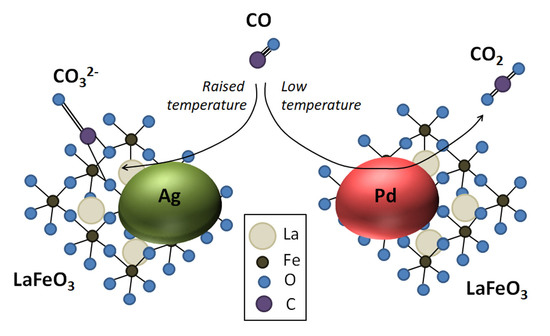
Distinct Roles of Additives in the Improved Sensitivity to CO of Ag- and Pd-Modified Nanosized LaFeO3
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
3. Results
3.1. Composition and Structure of Materials
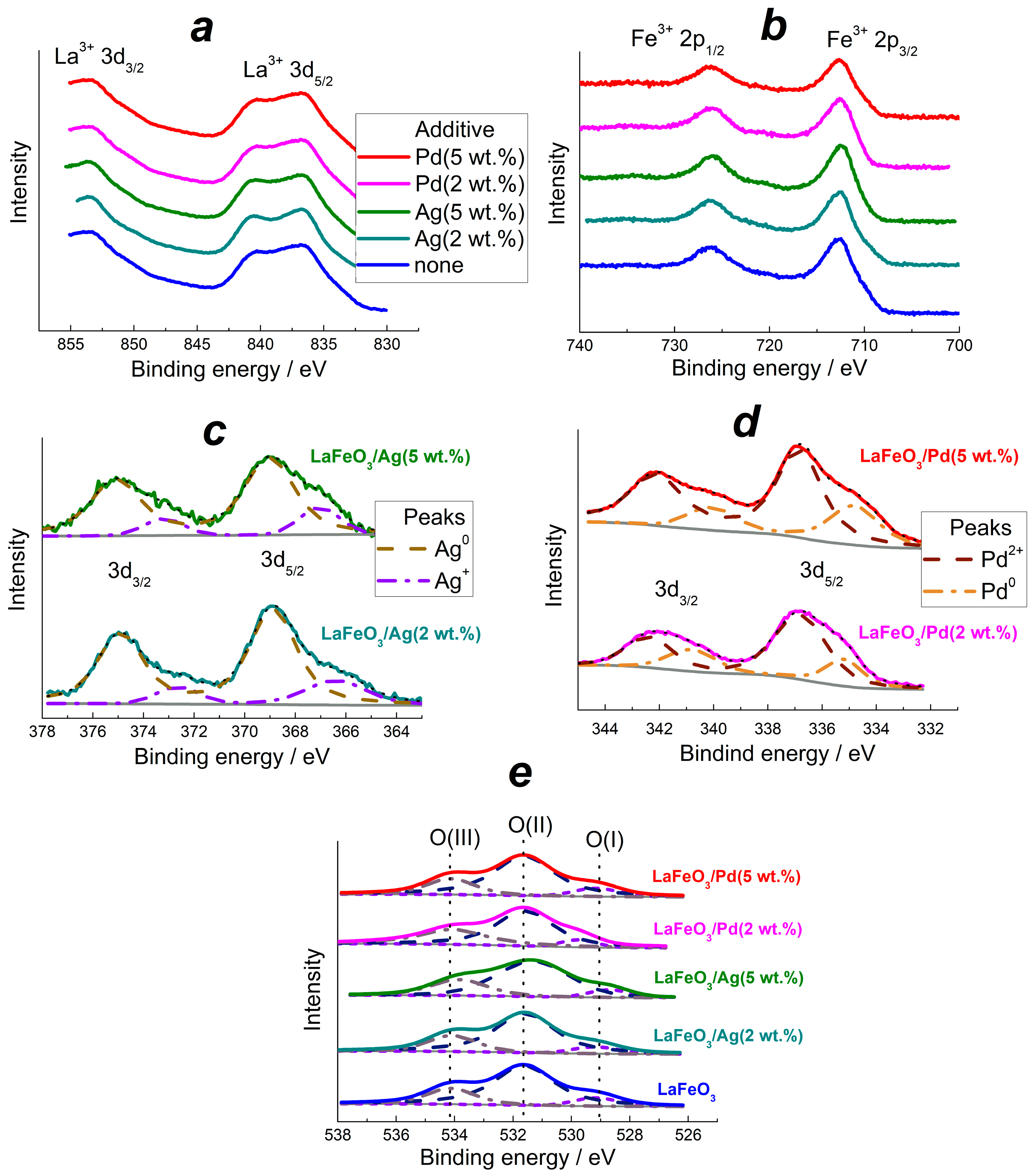
3.2. Oxidation States of Elements and Surface Species in the Materials
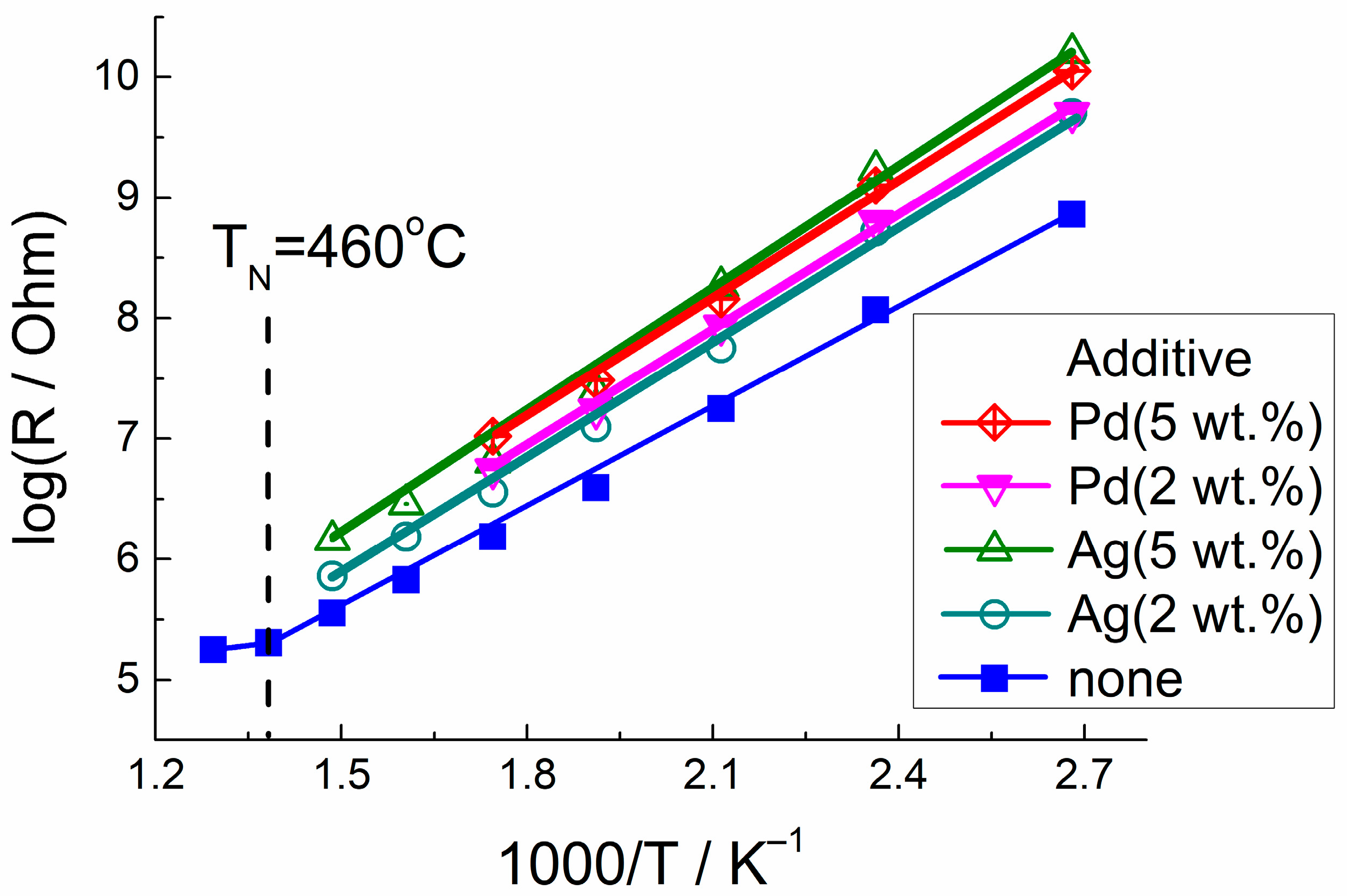
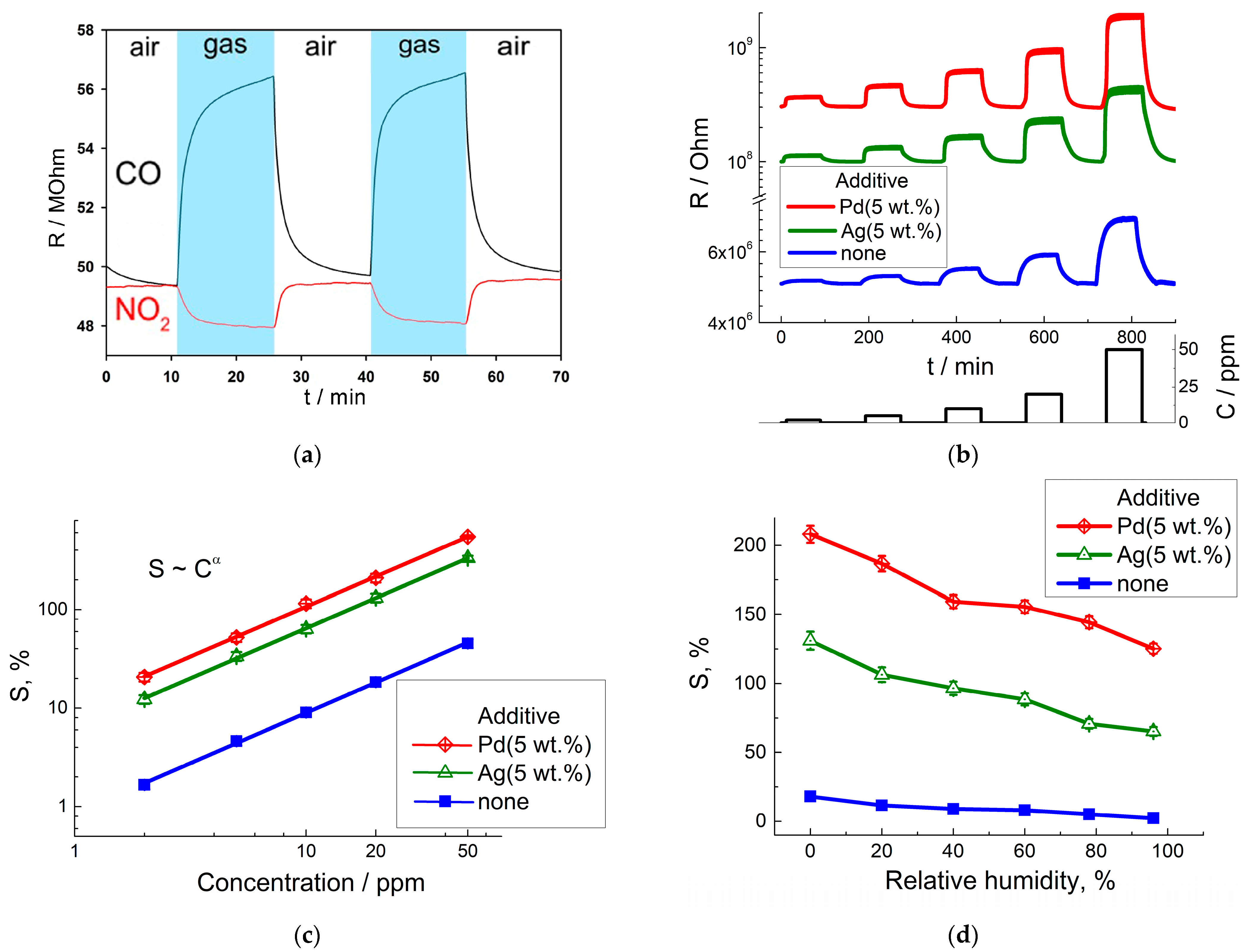
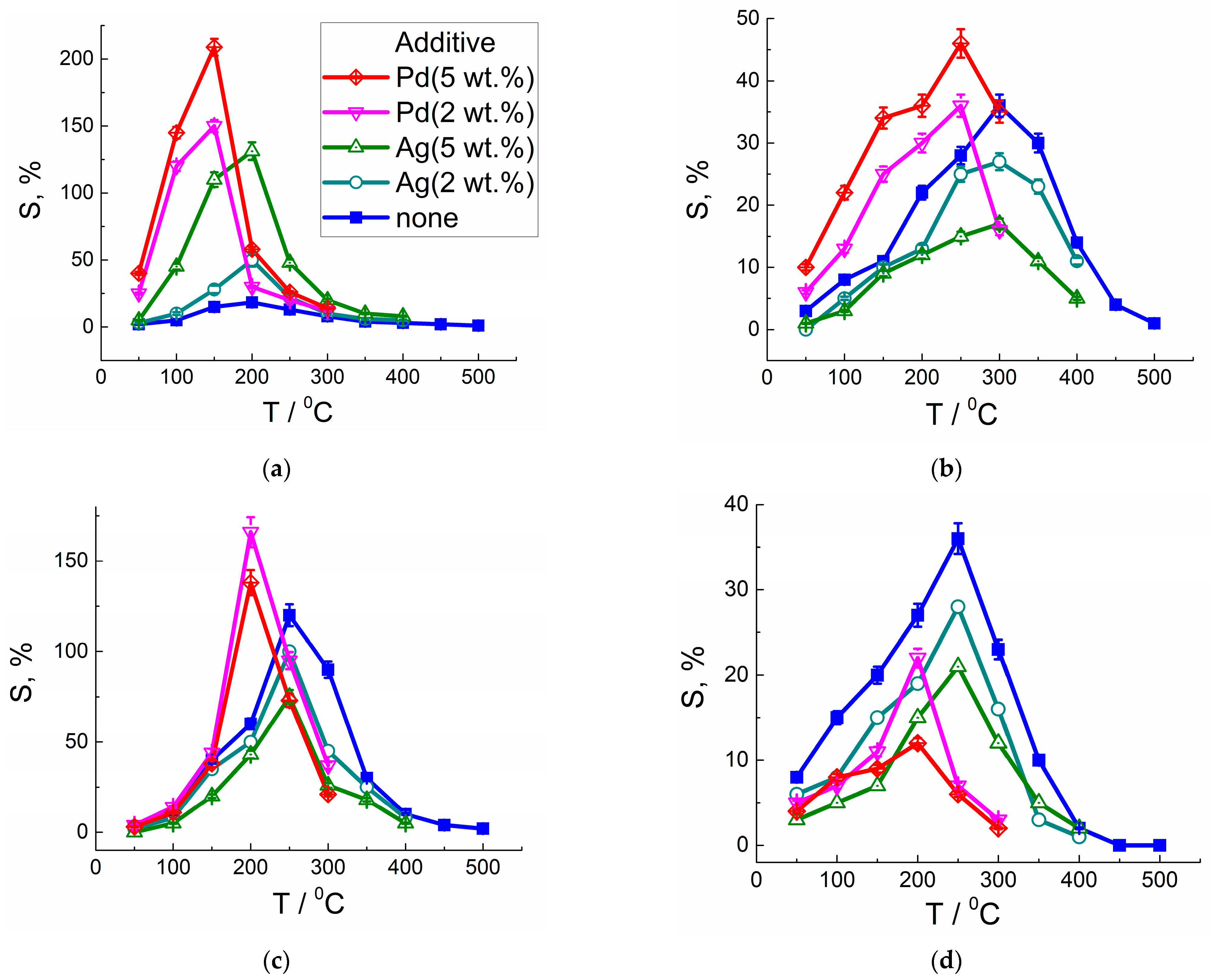
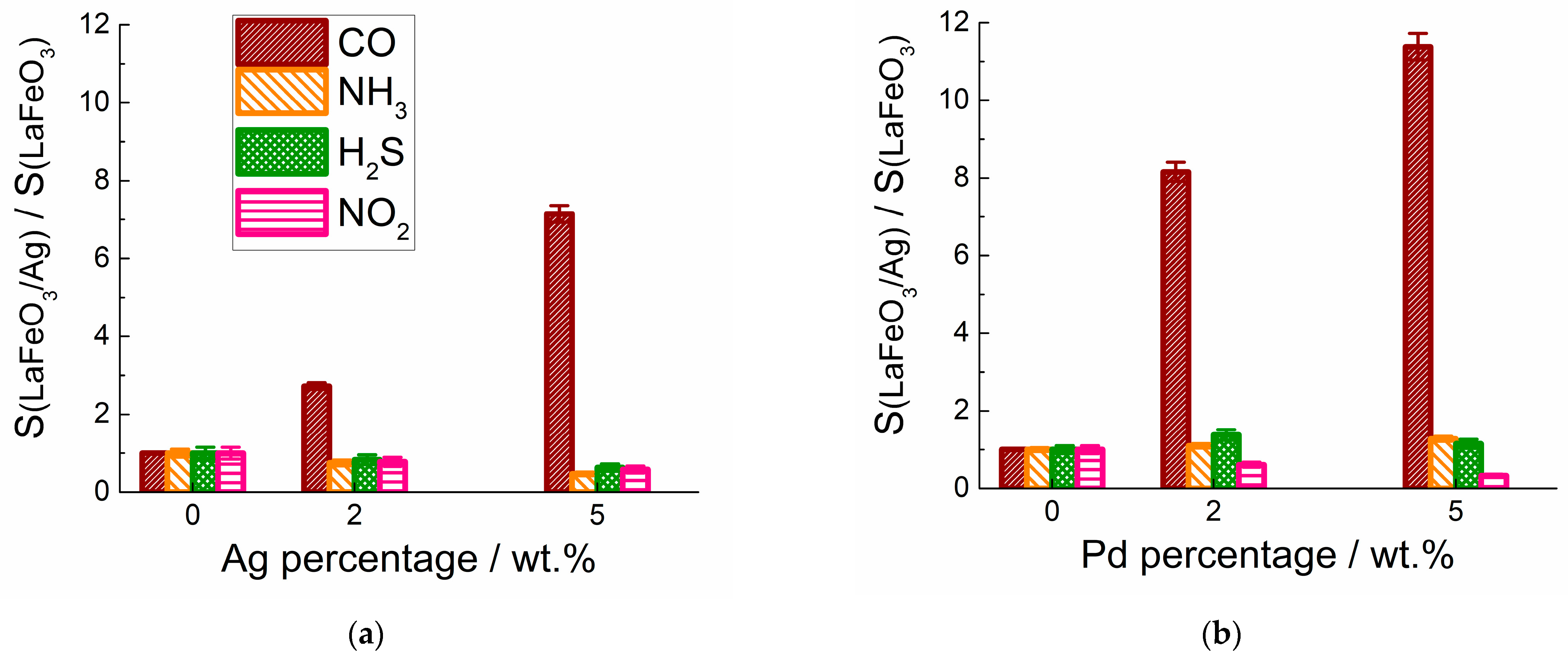
3.3. Electrophysical and Gas-Sensing Properties
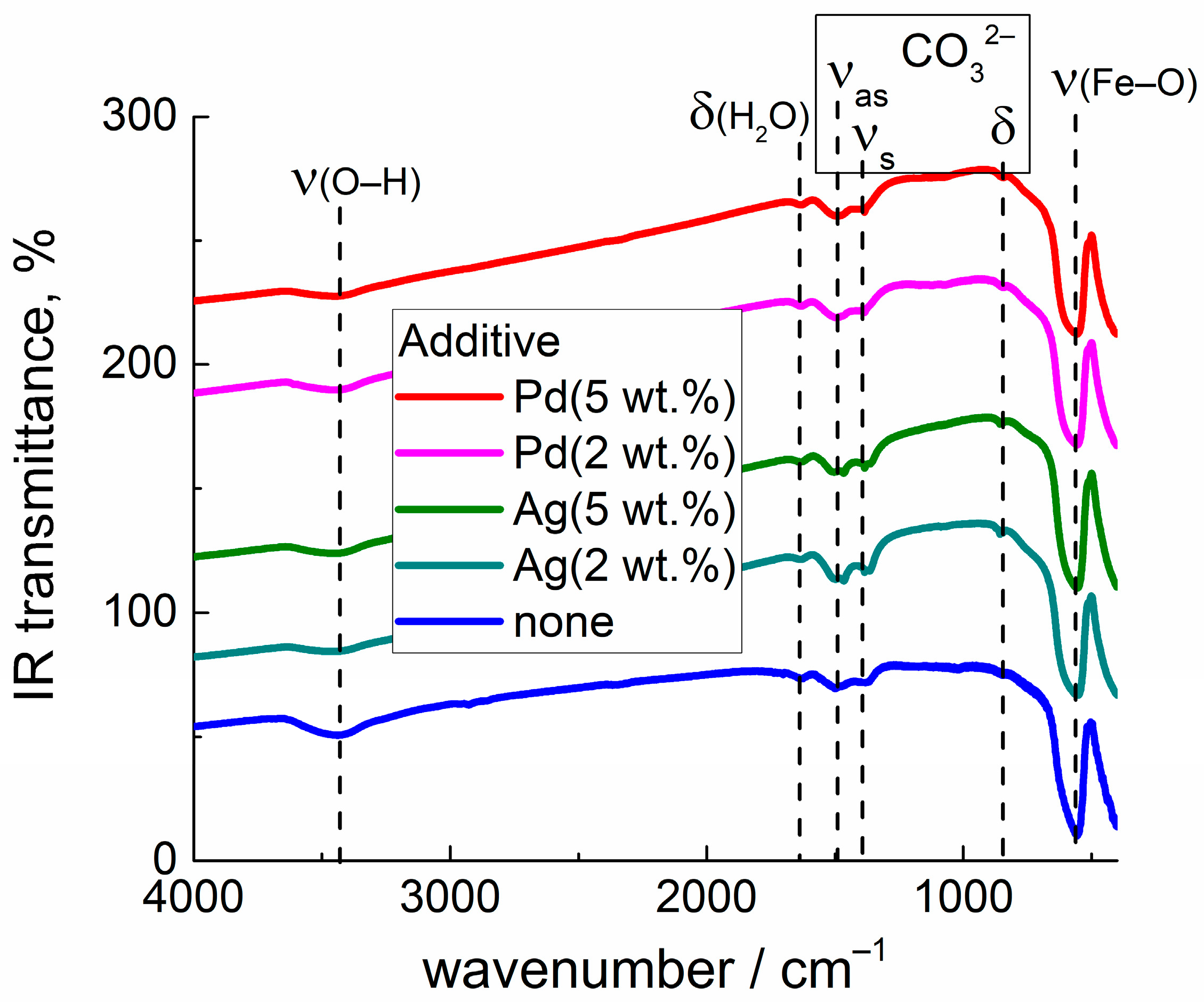
3.4. DRIFT Spectroscopy Study of Material Interaction with CO
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopel, W. Chemisorption and charge transfer at ionic semiconductor surfaces: Implications in designing gas sensors. Prog. Surf. Sci. 1985, 20, 9–103. