Novel Nitrate Ion-Selective Microsensor Fabricated by Means of Direct Ink Writing
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. PVC-ISM and PVB-NaClsaturated Membrane Preparation
2.3. Instrumentation
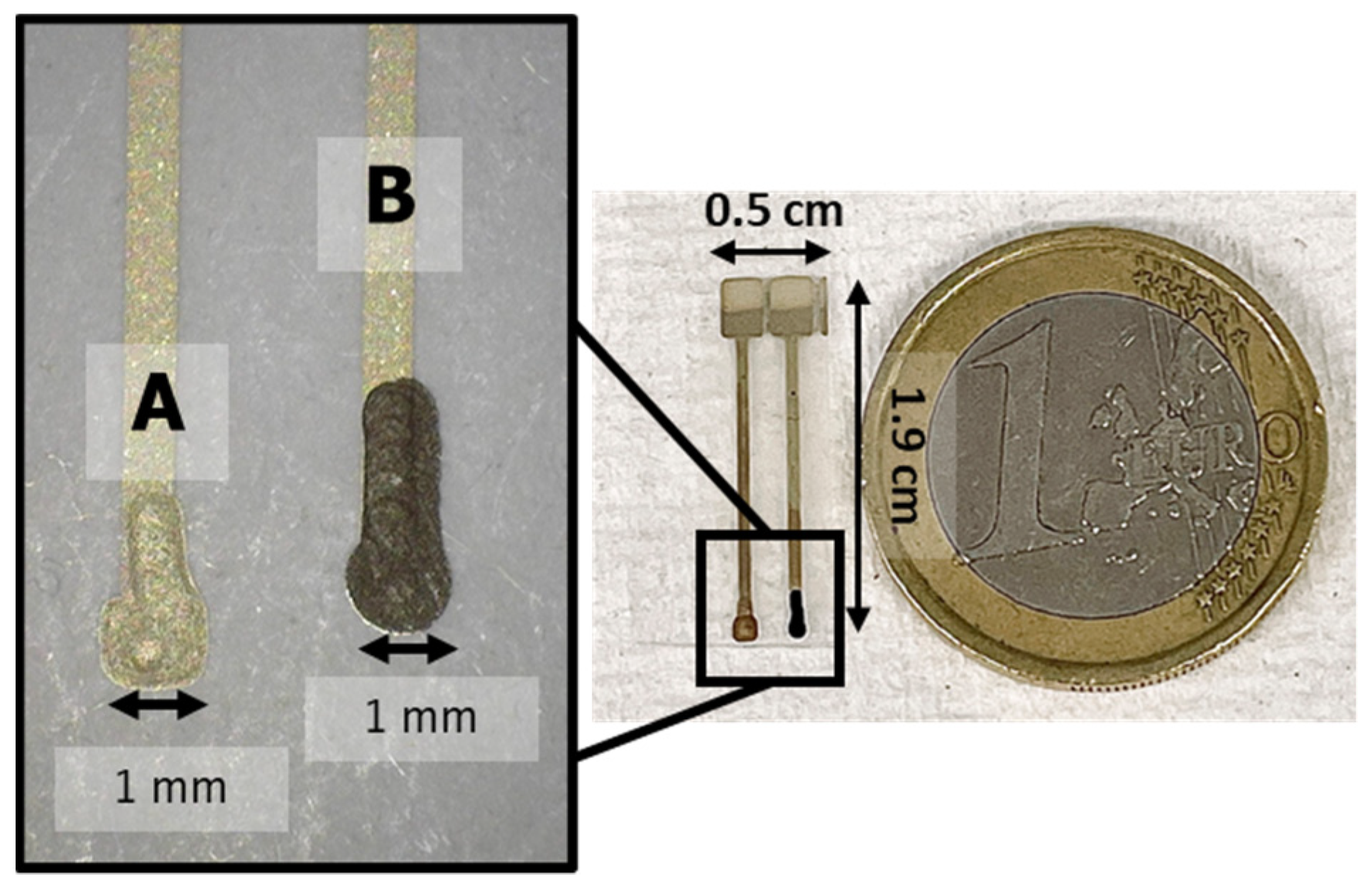
2.4. Electrode Fabrication
2.5. Membrane Deposition
2.6. Electrode Characterization
2.7. Interference Study
2.8. Sample Analysis
3. Results
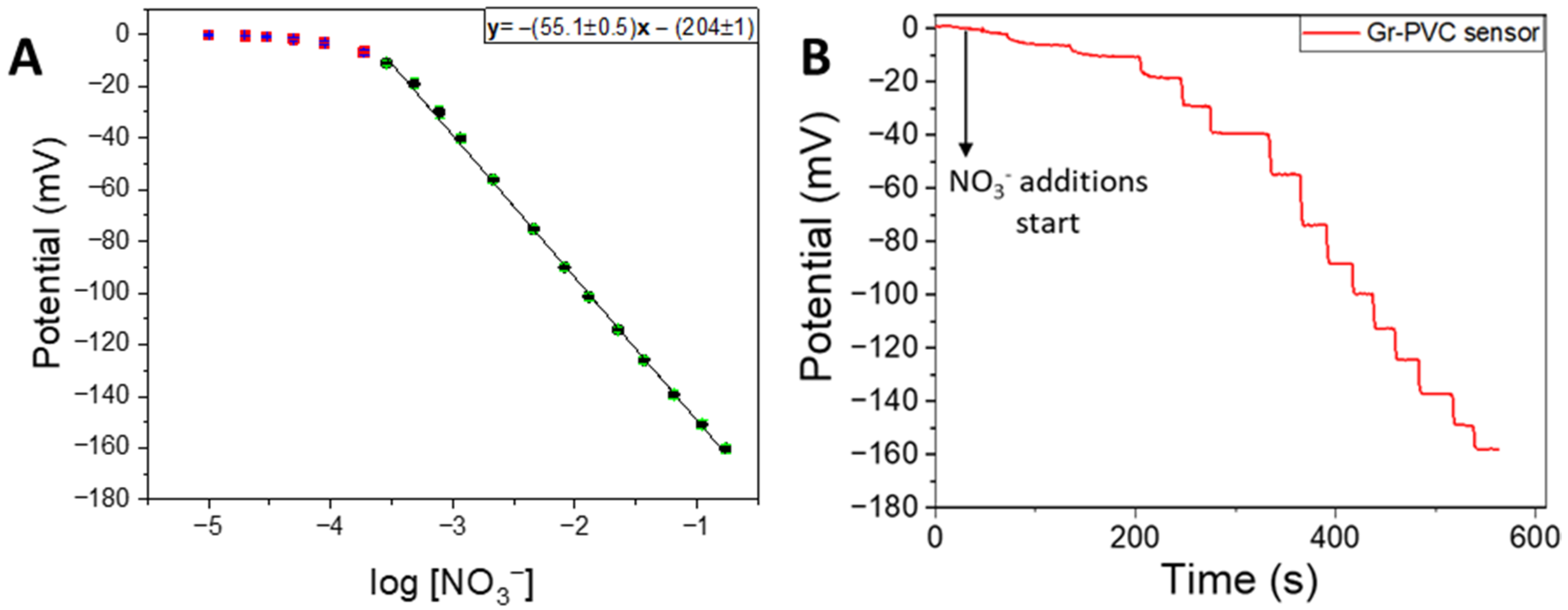
3.1. Electroanalytical Performance Evaluation
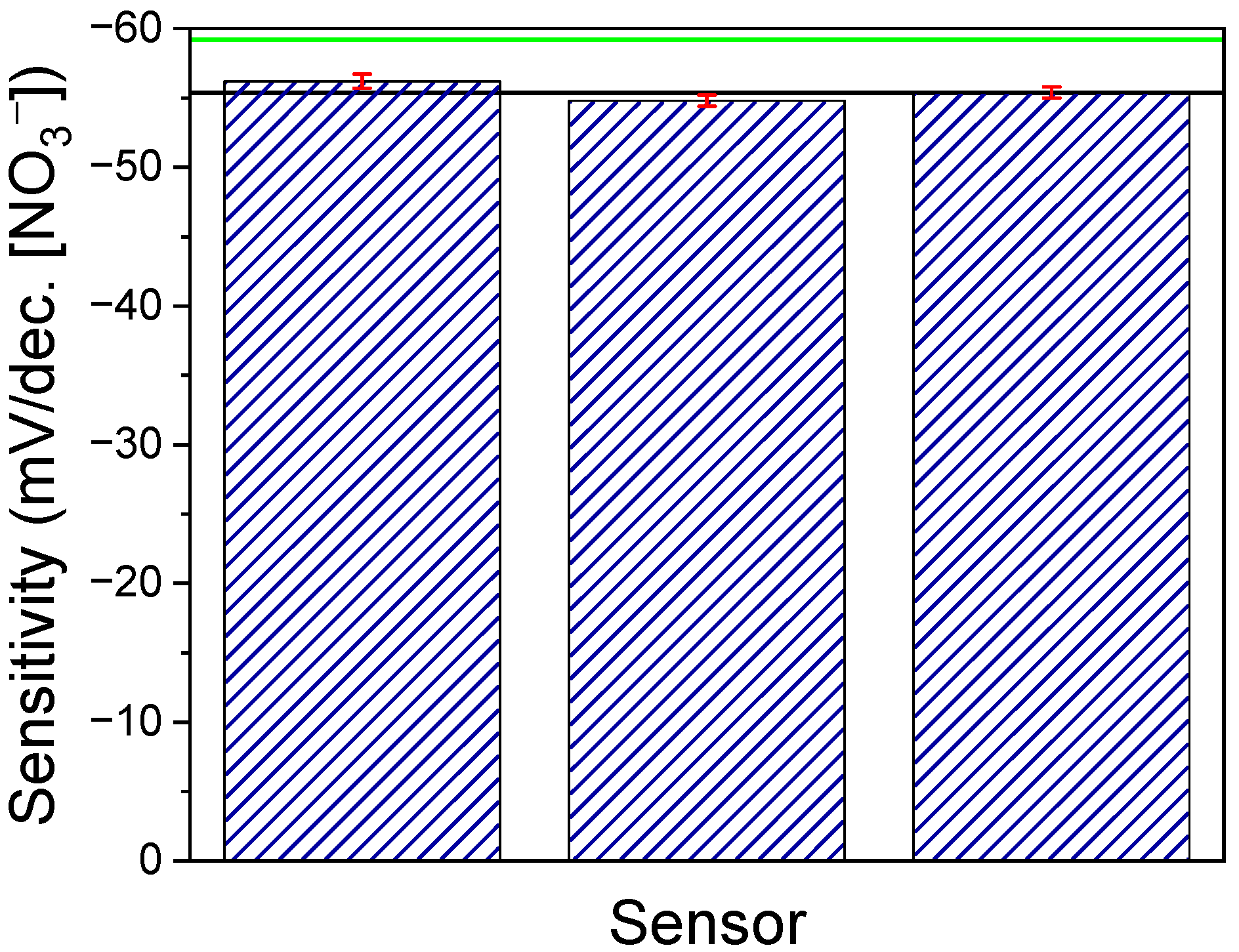
3.2. Response Variation among Sensors
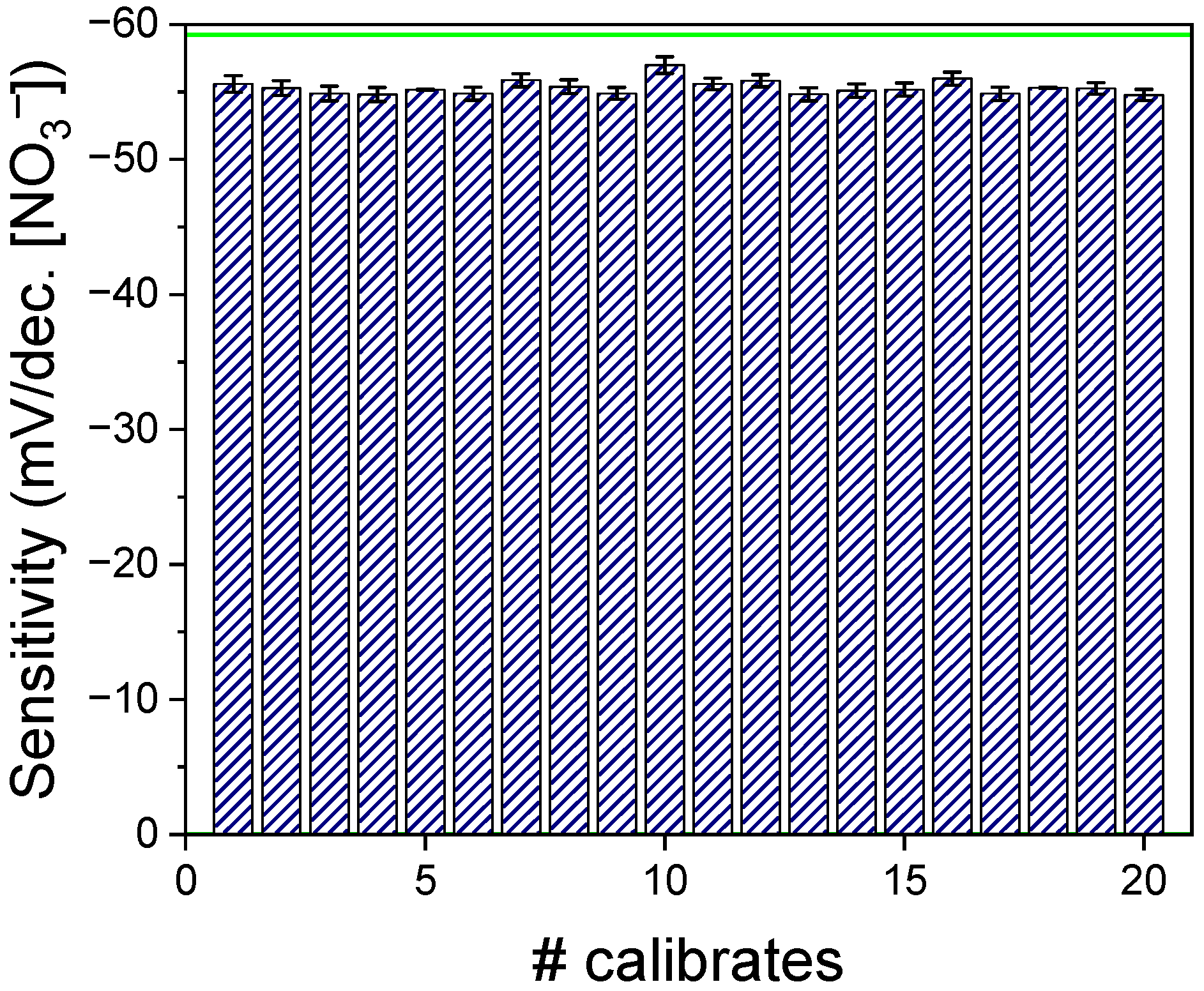
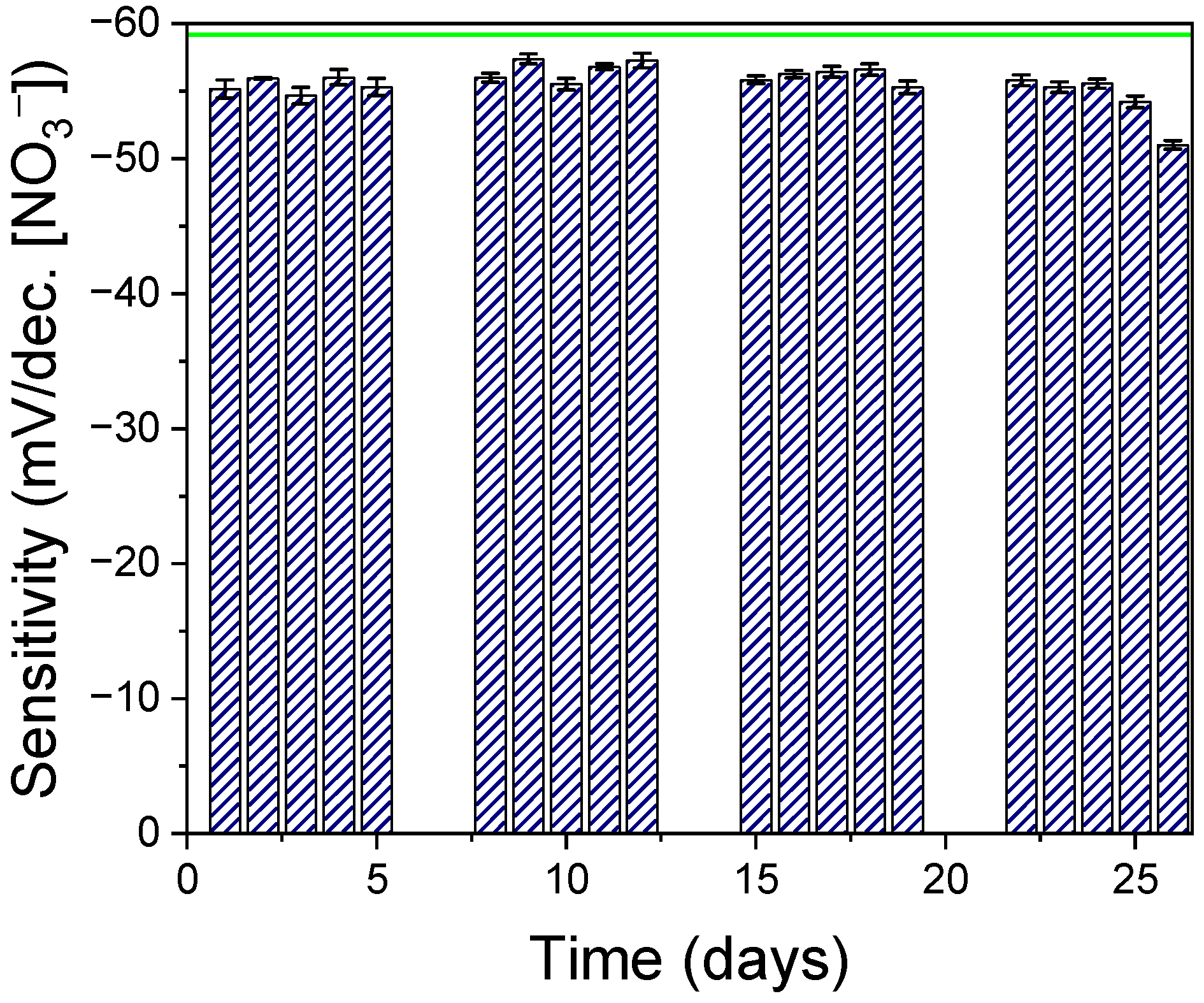
3.3. Stability of the Sensor
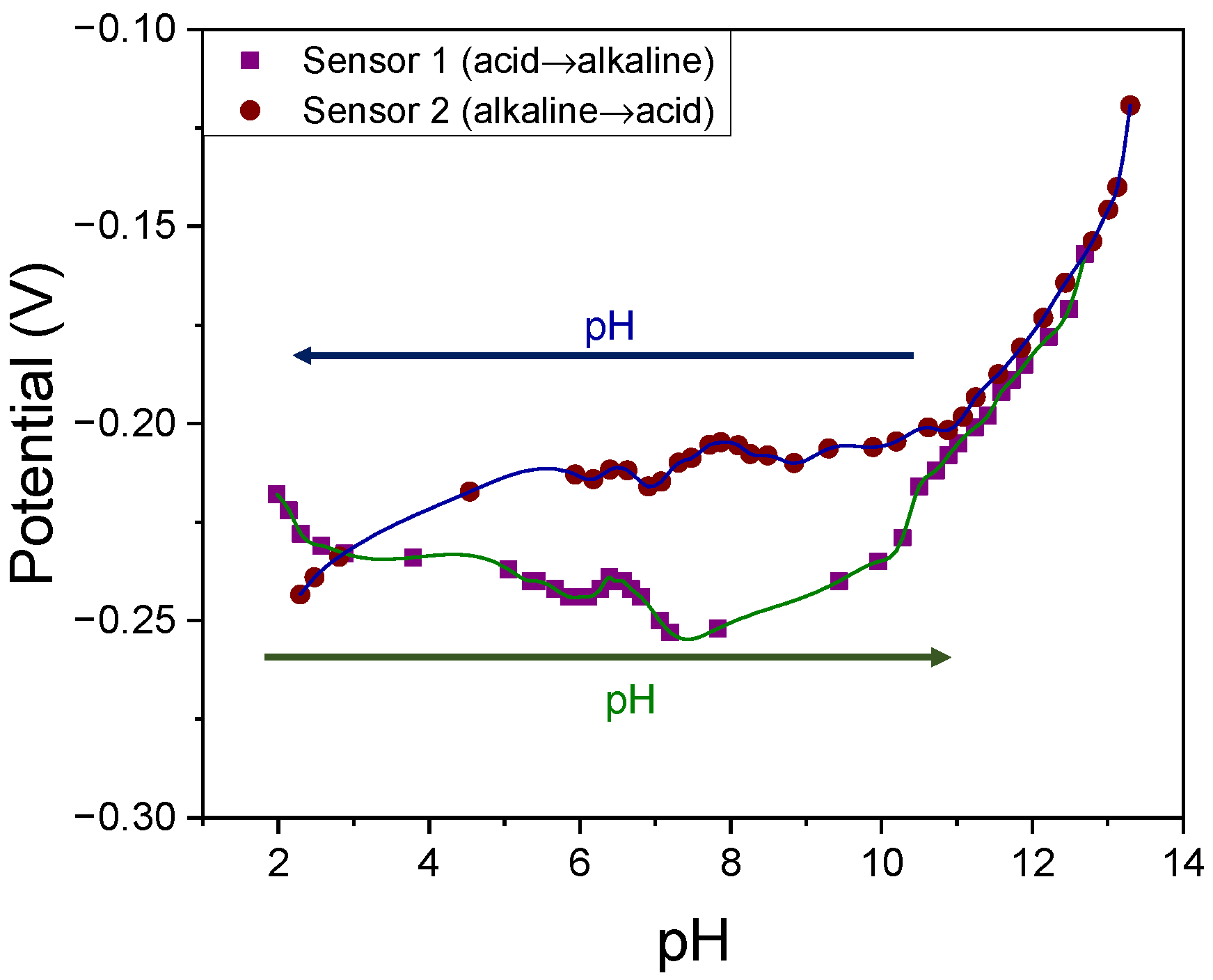
3.4. Robustness against pH
3.5. Change in Response with Temperature
3.6. Study of Interferents
3.7. Real-Sample Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolders, A.J.P.; Lucassen, E.C.H.E.T.; Bobbink, R.; Roelofs, J.G.M.; Lamers, L.P.M. How nitrate leaching from agricultural lands provokes phosphate eutrophication in groundwater fed wetlands: The sulphur bridge. Biogeochemistry 2009, 98, 1–7. [Google Scholar] [CrossRef]
