Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications
Abstract
:1. Introduction
2. SPEs: A Brief Overview
2.1. Construction of SPEs

2.2. Methodologies of Modification

2.3. Applications of SPEs in Electrochemical Biosensing
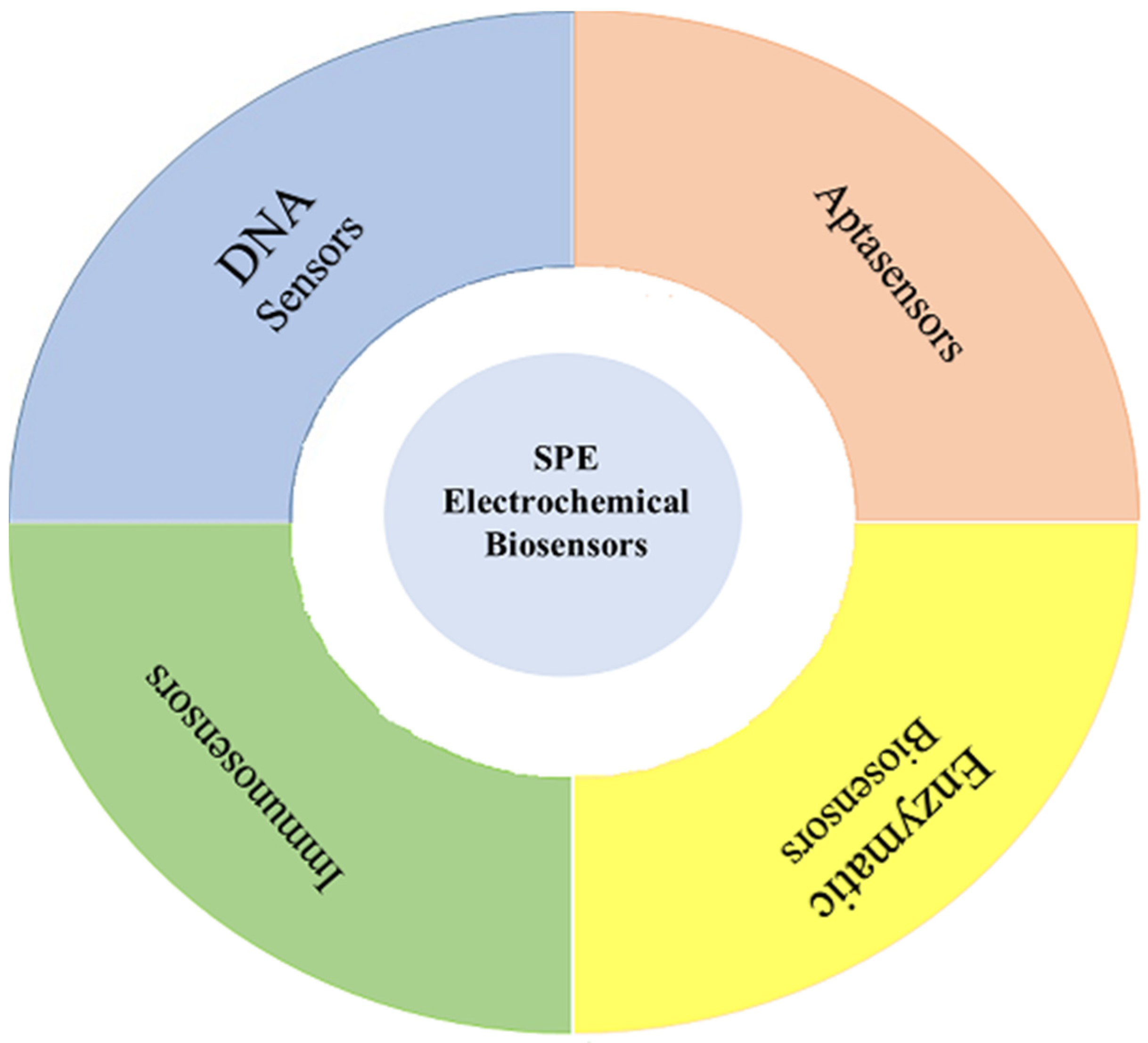
3. Biosensors
3.1. DNA Sensors
| Sensor Construction | Technique and Method | Detection | Analytical Characteristics | Analyte/Sample | Ref. |
|---|---|---|---|---|---|
| ASV-QD DNA assay | The inserted bismuth citrate was simultaneously transformed in situ to bismuth NPs by Pb electrolytic accumulation on the surface of the sensor | ASV | L.R.: 0.1 pM–10 nM LOD: 0.03 pM | Pb (II)/N/A | [66] |
| SiNWs/AuNPs-SPGE | SiNWs/AuNPs and MB (redox indicator) were used to increase the SPGE conductivity, as well as to produce a suitable site for immobilization and hybridization of the DNA probe | CV/DPV | L.R.: 0.1 pM–100 nM LOD: 1.63 pM | DNA oligomers related to dengue virus/N/A | [67] |
| Au/polythymine/MB/SPE | The Hg2+ detection was performed with the Thymine–Hg–Thymine (T–Hg–T) complex formation | SWV | L.R.: 0.2–100 nM LOD: 0.1 nM | Hg2+ ions/Waters and fishes | [68] |
| CNF/SPE | The sequence-selective DNA hybridization was performed following the bonding amino miRNA-34a inosine, which substituted the DNA probe at the CNF-SPE surface | EIS (Fe (CN)63−/4−) /DPV | L.R.: 25–100 μg/mL LOD: 10.98 μg/mL | miRNA-34a target RNA/N/A | [69] |
| PDA/SPCE | Covalent immobilization of amino-terminated probe DNA was executed on the surface of the sensor’s Schiff base: reaction of the quinones in PDA and the amino group of the probe DNA was based on the sandwich-type hybridization. Finally, the AuNP-labeled reporter DNA was bound onto the sensor’s surface to increase the signal | EIS (Fe (CN)63−/4−)/LSV | L.R.: 1.0–70 pM LOD: 0.3 pM. | Target DNA/N/A | [70] |
| Au/SH-ssDNA/MCH/SPGE | The response of this sensor was based on the ion channel mechanism | CV/OSWV | LOD for 280-mer RNA: 1 pM | Specific DNA and RNA sequences derived from Avian Influenza Virus H5N1/N/A | [71] |
| PMCSPE | MB was employed as the hybridization indicator; the –COOH groups of PBA were reused to immobilize oligonucleotides based on covalent bonding among the –NH2 groups of oligonucleotides and –COOH groups of PBA | DPV | L.R.: 1.0 aM–10 nM and 1 aM–0.1 nM LOD: 0.11 and 0.24 aM | M268T mutation of angiotensinogen gene/human blood samples | [72] |
| SH-probe/SPGE | The high selectivity of this biosensor in detecting the specific target DNA oligo in the real biological environment of unspecific DNA sequences was due to the considerable variation in the signal of the accumulated hematoxylin, between nonspecific oligos and target DNA oligo | EIS (Fe (CN)63−/4−)/CV | L.R.: 20 pM–150 nM LOD: 8.5 pM | PAH/N/A | [82] |
| DNA biosensor | Ebola virus DNA, diagnosable by enzyme-amplified detection | EIS (Fe (CN)63−/4−)/DPV | N/A | Ebola virus DNA/N/A | [73] |
| PANI/AuNP/avidin/SPCE | The sensing mechanism was based on an enzymatic reaction (interaction between HRP enzyme and TMB/H2O2). HRP converted a nonelectroactive substrate into an electroactive substrate | CV | L.R.: 0.001–1000 pM LOD: 0.5 fM | E. coli/Urine sample | [74] |
| DNA/sgRNA/dCas9/PAMAM/Cys/AuE | A practical, sensitive, and fast impedimetric/capacitive biosensor with CRISPR-dCas9 was modified by sgRNA to assess the most common IDH mutation in glioblastomas | EIS (Fe (CN)63−/4−) | L.R.: 100–1000 fM LOD: 33.96 fM | Glioblastoma (target mutant DNA) | [75] |
| ds-DNA/PtNPs/AgNPs/SPE | Interaction between dsDNA and three anthracyclines: EPI, IDA, and DOX by DPV | DPV | L.R.: 0.3–1.3 ppm for EPI 0.1–1.0 ppm for IDA/DOX LOD: N/A | Interaction between DNA and three intercalating anthracyclines | [76] |
| DNA/Gold-plated silver and DNA/SPE | An enzyme-amplified electrochemical assay permitted the PIK3CA point-mutations detection | Chronoamperometric | L.R.: 1–100 nM LOD: 10 pM | PIK3CA point-mutation (H1047R)/Plasma | [77] |
| DNA−MnO2 nanosheets/SPE | ctDNA analysis is performed by controlling the adsorption and desorption of DNA strands on MnO2 nanosheets | SWV | L.R.: 1 fM–1 nM LOD: 0.1 fM | ctDNA/Fetal bovine serum samples | [78] |
| HP-QDs-SPGE | The “turn-off” reaction of a hairpin DNA probe binds with a mismatched target and Hg2+ through the formation of T–Hg2+–T coordination | CV/DPV | L.R.: 10 pM–1 mM LOD: 0.11 pM | Hg2+ ions/Deionized water, tap water, groundwater, and urine samples | [79] |
| Fe3O4@SiO2/DABCO/SPE | The DPV signals of the hemin reduction and the guanine oxidation as an electrochemical indicator with indirect and direct methods, respectively, were applied to detect the hybridization process | DPV | L.R.: 10 pM–2 µM for guanine oxidation 7.5 pM–2 µM for hemin reduction LOD: 8 pM for guanine oxidation 6.4 pM for hemin reduction | Short-sequence DNA of PCa/N/A | [80] |
| Glucose/O2 biofuel cell | The biofuel cell was constructed by coupling a biocathode for O2 transformation based on a BOD-modified gas diffusion electrode with a bioanode for glucose conversion, made of PQQ–GDH embedded into an Os-complex-modified redox polymer | Chronoamperometry/CV | N/A | Glucose/ N/A | [81] |
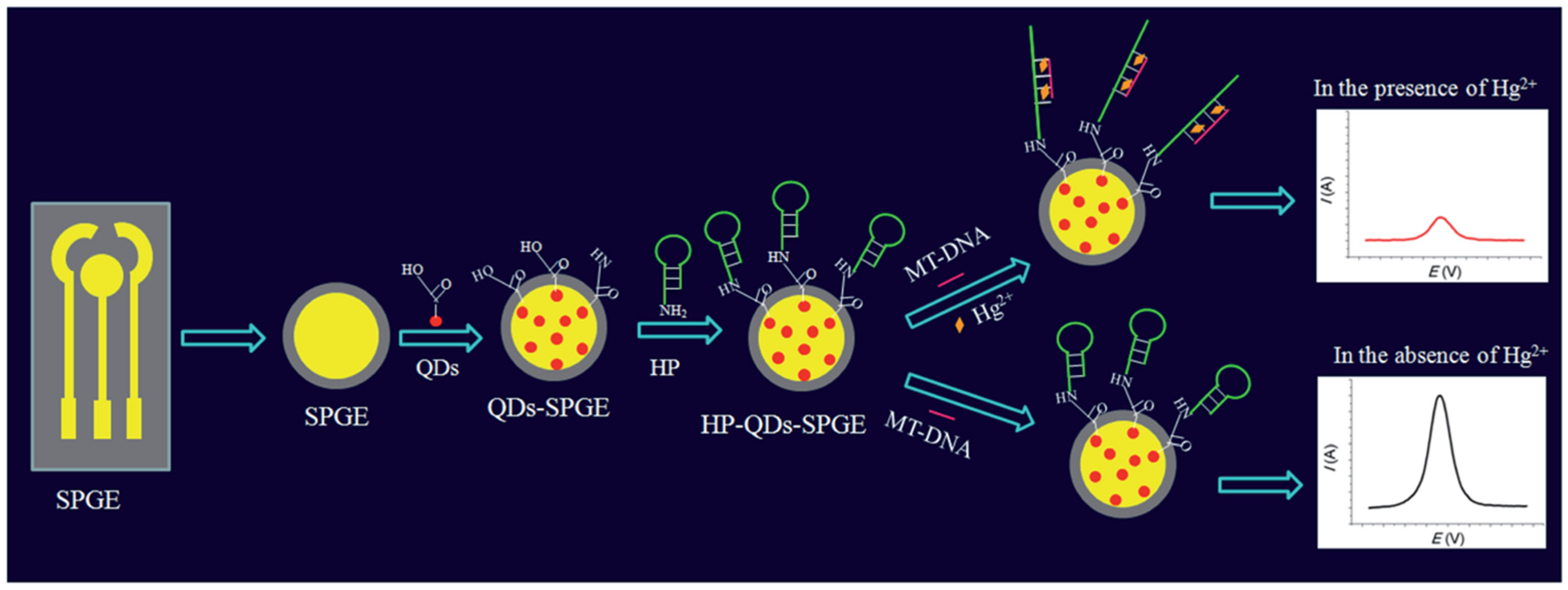
3.2. Aptasensors
| Sensor Construction | Technique and Method | Detection | Analytical Characteristics | Analyte/Sample | Ref. |
|---|---|---|---|---|---|
| Apt/AuNPs/SPCE | High affinity between FB1 and its aptamer by a small association constant (Ka), calculated by the Langmuir adsorption isotherm | EIS (Fe (CN)63−/4−)/CV | L.R.: 0.01–50 ng/mL LOD: 3.4 pg/mL | FB1/Corn | [88] |
| 4-MPBA/Au NFs/SPCE | Label-free and quantitative HbA1c electrochemical bioanalysis based on the catalytic property of HbA1c | CV | L.R.: 5–1000 μg/mL LOD: N/A | HbA1c/Serum | [89] |
| CcR/SAM-GNP/PPy/SPCE | Covalent coupling of CcR with SAM–GNP–PPy onto the SPCE | CV | L.R.: 0.1–1600 μM LOD: 60 nM | Nitrite/Hypoxia-induced cardiac cell lines | [90] |
| Carbon nanomaterial (C, SWCNT, MWCNT and CNF)/SPE | Noncovalent immobilization of aptamers on the nanomaterial electrodes via π–π stacking interactions between the DNA nucleobases and the surface | SWV/CV | L.R.: 0.0001–1000 ng/mL LOD: 0.03 pg/mL | HbA1c/Human whole blood | [91] |
| TNT-specific peptide/SPE | A portable smartphone-based biosensing platform for TNT detection was developed with impedance monitoring on SPE | EIS (Fe (CN)63−/4−) | L.R.: N/A LOD: N/A | TNT/N/A | [92] |
| CdTiPNPs-NTV/SPCE | Binding free biotin to CdTiPNPs–NTV and preventing their reaction with the sensor surface (Alb–BT) | SWASV | L.R.: 2–40 nM LOD: 1 nM | Biotin/Multivitamin tablets | [93] |
| Aptamer/SPE | Label-free aptasensor based on an SPE-specific adsorption to Cd2+ solution because of the key aptamer’s high affinity for Cd2+ | CV/DPV | L.R.: 0.1–1000 ng/mL LOD: 0.05 ng/mL | Cadmium (II) ions/River water | [94] |
| MoS2 NFs/CM/APTES/SPE | Physical and chemical reactions occurred in every step of the device surface modification to provide a higher binding affinity platform for the probe immobilization, which enhances a large number of immobilizations of biotin-linked aptamers on STVD | EIS (Fe (CN)63−/4−) | L.R.: 10 fM to 1 nM LOD: 10 fM | AMI biomarker (troponin I)/Human serum | [95] |
| Hydrazine-modified aptamer/TTCA monomer/AuNPs/SPCE | Sandwich aptamer detection was accomplished via a specific interaction between aptamers and cTnI | EIS (Fe (CN)63−/4−)/CV | L.R.: 1–100 pM LOD: 1 pM | cTnI/Human serum | [96] |
| Aptamer/AuNCs-Cys/SPGE | A label-free electrochemical aptasensor for selective CAP detection | EIS (Fe (CN)63−/4−)/CV/SWV | L.R.: 0.03–6.0 µM LOD: 4.0 nM | CAP/Human blood serum | [97] |
| Aptamer/rGO-PAMAM/Aunano/SPE | Selective interaction of CYC with W1/rGO-PAMAM-FAD/Aunano/Anti-ptamerCYC and VEGF165 with W2/rGO-PAMAM-Th/Aunano/Anti-ptamerVEGF165 | CV/DPV | L.R.: 2.5–320.0 pM LOD: 1.0 pM for CYC and 0.7 pM for VEGF165 | CYC and VEGF165 tumor markers/Human serum | [98] |
| AuNPs/Fe3O4@SiO2/DABCO/SPE | Label-free electrochemical aptasensor for the selective detection of epirubicin based on the specific interaction of aptamers with epirubicin and formation of the epirubicin–aptamer complex | EIS (Fe (CN)63−/4−)/CV/LSV | L.R.: 0.07μM to 1.0 μM and 1.0 μM to 21.0 μM LOD: 0.04 µM | Epirubicin/Human blood serum | [99] |
| Zr-MOF/Fe3O4(TMC)/AuNCs/SPE | Antibody-labeled Zr-MOF/Fe3O4(TMC)/ AuNCs as the signal amplification unit and rGO/APBA/SPE as the sensing platform | ECL/CV | L.R.: 2–18% LOD: 0.072% | HbA1c/Human whole blood | [100] |
| TBA-SWCNT/SPCE | Competitive interaction with the TBA to thrombin and SWCNT is a key role in this sensor system, which is applicable to label-free faradic impedance detection | EIS (Fe (CN)63−/4−) | L.R.: 0.0001–1.0 µM LOD: 0.02 nM | Thrombin | [101] |
| Hemin-aptamer/PEG- Au/SPE | Thrombin binding to the aptamer and formation of the DNAzyme—the G4 structure with intercalated hemin—underwent direct electron transfer (ET) | CV | L.R.: 0.5–100 fM LOD: 0.5 fM | Thrombin | [102] |
