Abstract
Organophosphates are mainly used as pesticides to protect crops from pests. Because organophosphate pesticides’ use has expanded dramatically worldwide, accurate monitoring of their concentrations in the environment and food has become of utmost importance. Once considered acutely toxic due to acetylcholinesterase inhibition, nowadays organophosphates are classified as extremely dangerous compounds, with a broad spectrum of toxicity types, by the World Health Organization. Having in mind their extensive use and diverse harmful effects, it is necessary to develop easy, rapid, and highly sensitive methods for organophosphate detection. Regardless of numerous conventional techniques for organophosphate detection, the construction of portable sensors is required to make routine analysis possible. Extensive literature on the different sensors for organophosphate detection is available. Many of them rely on the use of various carbon materials. There are many classes of carbon materials used in sensing element construction, as well as supporting materials. This review focuses on electrochemical and optical sensors based on carbon materials. Special attention is paid to the selectivity, sensitivity, stability, and reusability of reviewed sensors.
1. Introduction
Pesticides are used by humans for pest control. Their classification can be diverse. Most often, pesticides are grouped according to their chemical structure. The best-known groups are organochlorines, organophosphorus, carbamates, pyrethroids, amides, anilines, and nitrogenous heterocyclic compounds. Among them, organophosphate pesticides (OPs) stand out with their harmful toxic effects and the incidence of use. OPs were the first class of pesticides with documented existing levels for allowable residues in food by the U.S. Environmental Protection Agency (EPA). By 2006, chemical-specific evaluations of almost all OP pesticides had been completed. This resulted in the termination of all but a few OP residential usages [1]. One of the most well-known OPs is chlorpyrifos, a popular insecticide prohibited by the EPA in 2016. Its use was later reversed, and it is still available today. The European Union (EU) stated on 6 December 2019, that sales of chlorpyrifos would be prohibited after 31 January 2020. The Standing Committee on Plants, Animals, Food, and Feed (PAFF Committee) approved two draft Implementing Regulations that would prevent chlorpyrifos and chlorpyrifos-methyl permits from being renewed [2,3]. Despite all mentioned facts above, it is still in use.
OPs are widely used in agricultural, commercial, and residential settings, making exposure to the general population ubiquitous. The harmful effects of OPs are mainly ascribed to the irreversible inhibition of acetylcholinesterase (AChE). Other enzymes, such as myeloperoxidase (MPO), are also affected by this group of pesticides [4]. The primary function of AChE is cholinergic, but this enzyme is also involved in other processes, such as the phenomenon of oxidative stress, programmed cell death (apoptosis), inflammation, and oncogenesis [5]. AChE regulates the level of acetylcholine, the neurotransmitter known to be involved in immune response control [5], so its role in the process of inflammation is essential [6,7].
OPs were known as highly toxic compounds, but the fact that they could cause cancer was not well-known. In the past ten years, many studies indicated the connection between occupational and environmental exposure to OPs and the development of various tumors. The link between chronic low-dose exposure to numerous OPs and the perturbation of several biological processes (oxidative stress, immunotoxicity) connected to oncogenesis was designated [8]. The association of OPs exposure with multiple internal organ cancer (prostate, lungs, colon, pancreas), as well as hematological types of cancer (multiple myeloma and leukemia), was demonstrated [8]. OPs have recently also been associated with many neurological disorders. Their ability to cause anxiety and depression needs to be thoroughly investigated. Individuals with chronic health conditions [9] and specific professions (farming, fishing, forestry) [10] are shown to have higher rates of depression. In addition, a connection between suicidal behavior, affective disorder, and exposure to OPs is unambiguously observed [11]. Nevertheless, long-term exposure to low levels of OPs and its influence on human health is still not fully addressed.
Many OPs, as artificial organic compounds, are not biodegradable. Due to bioaccumulation, they can enter the food chain and affect the entire environment [12]. Having all their harmful effects and the extent of their use in mind, it is essential to control, monitor, and remove OPs from the environment.
Adequate materials for application in sensors have been researched extensively. Carbon materials have been used for sensing applications for many years, but they are still attractive. The work on their improvement is continual. The physical and chemical properties of carbon materials enabled them to retain a leading place in sensor applications for many years. Manufacturing processes are relatively simple. They also have the advantage of yielding an appropriate quantity of material with low densification defects. Furthermore, carbon-based materials are cheap and are considered an alternative to many expensive materials. They are also known to be environment-friendly materials [13].
Most often, carbonaceous materials are employed in electrochemical sensors. For electrode construction, the material must be inert without any electroactive species and conductive over a wide potential window. Carbon-based electrodes address sensitivity and specificity, the crucial challenges in electroanalytical performance improvement. Carbon is common in sensor applications due to its relatively inert electrochemistry, electrocatalytic diversity, and low price [14,15]. The carbon-based materials employed as electrode and supporting substrates are glassy carbon, graphite, carbon black, 0D fullerenes, 1D carbon nanotubes, 2D graphene-related materials, and 3D nanostructured porous carbon materials [15,16]. The use of carbon-based materials is also common in optical sensors.
Carbon nanostructures possess inimitable optoelectronic and physiochemical properties. Due to this, materials such as carbon nanotubes, graphene, and carbon dots have been used to develop opto-chemical sensors for many environmental contaminants (pesticides, microbiological pathogens, pharmaceutics) [17]. Remarkable properties and various carbon materials made them unavoidable in developing powerful sensor devices nowadays.
This contribution provides an overview of the optical and electrochemical carbon-based sensors for organophosphate pesticide detection. The outstanding advancements in carbon materials-based sensors are summarized and discussed. First, a basic background of sensor components and important characteristics are presented. Then, special attention is given to the carbon materials’ properties enabling them to be successfully used in robust sensor device construction. There are recent articles covering this important and attractive topic [18,19,20]. However, they mainly focus on carbon material-based biosensors that detect single pesticides. This review offers a fresh, broad perspective by covering most electrochemical and optical chemo- and biosensors for various organophosphate pesticides available in the literature. Moreover, it discusses in detail the properties of different carbon materials, enabling their use in electrochemical and optical sensor construction. In this way, it summarizes the essential information and allows new insight into this field. This work focuses on sensors’ most important properties, such as selectivity, sensitivity, stability, and reusability. Beyond an assessment of their performance, the paper discusses the current challenges, as well as future trends, for organophosphate monitoring. Hopefully, it will provide guidance for the future application of carbon materials in electrochemical and optical sensors for organophosphate pesticide construction.
2. Sensor Components
The International Union of Pure and Applied Chemistry defines a chemical sensor as, “a device that transforms chemical information, ranging from the concentration of a specific sample component to total composition analysis, into an analytically useful signal [21]”. A typical chemical sensor contains two basic functional units, namely, a receptor and a transducer (Figure 1). The receptor transforms chemical information contained in the analyte into a form of energy that the transducer may measure. In addition, the receptor has the role of providing high selectivity towards the desired analyte in the presence of potentially interfering chemical species, avoiding false-positive results [22]. The transducer is a device capable of transforming the energy carrying the chemical information about the analyte into an analytical signal and generally does not influence selectivity [21].
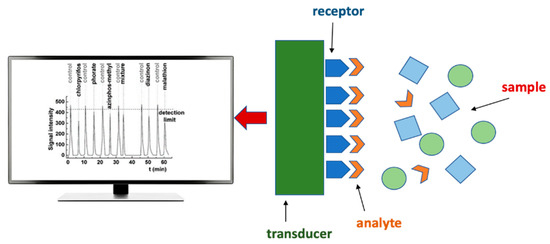
Figure 1.
Schematic representation of typical sensor components.
The receptor part of chemical sensors may be based on physical, chemical, or biochemical principles. In the case of receptors based on physical principles, there is no chemical reaction. Some examples are receptors measuring absorbance, refractive index, conductivity, temperature, or mass change. In chemical receptors, the chemical reaction of an analyte leads to the analytical signal. Sensors with biochemical receptor elements are called biosensors and represent a subgroup of chemical sensors. Within this group, a biochemical process is the source of the analytical signal [21]. The biosensors are not presented as a special class because the process on which they are based is generally common to chemical sensors, with the difference in participation of biological sensor elements. They may also be divided into subgroups according to the biological elements used in the receptor: organisms, tissues, cells, organelles, membranes, enzymes, antibodies, DNA, etc. It is common that biosensors have several enzymatic systems coupled in order to amplify the signal [21].
Chemical sensors may be classified with respect to the operating principle of the transducer as follows:
- Electrochemical sensors, which transform the result of an electrochemical interaction (spontaneous or stimulated electrically) between the analyte and electrode into a signal. There are three main subgroups of electrochemical sensors: voltammetric/amperometric, potentiometric, and impedimetric.
- Electrical sensors with no electrochemical processes taking place. Instead, the signal comes from the change in the electrical properties of the analyte caused by the interaction with the receptor. Some of them are metal oxide semiconductor sensors, organic semiconductor sensors, electrolytic conductivity sensors, and electric permittivity sensors.
- Optical sensors, which transform optical changes created as a result of an interaction of the analyte with the receptor into a useful signal. The type of optical phenomena measured can be used for their subdivision into absorbance, reflectance, luminescence, fluorescence, refractive index, optothermal effect, or light scattering.
- Mass-sensitive sensors, which transform the change of mass caused by the accumulation of the analyte on a special surface able to change some of its properties because of that accumulation. In this group, there are piezoelectric and surface acoustic wave sensors.
- Magnetic sensors, which measure a change in the paramagnetic properties of an analyte.
- Thermometric sensors, which measure the heat effects of a specific chemical reaction or adsorption taking place with the participation of an analyte [21].
3. Sensor Characteristics
Sensors must fulfill different conditions in order to be successfully applied. Many sensors are developed, but only a small portion are commercialized due to the rigorous requirements that are not easily met. The most important characteristics of sensors include selectivity, sensitivity, stability, resolution, linearity, range, repeatability, and reproducibility. These properties will be discussed below.
3.1. What Makes a Sensor Selective?
When a sensor is used as an analytical tool, a few important aspects must be satisfied, namely, the sensor must be selective, sensitive, and stable. The molecular recognition between the analyte and the sensing molecule represents a base for analytical sensor development. This recognition usually involves non-covalent interactions. They provide the reversible nature of the analyte-sensing molecule reaction. Therefore, the specificity and sensitivity of analytical sensors are dependent on the equilibrium binding constant for this reaction. Very high equilibrium binding constants (above 10–100 µM) will result in a drop in sensitivity and the low specificity of a sensor. On the other hand, very low equilibrium binding constants (below 10–100 pM) will assure high sensitivity, while strong conditions (pH, solvent change, ionic strength) for the dissociation of the analyte (for the sensor reuse) could be necessary, decreasing the sensor’s stability dramatically [23,24]. Sensors with good performance should have the equilibrium binding constant in a specific range to obtain optimal functioning.
There are no samples without interference. Selectivity is, therefore, the most important feature of a sensor. The linear range is narrow when an interfering compound is present in a high concentration. In addition, detection limits are higher. Consequently, receptors with high selectivity are essential for sensor development [25]. Biological and biomimetic sensor elements have shown the highest selectivity. In biosensors, the receptor could consist of enzymes, antigens or antibodies, DNA probes, tissue, cells, and cell organelles [26].
The properties of enzyme biosensors are based on the ability of natural enzymes to participate in catalytic reactions with the analyte of interest [27]. Enzymes are a specialized class of proteins with the ability to catalyze chemical reactions. They contain a substrate-binding site and an active site. Some enzymes have a cofactor in their structure, which is a non-protein component responsible for catalysis [26,28]. Enzymes differ from ordinary chemical catalysts in several important aspects. The enzymatically catalyzed reaction rates are significantly higher than the corresponding chemically catalyzed reactions. In addition, enzymes have a considerably greater degree of specificity with respect to their substrates than chemical catalysts. There is a well-known lock-and-key hypothesis describing enzyme activity: “The specificity of an enzyme (the lock) for its substrate (the key) arises from their geometrically complementary shapes” [28]. The activity of an enzyme is highly dependent on environmental conditions (temperature, ionic strength, and pH of the solution, and the presence or absence of inhibitors) [26,29]. On the other hand, these properties lead to low stability, short life span, and the high price of enzyme sensors. Tissue and cells can be used as receptors in biosensors, while the enzymes within them perform reactions [30]. These modifications are usually favorable regarding the stability and cost-effectiveness of a sensor.