- Romanelli, A.; Soto, D.X.; Matiatos, I.; Martínez, D.E.; Esquius, S. A biological and nitrate isotopic assessment framework to understand eutrophication in aquatic ecosystems. Sci. Total. Environ. 2020, 715, 136909. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P.; Molot, L. The role of ammonium and nitrate retention in the acidification of lakes and forested catchments. Biogeochemistry 1990, 11, 23–43. [Google Scholar] [CrossRef]
- Real Decreto 47/2022, de 18 de enero, sobre protección de las aguas contra la contaminación difusa producida por los nitratos procedentes de fuentes agrarias. Available online: https://www.boe.es/eli/es/rd/2022/01/18/47 (accessed on 1 February 2023).
- Moshoeshoe, M.N.; Obuseng, V. Simultaneous Determination of Nitrate, Nitrite and Phosphate in Environmental Samples by High Performance Liquid Chromatography with UV Detection. South Afr. J. Chem. 2018, 71, 79–85. [Google Scholar] [CrossRef]
- Nightingale, A.M.; Hassan, S.-U.; Warren, B.M.; Makris, K.; Evans, G.W.H.; Papadopoulou, E.; Coleman, S.; Niu, X. A Droplet Microfluidic-Based Sensor for Simultaneous in Situ Monitoring of Nitrate and Nitrite in Natural Waters. Environ. Sci. Technol. 2019, 53, 9677–9685. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, B.; Yin, T.; Qin, W. Alternative coulometric signal readout based on a solid-contact ion-selective electrode for detection of nitrate. Anal. Chim. Acta 2020, 1129, 136–142. [Google Scholar] [CrossRef]