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Shankar, P.; Bosco, J.; Rayappan, B. Gas sensing mechanism of metal oxides: The role of ambient atmosphere, type of semiconductor and gases—A review. ScienceJet 2015, 4, 126. [Google Scholar]
- Enhessari, M.; Salehabadi, A. Perovskite-based materials for chemical sensors. In Progresses in Chemical Sensor; Wang, W., Ed.; IntechOpen: London, UK, 2016; pp. 59–91. [Google Scholar]
- Fergus, J.W. Perovskite oxides for semiconductor-based gas sensors. Sens. Actuators B Chem. 2007, 123, 1169–1179. [Google Scholar] [CrossRef]
- Pena, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G. (Eds.) Properties and Applications of Perovskite-Type Oxides, 1st ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 241–267. [Google Scholar]
- Balamurugan, C.; Song, S.-J.; Kim, H.-S. Enhancing Gas Response Characteristics of Mixed Metal Oxide Gas Sensors. J. Korean Ceram. Soc. 2018, 55, 1–20. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, S.; Ge, M.; Zhang, Z.; Huang, J.; Tang, Y.; Sun, L.; Lin, C.; Lai, Y. Rational Construction of LaFeO3 Perovskite Nanoparticle-Modified TiO2 Nanotube Arrays for Visible-Light Driven Photocatalytic Activity. Coatings 2018, 8, 374–384. [Google Scholar] [CrossRef]
- Wheeler, G.; Choi, K.-S. Photoelectrochemical Properties and Stability of Nanoporous p-Type LaFeO3 Photoelectrodes Prepared by Electrodeposition. ACS Energy Lett. 2017, 2, 2378–2382. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Lu, C.; Li, D.; Li, Z.; Gao, J.; Wei, J.; Li, K. Enhanced performance of LaFeO3 oxygen carriers by NiO for chemical looping partial oxidation of methane. Fuel Process. Technol. 