When it comes to organophosphate pesticide detection, most enzymatic biosensors employ acetylcholinesterase. Since AChE is a primary target in the animal organism for this group of pesticides, its involvement in sensor construction is not unexpected. AChE is a serine hydrolase found predominantly in neural tissue [31]. Its prominent role is the termination of impulse transmission in the brain due to the hydrolysis of acetylcholine. The active site of AChE is a hydrophobic gorge divided into several subsites. There is a catalytic active site at the bottom of the gorge, an anionic subsite, an acyl-binding pocket, and an oxyanion hole. In addition, there is a peripheral anionic subsite located at the gorge’s rim, which is not part of the catalytic site but is essential for the binding of AChE inhibitors. Several allosteric sites can be found at the surface of the enzyme [32]. Inhibitors can bind any of these sites and disturb the enzyme’s conformation, preventing it from exerting its physiological function. OPs are irreversible inhibitors of AChE and exert their toxicological effects through esterase phosphorylation. OPs enter the active site of AChE and covalently bind the -OH group of serine. Dephosphorylation is very slow, so the enzyme remains inactive for days [31]. The specific recognition of an enzyme and the organophosphates and strong bonding are the main reasons for AChE employment in sensors for OP detection.
The formation of complementary complexes is achieved by reversible biochemical processes and is used in affinity biosensors. DNA and immunosensors belong to this group. The receptor in DNA sensors is a single-stranded DNA fragment, such as DNA probes and primers [33]. In an immunoassay, an antibody or an antigen plays the role of a receptor. Variable regions in the antibody’s structure are energetically and conformationally complementary to the antigen [34]. Nowadays, the library of antibodies and DNA probes is extensive, and the processes for obtaining such receptors are automated. In addition, DNA primers and antibodies are more stable and universal than enzymes [26].
In a sensor without a biological origin receptor (biomimetic receptor), a key role in the recognition of an analyte is played by synthetic molecules having, in their structure, functional groups able to selectively interact with that particular analyte. In this type of sensor, receptors are artificial and consist of the following types of compounds: non-protein catalysts, calixarenes, molecularly imprinted polymers, aptamers, and nanomaterials. In the case of biomimetic materials, the host–guest principle is crucial for their role as a receptor. Host–guest chemistry describes the formation of supramolecular complexes between two or more molecules or ions via non-covalent interactions (hydrogen-bonding interaction, π−π stacking interaction, electrostatic interaction, van der Waals force, and hydrophobic/hydrophilic attraction) [35]. Supramolecular materials are characterized by reversibility due to the weak non-covalent interactions, which allow easy dissociation and rebuilding of the supramolecular systems at a low energy cost [36]. Therefore, the supramolecular materials can be recycled and self-repaired, which is particularly useful for sensor functioning [37].
Non-protein catalysts are non-biological molecules that mimic the active sites of enzyme–redox-active metal centers and suitable ligand environments. They enable electrocatalytic processes by simulating the action of enzymes [38]. A large number of natural enzymes contain metal ions, which are important for catalysis. To ensure selectivity in non-enzyme sensors, materials capable of molecular recognition are necessary. The second part of the synthesis of non-biological catalysts is mimicking an enzyme’s active site. In addition, the topic of the catalytic activity of organic polymers without metal in their structure has been extensively investigated, but their catalysis efficiency is relatively low, so they are not widely used as biomimetics [26]. Calixarenes are a family of macrocyclic compounds with a variable number of phenol units linked by methylene bridges in the ortho position [39,40]. Via non-covalent interactions, calixarenes are recognizing guest molecules during host–guest complexation [41]. They can be synthesized easily and altered to obtain molecules according to the guest that should be complexed. The synthesis process is cheap and offers good yield, while the formed structures are not toxic nor immunogenic [41]. There are many sensors containing calixarenes as receptors described in the literature, which exert high selectivity and stable performance for long-term reuse, and provide rapid detection of multiple analytes in a cheap, eco-friendly, and highly efficient manner, and with a much lower detection limit in comparison with other alternatives [42,43]. The main problem with the calixarenes-based sensors is that they are used in non-aqueous mediums. As most chemical species of interest to analysts are to be found in aqueous samples, it is clear that more work needs to be carried out in order to put calixarenes on the map of considerable receptor candidates for environmental sensors [43].
Molecularly imprinted polymers (MIPs) serve as an alternative to enzymes when a specific analyte requires extraction from a sample matrix. MIPs can create recognition sites in synthetic polymers, such as cavities corresponding to the target molecule in shape, size, and energy. In this way, exceptional selectivity for the separation of the target molecules is provided. Organic monomers and cross-linking can be used to synthesize MIPs, together with the micro- or nanoparticles of an inorganic material coated with a polymer shell [44,45].
Aptamers are synthetic single-stranded DNA or RNA fragments of several tens of nucleic acids in length, able to provide selectivity for non-enzymatic sensors. In their structure, aptamers have clefts and grooves of target molecules, a higher receptor density, and a smaller spatial blockage that leads to higher binding efficiency. They are more stable under extreme storage conditions compared to proteins and can be synthesized chemically, which makes them affordable (unlike monoclonal antibodies). They can also bind large organic ligands and low-molecular compounds [26].
Nowadays, nanomaterials are often used to modify transducers’ layers to improve sensors’ analytical characteristics. The creation of this additional layer on the transducer’s surface opens a wide range of possibilities for the immobilization of various compounds, which increases the sensor’s sensitivity. Considering electrochemical sensors based on different nanoparticles, from metals to oxides and carbons, or any combination of these materials, the charge transfer process is inevitably connected to the generation of an analytical signal. From the fundamental point of view, this relates to the close interaction between analyte and nanoparticle, in most cases adsorption. In this sense, selectivity can be achieved by the rational choice of nanomaterials that selectively interact with analyte molecules. Due to a large number of low coordinated sites at nanoparticle surfaces, it cannot be expected that selective interactions with different chemically similar compounds are something that is easily achieved in the scenario where the transducer also serves as a receptor. Nanostructured materials of various geometries can be synthesized nowadays. They can be conjugated with various bio-molecules. Quantum dots, carbon nanomaterials, and metal nanoparticles are the most common. The hydrophobicity of carbon materials and their tendency to aggregate are avoided by surface functionalization techniques or reducing particle dimensions. On the other hand, metal nanoparticles and their oxides possess electrochemical activity and a large surface. Due to that, they can be used as signal-forming labels, carriers for the receptor layer, transducer components, and improve the analytical characteristics of the sensors. However, the problem is that the suspensions of nanoparticles are often unstable. This problem is eradicated mainly by nanoparticle surface modification [26].
3.2. What Makes a Sensor Sensitive?
The sensitivity of a sensor is defined as the slope of the output characteristic curve or, more generally, the minimum input of physical parameters that will produce a detectable output change [46]. Therefore, the sensitivity of a sensor is dependent on both the receptor and transducer. The favorable combination of two parts is essential to have the best possible sensor sensitivity. The receptor affects sensitivity via its natural properties and should be carefully chosen. The choice of the transducer part has to be made in accordance with the receptor’s nature. The response of the transducer can be amplified if it is necessary.
When speaking of electrochemical sensors, voltammetric and amperometric, charge transfer is, again, the primary process underlying signal generation. This means that the electrode used for detection must be active for the electrochemical reaction. This inevitably brings us to the relevance of electrocatalysis in the field of electrochemical sensors, as, in general, the rate of an electrochemical reaction depends on the material of the electrode on which it takes place. As in electrochemistry, the rate of the electrochemical reaction is directly connected to the measured current, in order to have high sensitivity, the best possible electrocatalyst for a given reaction should be chosen. Naturally, electrocatalysis is also closely connected to the selectivity of a voltammetric/amperometric sensor, but in a more complicated way than sensitivity.
3.3. What Makes a Sensor Stable?
The stability of a sensor is a measure of the sensor’s characteristics remaining constant over time. Changes in the stability of a sensor are known as sensor drift and can be caused by components aging, a decrease in sensitivity of the components, a change in the signal-to-noise ratio, etc. This stands for both the receptor and transducer parts. Receptors with biological origins are severely sensitive to aging. They should be kept under rigorously controlled conditions in order to extend the period within which they can be in use (temperature, pH, light, presence of inhibitors, and other factors).
Artificial receptors are more stable than biological ones and bind more firmly to the analyte [26]. Using supramolecular compounds, nanomaterials, MIPs, and aptamers as biomimetics makes non-enzymatic sensors an excellent alternative to traditional enzyme sensors. It also enables them to exceed the enzymatic sensors in terms of universality, tolerance to the environment, and life span. However, biomimetics are still, and probably will always be, inferior to natural enzymes in terms of their catalysis efficiency and selectivity [26].
Besides the stability of the receptor, the chemical stability of the transducer is also a very important issue. In principle, the conditions of electrochemical reactions are rather harsh, and under potentiodynamic conditions of voltammetric/amperometric sensors, different electrochemical processes can cause irreversible changes in transducing components. For this reason, it is necessary to carefully control the electrochemical window in which measurements are performed and also conditions, such as pH, the presence of gases, such as O2, and so on. In addition, storing a sensor correctly is important in order to prevent corrosion and other irreversible changes that could cause a change in the sensor’s characteristics.
3.4. Other Relevant Properties
Besides selectivity, sensitivity, and stability, there are other important properties of a sensor. They may not be as fundamental as the aforementioned ones, but they are equally important for the proper functioning of the sensor. The sensor range is the maximum and minimum values of the applied parameter that can be measured. Usually, it is of interest to have the range as wide as possible. The precision denotes the degree of reproducibility of a measurement. Resolution is the smallest detectable incremental change of input parameter that can be detected in the output signal, and this can be expressed either as a proportion of the reading or in absolute terms. The sensor’s accuracy represents the maximum difference between the actual value and the indicated value at the sensor’s output. The offset error of a transducer is defined as the output that will exist when it should be zero or, on the other hand, the difference between the actual output value and the specified output value under some particular set of conditions [46]. Other important peculiarities in sensor design include short response times, high mechanical strength and chemical resistance to changes in operating matrix (connected with stability). Many sensors are able to operate under critical conditions due to the high selectivity of biological receptors, compensating for the device’s weak mechanical and chemical strength [25].
4. Carbon Materials in Sensors—Underlying Properties Enabling Their Use
Carbon materials are present in a great variety in chemical laboratories. In recent years, the classical view of two carbon allotropes, diamond and graphite, has been extended with carbon structures of different dimensionality (Figure 2). This classification is closely related to the dimensions of carbon structures at the nanoscale and the definition of nanomaterials itself. Depending on the lateral dimension of carbon structures, the materials are classified as 0D, 1D, 2D, or 3D materials [47].
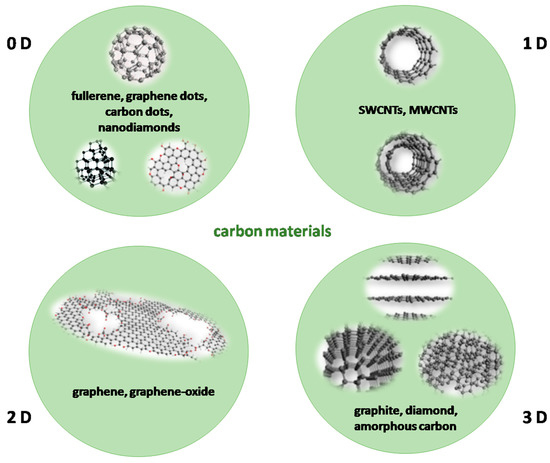
Figure 2.
Classification of carbon materials according to their dimensionality.
Formally speaking, 0D materials have all three dimensions under 100 nm. However, typical 0D carbon materials have much smaller dimensions (usually just a few nanometers). These include fullerenes [48,49], graphene quantum dots (GQDs) [50,51], carbon quantum dots (CQDs) [52,53], and nanodiamonds [54]. Small dimensions ensure that most carbon atoms are exposed to the 0D material’s surface and that the surface-to-volume ratio is exceptionally high.
Typical examples of 1D carbon nanomaterials are single-walled and multi-walled carbon nanotubes (SWCNTs and MWCNTs), with additions to this family such as unzipped carbon nanotubes, carbon nanoribbons, and others [55]. One dimension of these materials, where we consider their length, is larger than 100 nm. These materials have a well-defined structure, described through the folding scheme of graphene sheets, with a well-defined diameter being constant along the entire nanotube. CNTs are widely commercially available, and reporting their qualities is a rather standardized procedure.
The evaluation of specific surface area (SSA), which is one of the most important properties of carbon materials in the case of well-defined CNTs, renders a solution to a geometrical problem [56]. For SWCNT, the effect of the nanotube diameter cancels out, and the SSA is constant—1315 m2 g−1. For MWCNTs, the addition of extra walls increases the mass more than the exposed surface, and the effect of the diameter is present. With every additional wall, the SSA decreases; for 20WCNTs, it reaches only ~100 m2 g−1 and decreases with increasing NT diameter [56]. By definition, CNTs have a honeycomb arrangement of carbon atoms [57], i.e., sp2 hybridization. Thus, the walls of CNTs are generally chemically inert.