- Yasin, M.; Irawati, N.; Harun, S.W.; Ahmad, F.; Khasanah, M. Sodium Nitrate (NaNO3) Sensor Based on Graphene Coated Microfiber. Meas. J. Int. Meas. Confed. 2019, 146, 208–214. [Google Scholar] [CrossRef]
- Isildak, O.; Yildiz, I.; Erenler, R.; Dag, B.; Isildak, I. Hg(II) Ion-Selective Electrodes with PVC Membranes Based on Bis-1,5-dimethyl-2-phenyl-1,2-dihydro-3H-pyrazol-3-one. Bull. Chem. Soc. Jpn. 2022, 95, 353–358. [Google Scholar] [CrossRef]
- Isildak, I.; Attar, A.; Demir, E.; Kemer, B.; Aboul-Enein, H.Y. A Novel all Solid-State Contact PVC-Membrane Beryllium-Selective Electrode Based on 4-Hydroxybenzo-15-Crown-5 Ether Ionophore. Curr. Anal. Chem. 2018, 14, 43–48. [Google Scholar] [CrossRef]
- Demir, E.; Kemer, B.; Bekircan, O.; Aboul-Enein, H. A Novel Iron(III)-Selective Membrane Potentiometric Sensor Based on 5-Chloro-3-[4-(trifluoromethoxy) phenylimino] Indolin-2-one. Curr. Anal. Chem. 2014, 11, 29–35. [Google Scholar] [CrossRef]