| Aptamer/CNFs-AuNPs/SPCE | After the incubation of SARS-CoV-2-RBD (64 nM) with the immobilized aptamer, the Rct increased due to the mass transfer limiting of Fe (CN)63−/4− to the electrode surface that is caused by SARS-CoV-2-RBD (~35 kDa) as a large molecule | EIS (Fe (CN)63−/4−) | L.R.: 0.01–64 nM LOD: 7.0 pM | SARS-CoV-2-RBD/Human saliva samples | [103] |
| Aptamer-SWCNT-SPEs | Binding-induced folding of the DNA aptamer in the presence of the target S1 protein leads to a concentration-dependent suppression in the registered amperometric signal | DPV | L.R.: 20−100 nM LOD: 7 nM | SARS-CoV- 2 spike protein S1 subunit/Other proteins | [104] |
| DZN-thiolated aptamer-Au NP-SPGE | Label-free electrochemical nano-aptasensor as portable devices would be a promising approach in the fast and precise detection of DZN | EIS (Fe (CN)63−/4−)/CV | L.R.: 0.1–1000 nM LOD: 0.0169 nM | Diazinon/Plasma of male Wistar rat | [105] |
| SAM: Aptamer + MCH-SPGEs Ternary SAM: Aptamer + HDT + MCH-SPGEs | Two different aptamer immobilization strategies (SAM and ternary SAM) were demonstrated for the detection of the HER2 protein biomarker in PBS diluted and undiluted serum using SPGEs | EIS (Fe (CN)63−/4−) | L.R.:1 pg/mL–1000 ng/mL LOD: 172 pg/mL | Breast cancer (HER2)/Human serum | [106] |
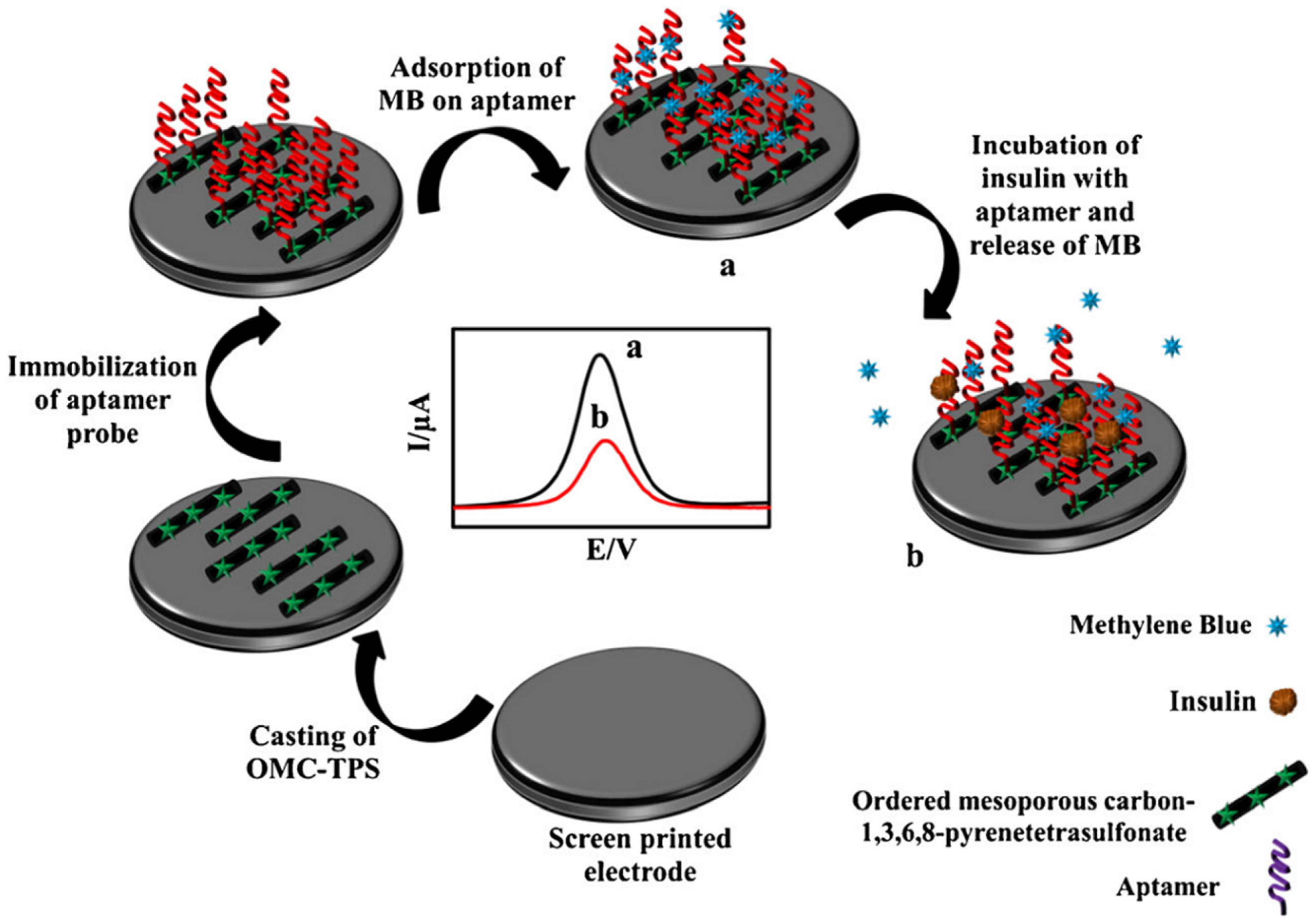
| Aptamer-MB/OMC-TPS CSPE | MB as a probe can bind to the DNA chain through the preferential binding between MB and guanine bases, and the decrease in peak current intensity of the DPV of intercalated MB was monitored | EIS (Fe (CN)63−/4−)/CV | L. R.:1.0 fM to 10.0 pM LOD: 0.18 fM | Insulin/Normal human serum | [107] |
| Cu (OH)2 NRs/SPCE | In the presence of SARS-CoV-2 spike glycoprotein, a decrease in Cu(OH)2 NR-associated peak current was observed that can be due to the target–aptamer complex formation and thus the blocking of the electron transfer of Cu(OH)2 NRs | SWV | L. R.: 0.1 fg/mL–1.2 μg/mL LOD: 0.03 fg/mL | SARS-CoV-2/Saliva and VTM samples | [108] |
| Apt-AuNPs/SPE | An amperometric aptasensor with a sandwich-type architecture for the specific detection of CRP through NPs as biorecognition and signaling elements | Amperometry | L.R.: 10 pg/mL–1.0 ng/mL LOD: 3.1 pg/mL | CRP/Human serum samples | [109] |
| Apt/Au/SPE | Signal switch-based detection was achieved using MB-modified insulin specific aptamer | SWV | L.R.: 25–150 pM LOD: 18.5 pM | Insulin hormone/Blood samples | [110] |
3.3. Immunosensors
| Sensor Construction | Technique and Method | Detection | Analytical Characteristics | Analyte/Sample | Ref. |
|---|---|---|---|---|---|
| Anti mAβ/AuNPs/DEP | Label-free impedimetric amyloid beta immunosensor on carbon DEP chip | EIS (Fe (CN)63−/4−) | L.R.: 1–200 µM LOD: 0.57 nM | Amyloid beta peptide/Human serum albumin | [120] |
| QD-STV/anti-H-IgA-BT/anti-tTG IgA/SPCE | A blocking-free one-step immunosensing strategy using eight-channel screen-printed arrays for the detection of anti-transglutaminase IgA antibodies | DPV | L.R.: 3–40 U/mL LOD: 2.7 U/mL | Anti-tTG IgA antibodies/Human serum | [121] |
| Ag/Ab/Fe3O4/SiO2/AuNPs/SPCE | A sandwich electrochemical immunoassay method | CV | L.R.: 102–106 CFU/mL LOD: 32 CFU/mL | S. pullorum and S. gallinarum/Food samples (chickens) | [122] |
| Anti-HSA/EDC + NHS/COOH-P-SPCE | A simple and sensitive electrochemical immunosensor based on carboxyl-enriched porous SPCE for detecting urinary albumin in the range of microalbuminuria | CV/CA | L.R.: 10–300 µg/mL LOD: 9.7 µg/mL | Microalbuminuria/Urine | [123] |
| BSA/HRP/Ab2/CEA/Ab1/EDC + NHS/AuNPs/rGO/SPEs | A sandwich type immunosensor to mimic the ELISA (enzyme-linked immunosorbent assay) immunoassay | CV | L.R.: 0.5–2000 ng/mL LOD: 0.28 ng/mL | CEA/N/A | [124] |
| PPY/CEA/Ag-SPE | Combination of the novel PCB-based SPEs comprising Ag tracks with the use of an antibody-like biomimetic material as a sensing element | CV/SWV/ EIS (Fe (CN)63−/4−) | L.R.: 0.05–1.25 pg/mL LOD: N/A | CEA/Urine | [125] |
| Ab/fG/SPE | A convenient graphene SPE platform for nonenzymatic label-free immunosensors | EIS (Fe (CN)63−/4−) | L.R.: 0.1–1000 ng/L LOD: 52 pg/L | Parathion/Tomato and carrot | [126] |
| Antibody SAL/EDC + NHS-activated MPA/AuNS/SPCEs | The high roughness and conductivity of AuNS allowed the immunosensor to have more immobilized antibodies and a smaller interface impedance, resulting in a lower LOD than the one using flat AuDEs | EIS (Fe (CN)63−/4−) | L.R.: 0.1 pg/mL–1 µg/mL LOD: 4 fg/mL | SAL/Serum samples | [127] |
| Ab/rGO-TEPA/AuNPs/SPE | A disposable sandwich immunosensor for sensitive electrochemical detection of AFP through the combination of SPEs and paper-based microfluidic channels | SWV | L.R.: 0.01–100 ng/mL LOD: 0.005 ng/mL | AFP/Serum samples | [54] |
| Ab/AgNPs-rGO/SPE | The sandwich-type immunosensor, which yielded a lower LOD than its nonsandwich counterpart | CV | L.R.: 0.05–0.40 µg/mL LOD: 0.042 µg/mL | CEA/N/A | [128] |
| HER2 Ag/Ab/SPE | Unmodified SPEs fabricated for HER2 detection antigen using the traditional sandwich ELISA protocol without compromising on the accuracy, precision, or sensitivity of the device | CV | L.R.: 5–20 ng/mL and 20–200 ng/mL LOD: 4 ng/mL and 5 ng/mL | HER2/Serum samples of invasive and non invasive breast cancer patients | [129] |
| AQ-2°Ab/Anti-1°Ab/L-Cys/Au/SPGE | A dual-working electrode was custom-designed to simultaneously compare the presence and absence of CRP to reduce the analysis time | DPV | L.R.: 0.01–150 µg/mL LOD: 1.5 ng/mL | CRP/Serum samples | [130] |
| (1) Ab/PoPD-Au/Pd-SA-AuNP/SPE (2) Ab/PMB-Au/Pd-SA-AuNP/SPE (3) Ab/PPPD-Au/Pd-SA-AuNP/SPE (4) Ab/PTMB-Au/Pd-SA AuNP/SPCE | Multiplexed label-free immunosensor, where one signal output channel could make the immunosensor be realized by a common single-channel electrochemical workstation | SWV | L.R.: 0.01–100 ng/mL for SCCA 0.01–100 ng/mL for Cyfra21-1 0.01–200 U/mL for CA125 0.01–200 ng/mL for NSE LOD: 5.5 pg/mL for SCCA 4.8 pg/mL for Cyfra21-1 0.0054 U/mL for CA125 2.3 pg/mL for NSE | SCCA, Cyfra21-1, CA125, NSE/Serum samples | [131] |
| (1) HC/BSA/PRF+1/SPCE (2) JIA/BSA/PRF+1/SPCE | The PRF+1 mimetic peptide used as a recognition biological element was successfully immobilized onto the SPCE surface, and a 15-fold increase in the current intensity was observed when compared to the bare electrode | DPV/ EIS (Fe (CN)63−/4−) | N/A | JIA/Serum samples | [132] |
| (1) AbEGFR Cd(II)@LP/MIP/DSP-SPE (2) AbVEGF-Cu(II) @LP/MIP/DSP-SPE | Development of electrochemical biosensors based on both MIP and antibodies for sandwich assays in the dual detection of EGFR and VEGF | EIS (Fe (CN)63−/4−) | L.R.: 0.05–50,000 pg/mL for EGFR 0.01–7000 pg/mL for VEGF LOD: 0.01 pg/mL for EGFR 0.005 pg/mL for VEGF | EGFR and VEGF | [133] |
| BSA/Ab2/NR-Au@Pt/rGO/E.coli O157:H7/BSA/Ab1/AuNPs/PANI/SPCE | The anti E. coli O157:H7 monoclonal antibody (Ab1) was automatically adsorbed on the AuNPs/PANI/SPCE platform through amino and AuNPs interaction. NR-Au@Pt/rGO as the nonenzyme signal label can enhance the performance of the immunoassay for the catalytic reduction of H2O2 | CV | L.R.: 8.9 × 103–8.9 × 109 CFU/mL LOD: 2.84 × 103 CFU/mL | E. coli O157:H7/Pork samples | [134] |
| Pt/rGO/P3ABA/SPCE | The biocompatible P3ABA contains an abundance of carboxylic groups, used as the matrix for the immobilization of enzymes (GOx or ChOx) via amide linkages to increase enzyme loading, to enhance the sensitivity and specificity, and to improve the stability of the modified electrode | CV/ EIS (Fe (CN)63−/4−)/Amperometry | L.R.: 0.25–6.00 mM for glucose 0.25–4.00 mM for cholesterol LOD: 44.3 µM for glucose 40.5 µM for cholesterol | Glucose and cholesterol/Serum samples | [135] |
| Au-Mab-hCG/hCG/Mab-FSH/SWCNTs/SPCE | A sandwich-type immunoassay, where the gold-linked second antibody (Au-Mab-hCG) was used as a label and the signal amplification strategy-using AuNPs as bio-trackers and SWCNT enhanced electron transfer nearly double that of bare SPCE | DPV | L.R.: 10–1000 pg/L LOD: 5 pg/L | hCG/N/A | [136] |
| PSA/anti-PSA/GO/SPCE | The sensing platform comprises a direct-type immunoassay which involves the selective interaction of PSA with anti-PSA | CV/ EIS (Fe (CN)63−/4−) | L.R.: 0.75–100 ng/mL LOD: 0.27 ng/mL | PSA/Human (male) blood serum sample | [137] |
| Ag/Ab/15 nm AuNPs-SPE | The surface modification of carbon SPEs with AuNPs could increase the electron transfer rate between the electrolytic solution and the modified electrode compared with that of bare SPE | CV/DPV/ EIS (Fe (CN)63−/4−) | L.R.: 10–106 CFU/mL LOD: 13 CFU/ml | MRSA/Pathogenic bacteria | [138] |
| MBs/anti-rabbit IgG-AP/anti-SARS-CoV antibody/CB/SPE | The electrochemical assay was conceived for spike (S) protein or nucleocapsid (N) protein detection using magnetic beads as the support of the immunological chain and the secondary antibody with alkaline phosphatase as the immunological label | DPV | L.R.: N/A LOD: 19 ng/mL in buffer solution and 8 ng/mL in untreated saliva | SARS-CoV-2/Saliva and nasopharyngeal swab samples | [139] |