In the 2D part of the carbon family, graphene and graphene oxide (GO) stand out as classic examples [58]. Graphene is an infinite sheet of carbon arranged into a hexagonal lattice and characterized by some exceptional properties (thermal, mechanical) [59]. Hexagonal structure is governed by the sp2 hybridization of carbon atoms, so the chemical bonds between carbon atoms are directed in-plane. The π electron cloud forms above and below the graphene plane. It makes graphene a zero-gap semi-metal [59]. Therefore, pristine graphene is enormously chemically inert [60]. The main chemical reactivity of graphene originates from the presence of defects and edges [61,62]. Determination of the SSA of graphene is also a geometrical problem, and for pristine graphene, the SSA amounts to 2630 m2 g−1. In practice, graphene can be synthesized using bottom-up and top-down approaches [63]. The best-known and most widely used one is the reduction of GO obtained via different modifications of the Hummers method [64], but strictly speaking, in this case, one obtains reduced graphene oxide (rGO).
GO is a highly oxidized graphene sheet with O content ranging from 30 to 50% [65]. It is a poor conductor or insulator, and its SSA has been measured from 2 to 2391 m2 g−1, depending on the applied approach [66]. It is commonly obtained via the exfoliation of graphite oxide, using the mentioned Hummers method or some other approach. Several models of GO exist in the literature, with the Lerf–Klinowski model now being widely accepted [67]. In this model, epoxy- and hydroxyl groups are found on the basal plane, while others, such as phenol, ether, and carboxyl, are located at the edges. GO can be controllably reduced to rGO with different amounts of oxygen functional groups using chemical, thermal or electrochemical methods [68,69]. Besides oxygen functional groups, commercial GO can contain small amounts of other heteroatoms (e.g., nitrogen or sulfur). Moreover, the structural disorder in these GO samples is typically very high, and the basal plane can be significantly damaged with a large number of holes reaching several nanometers in diameter [70].
Graphite and diamond represent two extremes of the 3D class of carbon allotropes family. Graphite has a hexagonal lattice and is comprised of graphene sheets stacked via weak Van der Walls forces. It is an exceptional conductor, but its conductivity is displayed only in the (0001) plane. In contrast, diamond has a cubic lattice governed by the sp3 hybridization of carbon atoms. It is an insulator and possesses exceptional hardness. Amorphous carbon does not possess long-range order in its lattice but, locally, contains nanometric domains with a graphite- or diamond-like structure. It mostly consists of sp2 hybridized carbon and it is usually conductive.
In the preceding discussion, carbon materials have been classified based on their dimensionality, but we considered the forms of carbon with well-defined crystal structures. However, terms such as carbon nanotubes (nanorods, wires, fibers, platelets, and others) are also used to describe the morphology of the carbon sample (with the addition of “nano” or, more precisely, “nanosized” if the dimensions are under 100 nm) irrespective of their crystal structure. A typical example is the conversion of organic materials with different morphologies to carbons, i.e., their carbonization, where the original morphology is preserved in the final carbon material. This scenario is quite usual when polymeric (nano)structures are carbonized. As a rule, the morphology of the polymeric precursor remains preserved [55,71]. The situation is the same when biomaterials with a particular morphology, such as fibers, are converted to carbons [72,73]. All these materials are generally amorphous carbons, but if produced at very high temperatures (>2500 °C), graphitization can take place [74]. Still, even in this case, the materials will consist of nanosized graphitic domains, and long-range ordering is not taking place.
So far, several types of nanosized carbon forms have been discussed. However, the material is also considered a nanomaterial if nanosized local structures can describe its internal structure. As most carbon materials have developed pore structures, and the dimensions of these pores are typically in a nanometric range, all carbons can be considered nanomaterials. However, as for 3D carbons, this description comes from their internal pore structure. Therefore, it is more accurate to refer to them as nanostructured materials. A large variety of suitable precursor materials is available to achieve the required carbon properties. In the last two decades, the interest in sustainable carbon precursors exploded, and all sorts of biomass, due to economic and ecological reasons, especially waste streams, have been tested as precursors for activated carbons [75,76,77,78,79]. In addition, several chemical and physical routes have been developed for increasing carbon surface areas and developing the pore network in carbon materials [80,81,82]. This process is called activation, and carbon material that has undergone activation is called activated carbon. All the mentioned classifications and attributes can be combined to name a given carbon material. For example, activated carbon fibers are carbon materials with fibrous morphology that have been activated using some of the possible activation routes [83]. Furthermore, if we speak about activated carbon nanofibers, this will also mean that the diameter of the fibers is under 100 nm.
When we talk about carbon materials suitable for electrochemical sensor construction, there are some important properties to be observed. In principle, carbon materials are good electronic conductors. They are inexpensive, profuse, easy to work with, and chemically inert. Carbon materials are suitable for making composites [84]. Although most materials would suffer from electrochemical transformations, carbon materials can take the many electrochemical reactions performed on them. On the other hand, this chemical inertness is a problem for electrochemical measurements. To perform electrocatalytic conversion and detection, it is necessary for the electrode material and the analyte to interact. Therefore, defects and functional groups present in carbon materials can be beneficial to overcome this issue. Glassy carbon (GC), boron-doped diamond (BDD), and graphite materials from the carbon black (CB) family are traditionally used in electrochemical laboratories. Nowadays, the new carbon-based nanomaterials are increasingly represented, especially in sensor construction. Carbon nanotubes and graphene-based materials are the leaders in this group [85].
Carbon materials are mostly used as a support for different metallic or oxide nanostructures and biologically active compounds in electrochemical sensors. Still, there are some papers reporting “only-carbon” electrodes for pesticide detection via their direct electrochemistry. In this case, more advanced electrochemical techniques, such as differential pulse voltammetry or square wave voltammetry, have to be used in order to improve the detection capabilities of sensing platforms. Besides, carbon-based materials are the most suitable for on-field use since they are generally very robust and rarely require special care [85].
In the case of optical sensors, carbon materials can also have diverse roles. They can be used as a supporting material, such as in electrochemical sensors. On the other hand, the unique opto-chemical properties of carbon-based nanostructures enable them to have many innovative applications in this field. Most often, they are used in plasmonic optical sensors. Different carbon-based nanomaterials can act as a plasmonic material, as the sensitivity enhancement material, and provide a large surface area as well as compatibility for the immobilization of various biomolecules (enzymes, DNA, antibodies, antigens) within the sensing layer [86].
Graphene-based materials and carbon nanotubes are the most widely used carbon-based nanomaterials for sensing applications. They have unique optical properties, in addition to high conductivity and a high surface-to-volume ratio, and they can be easily functionalized with various biomolecules and polymers for better selectivity, chemical stability, and biocompatibility. In the last few decades, carbon dots and nanodiamonds are becoming very interesting in this area, too [86].
In addition to their unique electronic properties, graphene-based materials have exceptional optical properties, and are being widely used for sensing applications. Although graphene, in its pristine condition, is a zero-bandgap material, GO possesses different functional groups, which display strong emissions from the UV to NIR range [87]. The emission originates from the electronic transition between the sp2 carbon domain and the functional groups positioned at the borders of the GO sheets [88]. In the presence of the target molecules, the fluorescence emission of GO can be enhanced or quenched [89,90].
The optical properties of CNTs are characterized by the hove singularities in the electronic density of states [91]. They exert near-infrared (NIR) photoluminescence upon photoexcitation. The great benefit of this phenomenon is it is tunable, photostable, and susceptible to the environment [92,93]. Recent discoveries showed that SWCNTs could be designed to obtain the chosen colors of emission. The emission is dependent on fluctuations of the dielectric constant around the SWCNTs. The sensitivity is so high that it allows perturbation detection at the surface of SWCNTs at the single-molecule level. This characteristic qualifies SWCNTs for use as molecular sensors. The emission could be obtained in NIR, and sensors such as this can be used successfully for in vivo applications since they work at wavelengths with maxim tissue penetration. Surface functionalization enables the specificity of the sensing method [94].
CQDs and GQDs are fluorescent carbon nanoparticles. Although they are similar, there are some noticeable physical differences between them. GQDs represent nanosized graphene monolayers, containing mainly sp2-hybridized carbon atoms [95]. In contrast, CQDs are spherical nanoparticles with cores with an sp3 or sp2-hybridized carbon configuration and a dimension below 10 nm [96,97,98,99]. Different functional groups can exist on the CQD surface. Since they are introduced during the synthesis process, their presence is highly dependent on the precursors used for the synthesis. Functional groups can modulate the optical and sensing properties of CQDs [100,101]. The sensitivity and selectivity of CQDs can also be achieved by their post-functionalization with different chelating groups or biomolecules [102]. In CQDs-based sensors, there are changes in fluorescence emission intensity in the presence of target molecules. This phenomenon can be ascribed to the inner filter effect, photo-induced electron transfer, and Forster resonance energy transfer [103]. For CQDs, the fluorescence emission wavelength is usually from 400 to 750 nm [104]. The intensity of fluorescence is dependent on the type of solvent [35], pH [36], temperature [105], and the concentration of CQDs [89,106].
Nanodiamonds (NDs) are 0D carbon materials that contain only sp3 carbon atoms and have a diamond-like morphology. Their dimensions vary from 2 to 20 nm [87]. NDs tend to aggregate [107]. The unique property qualifying NDs for use in sensing is the presence of the fluorescent nitrogen-vacancy (N-V) defect center. The N-V center is a defect in the crystal structure of NDs, where one of two carbon atoms is substituted with a nitrogen atom, while the other is a vacancy without replacement. The N-V centers can be optically excited and, subsequently, transit between the ground and electronically excited states. The N-V centers can relax to the ground state via radiative and nonradiative pathways. These futures are used in optical sensing [89,108,109].
Figure 3 recaps the properties of various allotropes of carbon.
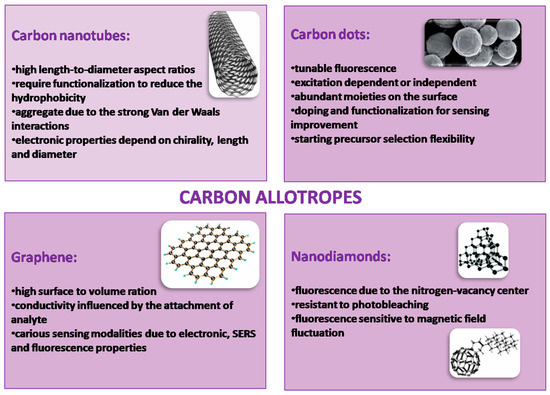
Figure 3.
Various carbon allotropes’ properties.
5. Electrochemical Sensors for Organophosphate Detection Based on Carbon Materials
Electrochemical sensors are devices that provide information about the composition of a system in real time by coupling a receptor to an electrochemical transducer, translating the chemical energy of the selective interaction between the analyte and the receptor into an analytical signal. They are the largest and the oldest group of chemical sensors, usually with simple instrumentation and a high potential for miniaturization and automatization without compromising their analytical characteristics [110]. What also makes electrochemical sensors suitable for diverse applications is their high performance and economic aspect. As already mentioned, electrochemical sensors can be divided into (1) voltammetric/amperometric, (2) potentiometric, and (3) impedimetric.
In voltammetric and amperometric sensors, information about an analyte is obtained by measuring the current while the potential is varied. A voltammetric measurement includes scanning the potential difference throughout an electrochemical cell and recording the cell’s current as a function of the applied potential. Amperometric measurement is made by recording the current in the cell at a single applied potential [111]. In both cases, the transducers have the ability to transfer the electrons to or from the analyte, making the charge transfer step to be the crucial process underlying detection. The flow of these electrons forms the output signal of the transducer. In their essence, the measurements are based on the electrochemical techniques, voltammetry and amperometry, which is relatively simpler but provides much less information due to the single potential at which the measurement is performed. Voltammetric and amperometric transducers are also capable of contributing to the selectivity of the overall sensing process due to the dependence of the measured current on the applied potential, which is a function of the standard potential of the redox couple that is analyzed. Voltammetric and amperometric transducers are also relatively simple and mainly consist of two or three electrodes immersed in a suitable electrolyte. These sensors are fast, sensitive, precise, and accurate. Furthermore, it is not necessary to wait until the thermodynamic equilibrium is achieved and the response is a linear function of the concentration of the analyte (irrespective of the nature of the charge transfer step) [112]. However, there are important factors that must be considered besides charge transfer, which provides the analytical signal. As the underlying voltammetry and amperometry are dynamic techniques, the mass transfer can influence the signal. In addition, current flow causes inevitable voltage losses in the electrolyte, so good conductivity of the electrolyte is needed to prevent large drifts in the measured current-potential responses.