- Patella, B.; Russo, R.R.; Aiello, G.; Sunseri, C.; Inguanta, R.; Parade, D. Copper Nanowire Array as Highly Selective Electrochemical Sensor of Nitrate Ions in Water. Talanta 2021, 221, 121643. [Google Scholar] [CrossRef] [PubMed]
- Bommireddy, N.; Palathedath, S.K. Templated Bimetallic Copper-Silver Nanostructures on Pencil Graphite for Amperometric Detection of Nitrate for Aquatic Monitoring. J. Electroanal. Chem. 2020, 856, 113660. [Google Scholar] [CrossRef]
- Mahmud, M.A.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbassi, R.; Razmjou, A.; Asadnia, M. Recent Progress in Sensing Nitrate, Nitrite, Phosphate, and Ammonium in Aquatic Environment; Elsevier Ltd.: Amsterdam, The Netherlands, 2020; Volume 259. [Google Scholar] [CrossRef]
- Veder, J.-P.; De Marco, R.; Clarke, G.; Chester, R.; Nelson, A.; Prince, K.; Pretsch, E.; Bakker, E. Elimination of Undesirable Water Layers in Solid-Contact Polymeric Ion-Selective Electrodes. Anal. Chem. 2008, 80, 6731–6740. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, M.; Wang, X.; Yang, Q.; Wang, M.; Liu, G.; Yao, L. An All-Solid-State Nitrate Ion-Selective Electrode Nutrient Monitoring. Sensors 2020, 20, 2270. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Huang, Y.; Linthicum, W.; Liu, F.; Beringhs, A.O.; Dang, Y.; Xu, Z.; Chang, S.