| AuDdrites/SPCE | A flexible and label-free immunosensor chip made with tree-like gold dendrites (AuDdrites) was electrochemically formed by selective desorption of l-cysteine (L-cys) on (111) gold planes | SWV | L.R.: 0.1–900 ng/mL LOD: 0.03 ng/mL | 25(OH)D3/Serum samples | [140] |
| GFAP/BSA/GFAP Ab/Au@ZIF-8@rGO/SPE | The concept of the immunosensor is to detect the signal perturbation obtained by measuring the changes in the load transfer resistance of the electrode by using Fe (CN)63−/4− measurements after binding the protein during incubation | CV/ EIS (Fe (CN)63−/4−) | L.R.: 50–10,000 fg/mL LOD: 50 fg/mL | GFAP/Urine samples | [141] |
| AFB1-mAb/MB-OVA-AFB1/CB/SPE | A user-friendly smartphone-based magneto-immunosensor on CB/SPE modified electrodes for point-of-care detection of aflatoxin B1 | CV/EIS (Fe (CN)63−/4−) | L.R.: N/A LOD: 13 pg/mL in buffer solutions and 24 pg/mL in corn samples | Aflatoxin B1/Corn samples | [142] |
| S1-IgG antibody and S1 protein/AuNP/SPE | A one-step and specific detection of SARS-CoV-2 virus from unprocessed clinical samples | SWV | L.R.: 0.1 fg/mL–100 pg/mL LOD: 7.62 fg/mL | SARS-CoV-2/Swab and blood samples | [143] |
| AbD/CYM/Au@MNPs/SPE | Modifications were set up to maximize the diffusion of the probe on the electrode surface, therefore amplifying the current decrease occurring after the 25(OH)D3 interaction due to both the steric hindrance and the lipophilic nature of molecule | DPV | L.R.: 7.4–70 ng/mL LOD: 2.4 ng/mL | Vitamin D3 (25-OHD3)/Untreated serum samples | [144] |
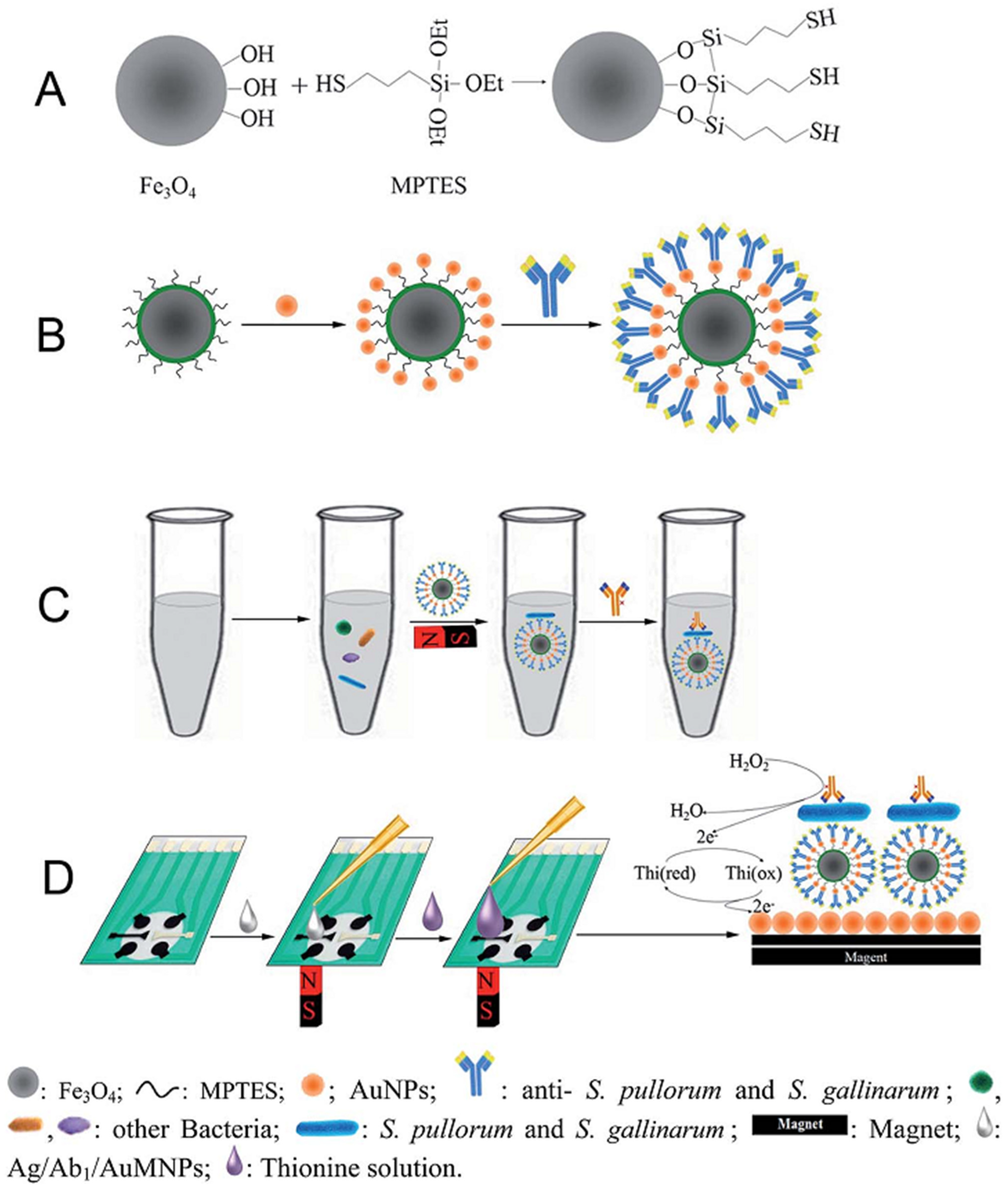
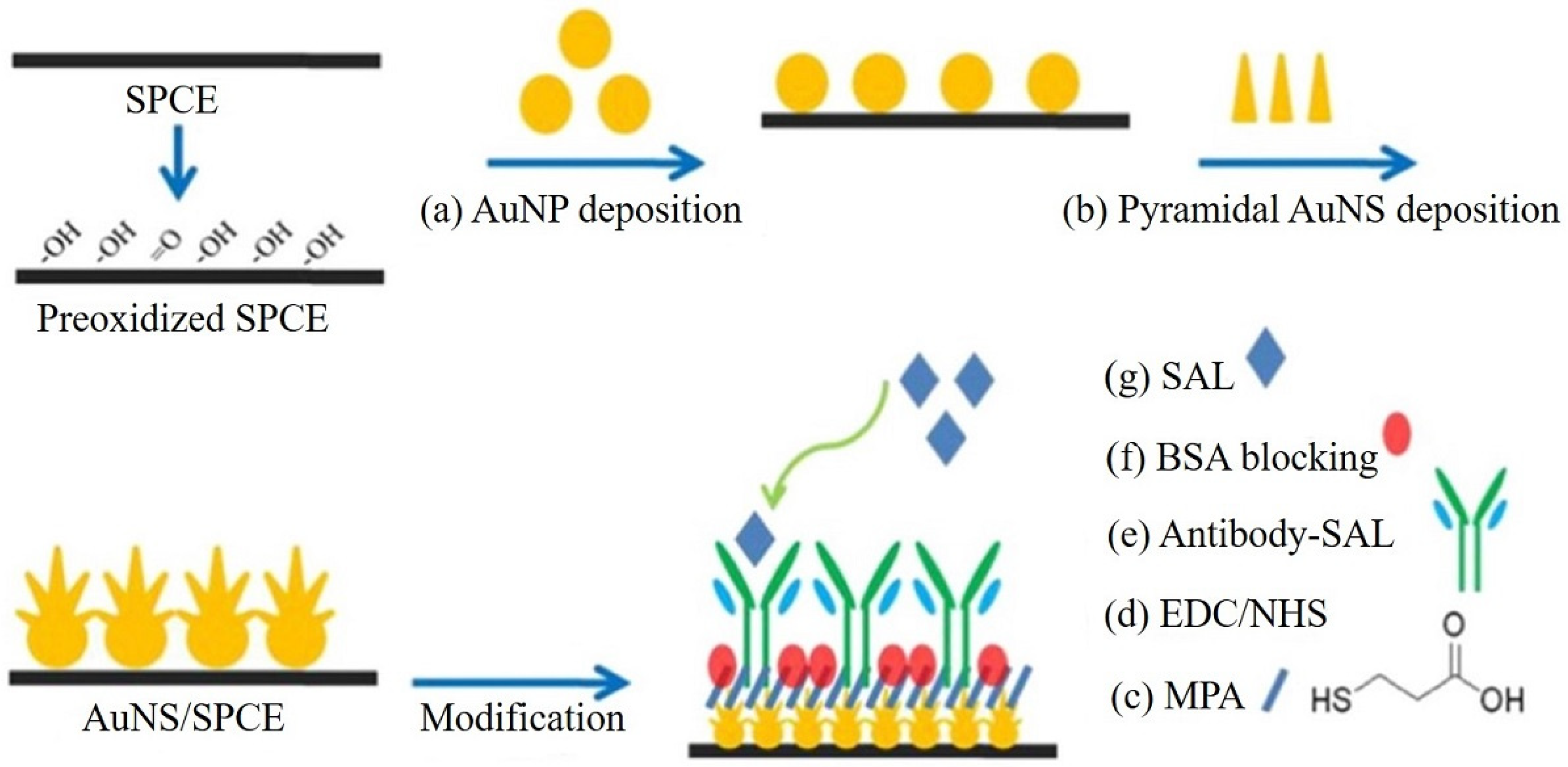
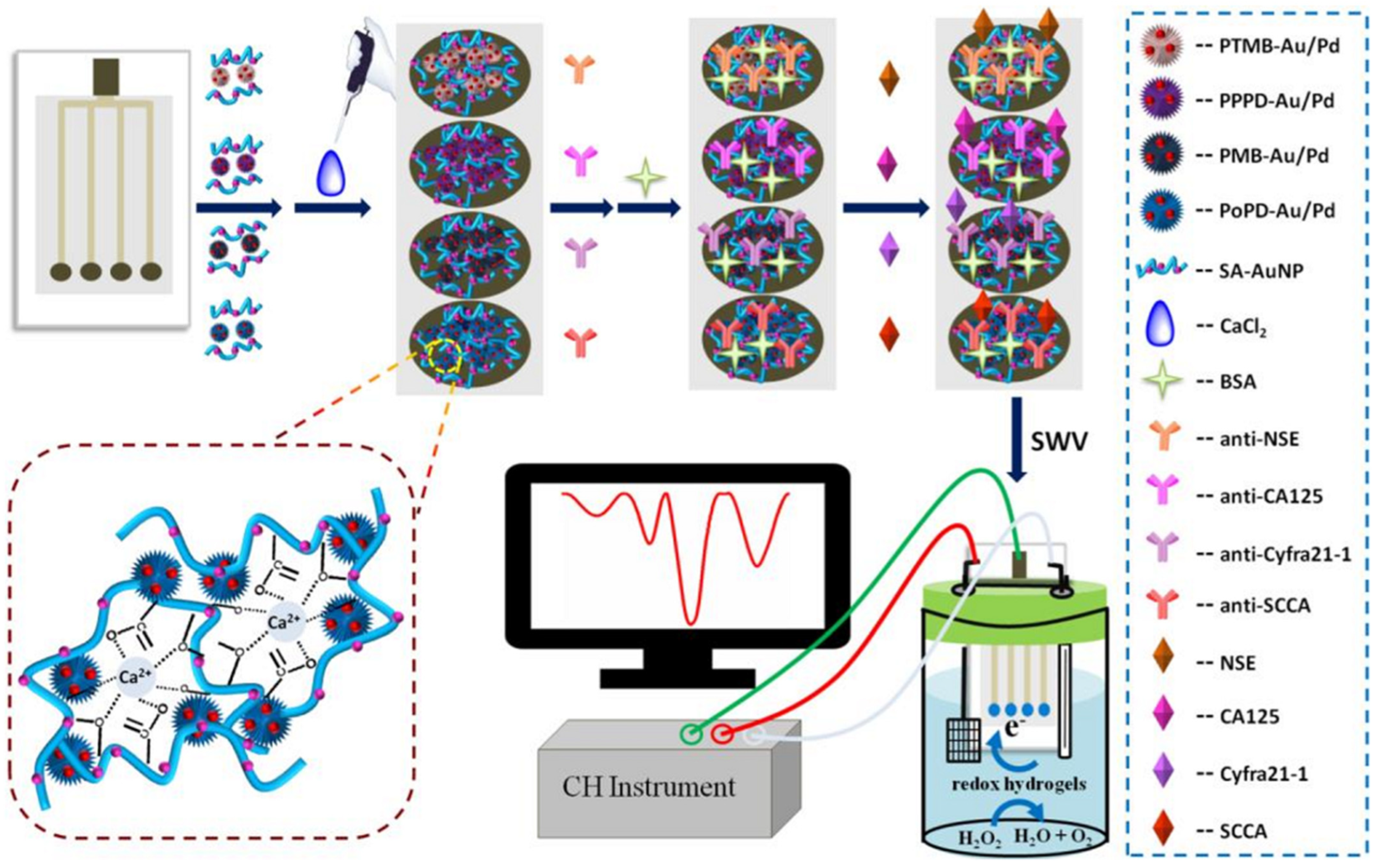
3.4. Enzyme Biosensors
| Sensor Construction | Technique and Method | Detection | Analytical Characteristics | Analyte/Sample | Ref. |
|---|---|---|---|---|---|
| ChOx/SiO2/AuSPE | ChOx/SiO2 exhibits the characteristics of the typical Michaelis–Menten kinetic mechanism with the signal saturation upon the addition of high choline concentrations | CV/Amperometry | L.R.: 0.02–0.6 mM LOD: 6 μM | Choline/Baby food samples | [155] |
| MWCNT-CHIT-MB/GLDH-NAD+-CHIT/MWCNT-CHIT/SPCE | A reagentless amperometric glutamate biosensor based on GLDH and NAD+ integrated with a disposable SPE | Amperometry | L.R.: 7.5–105 µM LOD: 3 μM | Glutamate/Food, serum and clinical samples | [151] |
| BSA-glutaraldehyde-uricase/PPD/SPE | The uricase as an enzyme on an SPE has been integrated onto a mouthguard platform along with anatomically miniaturized instrumentation electronics featuring a potentiostat, microcontroller, and a BLE transceiver | Amperometry | L.R.: 100–250 µM LOD: 2.32 μM | SUA/Human saliva samples | [156] |
| Ty-SWCNT-COOH/SPE | The -COOH functionalized SWCNT provides a suitable microenvironment for the immobilization of enzymes retaining the bioactivity of Ty | Amperometry | L.R.: 5–180 µM LOD: 0.62 μM | Tyramine/Pickled and smoked fish samples | [157] |
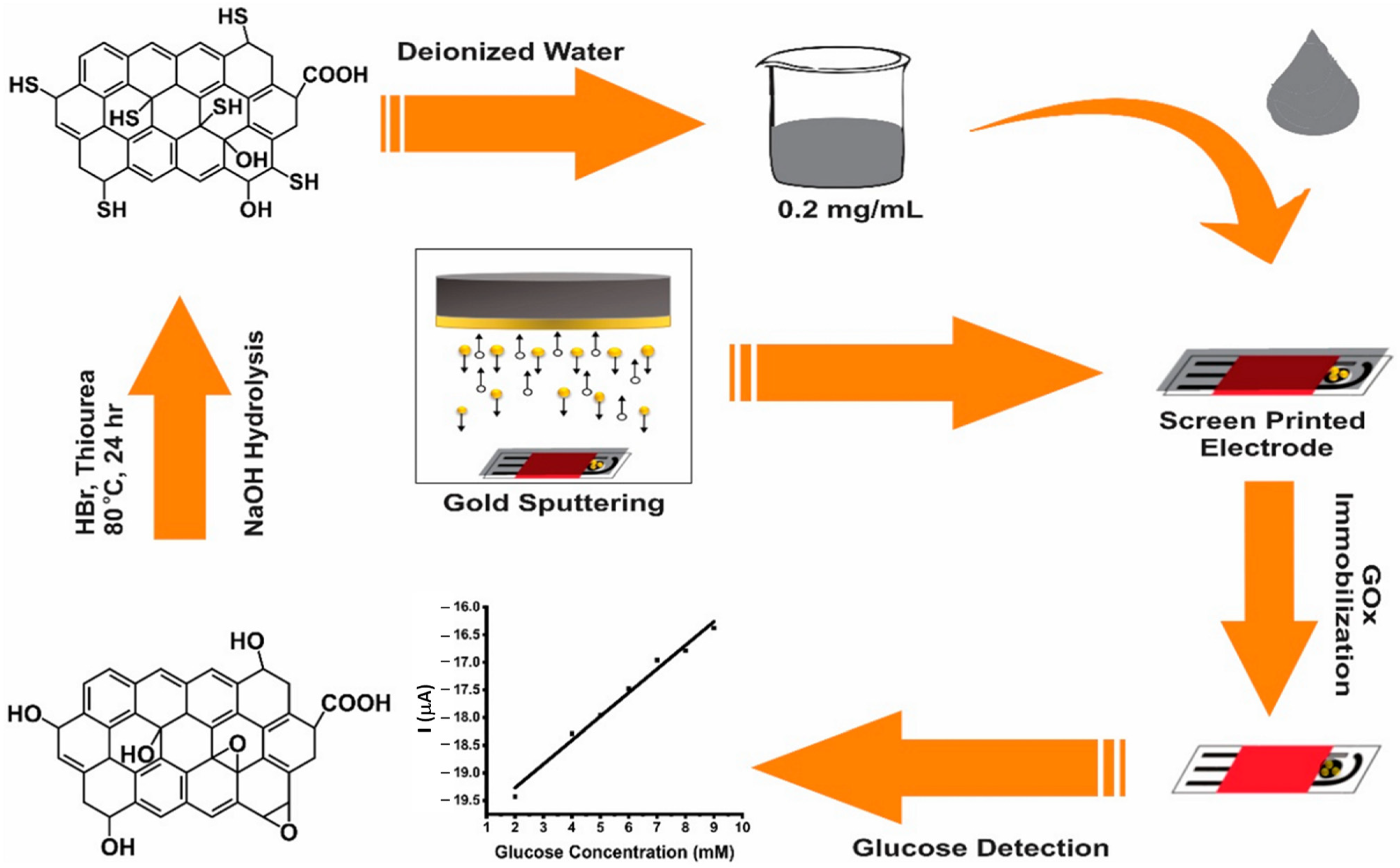
| GOx/4-APBA/SPCE | Glucose reacts with oxygen to generate hydrogen peroxide and gluconic acid | CV/Amperometry | L.R.: 0.05–100 mM LOD: 0.86 µM | Glucose/Blood serum, soft drink, sweet tea, and apple juice samples | [158] |
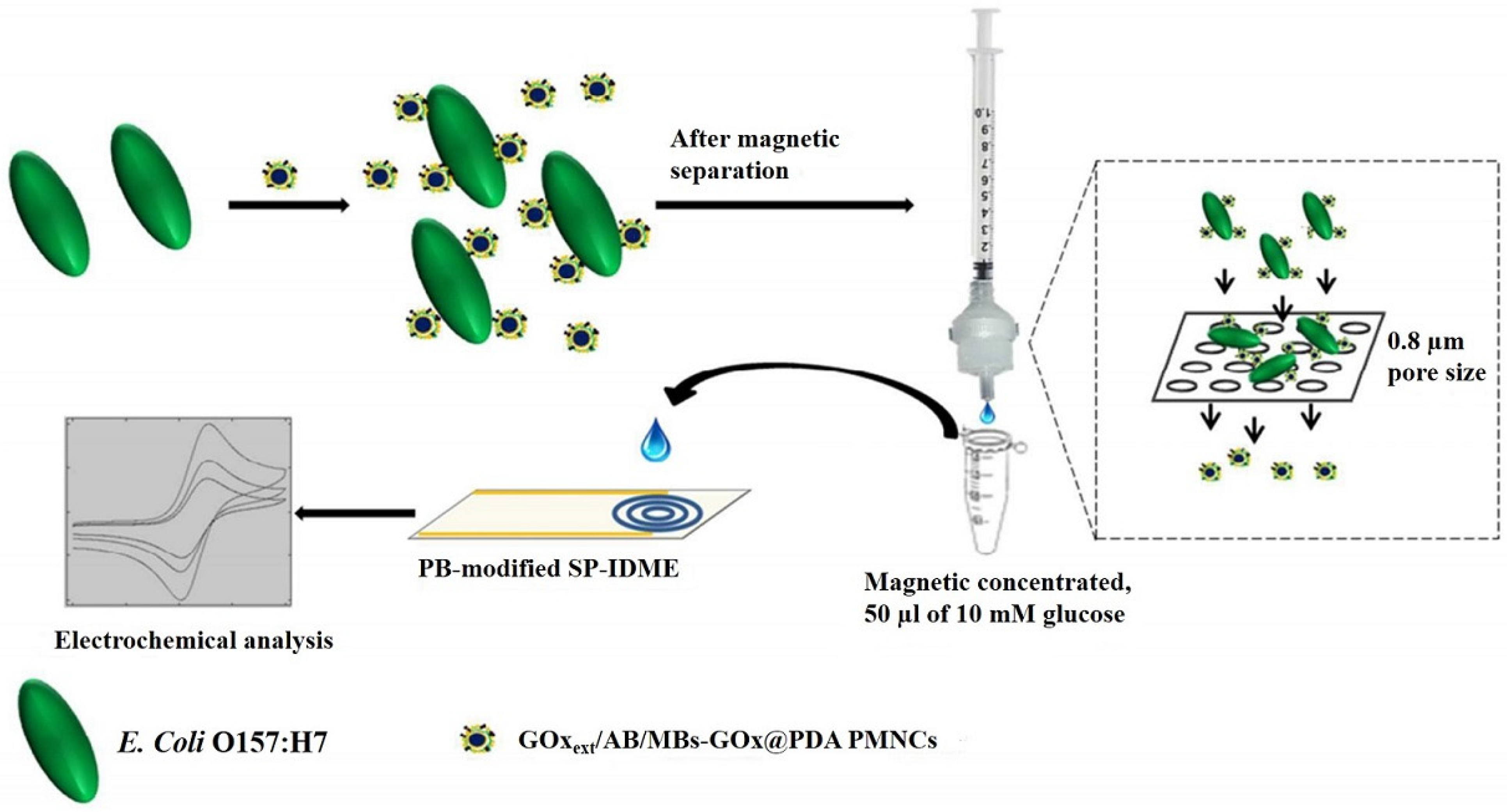
| ABs/GOxext/AuNPs/MBs-GOx@PDA PMNCs/SPE | The bifunctional PMNCs contain a high load of enzyme and can optimally utilize the binding sites on bacterial cells, which efficiently amplify the signal | CV/Amperometry | L.R.: 103–106 cfu/g LOD: 190 cfu/g | E. coli O157:H7/Foodborne pathogens | [152] |
| GA/ADH/PNR/AuNPs/MWCNTs/SPCE | Investigation of changes in conductivity and the electrocatalytic activity of the electrodes upon modifications | Amperometry | L.R.: 320.2–1000 μM LOD: 96.1 μM | Ethanol/Commercial alcoholic beverages | [159] |
| MWCNT/FcMe/CS/HRP/BSA/LOx/SPBGE | Potentially utilized as a nonintrusive point of care sensor | Amperometry | L.R.: 30.4–243.9 μM LOD: 22.6 μM | L-lactate/ Embryonic cell culture | [160] |
| AChE/MWCNTs/DCHP/SPE | The CV responses were associated with the inhibition of AChE activity based on the amount of the added pesticide | CV | L.R.: 0.05–105 μM LOD: 0.05 μM | Chlorpyrifos/Vegetable samples. | [161] |
| ε-FK/FAOx/Ru-complex/SPE | A disposable electrochemical enzyme sensor strip for the measurement of GA using FAOx, and hexaammineruthenium (III) chloride (Ru complex) as the electron mediator | Amperometry | L.R.: 0.05–105 μM LOD: 0.05 μM | GA/Albumin | [162] |
| GOx/PBNCs/AgNWs/SPE | The combination of high electrocatalysis of PBNCs and fast conductivity of AgNWs to exhibit the synergic effects in the electrocatalytic detections | Amperometry | L.R.: 0.01–1.3 mM LOD: 5 μM | Glucose/Blood serum sample | [163] |
| ChOx/NiO/SPE-Au | This electrode was assembled with ChOx to develop a first-generation cholesterol biosensor where the enzymatically generated H2O2 was used to sense the cholesterol concentration | CV/Amperometry | L.R.: 0.067–0.6 mM LOD: 20 μM | Cholesterol/N/A | [164] |
| ADH/RuO2-GNR/SPCE | This approach allowed increased communication and electron transfer between the electrode surface and redox centers in the ADH | CV/EIS/Amperometry | L.R.: 1–1800 μM for ethanol 1 to 1300 μM for NADH LOD: 0.19 μM for ethanol 0.52 μM for NADH | Ethanol and NADH/Commercial alcoholic beverages | [165] |
| GA/ADH/AuNPs/PNR/MWCNTs/SPCE for ADH and GA/G/AOx/AuNPs/PNR/MWCNTs/SPCE for AOx | The first biosensor based on ADH responds only to ethanol, whereas the second biosensor based on AOx responds to both methanol and ethanol | CV | L.R.: 178.5–1000 μM for ethanol 335.9–1000 μM for methanol LOD: 53.5 μM for ethanol 100.8 μM for methanol | Ethanol, methanol and their mixtures/ Commercial alcoholic drink | [166] |
| LOx–Cu-MOF/CS/Pt/SPCE | A LOx-based biosensor to determine lactate in a wide concentration range | Amperometry | L.R.: 0.75 μM–1 mM LOD: 0.75 μM | Lactate/Sweat and saliva | [167] |
| Tyr/AuNPs/SPCE | Catechol, phenol, caffeic acid, and tyrosol were analyzed individually, and adequate analytical and kinetic performances were obtained | Amperometry | L.R.: 2.5–20 μM LOD: 0.4 nM for catechol and 0.5 μM for phenol | Total phenolic content/Commercial beers | [168] |
| ADH/RA/SPCE | RA/SPCE was found to facilitate the electrocatalytic oxidation of NADH by the action of RA as a natural antioxidant mediator | CV/Amperometry | L.R.: 23.71–1000 μM LOD: 7.1 μM | Ethanol and NADH/Commercial alcoholic drink | [169] |
| GOx/GO-SH/Au/SPE | The enhanced electrochemical performance is originated from sputtered morphology of Au and the bifunctionality of the graphene backbone | CV | L.R.: 3–9 mM LOD: 0.3194 mM | Glucose/Various biomolecules such as cholesterol and D-alanine | [153] |
| PDA@ChOx/MWCNTox/ PB/SPE | Combination of electrocatalytic properties of surfactant-modified PB films and the large high surface-to-volume ratio of CNTs | CV/EIS/Amperometry | L.R.: 0–400 µM LOD: 11 µM | Cholesterol/Biological matrices | [170] |
| GOx/AuNP/PANI/rGO/NH2-MWCNTs/SPCE | The electrochemical analysis has been followed at different stages of glucose oxidase coating on modified SPCE using cyclic voltammetry | Amperometry | L.R.: 1–10 mM LOD: 64 µM | Glucose/Human blood serum samples | [171] |
| GOx/SiO2-ATO/PB/SPE | The used PB pigment is prepared by chemically growing a thin PB layer on the surface of SiO2 particles covered by the thin shell of ATO, which was combined with a Viton® binder system | Amperometry | L.R.: 0.1–1mM LOD: 54.1 μM | Glucose/ N/A | [172] |
| GGP/GA/ZnONPs/PtSPE | The PtSPE was modified with less than 5 nm ZnONPs and glutaraldehyde as a linker agent; GGPs as a biological recognition element exhibited sufficient catalytic activity towards H2O2 reduction | CV/Amperometry | L.R.: N/A LOD: 84 μM | H2O2/ N/A | [173] |
| (1) DAOx/PVF/GRO/SPCE (2) MAOx/PVF/GRO/SPCE | MAOx/PVF/GRO/SPCE showed higher sensitivity for tyramine determination in comparison with the DAOx/PVF/GRO/SPCE | CV | L.R.: 0.99–120 µM for DAOx 0.99–110 µM for MAOx LOD: 0.41 µM for DAOx 0.61 µM for MAOx | Tyramine/Cheese sample | [174] |
| CB/PBNPs/SPE | The versatile analysis of different pesticides was carried out by folding and unfolding the filter paper-based structure, without adding any reagents and multiple sample treatment | Amperometry | L.R.: 2–100 ppb for paraoxon 100 and 1000 ppb for 2,4-dichlorophenoxyacetic acid 10 and 100 ppb for atrazine LOD: 2 ppb for paraoxon 50 ppb for 2,4-dichlorophenoxyacetic acid for atrazine 5 ppb for atrazine | Pesticides (paraoxon, 2,4-dichlorophenoxyacetic acid, and atrazine)/Water sample | [175] |
| CA/Enzymes/GO+cofactors/SPE | NAD+ and Fe(CN)63− as cofactors for ADH, DIA, FDH, DLDH, and L-lactate dehydrogenase (L-LDH) enzymes | Amperometry | L.R.: 0.25–4 mM for L-lactate, D-lactate and formate 0.05–2 mm for ethanol LOD: N/A | L-lactate, D-lactate, ethanol and formate | [154] |
