of aquatic products. Sens. Actuators B Chem. 2022, 374, 132844. [Google Scholar] [CrossRef]
- Din, S.U.; Ul Haq, M.; Sajid, M.; Khatoon, R.; Chen, X.; Li, L.; Zhang, M.; Zhu, L. Development of high-performance sensor based on NiO/SnO2 heterostructures to study sensing properties towards various reducing gases. Nanotechnology 2020, 31, 395502. [Google Scholar] [CrossRef]
- Dai, H.; Ding, J.; Chen, H.; Fu, H. Improvement of ethanolamine sensing performance based on Au-modified ZnO rod-like nanoflowers. Mater. Lett. 2023, 340, 134183. [Google Scholar] [CrossRef]
- Zou, Z.; Zhao, Z.; Zhang, Z.; Tian, W.; Yang, C.; Jin, X.; Zhang, K. Room-temperature optoelectronic gas sensor based on core-shellg-C33N4@WO3 heterocomposites for efficient ammonia detection. Anal. Chem. 2023, 95, 2110–2118. [Google Scholar] [CrossRef]
- Bai, K.; Cui, Z.; Li, E.; Ding, Y.; Zheng, J.; Liu, C.; Zheng, Y. Adsorption of gas molecules on group III atoms adsorbed g-C3N4: A first-principles study. Vacuum 2020, 175, 109293. [Google Scholar] [CrossRef]
- Chen, M.; Guo, C.; Hou, S.; Lv, J.; Xu, J. A novel Z-scheme AgBr/P-g-C3N4 heterojunction photocatalyst: Excellent photocatalytic performance and photocatalytic mechanism for ephedrine degradation. Appl. Catal. B-Environ. 2020, 266, 118614. [Google Scholar] [CrossRef]
- Li, L.; Huang, Z.; Li, Z.; Li, H.; Wang, A. Defect-rich porous g-C3N4 nanosheets photocatalyst with enhanced photocatalytic activity. J. Mater. Sci.-Mater. Electron. 2021, 32, 6465–6474. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Z.; Hao, R.; Huang, Y.; Liu, K. Ultra-highly stable zinc metal anode via 3D-printed g-C3N4 modulating interface for long life energy storage systems. Chem. Eng. J. 2020, 403, 126425. [Google Scholar] [CrossRef]
- Lin, Z. Preparation of ZnO/layered g-C3N4 and their n-butanol gas sensing properties. Mater. Sci. 2020, 10, 278–286. [Google Scholar]
- Yuan, B.; Wang, Y.; Elnaggar, A.Y.; Azab, I.H.E.; Huang, M.; Mahmoud, M.H.H.; El-Bahy, S.M.; Guo, M. Physical vapor deposition of graphitic carbon nitride (g-C3N4) films on biomass substrate: Optoelectronic performance evaluation and life cycle assessment. Adv. Compos. Hybrid. Mater. 2022, 5, 813–822. [Google Scholar] [CrossRef]
- Cao, J.; Qin, C.; Wang, Y.; Zhang, B.; Gong, Y.; Zhang, H.; Sun, G.; Bala, H.; Zhang, Z. Calcination method synthesis of SnO2/g-C3N4 composites for a high-performance ethanol gas sensing application. Nanomaterials 2017, 7, 98. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.; Dai, M.; Liu, K.; Liu, Y.; Zhou, L.; Liu, F.; Li, F.; Gao, Y.; Yan, X.; et al. Visible light activated excellent NO2 sensing based on 2D/2D ZnO/g-C3N4 heterojunction composites. Sens. Actuators B Chem. 2020, 304, 127287. [Google Scholar] [CrossRef]
- Niu, J.; Wang, L.; Xu, J.; Jin, H.; Hong, B.; Jin, D.; Peng, X.; Ge, H.; Wang, X. Mesoporous Co3O4 nanowires decorated with g-C3N4 nanosheets for high performance toluene gas sensors based on p-n heterojunction. Mater. Chem. Phys. 2023, 293, 126980. [Google Scholar] [CrossRef]
- Xie, K.; Wang, Y.; Zhang, K.; Zhao, R.; Chai, Z.; Du, J.; Li, J. Controllable band structure of ZnO/g-C3N4 aggregation to enhance gas sensing for the dimethylamine detection. Sens. Actuators Rep. 2022, 4, 100084. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Y.; Liang, H.; Kang, Y.; Liu, B.; Zhao, R.; Wang, Y.; Shuai, X.; Shang, Y.; Guo, T.; et al. Facile template-free preparation of hierarchically porous graphitic carbon nitrides as high-performance photocatalyst for degradation of methyl violet dye. ChemistrySelect 2021, 6, 7130–7135. [Google Scholar] [CrossRef]
- Stefan, K.; Chris, B. Atomistic Descriptions of gas–surface interactions on tin dioxide. Chemosensors 2021, 9, 270. [Google Scholar]
- Wang, V.; Xu, N.; Liu, J.C. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Computer Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Liangruksa, M.; Sukpoonprom, P.; Junkaew, A.; Photaram, W.; Siriwong, C. Gas sensing properties of palladium-modified zinc oxide nanofilms: A DFT study. Appl. Surf. Sci. 2021, 544, 148868. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).