-Y.; Ling, J.; Huey, B.D.; et al. Toward Long-Term Accurate and Continuous Monitoring of Nitrate in Wastewater Using Poly(tetrafluoroethylene) (PTFE)–Solid-State Ion-Selective Electrodes (S-ISEs). ACS Sens. 2020, 5, 3182–3193. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Meng, Z.; Qin, Y.; Jiang, D.; Xi, K.; Wang, P. Thiol-Functionalized Reduced Graphene Oxide as Self-Assembled Ion-to- Electron Transducer for Durable Solid-Contact Ion-Selective Electrodes. Talanta 2020, 208, 120374. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Eldin, A.G.; Amr, A.E.-G.E.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Improved Solid-Contact Nitrate Ion Selective Electrodes Based on Multi-Walled Carbon Nanotubes (MWCNTs) as an Ion-to-Electron Transducer. Sensors 2019, 19, 3891. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895. [Google Scholar] [CrossRef]
- Saha, P.; Akter, R.; Shah, S.S.; Mahfoz, W.; Aziz, M.A.; Ahammad, A.J.S. Gold Nanomaterials and their Composites as Electrochemical Sensing Platforms for Nitrite Detection. Chem. Asian J. 2022, 17, e202200823. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Bressi, V.; Espro, C.; Iannazzo, D.; Piperopoulos, E.; Neri, G. Electrochemical determination of nitrites and sulfites by using waste-derived nanobiochar. J. Electroanal. Chem. 2023, 928, 117071. [Google Scholar] [CrossRef]