| Uricase/Chi-Gr cry/PB/SPCE | Amperometric detection of UA catalyzed by uricase was based on the change in the cathodic current of PB at a potential of 0.00 V in a flow injection system | CV/Amperometry | L.R.: 0.0025–0.40 mM LOD: 2.5 μM | UA/Human serum samples | [176] |
| e-Lac/CB/SPE | ESD process was exploited for the immobilization of laccase enzyme on CB/SPE | CV/Amperometry | L.R.: 2.5–50 µM LOD: 2.0 µM | Phenolic compound/ drinking, surface, and wastewater | [177] |
| LOx/PBNcs/SPE-BC | Handmade SPE was prepared on oxidized BC substrate and was modified with PBNcs as an electrochemical mediator to facilitate the electron transfer capability and enhance the biosensor sensitivity | CV/Amperometry | L.R.: 1–24 mM LOD: 1.31 mM | Lactate/ artificial sweat | [178] |
| LOx/GMgOC/SPE | The lactate sensing system features an integrated microfluidic sweat collector fabricated from polydimethylsiloxane | CV/Amperometry | L.R.: 0.1–100 mM LOD: 0.3 mM | Lactate/Sweat | [179] |
| E/NPs/SPCEs | Acetylthiocholine iodide, serotonin, and β-D-phenolphthalein glucuronide as E gold nanoparticles and carbon nanotubes as NPs | Amperometry | L.R.: 0.18–1.60 μg/L for AB Fubinaca 0.18–2.00 μg/L for AB Pinaca LOD: (0.07–0.10) μg/L for AB Fubinaca (0.08–0.09) μg/L for AB Pinaca | AB-Fubinaca and AB-Pinaca/Water matrixes | [180] |


4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renedo, O.D.; Alonso-Lomillo, M.; Martínez, M.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.G.-M.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Hart, J.P.; Wring, S.A. Recent developments in the design and application of screen-printed electrochemical sensors for biomedical, environmental and industrial analyses. TrAC Trends Anal. Chem. 1997, 16, 89–103. [Google Scholar] [CrossRef]
- Mincu, N.-B.; Lazar, V.; Stan, D.; Mihailescu, C.M.; Iosub, R.; Mateescu, A.L. Screen-Printed Electrodes (SPE) for in vitro diagnostic purpose. Diagnostics 2020, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S. Screen-Printed Carbon Electrodes. In Electrochemistry of Carbon Electrodes; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; pp. 425–444. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.-T.; Li, D.-W.; Long, Y.-T. Recent developments and applications of screen-printed electrodes in environmental assays—A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef]
- Foster, C.W.; Kadara, R.O.; Banks, C.E. Fundamentals of screen-printing electrochemical architectures. In Screen-Printing Electrochemical Architectures; Springer: Cham, Switzerland, 2016; pp. 13–23. [Google Scholar] [CrossRef]
- Couto, R.; Lima, J.; Quinaz, M. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef]
- Rama, E.C.; Costa-García, A. Screen-printed electrochemical immunosensors for the detection of cancer and cardiovascular biomarkers. Electroanalysis 2016, 28, 1700–1715. [Google Scholar] [CrossRef]
- Smart, A.; Crew, A.; Pemberton, R.; Hughes, G.; Doran, O.; Hart, J. Screen-printed carbon based biosensors and their applications in agri-food safety. TrAC Trends Anal. Chem. 2020, 127, 115898. [Google Scholar] [CrossRef]
- Vasilescu, A.; Nunes, G.; Hayat, A.; Latif, U.; Marty, J.-L. Electrochemical affinity biosensors based on disposable screen-printed electrodes for detection of food allergens. Sensors 2016, 16, 1863. [Google Scholar] [CrossRef]
- Mishra, R.; Nunes, G.; Souto, L.; Marty, J. Screen printed technology—An application towards biosensor development. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 487–498. [Google Scholar]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.M. Screen-printed disposable electrodes: Pharmaceutical applications and recent developments. TrAC Trends Anal. Chem. 2016, 82, 1–11. [Google Scholar] [CrossRef]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.-W.; Xiu, G.; Long, Y.-T. Applications of screen-printed electrodes in current environmental analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Alonso-Lomillo, M.; Domínguez-Renedo, O.; Arcos-Martínez, M. Screen-printed biosensors in microbiology; A review. Talanta 2010, 82, 1629–1636. [Google Scholar] [CrossRef]
- Barton, J.; García, M.B.G.; Santos, D.H.; Fanjul-Bolado, P.; Ribotti, A.; McCaul, M.; Diamond, D.; Magni, P. Screen-printed electrodes for environmental monitoring of heavy metal ions: A review. Microchim. Acta 2016, 183, 503–517. [Google Scholar] [CrossRef]
- Squissato, A.L.; Almeida, E.S.; Silva, S.G.; Richter, E.M.; Batista, A.D.; Munoz, R.A. Screen-printed electrodes for quality control of liquid (Bio) fuels. TrAC Trends Anal. Chem. 2018, 108, 210–220. [Google Scholar] [CrossRef]
- Squissato, A.L.; Munoz, R.A.; Banks, C.E.; Richter, E.M. An overview of recent electroanalytical applications utilizing screen-printed electrodes within flow systems. ChemElectroChem 2020, 7, 2211–2221. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Costa-García, A.; Muñiz, A.d.l.E. Electrochemical (bio) sensors for pesticides detection using screen-printed electrodes. Biosensors 2020, 10, 32. [Google Scholar] [CrossRef]
- Torre, R.; Costa-Rama, E.; Nouws, H.P.; Delerue-Matos, C. Screen-printed electrode-based sensors for food spoilage control: Bacteria and biogenic amines detection. Biosensors 2020, 10, 139. [Google Scholar] [CrossRef]
- Cano-Raya, C.; Denchev, Z.Z.; Cruz, S.F.; Viana, J.C. Chemistry of solid metal-based inks and pastes for printed electronics–A review. Appl. Mater. Today 2019, 15, 416–430. [Google Scholar] [CrossRef]
- Lanyon, Y.H.; Tothill, I.E.; Mascini, M. An amperometric bacterial biosensor based on gold screen-printed electrodes for the detection of benzene. Anal. Lett. 2006, 39, 1669–1681. [Google Scholar] [CrossRef]
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H. A review on amperometric-type immunosensors based on screen-printed electrodes. Analyst 2014, 139, 2289–2311. [Google Scholar] [CrossRef] [PubMed]
- Bigeleisen, J. Screen Printing: A Contemporary Guide to the Technique of Screen Printing for Artists, Designers, and Craftsmen; Watson-Guptill Publications Inc.: New York, NY, USA, 1971. [Google Scholar]
- Wang, J.; Pamidi, P.V.; Rogers, K.R. Sol–gel-derived thick-film amperoEetric immunosensors. Anal. Chem. 1998, 70, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Sanati, A.; Jalali, M.; Raeissi, K.; Karimzadeh, F.; Kharaziha, M.; Mahshid, S.S.; Mahshid, S. A review on recent advancements in electrochemical biosensing using carbonaceous nanomaterials. Microchim. Acta 2019, 186, 773. [Google Scholar] [CrossRef]
- Paimard, G.; Shamsipur, M.; Gholivand, M.B. A three-dimensional hybrid of CdS quantum dots/chitosan/reduced graphene oxide-based sensor for the amperometric detection of ceftazidime. Chem. Pap. 2022, 77, 437–449. [Google Scholar] [CrossRef]
- Paimard, G.; Gholivand, M.B.; Shamsipur, M.; Ahmadi, E.; Shahlaei, M. Introduction of a thrombin sensor based on its interaction with dabigatran as an oral direct thrombin inhibitor. Mater. Sci. Eng. C 2021, 119, 111417. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Ho, J.C.; Takahashi, T.; Yerushalmi, R.; Takei, K.; Ford, A.C.; Chueh, Y.L.; Javey, A. Toward the development of printable nanowire electronics and sensors. Adv. Mater. 2009, 21, 3730–3743. [Google Scholar] [CrossRef]
- Khairy, M.; Kadara, R.O.; Banks, C.E. Electroanalytical sensing of nitrite at shallow recessed screen printed microelectrode arrays. Anal. Methods 2010, 2, 851–854. [Google Scholar] [CrossRef]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Screen printed recessed microelectrode arrays. Sens. Actuators B Chem. 2009, 142, 342–346. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Hernández-Santos, D.; Lamas-Ardisana, P.J.; Martín-Pernía, A.; Costa-García, A. Electrochemical characterization of screen-printed and conventional carbon paste electrodes. Electrochim. Acta 2008, 53, 3635–3642. [Google Scholar] [CrossRef]
- Foulger, S.H. Electrical properties of composites in the vicinity of the percolation threshold. J. Appl. Polym. Sci. 1999, 72, 1573–1582. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hossain, M.M.; Safavieh, M.; Wong, Y.L.; Rahman, I.A.; Zourob, M.; Tamiya, E. Toward the development of smart and low cost point-of-care biosensors based on screen printed electrodes. Crit. Rev. Biotechnol. 2016, 36, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, P.; Chen, T.-W.; Chen, S.-M.; Tseng, T.-W.; Liu, X. Electrochemical activation of screen printed carbon electrode for the determination of antibiotic drug metronidazole. Int. J. Electrochem. Sci. 2018, 13, 1441–1451. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Rong, S.; Zhang, G.; Zhang, Y.; Zhou, Q.; Liu, F.; Li, M.; Chang, D.; Pan, H. Amperometric determination of dopamine using activated screen-printed carbon electrodes. Electrochemistry 2015, 83, 725–729. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef]
- Scognamiglio, V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosens. Bioelectron. 2013, 47, 12–25. [Google Scholar] [CrossRef]