2022, 236, 107396. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, B.; Li, Y.; Xu, X.; Sun, G.; Cao, J.; Wang, Y. Synthesis of spindle-like Co-doped LaFeO3 porous microstructure for high performance n-butanol sensor. Sens. Actuators B Chem. 2021, 343, 130125. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W.-L.; Cai, Z.-X.; Li, Y.-K.; Yamauchi, Y.; Guo, X. LaFeO3 porous hollow micro-spindles for NO2 sensing. Ceram. Int. 2019, 45, 5240–5248. [Google Scholar] [CrossRef]
- Bai, S.; Shi, B.; Ma, L.; Yang, P.; Liu, Z.; Li, D.; Chen, A. Synthesis of LaFeO3 catalytic materials and their sensing properties. Sci. China Ser. B Chem. 2009, 52, 2106–2113. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, T.; Xu, X.; Lu, N. Fabrication of N-type Fe2O3 and P-type LaFeO3 nanobelts by electrospinning and determination of gas-sensing properties. Sens. Actuators B Chem. 2011, 153, 83–88. [Google Scholar] [CrossRef]
- Queralto, A.; Graf, D.; Frohnhoven, R.; Fischer, T.; Vanrompay, H.; Bals, S.; Bartasyte, A.; Mathur, S. LaFeO3 nanofibers for high detection of sulfur-containing gas. ACS Sustain. Chem. Eng. 2019, 7, 6023–6032. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Lin, Y.T.; Chen, J.L.; Zhang, J.; Zhu, Z.Q.; Liu, Q.J. A high sensitivity gas sensor for formaldehyde based on silver doped lanthanum ferrite. Sens. Actuators B Chem. 2014, 190, 171–176. [Google Scholar] [CrossRef]
- Wang, C.; Rong, Q.; Zhang, Y.; Hu, J.; Zi, B.; Zhu, Z.; Zhang, J.; Liu, Q. Molecular imprinting Ag-LaFeO3 spheres for highly sensitive acetone gas detection. Mater. Res. Bull. 2019, 109, 265–272. [Google Scholar] [CrossRef]