- Gross, B.; Lockwood, S.Y.; Spence, D.M. Recent Advances in Analytical Chemistry by 3D Printing. Anal. Chem. 2017, 89, 57–70. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Li, Q.; Li, G.; Tian, X.; Luo, Z. Review of Printed Electrodes for Flexible Devices. Front. Mater. 2019, 5, 77. [Google Scholar] [CrossRef]
- Bao, C.; Kim, W.S. Perspective of Printed Solid-State Ion Sensors toward High Sensitivity and Selectivity. Adv. Eng. Mater. 2020, 22, 2000116. [Google Scholar] [CrossRef]
- Palenzuela, C.L.M.; Pumera, M. (Bio)Analytical Chemistry Enabled by 3D Printing: Sensors and Biosensors. Trends Anal. Chem. 2018, 103, 110–118. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, W.; Waimin, J.F.; Glassmaker, N.; Raghunathan, N.; Jiang, X.; Ziaie, B.; Rahimi, R. Inkjet-Printed Solid-State Potentiometric Nitrate Ion Selective Electrodes for Agricultural Application. Proc. IEEE Sensors 2019, 2019, 19–22. [Google Scholar] [CrossRef]
- Umezawa, Y.; Umezawa, K.; Tohda, K.; Amemiya, S. Potentiometric Selectivity Coefficients of Ion-Selective Electrodes. Int. Union Pure Appl. Chem. 2000, 72, 1851–2082. [Google Scholar] [CrossRef]