In contrast to voltammetric/amperometric sensors, potentiometric sensors measure the potential difference between two electrodes under the conditions of no current flow, i.e., the electromotive force of the cell [113]. As a rule, we only look into the signal of the sensing electrode, while the other one is a suitably chosen reference electrode with stable and well-known potential. Hence, variation of the sensing electrode’s potential should reflect the change in the concentration (activity) of the analyte. The signal of a general potentiometric sensor is based on the Nernst equation, which, in a perfect world, depends only on the activities of species forming a redox couple of analytes [113].
However, in real life, there are additional terms to the logarithmic terms giving an interference contribution to the potential of the sensing electrode. In contrast to the first mentioned class of electrochemical sensors, the signal depends linearly on the logarithm of activity (concentration) of the analyte, making this type of sensor more suitable for low concentrations of analytes. However, the Nernstian response is not available for an unlimited range of concentrations, and typically, there are several decades of concentrations in which the sensor is operative. Another point is that the O/R redox couple should be reversible, which is rarely the case with complex molecules (but also simple ones, such as the O2/H2O couple). Nevertheless, as there is no current flow, the high electrical conductivity of the electrolyte is not a prerequisite to performing measurements (but some conductivity must be assured so that the circuit is not open).
Potentiometric sensors are one of the lowest-cost analytical devices available today. Nowadays, the main obstacle with potentiometric sensors is obtaining better limits of detection and selectivity. Innovative developments in this field include sensor arrays, new ionophores, improvement of the detection limit, and new electrodes for miniaturization [112].
Impedimetric measurement can be, in general, carried out with electrodes of a certain functionalization, e.g., polymer, nanomaterials, and could also include a kind of bioreception. Impedimetric techniques are widely used tools in electrochemistry for the detection and quantification of chemical targets by measuring the changes in the complex impedance that are specific to the combination of the electrode materials and target analytes. Impedimetric sensors register changes in the electrical properties at their surface (either capacitance or resistance or a combination of these properties) [114]. Electrochemical impedance spectroscopy gives information about chemical reactions at the electrode liquid interfaces and provides an accurate conductivity measurement at the same time. The impedance spectra are usually fitted to an equivalent circuit model, which enables the separation of different effects based on their frequency-dependent behavior. They give information about the different processes occurring in the system. Electrochemical impedance spectroscopy can also be performed in conjunction with other electrochemical methods on the same measurement system [114], e.g., to characterize electrodes, detect analytes of interest, and investigate reaction mechanisms. Thereby impedance spectroscopy has, in its basic form, a weakness considering the chemical selectivity.
These kinds of sensors have the advantage of low cost. They are user-friendly and potentially portable. In addition, they are a powerful tool for biosensing at the modified electrode surfaces. Impedimetric sensors are less destructive as compared to other electrochemical methods. Moreover, they give direct electrical signals and do not require a label or other pre-treatment process, which is especially important for the detection of environmental toxins [115].
The list of carbon-based electrochemical sensors and their characteristics are given in Table 1.

Table 1.
Electrochemical sensors for organophosphate detection based on carbon materials.
As seen in Table 1, electrochemical sensors for organophosphate detection based on carbon materials have been examined in detail. In these studies, among the electrochemical methods used, CV, DPV, SWV, and EIS were the most preferred to determine analytes. Electrodes modified with carbon-based nanomaterials, composites, metal oxides, polymers, and metal nanoparticles were employed. Additionally, the analyzed organophosphates, in most cases, were fenitrothion, chlorpyrifos, methyl parathion, and parathion. In most cases, the LODs for the chemosensors presented in Table 1 were around the nM level. Considering the allowed concentrations of pesticides in the environment [151], this is not low enough. This is when the biosensors and MIP-based sensors show their advantages. Besides having higher selectivity, their sensitivity was much better than the other sensors reviewed. The biomimetic principle of recognition offers great potential.
Historically, one of the first types of sensors employed for organophosphate detection were electrochemical biosensors. The first investigations encompassed amperometric detection and employed acetylcholine and butyrylcholine esterases [85,152]. As can be seen from the data presented in Table 1, their performances were good. The enzyme provided the necessary selectivity, and the limit of detection was lower compared to chemosensors. Moreover, immunosensors and aptasensors showed similar characteristics. Still, the main trouble is the handling of the biological recognition element. Biomolecules should be used with great care since they acquire special conditions to retain their biological activity. An additional issue is their high price.
With MIP-based sensors, selectivity increases while consecutive electrochemical transformations of recognized analytes enable high sensitivity and low detection limits. Since conductivity is crucial in electrochemistry, conductive polymers are used for MIP synthesis. Although the selectivity and sensitivity of MIP-based sensors are similar to that of immuno-based and aptasensors, an important difference is that MIP technology is affordable and less complicated to use. Still, there are some important limitations of MIPs for electrochemical detection. These polymers are sometimes also electrochemically active, and their signal could mask the signal from the analyte [153]. Besides, the possibility of irreversible electrochemical transformations of polymers and their pH sensitivity is an important issue [85]. It could lead to irreversible losses of electroactivity, conductivity, and conformational changes, which might disrupt the recognition moieties of MIPs [85,154]. These issues could compromise the overall performance of the sensor.
Biomimetic sensors are one of the newest sensor technologies. They demonstrated amazing performance, but there are only a few examples (Table 1). Polymers used for their construction mainly mimic AChE and organophosphate hydrolase. Unlike enzymatic biosensors, they are easier to handle and have no issues with interference.
Recently, Cesana et al. reported on the new chemosensor for fenitrothion detection [155]. It is based on fluorescent Cdots(N)-Silica composites. Carbon quantum dots with an N heteroatom in their structure (Cdots(N)) were synthesized electrochemically. After that, the Cdots(N)-Silica composites were synthesized via a sol-gel process without the use of acid/base catalysts. Previously polished GCE was modified with Cdots(N)-Silica composite by adding the ethanolic suspension of the composite and subsequent drying. The electrode, modified in this way, was further used in electroanalytical analysis without pre-treatment in order to detect fenitrothion by means of DPV. The detection limit of the reported sensor was 1.05 × 10−9 mol L−1. Having in mind the maximum residue limit (MRL) for fenitrothion in fruits is 3.61 μmol L− 1, this sensor offered a satisfactory result. Besides, it was successfully applied in real samples with high repeatability in the presence of possible interferents. The reproducibility of the modification of GCE with the Cdots(N)-Silica composite was 100%, and they remained stable for over a month.
Akyüz et al. reported on sensors for fenitrothion detection based on electropolymerized metallophthalocyanines modified GCE [156]. New metallophthalocyanines (MPcs) were designed with redox active metal centers (Co(II), Cl-Mn(III), and Ti(IV)O) and morpholine and amino bearing substituents (ma). Redox-active metal centers enhanced the redox activity of the complexes, and redox-active and electropolymerizable [2-(4-phenyl)ethoxy] substituents triggered the coating of MPcs with the oxidative electropolymerizations. All complexes were used for GCE modification, and the electrodes were studied as potential fenitrothion sensors. The GCE/CoPc(ma) electrode showed good selectivity and sensitivity, with a linear range between 1.20 and 42.0 μM and a detection limit of 0.46 μM. The GCE/TiOPc(ma) electrode also showed good selectivity, but its linear range was very narrow. The GCE/Cl-MnPc(ma) electrode had poor selectivity and good sensitivity.
Ivanov et al. reported on a flow-injection system with an integrated amperometric biosensor with immobilized AChE [157,158]. AChE was immobilized on MWCNT and an acrylonitrile-methylmethacrylate-sodium vinylsulfonate membrane. The chemical modification of MWCNTs was achieved by treatment with concentrated nitric acid in order to introduce carboxylic groups. The carboxylated nanotubes were then washed and treated with ethylene-diamine for 1 h to introduce amino groups, followed by washing again. The aminated nanotubes were mixed with bovine serum albumin (BSA). AChE immobilization was conducted in multiple steps. The first step was to activate the amino groups of the modified membrane with glutaraldehyde and immerse it in a mixture of modified MWCN and BSA. The next step was to immerse the membrane into glutaraldehyde again and wash it. The activated membrane was then incubated with concanavalin A for 1 h. Finally, the membrane was immersed in AChE solution for 24 h and washed afterward. The sensor showed a low detection limit for three OPs: paraoxon ethyl (0.9 × 10−12 M), monocrotophos (1.8 × 10−12 M), and dichlorvos (2.0 × 10−12 M). It was found that the biosensor can be reused in 15 operation cycles. The prepared biosensor showed good precision, reproducibility, and stability. Still, its selectivity was questionable since AChE is sensitive to many OPs.
When it comes to electrochemical sensors for organophosphate detection based on carbon materials, it is obvious that biosensors offer great characteristics but require special care. A person without proper training could not work with bio-based sensors in the field. On the other hand, new technologies offer robust solutions, such as MIP-based, biomimetic, and aptasensors [159]. They are highly sensitive and have no problem with interference, unlike enzymatic biosensors, for instance. However, they are expensive, which makes their wide application unlikely in the next few years.
6. Optical Sensors for Organophosphate Detection Based on Carbon Materials
Optical sensors detect the wavelength, frequency, or polarization of light and convert it into an electric signal due to the photoelectric effect. They can be based on the absorption and emission of electromagnetic waves. Absorption detection methods measure the radiation that has passed through the analyte. In emission-based methods, the electromagnetic radiation emitted by the analyte after excitation is measured in an appropriate manner. Optical sensors based on UV-visible absorption, fluorescence, and plasmonic techniques are commonly used to detect organophosphates [160].
Optical sensors with UV-Vis detection are mostly based on colorimetric assays and used for the qualitatively and quantitatively analysis of organophosphates. These systems are based on Beer Lambert’s law, and the measurements of the intensity and color of the samples before and after the complexation [161]. The visual detection of the color change can be used for qualitative analysis. For quantification, it is necessary to use a UV-Vis spectrophotometer and to determine the concentration of organophosphates in samples by measuring the change in intensity of color or absorption. There are two types of colorimetric sensors: enzymatic (biosensors) and non-enzymatic (chemosensors). Most sensors for organophosphate detection are enzymatic and rely on the inhibition of acetylcholinesterase and butyrylcholinesterase [162,163].
Fluorescent-based sensors for organophosphate detection have been gaining immense importance in the last few decades [164]. The need for a high concentration of fluorophore is the most significant limitation of this technique since it further leads to quenching due to aggregation. Some modifications have been carried out to overcome this problem, such as sensors based on Fluorescence Resonance Energy Transfer (FRET) [165]. In various studies, carbon materials such as carbon dots are used as the donor atoms, while metal nanoparticles or Ellman’s reagents are used as the acceptor [166,167]. Many fluorescent sensors developed for organophosphate detection rely on the interaction with enzymes (acetylcholinesterase, organophosphate hydrolase, alkaline phosphatase, choline oxidase, butyrylcholinesterase) [163,168].
Plasmonic-based optical sensors have been under development for a long time. They have many advantages compared to conventional sensors. The most important one is that plasmonic-based sensors are capable of real-time monitoring. In addition, they allow label-free detection and high reusability, short response time, and simple sample treatments. In contrast, plasmonic sensors are not specific to the binding surface. Their further limitations are mass transportation, susceptibility to steric hindrance, and data misinterpretation risk [169]. Plasmonic-based methods comprise surface plasmon resonance (SPR), localized surface plasmon resonance (LSPR), surface-enhanced Raman scattering (SERS), surface-enhanced fluorescence (SEF), and surface-enhanced infrared absorption spectroscopy (SEIRA) [170]. When it comes to organophosphate detection, SERS offers the most promising results. Via the optical and chemical properties of accessible plasmonic nanomaterial, SERS technology enhanced the naturally weak Raman signal. This type of sensing offers excellent selectivity due to the unique fingerprint of the analyte. There is no interference issue, so the preparation of the sample is easy. In some cases, it is possible to detect a single molecule [153].
Optical sensors are infamous for their unenviable sensitivity. However, historically, these have been important in pesticide detection. Every year, many articles are published on this topic. Scientists make an effort to achieve better results with newly available techniques. Outdated colorimetric sensors have been superseded by those based on the application of fluorescence and plasmonic-based techniques. The list and characteristics of optical sensors for organophosphate detection based on carbon materials are given in Table 2.

Table 2.
Optical sensors for organophosphate detection based on carbon materials.