- Arduini, F.; Forchielli, M.; Amine, A.; Neagu, D.; Cacciotti, I.; Nanni, F.; Moscone, D.; Palleschi, G. Screen-printed biosensor modified with carbon black nanoparticles for the determination of paraoxon based on the inhibition of butyrylcholinesterase. Microchim. Acta 2015, 182, 643–651. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Carbone, M.; Sansone, L.; Cacciotti, I.; Moscone, D.; Palleschi, G. Screen-printed electrodes modified with carbon nanomaterials: A comparison among carbon black, carbon nanotubes and graphene. Electroanalysis 2015, 27, 2230–2238. [Google Scholar] [CrossRef]
- Putzbach, W.; Ronkainen, N.J. Immobilization techniques in the fabrication of nanomaterial-based electrochemical biosensors: A review. Sensors 2013, 13, 4811–4840. [Google Scholar] [CrossRef]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-printed electrodes modified with metal nanoparticles for small molecule sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Duffy, G.; Moore, E. Electrochemical immunosensors for food analysis: A review of recent developments. Anal. Lett. 2017, 50, 1–32. [Google Scholar] [CrossRef]
- Chu, Z.; Peng, J.; Jin, W. Advanced nanomaterial inks for screen-printed chemical sensors. Sens. Actuators B Chem. 2017, 243, 919–926. [Google Scholar] [CrossRef]
- Thiyagarajan, N.; Chang, J.-L.; Senthilkumar, K.; Zen, J.-M. Disposable electrochemical sensors: A mini review. Electrochem. Commun. 2014, 38, 86–90. [Google Scholar] [CrossRef]
- Banks, C.E.; Crossley, A.; Salter, C.; Wilkins, S.J.; Compton, R.G. Carbon nanotubes contain metal impurities which are responsible for the “electrocatalysis” seen at some nanotube-modified electrodes. Angew. Chem. Int. Ed. 2006, 45, 2533–2537. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Díaz-Cruz, J.M.; Serrano, N.; Pérez-Ràfols, C.; Ariño, C.; Esteban, M. Electroanalysis from the past to the twenty-first century: Challenges and perspectives. J. Solid State Electrochem. 2020, 24, 2653–2661. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; Wang, J. Wearable non-invasive epidermal glucose sensors: A review. Talanta 2018, 177, 163–170. [Google Scholar] [CrossRef]
- Campbell, A.S.; Kim, J.; Wang, J. Wearable electrochemical alcohol biosensors. Curr. Opin. Electrochem. 2018, 10, 126–135. [Google Scholar] [CrossRef]
- Cao, L.; Fang, C.; Zeng, R.; Zhao, X.; Zhao, F.; Jiang, Y.; Chen, Z. A disposable paper-based microfluidic immunosensor based on reduced graphene oxide-tetraethylene pentamine/Au nanocomposite decorated carbon screen-printed electrodes. Sens. Actuators B Chem. 2017, 252, 44–54. [Google Scholar] [CrossRef]
- Shriver-Lake, L.C.; Zabetakis, D.; Dressick, W.J.; Stenger, D.A.; Trammell, S.A. based electrochemical detection of chlorate. Sensors 2018, 18, 328. [Google Scholar] [CrossRef] [PubMed]
- Colozza, N.; Kehe, K.; Dionisi, G.; Popp, T.; Tsoutsoulopoulos, A.; Steinritz, D.; Moscone, D.; Arduini, F. A wearable origami-like paper-based electrochemical biosensor for sulfur mustard detection. Biosens. Bioelectron. 2019, 129, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Figueredo, F.; Jesús González-Pabón, M.; Cortón, E. Low Cost Layer by Layer Construction of CNT/Chitosan Flexible Paper-based Electrodes: A Versatile Electrochemical Platform for Point of Care and Point of Need Testing. Electroanalysis 2018, 30, 497–508. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Zhou, F.; Yang, G.; Qu, L.; Miao, X. Development of a paper-based, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochem. Commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tiwari, I. Recent build outs in electroanalytical biosensors based on carbon-nanomaterial modified screen printed electrode platforms. Anal. Methods 2017, 9, 3895–3907. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Honeychurch, K.C.; Hart, J.P. Screen-printed electrochemical sensors for monitoring metal pollutants. TrAC Trends Anal. Chem. 2003, 22, 456–469. [Google Scholar] [CrossRef]
- Lucarelli, F.; Marrazza, G.; Turner, A.P.; Mascini, M. Carbon and gold electrodes as electrochemical transducers for DNA hybridisation sensors. Biosens. Bioelectron. 2004, 19, 515–530. [Google Scholar] [CrossRef]
- Ricci, F.; Plaxco, K.W. E-DNA sensors for convenient, label-free electrochemical detection of hybridization. Microchim. Acta 2008, 163, 149–155. [Google Scholar] [CrossRef]
- Palchetti, I.; Mascini, M. Electrochemical nanomaterial-based nucleic acid aptasensors. Anal. Bioanal. Chem. 2012, 402, 3103–3114. [Google Scholar] [CrossRef]
- Biagiotti, V.; Porchetta, A.; Desiderati, S.; Plaxco, K.W.; Palleschi, G.; Ricci, F. Probe accessibility effects on the performance of electrochemical biosensors employing DNA monolayers. Anal. Bioanal. Chem. 2012, 402, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Prodromidis, M.; Economou, A.; Petrou, P.; Kakabakos, S. Quantum dot-based electrochemical DNA biosensor using a screen-printed graphite surface with embedded bismuth precursor. Electrochem. Commun. 2015, 60, 47–51. [Google Scholar] [CrossRef]
- Abd Rashid, J.I.; Yusof, N.A.; Abdullah, J.; Hashim, U.; Hajian, R. A novel disposable biosensor based on SiNWs/AuNPs modified-screen printed electrode for dengue virus DNA oligomer detection. IEEE Sens. J. 2015, 15, 4420–4427. [Google Scholar] [CrossRef]
- Tortolini, C.; Bollella, P.; Antonelli, M.L.; Antiochia, R.; Mazzei, F.; Favero, G. DNA-based biosensors for Hg2+ determination by polythymine–methylene blue modified electrodes. Biosens. Bioelectron. 2015, 67, 524–531. [Google Scholar] [CrossRef]
- Erdem, A.; Eksin, E.; Congur, G. Indicator-free electrochemical biosensor for microRNA detection based on carbon nanofibers modified screen printed electrodes. J. Electroanal. Chem. 2015, 755, 167–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, X.; Ai, J.; Gao, Q.; Qi, H.; Zhang, C. Signal amplification detection of DNA using a sensor fabricated by one-step covalent immobilization of amino-terminated probe DNA onto the polydopamine-modified screen-printed carbon electrode. Sens. Actuators B Chem. 2015, 221, 1535–1541. [Google Scholar] [CrossRef]
- Malecka, K.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecka, H.; Radecki, J. Electrochemical genosensor based on disc and screen printed gold electrodes for detection of specific DNA and RNA sequences derived from Avian Influenza Virus H5N1. Sens. Actuators B Chem. 2016, 224, 290–297. [Google Scholar] [CrossRef]
- Mazloum-Ardakani, M.; Hosseinzadeh, L.; Heidari, M.M. Detection of the M268T Angiotensinogen A3B2 mutation gene based on screen-printed electrodes modified with a nanocomposite: Application to human genomic samples. Microchim. Acta 2016, 183, 219–227. [Google Scholar] [CrossRef]
- Ilkhani, H.; Farhad, S. A novel electrochemical DNA biosensor for Ebola virus detection. Anal. Biochem. 2018, 557, 151–155. [Google Scholar] [CrossRef]
- Shoaie, N.; Forouzandeh, M.; Omidfar, K. Voltammetric determination of the Escherichia coli DNA using a screen-printed carbon electrode modified with polyaniline and gold nanoparticles. Microchim. Acta 2018, 185, 217. [Google Scholar] [CrossRef]
- Uygun, Z.O.; Atay, S. Label-free highly sensitive detection of DNA approximate length and concentration by impedimetric CRISPR-dCas9 based biosensor technology. Bioelectrochemistry 2021, 140, 107812. [Google Scholar] [CrossRef] [PubMed]
- Karadurmus, L.; Dogan-Topal, B.; Kurbanoglu, S.; Shah, A.; Ozkan, S.A. The interaction between DNA and three intercalating anthracyclines using electrochemical DNA nanobiosensor based on metal nanoparticles modified screen-printed electrode. Micromachines 2021, 12, 1337. [Google Scholar] [CrossRef] [PubMed]
- Thoeny, V.; Melnik, E.; Asadi, M.; Mehrabi, P.; Schalkhammer, T.; Pulverer, W.; Maier, T.; Mutinati, G.C.; Lieberzeit, P.; Hainberger, R. Detection of breast cancer-related point-mutations using screen-printed and gold-plated electrochemical sensor arrays suitable for point-of-care applications. Talanta Open 2022, 6, 100150. [Google Scholar] [CrossRef]
- Chai, H.; Ma, X.; Sun, H.; Miao, P. DNA–MnO2 Nanoconjugates Investigation and Application for Electrochemical Polymerase Chain Reaction. Anal. Chem. 2022, 94, 4565–4569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, P.; Liang, Y.; Cheng, W.; Li, L.; Wang, H.; Yu, Z.; Liu, Y.; Zhang, X. Rapid electrochemical quantification of trace Hg2+ using a hairpin DNA probe and quantum dot modified screen-printed gold electrodes. RSC Adv. 2022, 12, 13448–13455. [Google Scholar] [CrossRef] [PubMed]
- Abedi, R.; Raoof, J.B.; Hashkavayi, A.B.; Asghari, M.; Azimi, R.; Hejazi, M.S. A novel genosensor based on Fe3O4@SiO2/DABCO-modified screen-printed graphite electrode for detection of prostate cancer gene sequence hybridization. J. Iran. Chem. Soc. 2022, 19, 2631–2640. [Google Scholar] [CrossRef]