- Moya, A.; Pol, R.; Martínez-Cuadrado, A.; Villa, R.; Gabriel, G.; Baeza, M. Stable Full-Inkjet-Printed Solid-State Ag/AgCl Reference Electrode. Anal. Chem. 2019, 91, 15539–15546. [Google Scholar] [CrossRef]
| Sensor | Sensitivity (mV/dec.) | LOD * (mM) | Stability | Linear Range (mM) | Samples | Integrated RE | Reference |
|---|---|---|---|---|---|---|---|
| PPy(NO3−) 1 | −50 | 6.3·10−3 | 65 days | 0.001–10 | Real (soil) | No | [13] |
| PTFE-PVC 2 | −58 | 0.2 | 20 days | 0.016–1 | Real | No | [14] |
| TRGO-PVC 3 | −60.0 ± 0.5 | 4·10−3 | 14 days | 0.004–100 | No samples | No | [15] |
| MWCNTs-PVC 4 | −55.1 ± 2.1 | 28·10−6 | Not reported | 0.00008–10 | Real | No | [16] |
| Ag-PVC | −52 ± 1.8 | Not reported | Not reported | 1–100 | Real (soil) | No | [21] |
| Gr-PVC | −55.1 ± 0.7 | 0.2 | 25 days | 0.3–170 | Spiked, real | Yes | This work |
| Interferent | Concentration (mM) | Kpot |
|---|---|---|
| Cl− | 8.5 | 7.3·10−2 |
| HCO3− | 4.9 | 3.35·10−2 |
| CH3COO− | 1 | 3·10−2 |
| NO2− | 1.56 | 8.1·10−2 |
| SO42− | 1 | 9.1·10−3 |
| Sample | Printed Sensor [NO3−] (mM) | IC [NO3−] (mM) | [Cl−] (mM) | [HCO3−] (mM) | Recovery % | t-Test (N = 3) |