As seen in Table 2, optical sensors for organophosphate detection based on carbon materials mainly employ fluorimetric and colorimetric techniques for analyte detection. These two techniques are often used in dual mode to improve sensitivity. When it comes to the carbon materials most frequently used, carbon dots and graphene oxide stand out. The obvious reason is their interesting optical properties, as mentioned before. These materials are combined with silver and gold nanoparticles or modified with different oxides and polymers. Additionally, in most cases, organophosphates analyzed by optical sensors based on carbon materials are fenitrothion, chlorpyrifos, paraoxon, and glyphosate.
From the data presented in Table 2, it is obvious that there are a significant number of optical biosensors for organophosphate detection based on the combination of carbon dots and AChE. These examples have the lowest detection limits (in the nanomolar range). Still, they are far from the necessary level for relevant organophosphate monitoring in environmental conditions. Besides, the fragility of the biological recognizing elements already discussed within the electrochemical sensors section can also be applied here with the same implications.
Enzyme-based biosensors have the leading position when it comes to the detection of organophosphate pesticides. Employing enzymes, combined with applying carbon materials and their exquisite optical properties, has great practical significance. Nevertheless, there are still many obstacles to overcome. Environmental factors (temperature, pH value) greatly influence enzymatic activity. In addition, the enzymes are expensive. Finally, enzymes used for organophosphate detection, such as AChE, are sensitive to the whole class of compounds, so interference is a big issue.
On the other hand, antibody-based immunosensors exert stronger specific recognition toward the target analyte. Still, there is an issue with mutation possibility and low modification efficiency. In addition, the detection time is too long, so rapid analysis is not possible. Integrating aptamer and carbon materials provides an effective platform for organophosphate detection. Still, high selectivity is not easy to achieve with the technologies available nowadays. MIPs combined with fluorescing carbon materials are a promising tool for organophosphate detection. They are low-cost and have good stability. On the other hand, there are issues with template leakage, uneven distribution of binding sites, and incompatibility in aqueous media.
The newest approach to optical sensing of pesticides is plasmonic-based techniques, such as SERS [185]. It is attractive and offers additional selectivity. Still, the performance of SERS-based sensors for organophosphate detection based on the use of carbon materials in terms of sensitivity are far from the potential of electrochemical detection.
Li et al. developed a fluorescence sensor for selective dimethoate detection based on fluorescence resonance energy transfer (FRET) between carbon dots and a dye-doped molecularly imprinted polymer. The CDs were synthesized using the one-step hydrothermal method at 180 ◦C for 5 h. The CDs were purified via dialysis and bonded onto the surface of dimethoate using the cross-linking agents 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide and N-hydroxysuccinimide. An indium tin oxide (ITO) electrode was cleaned and then used for the direct electropolymerization of the MIP. The polymer solution contained o-phenylenediamine and dimethoate. The MIP was formed on an ITO substrate via 30 cycles of cyclic voltammetry. When OP is present, a competitive reaction between dimethoate and CDs labeled dimethoate for binding to dye-doped MIP occurs. The FRET-based sensor using CDs provided high selectivity and good sensitivity with a detection limit of 1.83 × 10−8 μM. The sensor also showed good stability and a short detection time [186].
It is evident that CDs-based sensors show lower limits of detection and better performances in general. They have low toxicity and a great potential for green synthesis. Still, they usually suffer from long detection times [159]. Having in mind that CDs have shown better overall performances compared to other carbon nanomaterials, it is worth optimizing their usage in optical sensor construction further.
7. Conclusions and Future Perspective
In order to meet the augmented need for food production, and therefore the increased use of pesticides, their monitoring must be easily accessible. The advancements in novel detection technologies for the efficient determination of OPs has become a topic of the highest importance. Despite the fact that current sensing techniques offer numerous benefits for detecting OPs, there is an urge to create field-deployable, adaptable, and cheap devices.
This paper summarizes optical and electrochemical sensors based on carbon materials for organophosphate pesticide detection. Their performances and characteristics are considered satisfactory. Carbon materials employed in sensor construction have offered great solutions for many issues. Customizable optical properties, large specific surface area, high surface free energy, and outstanding biocompatibility make carbon materials ideal for covering different roles in many types of sensors. They exert highly selective and efficient optical sensing behaviors that are principally dependent upon the signal transduction mode pathway. In addition, the suitability of carbon materials for use in electrochemical sensor construction is unquestionable.
Combining carbon materials with different biological recognition elements, such as enzymes, antibodies, and aptamers, has provided excellent results regarding the selectivity of available sensors. Still, significant improvements regarding the novel or modified bio-recognition elements are required to achieve their stability, easy preparation, and an affordable price. Portability and miniaturization are additional problems.
To date, a sensing system for OP detection with desirable sensitivity and stability has not yet been designed. Biosensors offer satisfactory selectivity but are not stable nor user-friendly. MIPs represent the best currently available choice to meet all of the mentioned criteria. They are highly selective towards the analyte, such as an enzyme or antibody, and their stability is much better. Regarding sensitivity, electrochemical detection looked promising until recently. However, today, we are aware that much lower concentrations of OPs need to be monitored, since long-term exposure to them can lead to many diseases. The symbiosis of electrochemical and plasmonic optical sensing could provide the solution for this challenge. The combination of electrochemistry and SERS is a powerful analytical approach. While using independent detection channels, these techniques could provide complementary information and cover the blind spots of each other, enabling a minimal risk of false results. The issues that still need to be addressed are the cost of the sensors and easier handling. With the currently available technology, their affordability and routine use in-field are not yet on the horizon.
Funding
The author acknowledges the support provided by the Serbian Ministry of Education, Science and Technological Development (Contract number: 451-03-68/2022-14/200017).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Clune, A.L.; Ryan, P.B.; Barr, D.B. Have regulatory efforts to reduce organophosphorus insecticide exposures been effective? Environ. Health Perspect. 2012, 120, 521–525. [Google Scholar] [CrossRef] [PubMed]
- European Union to Ban Chlorpyrifos after January 31. 2020. Available online: https://www.natlawreview.com/article/european-union-to-ban-chlorpyrifos-after-january-31-2020 (accessed on 15 December 2022.).
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide regulations and their malpractice implications on food and environment safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Lazarevic-Pasti, T.; Leskovac, A.; Vasic, V. Myeloperoxidase inhibitors as potential drugs. Curr. Drug Metab. 2015, 16, 168–190. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic-Pasti, T.; Leskovac, A.; Momic, T.; Petrovic, S.; Vasic, V. Modulators of acetylcholinesterase activity: From Alzheimer’s disease to anti-cancer drugs. Curr. Med. Chem. 2017, 24, 3283–3309. [Google Scholar] [CrossRef] [PubMed]
- Gilboa-Geffen, A.; Hartmann, G.; Soreq, H. Stressing hematopoiesis and immunity: An acetylcholinesterase window into nervous and immune system interactions. Front. Mol. Neurosci. 2012, 5, 30. [Google Scholar] [CrossRef]
- Hernández, A.F.; Menéndez, P. Linking pesticide exposure with pediatric leukemia: Potential underlying mechanisms. Int. J. Mol. Sci. 2016, 17, 461. [Google Scholar] [CrossRef]
- Alavanja, M.C.; Bonner, M.R. Occupational pesticide exposures and cancer risk: A review. J. Toxicol. Environ. Health Part B 2012, 15, 238–263. [Google Scholar] [CrossRef]
- National Collaborating Centre for Mental Health. Depression in Adults with a Chronic Physical Health Problem: Treatment and Management; National Collaborating Centre for Mental Health: London, UK, 2010. [Google Scholar]
- Sanne, B.; Mykletun, A.; Dahl, A.A.; Moen, B.E.; Tell, G.S. Occupational differences in levels of anxiety and depression: The Hordaland Health Study. J. Occup. Environ. Med. 2003, 45, 628–638. [Google Scholar] [CrossRef]
- Harrison, V.; Ross, S.M. Anxiety and depression following cumulative low-level exposure to organophosphate pesticides. Environ. Res. 2016, 151, 528–536. [Google Scholar] [CrossRef]
- Ding, Y.; Han, M.; Wu, Z.; Zhang, R.; Li, A.; Yu, K.; Wang, Y.; Huang, W.; Zheng, X.; Mai, B. Bioaccumulation and trophic transfer of organophosphate esters in tropical marine food web, South China Sea. Environ. Int. 2020, 143, 105919. [Google Scholar] [CrossRef]
- Peng, L.-M.; Zhang, Z.; Wang, S. Carbon nanotube electronics: Recent advances. Mater. Today 2014, 17, 433–442. [Google Scholar] [CrossRef]
- Walcarius, A. Recent Trends on Electrochemical Sensors Based on Ordered Mesoporous Carbon. Sensors 2017, 17, 1863. [Google Scholar] [CrossRef]
- Bhat, V.S.; Supriya, S.; Hegde, G. Biomass derived carbon materials for electrochemical sensors. J. Electrochem. Soc. 2019, 167, 037526. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- Gupta, A.; Kumari, A.; Kaushal, N.; Saifi, A.; Mohanta, G.; Sachdev, A.; Kumar, K.; Deep, A.; Saha, A. Recent Advances in the Applications of Carbon Nanostructures on Optical Sensing of Emerging Aquatic Pollutants. ChemNanoMat 2022, 8, e202200011. [Google Scholar] [CrossRef]
- Pundir, C.S.; Malik, A.; Pretty. Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 111348. [Google Scholar] [CrossRef]
- Christopher, F.C.; Kumar, P.S.; Christopher, F.J.; Joshiba, G.J.; Madhesh, P. Recent advancements in rapid analysis of pesticides using nano biosensors: A present and future perspective. J. Clean. Prod. 2020, 269, 122356. [Google Scholar] [CrossRef]
- Sradha S, A.; George, L.; P, K.; Varghese, A. Recent advances in electrochemical and optical sensing of the organophosphate chlorpyrifos: A review. Crit. Rev. Toxicol. 2022, 52, 431–448. [Google Scholar] [CrossRef]
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Yetisen, A.K. Commercialization of microfluidic devices. Trends Biotechnol. 2014, 32, 347–350. [Google Scholar] [CrossRef]
- Winzor, D.J.; Sawyer, W.H. Quantitative Characterization of Ligand Binding; Wiley: Hoboken, NJ, USA, 1995. [Google Scholar]