- Becker, J.M.; Lielpetere, A.; Szczesny, J.; Ruff, A.; Conzuelo, F.; Schuhmann, W. Assembling a Low-volume Biofuel Cell on a Screen-printed Electrode for Glucose Sensing. Electroanalysis 2022, 34, 1629–1637. [Google Scholar] [CrossRef]
- Aghaei, F.; Seifati, S.M.; Nasirizadeh, N. Development of a DNA biosensor for the detection of phenylketonuria based on a screen-printed gold electrode and hematoxylin. Anal. Methods 2017, 9, 966–973. [Google Scholar] [CrossRef]
- Huang, K.-J.; Liu, Y.-J.; Zhang, J.-Z.; Liu, Y.-M. A novel aptamer sensor based on layered tungsten disulfide nanosheets and Au nanoparticles amplification for 17β-estradiol detection. Anal. Methods 2014, 6, 8011–8017. [Google Scholar] [CrossRef]
- Mirian, M.; Khanahmad, H.; Darzi, L.; Salehi, M.; Sadeghi-Aliabadi, H. Oligonucleotide aptamers: Potential novel molecules against viral hepatitis. Res. Pharm. Sci. 2017, 12, 88. [Google Scholar] [CrossRef]
- Ilgu, M.; Nilsen-Hamilton, M. Aptamers in analytics. Analyst 2016, 141, 1551–1568. [Google Scholar] [CrossRef] [PubMed]
- Douaki, A.; Garoli, D.; Inam, A.S.; Angeli, M.A.C.; Cantarella, G.; Rocchia, W.; Wang, J.; Petti, L.; Lugli, P. Smart Approach for the Design of Highly Selective Aptamer-Based Biosensors. Biosensors 2022, 12, 574. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.K.; Sharma, V.; Mishra, R.K. Electrochemical aptasensors for food and environmental safeguarding: A review. Biosensors 2018, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Li, H.; Lu, X.; Qian, J.; Zhu, M.; Chen, W.; Liu, Q.; Hao, N.; Li, H.; Wang, K. A disposable aptasensing device for label-free detection of fumonisin B1 by integrating PDMS film-based micro-cell and screen-printed carbon electrode. Sens. Actuators B Chem. 2017, 251, 192–199. [Google Scholar] [CrossRef]
- Wang, X.; Su, J.; Zeng, D.; Liu, G.; Liu, L.; Xu, Y.; Wang, C.; Liu, X.; Wang, L.; Mi, X. Gold nano-flowers (Au NFs) modified screen-printed carbon electrode electrochemical biosensor for label-free and quantitative detection of glycated hemoglobin. Talanta 2019, 201, 119–125. [Google Scholar] [CrossRef]
- Santharaman, P.; Venkatesh, K.A.; Vairamani, K.; Benjamin, A.R.; Sethy, N.K.; Bhargava, K.; Karunakaran, C. ARM-microcontroller based portable nitrite electrochemical analyzer using cytochrome c reductase biofunctionalized onto screen printed carbon electrode. Biosens. Bioelectron. 2017, 90, 410–417. [Google Scholar] [CrossRef]
- Eissa, S.; Almusharraf, A.Y.; Zourob, M. A comparison of the performance of voltammetric aptasensors for glycated haemoglobin on different carbon nanomaterials-modified screen printed electrodes. Mater. Sci. Eng. C 2019, 101, 423–430. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, J.; Chen, J.; Zhang, Q.; Lu, Y.; Yao, Y.; Li, S.; Liu, G.L.; Liu, Q. Smartphone-based portable biosensing system using impedance measurement with printed electrodes for 2,4,6-trinitrotoluene (TNT) detection. Biosens. Bioelectron. 2015, 70, 81–88. [Google Scholar] [CrossRef]
- Martín-Yerga, D.; Carrasco-Rodríguez, J.; Alonso, F.J.G.; Costa-García, A. Competitive electrochemical biosensing of biotin using cadmium-modified titanium phosphate nanoparticles and 8-channel screen-printed disposable electrodes. Anal. Methods 2017, 9, 3983–3991. [Google Scholar] [CrossRef]
- Li, Y.; Ran, G.; Lu, G.; Ni, X.; Liu, D.; Sun, J.; Xie, C.; Yao, D.; Bai, W. Highly sensitive label-free electrochemical aptasensor based on screen-printed electrode for detection of cadmium (II) ions. J. Electrochem. Soc. 2019, 166, B449. [Google Scholar] [CrossRef]
- Vasudevan, M.; Tai, M.J.; Perumal, V.; Gopinath, S.C.; Murthe, S.S.; Ovinis, M.; Mohamed, N.M.; Joshi, N. Highly sensitive and selective acute myocardial infarction detection using aptamer-tethered MoS2 nanoflower and screen-printed electrodes. Biotechnol. Appl. Biochem. 2021, 68, 1386–1395. [Google Scholar]
- Jo, H.; Her, J.; Lee, H.; Shim, Y.-B.; Ban, C. Highly sensitive amperometric detection of cardiac troponin I using sandwich aptamers and screen-printed carbon electrodes. Talanta 2017, 165, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Bagheri Hashkavayi, A.; Bakhsh Raoof, J.; Ojani, R.; Hamidi Asl, E. Label-free electrochemical aptasensor for determination of chloramphenicol based on gold nanocubes-modified screen-printed gold electrode. Electroanalysis 2015, 27, 1449–1456. [Google Scholar] [CrossRef]
- Tabrizi, M.A.; Shamsipur, M.; Saber, R.; Sarkar, S. Simultaneous determination of CYC and VEGF165 tumor markers based on immobilization of flavin adenine dinucleotide and thionine as probes on reduced graphene oxide-poly (amidoamine)/gold nanocomposite modified dual working screen-printed electrode. Sens. Actuators B Chem. 2017, 240, 1174–1181. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.B. Design an aptasensor based on structure-switching aptamer on dendritic gold nanostructures/Fe3O4@SiO2/DABCO modified screen printed electrode for highly selective detection of epirubicin. Biosens. Bioelectron. 2017, 91, 650–657. [Google Scholar] [CrossRef]
- Ahmadi, A.; Khoshfetrat, S.M.; Kabiri, S.; Dorraji, P.S.; Larijani, B.; Omidfar, K. Electrochemiluminescence paper-based screen-printed electrode for HbA1c detection using two-dimensional zirconium metal-organic framework/Fe3O4 nanosheet composites decorated with Au nanoclusters. Microchim. Acta 2021, 188, 296. [Google Scholar] [CrossRef]
- Park, K. Impedance Technique-Based Label-Free Electrochemical Aptasensor for Thrombin Using Single-Walled Carbon Nanotubes-Casted Screen-Printed Carbon Electrode. Sensors 2022, 22, 2699. [Google Scholar] [CrossRef]
- Gómez-Arconada, L.; Díaz-Fernández, A.; Ferapontova, E.E. Ultrasensitive disposable apatasensor for reagentless electrocatalytic detection of thrombin: An O2-Dependent hemin-G4-aptamer assay on gold screen-printed electrodes. Talanta 2022, 245, 123456. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Acedo, P. An electrochemical impedance spectroscopy-based aptasensor for the determination of SARS-CoV-2-RBD using a carbon nanofiber–gold nanocomposite modified screen-printed electrode. Biosensors 2022, 12, 142. [Google Scholar] [CrossRef]
- Curti, F.; Fortunati, S.; Knoll, W.; Giannetto, M.; Corradini, R.; Bertucci, A.; Careri, M. A Folding-Based Electrochemical Aptasensor for the Single-Step Detection of the SARS-CoV-2 Spike Protein. ACS Appl. Mater. Interfaces 2022, 14, 19204–19211. [Google Scholar] [CrossRef]
- Hassani, S.; Akmal, M.R.; Salek-Maghsoudi, A.; Rahmani, S.; Ganjali, M.R.; Norouzi, P.; Abdollahi, M. Novel label-free electrochemical aptasensor for determination of Diazinon using gold nanoparticles-modified screen-printed gold electrode. Biosens. Bioelectron. 2018, 120, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.C.; Batistuti, M.R.; Junior, B.B.; Mulato, M. Aptasensor based on screen-printed electrode for breast cancer detection in undiluted human serum. Bioelectrochemistry 2021, 137, 107586. [Google Scholar] [CrossRef] [PubMed]
- Amouzadeh Tabrizi, M.; Shamsipur, M.; Saber, R.; Sarkar, S.; Besharati, M. An electrochemical aptamer-based assay for femtomolar determination of insulin using a screen printed electrode modified with mesoporous carbon and 1,3,6,8-pyrenetetrasulfonate. Microchim. Acta 2018, 185, 59. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. Label-free electrochemical aptasensor for rapid detection of SARS-CoV-2 spike glycoprotein based on the composite of Cu(OH)2 nanorods arrays as a high-performance surface substrate. Bioelectrochemistry 2022, 146, 108106. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, A.; Sánchez, A.; Vilela, D.; Mayol, B.; Martínez-Ruíz, P.; Villalonga, R. Electrochemical aptasensor based on anisotropically modified (Janus-type) gold nanoparticles for determination of C-reactive protein. Microchim. Acta 2022, 189, 309. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Kaya, Ş.; Üstündağ, Z.; Caglayan, M.O. An electrochemical signal switch–based (on–off) aptasensor for sensitive detection of insulin on gold-deposited screen-printed electrodes. J. Solid State Electrochem. 2022, 26, 907–915. [Google Scholar] [CrossRef]
- Pakzad, M.; Fouladdel, S.; Nili-Ahmadabadi, A.; Pourkhalili, N.; Baeeri, M.; Azizi, E.; Sabzevari, O.; Ostad, S.N.; Abdollahi, M. Sublethal exposures of diazinon alters glucose homostasis in Wistar rats: Biochemical and molecular evidences of oxidative stress in adipose tissues. Pestic. Biochem. Physiol. 2013, 105, 57–61. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, X.; Gao, R.; Hu, J. Mechanism and kinetic properties for OH-initiated atmospheric degradation of the organophosphorus pesticide diazinon. Atmos. Environ. 2011, 45, 3141–3148. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Fakhruddin, A.; Chowdhury, M.; Rahman, M.; Alam, M. Monitoring of selected pesticides residue levels in water samples of paddy fields and removal of cypermethrin and chlorpyrifos residues from water using rice bran. Bull. Environ. Contam. Toxicol. 2012, 89, 348–353. [Google Scholar] [CrossRef]