|---|---|---|---|---|---|---|
| Milli-Q water | 97 ± 1 | 91 ± 4 | --- | --- | 106 | 2.52 |
| Bottled water | 102 ± 6 | 97 ± 4 | 0.17 | 1.86 | 106 | 1.2 |
| Tap water | 97 ± 2 | 92 ± 3 | 2.35 | 2.03 | 105 | 2.4 |
| Reactor medium | 0.53 ± 0.01 | 0.58 ± 0.03 | 5.26 | --- | 93 | 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paré, F.; Visús, A.; Gabriel, G.; Baeza, M. Novel Nitrate Ion-Selective Microsensor Fabricated by Means of Direct Ink Writing. Chemosensors 2023, 11, 174. https://doi.org/10.3390/chemosensors11030174
Paré F, Visús A, Gabriel G, Baeza M. Novel Nitrate Ion-Selective Microsensor Fabricated by Means of Direct Ink Writing. Chemosensors. 2023; 11(3):174. https://doi.org/10.3390/chemosensors11030174
Chicago/Turabian StyleParé, Franc, Aida Visús, Gemma Gabriel, and Mireia Baeza. 2023. "Novel Nitrate Ion-Selective Microsensor Fabricated by Means of Direct Ink Writing" Chemosensors 11, no. 3: 174. https://doi.org/10.3390/chemosensors11030174
APA StyleParé, F., Visús, A., Gabriel, G., & Baeza, M. (2023). Novel Nitrate Ion-Selective Microsensor Fabricated by Means of Direct Ink Writing. Chemosensors, 11(3), 174. https://doi.org/10.3390/chemosensors11030174