- Wyman, J.; Gill, S.J. Binding and Linkage: Functional Chemistry of Biological Macromolecules; University Science Books: Sausalito, CA, USA, 1990. [Google Scholar]
- Jiménez, D.; Díaz-Díaz, G.; Blanco-López, M.C.; Lobo-Castañón, M.J.; Miranda, A.; Tuñón-Blanco, P. Chapter 1—Molecularly Imprinted Electrochemical Sensors: Past, Present, and Future; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–34. [Google Scholar]
- Kozitsina, A.N.; Svalova, T.S.; Malysheva, N.N.; Okhokhonin, A.V.; Vidrevich, M.B.; Brainina, K.Z. Sensors Based on Bio and Biomimetic Receptors in Medical Diagnostic, Environment, and Food Analysis. Biosensors 2018, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Dzyadevych, S.; Arkhypova, V.; Soldatkin, A.; El’Skaya, A.; Martelet, C.; Jaffrezic-Renault, N. Amperometric enzyme biosensors: Past, present and future. Irbm 2008, 29, 171–180. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C. Enzymatic and whole cell catalysis: Finding new strategies for old processes. Biotechnol. Adv. 2011, 29, 75–83. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Lazarević-Pašti, T.D.; Leskovac, A.R.; Petrović, S.; Čolović, M.B.; Parac-Vogt, T.N.; Janjić, G.V. A new acetylcholinesterase allosteric site responsible for binding voluminous negatively charged molecules—The role in the mechanism of AChE inhibition. Eur. J. Pharm. Sci. 2020, 151, 105376. [Google Scholar] [CrossRef]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio-Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Harris, L.J.; Larson, S.B.; Hasel, K.W.; McPherson, A. Refined structure of an intact IgG2a monoclonal antibody. Biochemistry 1997, 36, 1581–1597. [Google Scholar] [CrossRef]
- Teyssandier, J.; De Feyter, S.; Mali, K.S. Host–guest chemistry in two-dimensional supramolecular networks. Chem. Commun. 2016, 52, 11465–11487. [Google Scholar] [CrossRef]
- Yu, G.; Chen, X. Host-guest chemistry in supramolecular theranostics. Theranostics 2019, 9, 3041. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, Y. Biomedical applications of supramolecular systems based on host–guest interactions. Chem. Rev. 2015, 115, 7794–7839. [Google Scholar] [CrossRef]
- Patil, A.; Saha, D.; Ganguly, S. A quantum biomimetic electronic nose sensor. Sci. Rep. 2018, 8, 128. [Google Scholar] [CrossRef]
- Li, G.; Song, X.; Yu, H.; Hu, C.; Liu, M.; Cai, J.; Zhao, L.; Chen, Y.; Yang, P. Supramolecular recognition of A-tracts DNA by calix [4] carbazole. Sens. Actuators B Chem. 2018, 259, 177–182. [Google Scholar] [CrossRef]
- Athar, M.; Lone, M.Y.; Jha, P.C. Recognition of anions using urea and thiourea substituted calixarenes: A density functional theory study of non-covalent interactions. Chem. Phys. 2018, 501, 68–77. [Google Scholar] [CrossRef]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and their role in improving the solubility, biocompatibility, stability, bioavailability, detection, and transport of biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef]
- Göde, C.; Yola, M.L.; Yılmaz, A.; Atar, N.; Wang, S. A novel electrochemical sensor based on calixarene functionalized reduced graphene oxide: Application to simultaneous determination of Fe (III), Cd (II) and Pb (II) ions. J. Colloid Interface Sci. 2017, 508, 525–531. [Google Scholar] [CrossRef]
- Oueslati, I.; Ghrairi, A.; Ribeiro, E.S.; Batista de Carvalho, L.A.E.; Gil, J.M.; Paixao, J.A. Calixarene functionalization of TiO2 nanoarrays: An effective strategy for enhancing the sensor versatility. J. Mater. Chem. A 2018, 6, 10649–10654. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, H.; Gao, J.; Liu, X.; Gao, X.; Lu, X.; Fang, G.; Wang, J.; Li, J. Upconversion particle@ Fe3O4@ molecularly imprinted polymer with controllable shell thickness as high-performance fluorescent probe for sensing quinolones. Talanta 2018, 181, 95–103. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Carr, J.J. Introduction to Biomedical Equipment Technology; Pearson Education: London, UK, 2001. [Google Scholar]
- Belenkov, E.A.; Greshnyakov, V.A. Classification schemes for carbon phases and nanostructures. New Carbon Mater. 2013, 28, 273–282. [Google Scholar] [CrossRef]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous amperometric detection of ascorbic acid and antioxidant capacity in orange, blueberry and kiwi juice, by a telemetric system coupled with a fullerene- or nanotubes-modified ascorbate subtractive biosensor. Biosens. Bioelectron. 2015, 67, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, J.; Zhang, Y.; Chen, J.; Zhao, Y.; Niu, Y.; Yu, C. Cerium dioxide-doped carboxyl fullerene as novel nanoprobe and catalyst in electrochemical biosensor for amperometric detection of the CYP2C19*2 allele in human serum. Biosens. Bioelectron. 2018, 102, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhou, L.; Chen, J.; Yan, F.; Liu, J.; Dong, X.; Xi, F.; Chen, P. Nanochannel-Confined Graphene Quantum Dots for Ultrasensitive Electrochemical Analysis of Complex Samples. ACS Nano 2018, 12, 12673–12681. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Ma, J.J.; Chen, J.R.; Feng, H. DNA nanosensor based on biocompatible graphene quantum dots and carbon nanotubes. Biosens. Bioelectron. 2014, 60, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, X.; Qiao, S.; Lu, G.; Su, X. Yellow-Emissive Carbon Dot-Based Optical Sensing Platforms: Cell Imaging and Analytical Applications for Biocatalytic Reactions. ACS Appl. Mater. Interfaces 2018, 10, 7737–7744. [Google Scholar] [CrossRef]
- Zheng, M.; Ruan, S.; Liu, S.; Sun, T.; Qu, D.; Zhao, H.; Xie, Z.; Gao, H.; Jing, X.; Sun, Z. Self-Targeting Fluorescent Carbon Dots for Diagnosis of Brain Cancer Cells. ACS Nano 2015, 9, 11455–11461. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; Liang, R.; Wei, M. Application of Zero-Dimensional Nanomaterials in Biosensing. Front. Chem. 2020, 8, 320. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G.; Pašti, I.; Mentus, S. One-dimensional nitrogen-containing carbon nanostructures. Prog. Mater. Sci. 2015, 69, 61–182. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Lehman, J.H.; Terrones, M.; Mansfield, E.; Hurst, K.E.; Meunier, V. Evaluating the characteristics of multiwall carbon nanotubes. Carbon 2011, 49, 2581–2602. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Bonaccorso, F.; Fal’ko, V.; Novoselov, K.S.; Roche, S.; Bøggild, P.; Borini, S.; Koppens, F.H.; Palermo, V.; Pugno, N.; et al. Science and technology roadmap for graphene, related two-dimensional crystals, and hybrid systems. Nanoscale 2015, 7, 4598–4810. [Google Scholar] [CrossRef]
- Pašti, I.A.; Jovanović, A.; Dobrota, A.S.; Mentus, S.V.; Johansson, B.; Skorodumova, N.V. Atomic adsorption on pristine graphene along the Periodic Table of Elements—From PBE to non-local functionals. Appl. Surf. Sci. 2018, 436, 433–440. [Google Scholar] [CrossRef]
- Pašti, I.A.; Jovanović, A.; Dobrota, A.S.; Mentus, S.V.; Johansson, B.; Skorodumova, N.V. Atomic adsorption on graphene with a single vacancy: Systematic DFT study through the periodic table of elements. Phys. Chem. Chem. Phys. 2018, 20, 858–865. [Google Scholar] [CrossRef]
- Yuan, W.; Zhou, Y.; Li, Y.; Li, C.; Peng, H.; Zhang, J.; Liu, Z.; Dai, L.; Shi, G. The edge- and basal-plane-specific electrochemistry of a single-layer graphene sheet. Sci. Rep. 2013, 3, 2248. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Dideikin, A.T.; Vul’, A.Y. Graphene Oxide and Derivatives: The Place in Graphene Family. Front. Phys. 2019, 6, 149. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kamarudin, S.K.; Daud, W.R.W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Graphene Oxide. Available online: https://www.graphenea.com/collections/graphene-oxide/products/graphene-oxide-4-mg-ml-water-dispersion-1000-ml (accessed on 15 December 2022.).
- Pašti, I.A.; Janošević Ležaić, A.; Gavrilov, N.M.; Ćirić-Marjanović, G.; Mentus, S.V. Nanocarbons derived from polymers for electrochemical energy conversion and storage—A review. Synth. Met. 2018, 246, 267–281. [Google Scholar] [CrossRef]
- Breitenbach, S.; Gavrilov, N.; Pašti, I.; Unterweger, C.; Duchoslav, J.; Stifter, D.; Hassel, A.W.; Fürst, C. Biomass-Derived Carbons as Versatile Materials for Energy-Related Applications: Capacitive Properties vs. Oxygen Reduction Reaction Catalysis. C 2021, 7, 55. [Google Scholar] [CrossRef]
- Breitenbach, S.; Unterweger, C.; Lumetzberger, A.; Duchoslav, J.; Stifter, D.; Hassel, A.W.; Fürst, C. Viscose-based porous carbon fibers: Improving yield and porosity through optimization of the carbonization process by design of experiment. J. Porous Mater. 2021, 28, 727–739. [Google Scholar] [CrossRef]
- Barbera, K.; Frusteri, L.; Italiano, G.; Spadaro, L.; Frusteri, F.; Perathoner, S.; Centi, G. Low-temperature graphitization of amorphous carbon nanospheres. Chin. J. Catal. 2014, 35, 869–876. [Google Scholar] [CrossRef]
- Bilal, M.; Shah, J.A.; Ashfaq, T.; Gardazi, S.M.; Tahir, A.A.; Pervez, A.; Haroon, H.; Mahmood, Q. Waste biomass adsorbents for copper removal from industrial wastewater—A review. J. Hazard. Mater. 2013, 263 Pt 2, 322–333. [Google Scholar] [CrossRef]
- Danish, M.; Ahmad, T. A review on utilization of wood biomass as a sustainable precursor for activated carbon production and application. Renew. Sustain. Energy Rev. 2018, 87, 1–21. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Lau, L.C.; Lee, K.T.; Mohamed, A. Synthesis of activated carbon from lignocellulosic biomass and its applications in air pollution control—A review. J. Environ. Chem. Eng. 2013, 1, 658–666. [Google Scholar] [CrossRef]
- Tay, T.; Ucar, S.; Karagöz, S. Preparation and characterization of activated carbon from waste biomass. J. Hazard. Mater. 2009, 165, 481–485. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M. Activated carbons from lignocellulosic materials by chemical and/or physical activation: An overview. Carbon 1992, 30, 1111–1118. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; Molina-Sabio, M.; González, M.T. The use of steam and CO2 as activating agents in the preparation of activated carbons. Carbon 1995, 33, 15–23. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Breitenbach, S.; Lumetzberger, A.; Hobisch, M.A.; Unterweger, C.; Spirk, S.; Stifter, D.; Fürst, C.; Hassel, A.W. Supercapacitor Electrodes from Viscose-Based Activated Carbon Fibers: Significant Yield and Performance Improvement Using Diammonium Hydrogen Phosphate as Impregnating Agent. C 2020, 6, 17. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Y.; Wan, X.; Yang, S.; Cai, Z.; Ye, Y.; Li, G. Morphology-dependent MnO2/nitrogen-doped graphene nanocomposites for simultaneous detection of trace dopamine and uric acid. Mater. Sci. Eng. C 2020, 109, 110615. [Google Scholar] [CrossRef]
- Kanoun, O.; Lazarević-Pašti, T.; Pašti, I.; Nasraoui, S.; Talbi, M.; Brahem, A.; Adiraju, A.; Sheremet, E.; Rodriguez, R.D.; Ben Ali, M.; et al. A Review of Nanocomposite-Modified Electrochemical Sensors for Water Quality Monitoring. Sensors 2021, 21, 4131. [Google Scholar] [CrossRef]
- Gupta, B.D.; Pathak, A.; Semwal, V. Carbon-Based Nanomaterials for Plasmonic Sensors: A Review. Sensors 2019, 19, 3536. [Google Scholar] [CrossRef]
- Hong, G.; Diao, S.; Antaris, A.L.; Dai, H. Carbon Nanomaterials for Biological Imaging and Nanomedicinal Therapy. Chem. Rev. 2015, 115, 10816–10906. [Google Scholar] [CrossRef]
- Shang, J.; Ma, L.; Li, J.; Ai, W.; Yu, T.; Gurzadyan, G.G. The Origin of Fluorescence from Graphene Oxide. Sci. Rep. 2012, 2, 792. [Google Scholar] [CrossRef] [PubMed]