- Akan, J.; Jafiya, L.; Mohammed, Z.; Abdulrahman, F. Organophosphorus pesticide residues in vegetables and soil samples from alau dam and gongulong agricultural areas, Borno State, Nigeria. Ecosystems 2013, 3, 58–64. [Google Scholar] [CrossRef]
- Yalow, R.S.; Berson, S.A. Assay of plasma insulin in human subjects by immunological methods. Nature 1959, 184, 1648–1649. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Ricci, F.; Volpe, G.; Micheli, L.; Palleschi, G. A review on novel developments and applications of immunosensors in food analysis. Anal. Chim. Acta 2007, 605, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Gómez, V.; Campuzano, S.; Pedrero, M.; Pingarrón, J.M. Gold screen-printed-based impedimetric immunobiosensors for direct and sensitive Escherichia coli quantisation. Biosens. Bioelectron. 2009, 24, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Lien, T.T.; Takamura, Y.; Tamiya, E.; Mun’delanji, C.V. Modified screen printed electrode for development of a highly sensitive label-free impedimetric immunosensor to detect amyloid beta peptides. Anal. Chim. Acta 2015, 892, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Martin-Yerga, D.; Costa-Garcia, A. Towards a blocking-free electrochemical immunosensing strategy for anti-transglutaminase antibodies using screen-printed electrodes. Bioelectrochemistry 2015, 105, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.; Dou, W.; Zhao, G. A sandwich electrochemical immunoassay for Salmonella pullorum and Salmonella gallinarum based on a AuNPs/SiO2/Fe3O4 adsorbing antibody and 4 channel screen printed carbon electrode electrodeposited gold nanoparticles. RSC Adv. 2015, 5, 74548–74556. [Google Scholar] [CrossRef]
- Tsai, J.-Z.; Chen, C.-J.; Settu, K.; Lin, Y.-F.; Chen, C.-L.; Liu, J.-T. Screen-printed carbon electrode-based electrochemical immunosensor for rapid detection of microalbuminuria. Biosens. Bioelectron. 2016, 77, 1175–1182. [Google Scholar] [CrossRef]
- Chan, K.; Lim, H.; Shams, N.; Jayabal, S.; Pandikumar, A.; Huang, N. Fabrication of graphene/gold-modified screen-printed electrode for detection of carcinoembryonic antigen. Mater. Sci. Eng. C 2016, 58, 666–674. [Google Scholar] [CrossRef]
- Moreira, F.T.; Ferreira, M.J.M.; Puga, J.R.; Sales, M.G.F. Screen-printed electrode produced by printed-circuit board technology. Application to cancer biomarker detection by means of plastic antibody as sensing material. Sens. Actuators B Chem. 2016, 223, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Vinayak, P.; Tuteja, S.K.; Chhabra, V.A.; Bhardwaj, N.; Paul, A.; Kim, K.-H.; Deep, A. Graphene modified screen printed immunosensor for highly sensitive detection of parathion. Biosens. Bioelectron. 2016, 83, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Wu, C.-C.; Kuo, Y.-F. A high sensitive impedimetric salbutamol immunosensor based on the gold nanostructure-deposited screen-printed carbon electrode. J. Electroanal. Chem. 2016, 768, 27–33. [Google Scholar] [CrossRef]
- Lee, S.; Lim, H.; Ibrahim, I.; Jamil, A.; Pandikumar, A.; Huang, N. Horseradish peroxidase-labeled silver/reduced graphene oxide thin film-modified screen-printed electrode for detection of carcinoembryonic antigen. Biosens. Bioelectron. 2017, 89, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Tallapragada, S.D.; Layek, K.; Mukherjee, R.; Mistry, K.K.; Ghosh, M. Development of screen-printed electrode based immunosensor for the detection of HER2 antigen in human serum samples. Bioelectrochemistry 2017, 118, 25–30. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Laocharoensuk, R.; Vilaivan, T.; Chailapakul, O. Electrochemical detection of c-reactive protein based on anthraquinone-labeled antibody using a screen-printed graphene electrode. Talanta 2018, 183, 311–319. [Google Scholar] [CrossRef]
- Zhao, L.; Han, H.; Ma, Z. Improved screen-printed carbon electrode for multiplexed label-free amperometric immuniosensor: Addressing its conductivity and reproducibility challenges. Biosens. Bioelectron. 2018, 101, 304–310. [Google Scholar] [CrossRef]
- Rodovalho, V.; Araujo, G.; Vaz, E.; Ueira-Vieira, C.; Goulart, L.; Madurro, J.; Brito-Madurro, A. Peptide-based electrochemical biosensor for juvenile idiopathic arthritis detection. Biosens. Bioelectron. 2018, 100, 577–582. [Google Scholar] [CrossRef]
- Johari-Ahar, M.; Karami, P.; Ghanei, M.; Afkhami, A.; Bagheri, H. Development of a molecularly imprinted polymer tailored on disposable screen-printed electrodes for dual detection of EGFR and VEGF using nano-liposomal amplification strategy. Biosens. Bioelectron. 2018, 107, 26–33. [Google Scholar] [CrossRef]
- Mo, X.; Wu, Z.; Huang, J.; Zhao, G.; Dou, W. A sensitive and regenerative electrochemical immunosensor for quantitative detection of Escherichia coli O157: H7 based on stable polyaniline coated screen-printed carbon electrode and rGO-NR-Au@Pt. Anal. Methods 2019, 11, 1475–1482. [Google Scholar] [CrossRef]
- Phetsang, S.; Jakmunee, J.; Mungkornasawakul, P.; Laocharoensuk, R.; Ounnunkad, K. Sensitive amperometric biosensors for detection of glucose and cholesterol using a platinum/reduced graphene oxide/poly (3-aminobenzoic acid) film-modified screen-printed carbon electrode. Bioelectrochemistry 2019, 127, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Viet, N.X.; Hoan, N.X.; Takamura, Y. Development of highly sensitive electrochemical immunosensor based on single-walled carbon nanotube modified screen-printed carbon electrode. Mater. Chem. Phys. 2019, 227, 123–129. [Google Scholar] [CrossRef]
- Thunkhamrak, C.; Chuntib, P.; Ounnunkad, K.; Banet, P.; Aubert, P.-H.; Saianand, G.; Gopalan, A.-I.; Jakmunee, J. Highly sensitive voltammetric immunosensor for the detection of prostate specific antigen based on silver nanoprobe assisted graphene oxide modified screen printed carbon electrode. Talanta 2020, 208, 120389. [Google Scholar] [CrossRef] [PubMed]
- Khue, V.Q.; Huy, T.Q.; Phan, V.N.; Tuan-Le, A.; Le, D.T.T.; Tonezzer, M.; Hanh, N.T.H. Electrochemical stability of screen-printed electrodes modified with Au nanoparticles for detection of methicillin-resistant Staphylococcus aureus. Mater. Chem. Phys. 2020, 255, 123562. [Google Scholar] [CrossRef]
- Fabiani, L.; Saroglia, M.; Galatà, G.; De Santis, R.; Fillo, S.; Luca, V.; Faggioni, G.; D’Amore, N.; Regalbuto, E.; Salvatori, P. Magnetic beads combined with carbon black-based screen-printed electrodes for COVID-19: A reliable and miniaturized electrochemical immunosensor for SARS-CoV-2 detection in saliva. Biosens. Bioelectron. 2021, 171, 112686. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.S.; Bott-Neto, J.L.; Machado, S.A.; Oliveira, O.N., Jr. Label-Free Electrochemical Immunosensor Made with Tree-like Gold Dendrites for Monitoring 25-Hydroxyvitamin D3 Metabolite. ACS Appl. Mater. Interfaces 2022, 14, 31455–31462. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Erk, E.E.; Soylak, M.; Erk, N.; Karimi, F. Metal–Organic Framework Based Electrochemical Immunosensor for Label-Free Detection of Glial Fibrillary Acidic Protein as a Biomarker. Ind. Eng. Chem. Res. 2022. [Google Scholar] [CrossRef]
- Jafari, S.; Burr, L.; Migliorelli, D.; Galve, R.; Marco, M.-P.; Campbell, K.; Elliott, C.; Suman, M.; Sturla, S.J.; Generelli, S. Smartphone-based magneto-immunosensor on carbon black modified screen-printed electrodes for point-of-need detection of aflatoxin B1 in cereals. Anal. Chim. Acta 2022, 1221, 340118. [Google Scholar] [CrossRef]
- Jiang, F.; Xiao, Z.; Wang, T.; Wang, J.; Bie, L.; Saleh, L.; Frey, K.; Zhang, L.; Wang, J. Rapid and sensitive multiplex detection of COVID-19 antigens and antibody using electrochemical immunosensor-/aptasensor-enabled biochips. Chem. Commun. 2022, 58, 7285–7288. [Google Scholar] [CrossRef]
- Polli, F.; D’Agostino, C.; Zumpano, R.; De Martino, V.; Favero, G.; Colangelo, L.; Minisola, S.; Mazzei, F. ASu@MNPs-based electrochemical immunosensor for vitamin D3 serum samples analysis. Talanta 2023, 251, 123755. [Google Scholar] [CrossRef]
- Du, D.; Wang, J.; Lu, D.; Dohnalkova, A.; Lin, Y. Multiplexed electrochemical immunoassay of phosphorylated proteins based on enzyme-functionalized gold nanorod labels and electric field-driven acceleration. Anal. Chem. 2011, 83, 6580–6585. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Zhou, Y.G.; Poudineh, M.; Safaei, T.S.; Mohamadi, R.M.; Sargent, E.H.; Kelley, S.O. Highly specific electrochemical analysis of cancer cells using multi-nanoparticle labeling. Angew. Chem. 2014, 126, 13361–13365. [Google Scholar] [CrossRef]
- Agrisuelas, J.; González-Sánchez, M.-I.; Valero, E. Hydrogen peroxide sensor based on in situ grown Pt nanoparticles from waste screen-printed electrodes. Sens. Actuators B Chem. 2017, 249, 499–505. [Google Scholar] [CrossRef]
- Sassolas, A.; Blum, L.J.; Leca-Bouvier, B.D. Immobilization strategies to develop enzymatic biosensors. Biotechnol. Adv. 2012, 30, 489–511. [Google Scholar] [CrossRef]