- Yap, S.H.K.; Chan, K.K.; Tjin, S.C.; Yong, K.-T. Carbon Allotrope-Based Optical Fibers for Environmental and Biological Sensing: A Review. Sensors 2020, 20, 2046. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, M.; Zamarreño, C.R.; Melendi-Espina, S.; Bird, L.R.; Mayes, A.G.; Arregui, F.J. Optical Fibre Sensors Using Graphene-Based Materials: A Review. Sensors 2017, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Wulf, V.; Bisker, G. Single-Walled Carbon Nanotubes as Fluorescent Probes for Monitoring the Self-Assembly and Morphology of Peptide/Polymer Hybrid Hydrogels. Nano Lett. 2022, 22, 9205–9214. [Google Scholar] [CrossRef] [PubMed]
- Nißler, R.; Ackermann, J.; Ma, C.; Kruss, S. Prospects of Fluorescent Single-Chirality Carbon Nanotube-Based Biosensors. Anal. Chem. 2022, 94, 9941–9951. [Google Scholar] [CrossRef]
- Bao, L.; Cui, X.; Wang, X.; Wu, J.; Guo, M.; Yan, N.; Chen, C. Carbon Nanotubes Promote the Development of Intestinal Organoids through Regulating Extracellular Matrix Viscoelasticity and Intracellular Energy Metabolism. ACS Nano 2021, 15, 15858–15873. [Google Scholar] [CrossRef]
- Farrera, C.; Torres Andón, F.; Feliu, N. Carbon Nanotubes as Optical Sensors in Biomedicine. ACS Nano 2017, 11, 10637–10643. [Google Scholar] [CrossRef]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac Billiard in Graphene Quantum Dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef]
- Jiang, J.; He, Y.; Li, S.; Cui, H. Amino acids as the source for producing carbon nanodots: Microwave assisted one-step synthesis, intrinsic photoluminescence property and intense chemiluminescence enhancement. Chem. Commun. 2012, 48, 9634–9636. [Google Scholar] [CrossRef]
- Hsu, P.-C.; Chang, H.-T. Synthesis of high-quality carbon nanodots from hydrophilic compounds: Role of functional groups. Chem. Commun. 2012, 48, 3984–3986. [Google Scholar] [CrossRef]
- Salinas-Castillo, A.; Ariza-Avidad, M.; Pritz, C.; Camprubí-Robles, M.; Fernández, B.; Ruedas-Rama, M.J.; Megia-Fernández, A.; Lapresta-Fernández, A.; Santoyo-Gonzalez, F.; Schrott-Fischer, A.; et al. Carbon dots for copper detection with down and upconversion fluorescent properties as excitation sources. Chem. Commun. 2013, 49, 1103–1105. [Google Scholar] [CrossRef]
- Hu, S.-L.; Niu, K.-Y.; Sun, J.; Yang, J.; Zhao, N.-Q.; Du, X.-W. One-step synthesis of fluorescent carbon nanoparticles by laser irradiation. J. Mater. Chem. 2009, 19, 484–488. [Google Scholar] [CrossRef]
- Sun, D.; Ban, R.; Zhang, P.-H.; Wu, G.-H.; Zhang, J.-R.; Zhu, J.-J. Hair fiber as a precursor for synthesizing of sulfur- and nitrogen-co-doped carbon dots with tunable luminescence properties. Carbon 2013, 64, 424–434. [Google Scholar] [CrossRef]
- Xu, Q.; Pu, P.; Zhao, J.; Dong, C.; Gao, C.; Chen, Y.; Chen, J.; Liu, Y.; Zhou, H. Preparation of highly photoluminescent sulfur-doped carbon dots for Fe(iii) detection. J. Mater. Chem. A 2015, 3, 542–546. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, T.; Li, X.; Wei, Z.; Du, X.; Wang, X. Boronic acid functionalized N-doped carbon quantum dots as fluorescent probe for selective and sensitive glucose determination. Mater. Res. Express 2014, 1, 025708. [Google Scholar] [CrossRef]
- Chan, K.K.; Yap, S.H.K.; Yong, K.-T. Biogreen Synthesis of Carbon Dots for Biotechnology and Nanomedicine Applications. Nano-Micro Lett. 2018, 10, 72. [Google Scholar] [CrossRef]
- Sun, Y.-P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.S.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Yu, P.; Wen, X.; Toh, Y.-R.; Tang, J. Temperature-Dependent Fluorescence in Carbon Dots. J. Phys. Chem. C 2012, 116, 25552–25557. [Google Scholar] [CrossRef]
- Meng, X.; Chang, Q.; Xue, C.; Yang, J.; Hu, S. Full-colour carbon dots: From energy-efficient synthesis to concentration-dependent photoluminescence properties. Chem. Commun. 2017, 53, 3074–3077. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Neitzel, I.; Etzold, B.J.M.; Peterson, A.; Palmese, G.; Gogotsi, Y. Covalent Incorporation of Aminated Nanodiamond into an Epoxy Polymer Network. ACS Nano 2011, 5, 7494–7502. [Google Scholar] [CrossRef]
- Plakhotnik, T.; Aman, H.; Chang, H.-C. All-optical single-nanoparticle ratiometric thermometry with a noise floor of 0.3 K Hz−1/2. Nanotechnology 2015, 26, 245501. [Google Scholar] [CrossRef] [PubMed]
- Plakhotnik, T.; Doherty, M.W.; Cole, J.H.; Chapman, R.; Manson, N.B. All-Optical Thermometry and Thermal Properties of the Optically Detected Spin Resonances of the NV—Center in Nanodiamond. Nano Lett. 2014, 14, 4989–4996. [Google Scholar] [CrossRef] [PubMed]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Edmonds, T.E. Voltammetric and amperometric transducers. In Chemical Sensors; Edmonds, T.E., Ed.; Springer: Dordrecht, The Netherlands, 1988; pp. 193–213. [Google Scholar]
- Mello, L.D.; Kubota, L.T. Review of the use of biosensors as analytical tools in the food and drink industries. Food Chem. 2002, 77, 237–256. [Google Scholar] [CrossRef]
- Picó, Y. Chemical Analysis of Food: Techniques and Applications; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Brosel-Oliu, S.; Uria, N.; Abramova, N.; Bratov, A. Impedimetric sensors for bacteria detection. In Biosensors-Micro and Nanoscale Applications; IntechOpen: London, UK, 2015; pp. 257–288. [Google Scholar]
- Bhand, S.; Bacher, G. Impedimetric sensors in environmental analysis: An overview. In Environmental, Chemical and Medical Sensors; Springer: Singapore, 2018; pp. 67–85. [Google Scholar]
- Pedrosa, V.A.; Miwa, D.; Machado, S.A.; Avaca, L.A. On the utilization of boron doped diamond electrode as a sensor for parathion and as an anode for electrochemical combustion of parathion. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2006, 18, 1590–1597. [Google Scholar] [CrossRef]
- Majidi, M.R.; Asadpour-Zeynali, K.; Nazarpur, M. Determination of fenitrothion in river water and commercial formulations by adsorptive stripping voltammetry with a carbon ceramic electrode. J. AOAC Int. 2009, 92, 548–554. [Google Scholar] [CrossRef]
- Okumura, L.L.; Saczk, A.A.; De Oliveira, M.F.; Fulgêncio, A.C.C.; Torrezani, L.; Gomes, P.E.N.; Peixoto, R.M. Electrochemical feasibility study of methyl parathion determination on graphite-modified basal plane pyrolytic graphite electrode. J. Braz. Chem. Soc. 2011, 22, 652–659. [Google Scholar] [CrossRef]
- Amare, M.; Abicho, S.; Admassie, S. Determination of fenitrothion in water using a voltammetric sensor based on a polymer-modified glassy carbon electrode. J. AOAC Int. 2014, 97, 580–585. [Google Scholar] [CrossRef]
- Bolat, G.; Abaci, S.; Vural, T.; Bozdogan, B.; Denkbas, E.B. Sensitive electrochemical detection of fenitrothion pesticide based on self-assembled peptide-nanotubes modified disposable pencil graphite electrode. J. Electroanal. Chem. 2018, 809, 88–95. [Google Scholar] [CrossRef]
- Itkes, M.P.; de Oliveira, G.G.; Silva, T.A.; Fatibello-Filho, O.; Janegitz, B.C. Voltammetric sensing of fenitrothion in natural water and orange juice samples using a single-walled carbon nanohorns and zein modified sensor. J. Electroanal. Chem. 2019, 840, 21–26. [Google Scholar] [CrossRef]
- Noyrod, P.; Chailapakul, O.; Wonsawat, W.; Chuanuwatanakul, S. The simultaneous determination of isoproturon and carbendazim pesticides by single drop analysis using a graphene-based electrochemical sensor. J. Electroanal. Chem. 2014, 719, 54–59. [Google Scholar] [CrossRef]
- Ma, H.; Wang, L.; Liu, Z.; Guo, Y. Ionic liquid–graphene hybrid nanosheets-based electrochemical sensor for sensitive detection of methyl parathion. Int. J. Environ. Anal. Chem. 2016, 96, 161–172. [Google Scholar] [CrossRef]
- Wei, P.; Gan, T.; Wu, K. N-methyl-2-pyrrolidone exfoliated graphene as highly sensitive analytical platform for carbendazim. Sens. Actuators B Chem. 2018, 274, 551–559. [Google Scholar] [CrossRef]
- Velusamy, V.; Palanisamy, S.; Chen, S.-W.; Balu, S.; Yang, T.C.; Banks, C.E. Novel electrochemical synthesis of cellulose microfiber entrapped reduced graphene oxide: A sensitive electrochemical assay for detection of fenitrothion organophosphorus pesticide. Talanta 2019, 192, 471–477. [Google Scholar] [CrossRef]
- Masibi, K.K.; Fayemi, O.E.; Adekunle, A.S.; Al-Mohaimeed, A.M.; Fahim, A.M.; Mamba, B.B.; Ebenso, E.E. Electrochemical detection of endosulfan using an AONP-PANI-SWCNT modified glassy carbon electrode. Materials 2021, 14, 723. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Wang, P.; Zhang, C.; Wu, M.; Yang, L. A stable biosensor for organophosphorus pesticide detection based on chitosan modified graphene. Biotechnol. Appl. Biochem. 2022, 69, 567–575. [Google Scholar] [CrossRef]
- Keerthika Devi, R.; Ganesan, M.; Chen, T.W.; Chen, S.M.; Lin, K.Y.; Akilarasan, M.; Al-Onazi, W.A.; Ahmed Rasheed, R.; Elshikh, M.S. Tailored architecture of molybdenum carbide/iron oxide micro flowers with graphitic carbon nitride: An electrochemical platform for nano-level detection of organophosphate pesticide in food samples. Food Chem. 2022, 397, 133791. [Google Scholar] [CrossRef]
- Saranya, S.; Deepa, P.N. Perforated oxidized graphitic carbon nitride with Nickel spikes as efficient material for electrochemical sensing of chlorpyrifos in water samples. Surf. Interfaces 2022, 32, 102170. [Google Scholar] [CrossRef]
- Khoshsafar, H.; Karimian, N.; Nguyen, T.A.; Fakhri, H.; Khanmohammadi, A.; Hajian, A.; Bagheri, H. Enzymeless voltammetric sensor for simultaneous determination of parathion and paraoxon based on Nd-based metal-organic framework. Chemosphere 2022, 292, 133440. [Google Scholar] [CrossRef]
- Maji, B.; Achary, L.S.K.; Barik, B.; Jyotsna Sahoo, S.; Mohanty, A.; Dash, P. MnCo2O4 decorated (2D/2D) rGO/g-C3N4-based Non-Enzymatic sensor for highly selective and sensitive detection of Chlorpyrifos in water and food samples. J. Electroanal. Chem. 2022, 909, 116115. [Google Scholar] [CrossRef]
- Jangid, K.; Sahu, R.P.; Sakib, S.; Zhitomirsky, I.; Puri, I.K. Surface-Modified Metal Oxides for Ultrasensitive Electrochemical Detection of Organophosphates, Heavy Metals, and Nutrients. ACS Appl. Nano Mater. 2022, 5, 17183–17193. [Google Scholar] [CrossRef]
- Khuntia, A.; Kumawat, A.S.; Kundu, M. Detection of pesticide using Cu-rGO modified electrochemical sensor. Mater. Today Proc. 2022, 62, 6227–6231. [Google Scholar] [CrossRef]
- An, Y.; Dong, S.; He, Z.; Xie, Q.; Huang, T. Heteroatom-doped Co-MOF derivative enhancing immobilization and activity of two enzymes for small-molecules electrochemical determination. Microchem. J. 2022, 172, 106942. [Google Scholar] [CrossRef]
- Huang, J.; Xiang, Y.; Li, J.; Kong, Q.; Zhai, H.; Xu, R.; Yang, F.; Sun, X.; Guo, Y. A novel electrochemiluminescence aptasensor based on copper-gold bimetallic nanoparticles and its applications. Biosens. Bioelectron. 2021, 194, 113601. [Google Scholar] [CrossRef] [PubMed]
- Radi, A.E.; Oreba, R.; Elshafey, R. Molecularly Imprinted Electrochemical Sensor for the Detection of Organophosphorus Pesticide Profenofos. Electroanalysis 2021, 33, 1945–1951. [Google Scholar] [CrossRef]
- Mersal, G.A.; El-Sheshtawy, H.S.; Amin, M.A.; Mostafa, N.Y.; Mezni, A.; Alharthi, S.; Boukherroub, R.; Ibrahim, M.M. Simultaneous Hydrolysis and Detection of Organophosphate by Benzimidazole Containing Ligand-Based Zinc (II) Complexes. Crystals 2021, 11, 714. [Google Scholar] [CrossRef]
- Jangid, K.; Sahu, R.P.; Pandey, R.; Chen, R.; Zhitomirsky, I.; Puri, I.K. Multiwalled Carbon Nanotubes Coated with Nitrogen–Sulfur Co-Doped Activated Carbon for Detecting Fenitrothion. ACS Appl. Nano Mater. 2021, 4, 4781–4789. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Shimizu, F.M.; Machado, S.A.; Oliveira, O.N., Jr. Selective and sensitive multiplexed detection of pesticides in food samples using wearable, flexible glove-embedded non-enzymatic sensors. Chem. Eng. J. 2021, 408, 127279. [Google Scholar] [CrossRef]
- Rajaji, U.; Chinnapaiyan, S.; Chen, T.-W.; Chen, S.-M.; Mani, G.; Mani, V.; Ali, M.A.; Al-Hemaid, F.M.; El-Shikh, M.S. Rational construction of novel strontium hexaferrite decorated graphitic carbon nitrides for highly sensitive detection of neurotoxic organophosphate pesticide in fruits. Electrochim. Acta 2021, 371, 137756. [Google Scholar] [CrossRef]
- Motaharian, A.; Motaharian, F.; Abnous, K.; Hosseini, M.R.M.; Hassanzadeh-Khayyat, M. Molecularly imprinted polymer nanoparticles-based electrochemical sensor for determination of diazinon pesticide in well water and apple fruit samples. Anal. Bioanal. Chem. 2016, 408, 6769–6779. [Google Scholar] [CrossRef]
- Khadem, M.; Faridbod, F.; Norouzi, P.; Rahimi Foroushani, A.; Ganjali, M.R.; Shahtaheri, S.J.; Yarahmadi, R. Modification of carbon paste electrode based on molecularly imprinted polymer for electrochemical determination of diazinon in biological and environmental samples. Electroanalysis 2017, 29, 708–715. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Abdallah, A. Molecular imprinted polymer based sensor for recognition and determination of profenofos organophosphorous insecticide. Biosens. Bioelectron. X 2019, 2, 100027. [Google Scholar] [CrossRef]
- Chauhan, N.; Pundir, C.S. An amperometric acetylcholinesterase sensor based on Fe3O4 nanoparticle/multi-walled carbon nanotube-modified ITO-coated glass plate for the detection of pesticides. Electrochim. Acta 2012, 67, 79–86. [Google Scholar] [CrossRef]
- Dong, P.; Jiang, B.; Zheng, J. A novel acetylcholinesterase biosensor based on gold nanoparticles obtained by electroless plating on three-dimensional graphene for detecting organophosphorus pesticides in water and vegetable samples. Anal. Methods 2019, 11, 2428–2434. [Google Scholar] [CrossRef]
- De Fátima Alves, M.; de Souza Corrêa, R.A.M.; da Cruz, F.S.; Franco, D.L.; Ferreira, L.F. Electrochemical enzymatic fenitrothion sensor based on a tyrosinase/poly (2-hydroxybenzamide)-modified graphite electrode. Anal. Biochem. 2018, 553, 15–23. [Google Scholar] [CrossRef]
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.; Kim, K.-H.; Deep, A. Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Tuteja, S.K.; Vinayak, P.; Paul, A.; Kim, K.-H.; Deep, A. Graphene quantum dot modified screen printed immunosensor for the determination of parathion. Anal. Biochem. 2017, 523, 1–9. [Google Scholar] [CrossRef]
- Fu, J.; Dong, H.; Zhao, Q.; Cheng, S.; Guo, Y.; Sun, X. Fabrication of refreshable aptasensor based on hydrophobic screen-printed carbon electrode interface. Sci. Total Environ. 2020, 712, 136410. [Google Scholar] [CrossRef]
- Sgobbi, L.F.; Machado, S.A. Functionalized polyacrylamide as an acetylcholinesterase-inspired biomimetic device for electrochemical sensing of organophosphorus pesticides. Biosens. Bioelectron. 2018, 100, 290–297. [Google Scholar] [CrossRef]
- Kruć-Fijałkowska, R.; Dragon, K.; Drożdżyński, D.; Górski, J. Seasonal variation of pesticides in surface water and drinking water wells in the annual cycle in western Poland, and potential health risk assessment. Sci. Rep. 2022, 12, 3317. [Google Scholar] [CrossRef]
- Palleschi, G.; Bernabei, M.; Cremisini, C.; Mascini, M. Determination of organophosphorus insecticides with a choline electrochemical biosensor. Sens. Actuators B Chem. 1992, 7, 513–517. [Google Scholar] [CrossRef]
- Lazarević-Pašti, T.; Tasić, T.; Milanković, V.; Potkonjak, N. Molecularly Imprinted Plasmonic-Based Sensors for Environmental Contaminants—Current State and Future Perspectives. Chemosensors 2023, 11, 35. [Google Scholar] [CrossRef]
- Sun, G.; Wang, P.; Ge, S.; Ge, L.; Yu, J.; Yan, M. Photoelectrochemical sensor for pentachlorophenol on microfluidic paper-based analytical device based on the molecular imprinting technique. Biosens. Bioelectron. 2014, 56, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Cesana, R.; Ferreira, J.H.A.; Gonçalves, J.M.; Gomes, D.; Nakamura, M.; Peres, R.M.; Toma, H.E.; Canevari, T.C. Fluorescent Cdots(N)-Silica composites: Direct synthesis and application as electrochemical sensor of fenitrothion pesticide. Mater. Sci. Eng. B 2021, 267, 115084. [Google Scholar] [CrossRef]
- Akyüz, D.; Keleş, T.; Biyiklioglu, Z.; Koca, A. Electrochemical pesticide sensors based on electropolymerized metallophthalocyanines. J. Electroanal. Chem. 2017, 804, 53–63. [Google Scholar] [CrossRef]
- Ivanov, Y.; Marinov, I.; Portaccio, M.; Lepore, M.; Mita, D.G.; Godjevargova, T. Flow-Injection System with Site-Specific Immobilization of Acetylcholinesterase Biosensor for Amperometric Detection of Organophosphate Pesticides. Biotechnol. Biotechnol. Equip. 2012, 26, 3044–3053. [Google Scholar] [CrossRef]
- Mishra, A.; Kumar, J.; Melo, J.S.; Sandaka, B.P. Progressive development in biosensors for detection of dichlorvos pesticide: A review. J. Environ. Chem. Eng. 2021, 9, 105067. [Google Scholar] [CrossRef]
- Majdinasab, M.; Daneshi, M.; Louis Marty, J. Recent developments in non-enzymatic (bio)sensors for detection of pesticide residues: Focusing on antibody, aptamer and molecularly imprinted polymer. Talanta 2021, 232, 122397. [Google Scholar] [CrossRef]
- İlktaç, R.; Henden, E. Molecularly Imprinted Polymer-Based Optical Sensors for Pesticide Determination. In Molecular Imprinting for Nanosensors and Other Sensing Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 93–115. [Google Scholar]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; da S Petruci, J.F.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Štěpánková, Š.; Vorčáková, K. Cholinesterase-based biosensors. J. Enzyme Inhib. Med. Chem. 2016, 31, 180–193. [Google Scholar] [CrossRef]
- Bhattu, M.; Verma, M.; Kathuria, D. Recent advancements in the detection of organophosphate pesticides: A review. Anal. Methods 2021, 13, 4390–4428. [Google Scholar] [CrossRef]
- Kaur, J.; Singh, P.K. Enzyme-based optical biosensors for organophosphate class of pesticide detection. Phys. Chem. Chem. Phys. 2020, 22, 15105–15119. [Google Scholar] [CrossRef]
- Wu, X.; Wang, P.; Hou, S.; Wu, P.; Xue, J. Fluorescence sensor for facile and visual detection of organophosphorus pesticides using AIE fluorogens-SiO(2)-MnO(2) sandwich nanocomposites. Talanta 2019, 198, 8–14. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, J.; Guo, L.; Luo, F.; Qiu, B.; Hong, G.; Lin, Z. Targets regulated formation of boron nitride quantum dots—Gold nanoparticles nanocomposites for ultrasensitive detection of acetylcholinesterase activity and its inhibitors. Sens. Actuators B Chem. 2019, 279, 61–68. [Google Scholar] [CrossRef]
- Long, Q.; Li, H.; Zhang, Y.; Yao, S. Upconversion nanoparticle-based fluorescence resonance energy transfer assay for organophosphorus pesticides. Biosens. Bioelectron. 2015, 68, 168–174. [Google Scholar] [CrossRef]
- Bucur, B.; Munteanu, F.-D.; Marty, J.-L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef]
- Helmerhorst, E.; Chandler, D.J.; Nussio, M.; Mamotte, C.D. Real-time and Label-free Bio-sensing of Molecular Interactions by Surface Plasmon Resonance: A Laboratory Medicine Perspective. Clin. Biochem. Rev. 2012, 33, 161–173. [Google Scholar]
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A comprehensive review on plasmonic-based biosensors used in viral diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef]
- Wang, M.; Li, M.; Lu, J.; Fan, B.; He, Y.; Huang, Y.; Wang, F. “Off–On” fluorescent sensing of organophosphate pesticides using a carbon dot–Au(iii) complex. RSC Adv. 2018, 8, 11551–11556. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Tian, Z.; Lu, J.; Wang, Q.; Ai, S.; Wang, M. A sensitive fluorescent sensor for selective determination of dichlorvos based on the recovered fluorescence of carbon dots-Cu(II) system. Food Chem. 2016, 202, 81–87. [Google Scholar] [CrossRef]
- Wu, X.; Song, Y.; Yan, X.; Zhu, C.; Ma, Y.; Du, D.; Lin, Y. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens. Bioelectron. 2017, 94, 292–297. [Google Scholar] [CrossRef]
- Kim, I.; Kim, G.H.; Kim, C.S.; Cha, H.J.; Lim, G. Optical detection of paraoxon using single-walled carbon nanotube films with attached organophosphorus hydrolase-expressed Escherichia coli. Sensors 2015, 15, 12513–12525. [Google Scholar] [CrossRef] [PubMed]
- Gong, N.C.; Li, Y.L.; Jiang, X.; Zheng, X.F.; Wang, Y.Y.; Huan, S.Y. Fluorescence Resonance Energy Transfer-based Biosensor Composed of Nitrogen-doped Carbon Dots and Gold Nanoparticles for the Highly Sensitive Detection of Organophosphorus Pesticides. Anal. Sci. 2016, 32, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi Tafreshi, F.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Yan, Y.; Guo, M.; Cao, Y.; Yu, Y.; Zhang, T.; Huang, Y.; Wu, D. Modification-free carbon dots as turn-on fluorescence probe for detection of organophosphorus pesticides. Food Chem. 2018, 245, 1176–1182. [Google Scholar] [CrossRef]
- Guan, M.; He, H.; Li, R.; Si, X.; Peng, X.; Yan, X.; Yang, Z.; Nien, E.; Lei, Y.; Luo, L. Lanthanum ions assisted non-enzymatic ratiometric fluorescence probe for monitoring fenthion residues in agro-product samples. Anal. Chim. Acta 2022, 1236, 340579. [Google Scholar] [CrossRef]
- Feng, Y.; Qu, Y.; Sun, X.; Pan, W.; Wang, J. Fluorimetric and ratiometric colorimetric dual-mode detection of organophosphorus pesticides based on carbon dots/DTNB. New J. Chem. 2022, 46, 8022–8028. [Google Scholar] [CrossRef]
- Gaviria, M.I.; Barrientos, K.; Arango, J.P.; Cano, J.B.; Peñuela, G.A. Highly Sensitive Fluorescent Biosensor Based on Acetylcholinesterase and Carbon Dots–Graphene Oxide Quenching Test for Analytical and Commercial Organophosphate Pesticide Detection. Front. Environ. Sci. 2022, 10, 1–13. [Google Scholar] [CrossRef]
- Wei, J.; Yang, Y.; Dong, J.; Wang, S.; Li, P. Fluorometric determination of pesticides and organophosphates using nanoceria as a phosphatase mimic and an inner filter effect on carbon nanodots. Microchim. Acta 2019, 186, 66. [Google Scholar] [CrossRef]
- Reshma; Gupta, B.; Sharma, R.; Ghosh, K.K. Facile and visual detection of acetylcholinesterase inhibitors by carbon quantum dots. New J. Chem. 2019, 43, 9924–9933. [Google Scholar] [CrossRef]
- Li, H.; Yan, X.; Lu, G.; Su, X. Carbon dot-based bioplatform for dual colorimetric and fluorometric sensing of organophosphate pesticides. Sens. Actuators B Chem. 2018, 260, 563–570. [Google Scholar] [CrossRef]
- Singh, P.; Kumar, S.; Verma, S.K. Development of fluorescent aptasensor for detection of acephate by utilizing graphene oxide platform. Talanta 2023, 252, 123843. [Google Scholar] [CrossRef]
- Butmee, P.; Samphao, A.; Tumcharern, G. Reduced graphene oxide on silver nanoparticle layers-decorated titanium dioxide nanotube arrays as SERS-based sensor for glyphosate direct detection in environmental water and soil. J. Hazard. Mater. 2022, 437, 129344. [Google Scholar] [CrossRef]
- Li, S.; Luo, J.; Yin, G.; Xu, Z.; Le, Y.; Wu, X.; Wu, N.; Zhang, Q. Selective determination of dimethoate via fluorescence resonance energy transfer between carbon dots and a dye-doped molecularly imprinted polymer. Sens. Actuators B Chem. 2015, 206, 14–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).