- Malmiri, H.J.; Jahanian, M.A.G.; Berenjian, A. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar]
- Qian, L.; Yang, X. Composite film of carbon nanotubes and chitosan for preparation of amperometric hydrogen peroxide biosensor. Talanta 2006, 68, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.; Pemberton, R.; Fielden, P.; Hart, J.P. Development of a novel reagentless, screen-printed amperometric biosensor based on glutamate dehydrogenase and NAD+, integrated with multi-walled carbon nanotubes for the determination of glutamate in food and clinical applications. Sens. Actuators B Chem. 2015, 216, 614–621. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Li, Y. An electrochemical biosensor for rapid detection of E. coli O157: H7 with highly efficient bi-functional glucose oxidase-polydopamine nanocomposites and Prussian blue modified screen-printed interdigitated electrodes. Analyst 2016, 141, 5441–5449. [Google Scholar] [CrossRef]
- Akhtar, M.A.; Batool, R.; Hayat, A.; Han, D.; Riaz, S.; Khan, S.U.; Nasir, M.; Nawaz, M.H.; Niu, L. Functionalized graphene oxide bridging between enzyme and Au-sputtered screen-printed interface for glucose detection. ACS Appl. Nano Mater. 2019, 2, 1589–1596. [Google Scholar] [CrossRef]
- Pilas, J.; Selmer, T.; Keusgen, M.; Schöning, M.J. Screen-printed carbon electrodes modified with graphene oxide for the design of a reagent-free NAD+-dependent biosensor array. Anal. Chem. 2019, 91, 15293–15299. [Google Scholar] [CrossRef]
- Mazurenko, I.; Tananaiko, O.; Biloivan, O.; Zhybak, M.; Pelyak, I.; Zaitsev, V.; Etienne, M.; Walcarius, A. Amperometric Biosensor for Choline Based on Gold Screen-Printed Electrode Modified with Electrochemically-Deposited Silica Biocomposite. Electroanalysis 2015, 27, 1685–1692. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.; Mercier, P.P.; Wang, J. Wearable salivary uric acid mouthguard biosensor with integrated wireless electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Apetrei, I.M.; Apetrei, C. The biocomposite screen-printed biosensor based on immobilization of tyrosinase onto the carboxyl functionalised carbon nanotube for assaying tyramine in fish products. J. Food Eng. 2015, 149, 1–8. [Google Scholar] [CrossRef]
- Rungsawang, T.; Punrat, E.; Adkins, J.; Henry, C.; Chailapakul, O. Development of Electrochemical Paper-based Glucose Sensor Using Cellulose-4-aminophenylboronic Acid-modified Screen-printed Carbon Electrode. Electroanalysis 2016, 28, 462–468. [Google Scholar] [CrossRef]
- Bilgi, M.; Ayranci, E. Biosensor application of screen-printed carbon electrodes modified with nanomaterials and a conducting polymer: Ethanol biosensors based on alcohol dehydrogenase. Sens. Actuators B Chem. 2016, 237, 849–855. [Google Scholar] [CrossRef]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Z.; Fu, J.; Guo, Y.; Sun, X.; Yang, Q.; Wang, X. Electrochemical acetylcholinesterase biosensor based on multi-walled carbon nanotubes/dicyclohexyl phthalate modified screen-printed electrode for detection of chlorpyrifos. J. Electroanal. Chem. 2017, 801, 185–191. [Google Scholar] [CrossRef]
- Hatada, M.; Tsugawa, W.; Kamio, E.; Loew, N.; Klonoff, D.C.; Sode, K. Development of a screen-printed carbon electrode based disposable enzyme sensor strip for the measurement of glycated albumin. Biosens. Bioelectron. 2017, 88, 167–173. [Google Scholar] [CrossRef]
- Yang, P.; Peng, J.; Chu, Z.; Jiang, D.; Jin, W. Facile synthesis of Prussian blue nanocubes/silver nanowires network as a water-based ink for the direct screen-printed flexible biosensor chips. Biosens. Bioelectron. 2017, 92, 709–717. [Google Scholar] [CrossRef]
- Salazar, P.; García-García, F.J.; González-Elipe, A.R. Sensing and biosensing with screen printed electrodes modified with nanostructured nickel oxide thin films prepared by magnetron sputtering at oblique angles. Electrochem. Commun. 2018, 94, 5–8. [Google Scholar] [CrossRef]
- Vukojević, V.; Djurdjić, S.; Ognjanović, M.; Antić, B.; Kalcher, K.; Mutić, J.; Stanković, D.M. RuO2/graphene nanoribbon composite supported on screen printed electrode with enhanced electrocatalytic performances toward ethanol and NADH biosensing. Biosens. Bioelectron. 2018, 117, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Bilgi, M.; Ayranci, E. Development of amperometric biosensors using screen-printed carbon electrodes modified with conducting polymer and nanomaterials for the analysis of ethanol, methanol and their mixtures. J. Electroanal. Chem. 2018, 823, 588–592. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Arcos-Martinez, M.J. Dual range lactate oxidase-based screen printed amperometric biosensor for analysis of lactate in diversified samples. Talanta 2018, 188, 779–787. [Google Scholar] [CrossRef]
- Cerrato-Alvarez, M.; Bernalte, E.; Bernalte-García, M.J.; Pinilla-Gil, E. Fast and direct amperometric analysis of polyphenols in beers using tyrosinase-modified screen-printed gold nanoparticles biosensors. Talanta 2019, 193, 93–99. [Google Scholar] [CrossRef]
- Bilgi, M.; Sahin, E.M.; Ayranci, E. Sensor and biosensor application of a new redox mediator: Rosmarinic acid modified screen-printed carbon electrode for electrochemical determination of NADH and ethanol. J. Electroanal. Chem. 2018, 813, 67–74. [Google Scholar] [CrossRef]
- Salazar, P.; Martín, M.; González-Mora, J.L. In situ electrodeposition of cholesterol oxidase-modified polydopamine thin film on nanostructured screen printed electrodes for free cholesterol determination. J. Electroanal. Chem. 2019, 837, 191–199. [Google Scholar] [CrossRef]
- Maity, D.; Minitha, C.; RT, R.K. Glucose oxidase immobilized amine terminated multiwall carbon nanotubes/reduced graphene oxide/polyaniline/gold nanoparticles modified screen-printed carbon electrode for highly sensitive amperometric glucose detection. Mater. Sci. Eng. C 2019, 105, 110075. [Google Scholar] [CrossRef] [PubMed]
- Aller-Pellitero, M.; Fremeau, J.; Villa, R.; Guirado, G.; Lakard, B.; Hihn, J.-Y.; del Campo, F.J. Electrochromic biosensors based on screen-printed Prussian Blue electrodes. Sens. Actuators B Chem. 2019, 290, 591–597. [Google Scholar] [CrossRef]
- Uribe, P.A.; Ortiz, C.C.; Centeno, D.A.; Castillo, J.J.; Blanco, S.I.; Gutierrez, J.A. Self-assembled Pt screen printed electrodes with a novel peroxidase Panicum maximum and zinc oxide nanoparticles for H2O2 detection. Colloids Surf. A Physicochem. Eng. Asp. 2019, 561, 18–24. [Google Scholar] [CrossRef]
- Erden, P.E.; Erdoğan, Z.Ö.; Öztürk, F.; Koçoğlu, İ.O.; Kılıç, E. Amperometric biosensors for tyramine determination based on graphene oxide and polyvinylferrocene modified screen-printed electrodes. Electroanalysis 2019, 31, 2368–2378. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Jirakunakorn, R.; Khumngern, S.; Choosang, J.; Thavarungkul, P.; Kanatharana, P.; Numnuam, A. Uric acid enzyme biosensor based on a screen-printed electrode coated with Prussian blue and modified with chitosan-graphene composite cryogel. Microchem. J. 2020, 154, 104624. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Cartoni, A.; Calandra, P.; Tempesta, E.; Giardi, M.T.; Antonacci, A.; Arduini, F. Electrospray deposition as a smart technique for laccase immobilisation on carbon black-nanomodified screen-printed electrodes. Biosens. Bioelectron. 2020, 163, 112299. [Google Scholar] [CrossRef]
- Gomes, N.O.; Carrilho, E.; Machado, S.A.S.; Sgobbi, L.F. Bacterial cellulose-based electrochemical sensing platform: A smart material for miniaturized biosensors. Electrochim. Acta 2020, 349, 136341. [Google Scholar] [CrossRef]
- Shitanda, I.; Mitsumoto, M.; Loew, N.; Yoshihara, Y.; Watanabe, H.; Mikawa, T.; Tsujimura, S.; Itagaki, M.; Motosuke, M. Continuous sweat lactate monitoring system with integrated screen-printed MgO-templated carbon-lactate oxidase biosensor and microfluidic sweat collector. Electrochim. Acta 2021, 368, 137620. [Google Scholar] [CrossRef]
- Brenes, J.P.; Arroyo-Mora, L.E.; Barquero-Quirós, M. Enzymatic inhibitive determination of AB-Fubinaca and AB-Pinaca on screen printed carbon tetratiofulvalene electrodes modified with nanoparticles and carbon nanotubes. Sens. Bio-Sens. Res. 2022, 38, 100515. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paimard, G.; Ghasali, E.; Baeza, M. Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors 2023, 11, 113. https://doi.org/10.3390/chemosensors11020113
Paimard G, Ghasali E, Baeza M. Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors. 2023; 11(2):113. https://doi.org/10.3390/chemosensors11020113
Chicago/Turabian StylePaimard, Giti, Ehsan Ghasali, and Mireia Baeza. 2023. "Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications" Chemosensors 11, no. 2: 113. https://doi.org/10.3390/chemosensors11020113
APA StylePaimard, G., Ghasali, E., & Baeza, M. (2023). Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors, 11(2), 113. https://doi.org/10.3390/chemosensors11020113