- Alharbi, A.A.; Sackmann, A.; Weimar, U.; Barsan, N. Acetylene- and Ethylene-Sensing Mechanism for LaFeO3-Based Gas Sensors: Operando Insights. J. Phys. Chem. C 2020, 124, 7317–7326. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Mastelaro, V.R.; Ponpandian, N. Surface Morphology-Dependent Room-Temperature LaFeO3 Nanostructure Thin Films as Selective NO2 Gas Sensor Prepared by Radio Frequency Magnetron Sputtering. ACS Appl. Mater. Interfaces 2014, 6, 13917–13927. [Google Scholar] [CrossRef]
- Ding, J.-C.; Li, H.-Y.; Cai, Z.-X.; Wang, X.-X.; Guo, X. Near room temperature CO sensing by mesoporous LaCoO3 nanowires functionalized with Pd nanodots. Sens. Actuators B Chem. 2016, 222, 517–524. [Google Scholar] [CrossRef]
- Navale, S.; Shahbaz, M.; Mirzaei, A.; Kim, S.S.; Kim, H.W. Effect of Ag Addition on the Gas-Sensing Properties of Nanostructured Resistive-Based Gas Sensors: An Overview. Sensors 2021, 21, 6454. [Google Scholar] [CrossRef]
- Thirumalairajan, S.; Girija, K.; Hebalkar, N.Y.; Mangalaraj, D.; Viswanathan, C.; Ponpandian, N. Shape evolution of perovskite LaFeO3 nanostructures: A systematic investigation of growth mechanism, properties and morphology dependent photocatalytic activities. RSC Adv. 2013, 3, 7549–7561. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Liu, X.; Li, S.; Zhao, M. XPS study on O(1s) and Fe(2p) for nanocrystalline composite oxide LaFeO3 with the perovskite structure. Mater. Chem. Phys. 1994, 38, 355–362. [Google Scholar] [CrossRef]
- Ferraria, A.M.; Carapeto, A.P.; Botelho do Rego, A.M. X-ray photoelectron spectroscopy: Silver salts revisited. Vacuum 2012, 86, 1988–1991. [Google Scholar] [CrossRef]
- Cao, K.; Cao, E.; Zhang, Y.; Hao, W.; Sun, L.; Peng, H. The influence of nonstoichiometry on electrical transport and ethanol sensing characteristics for nanocrystalline LaFexO3−δ sensors. Sens. Actuators B Chem. 2016, 230, 592–599. [Google Scholar] [CrossRef]
- Sharma, N.; Kushwaha, H.S.; Sharma, S.K.; Sachdev, K. Fabrication of LaFeO3 and rGO-LaFeO3 microspheres based gas sensors for detection of NO2 and CO. RSC Adv. 2020, 10, 1297–1308. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I.; Vayssilov, G.N. Characterization of Oxide Surfaces and Zeolites by Carbon Monoxide as an IR Probe Molecule. Adv. Catal. 2002, 47, 307–511. [Google Scholar]
- Koferstein, R.; Jager, L.; Ebbinghaus, S.G. Magnetic and optical investigations on LaFeO3 powders with different particle sizes and corresponding ceramics. Solid State Ion. 2013, 249–250, 1–5. [Google Scholar] [CrossRef]
- Seo, J.W.; Fullerton, E.E.; Nolting, F.; Scholl, A.; Fompeyrine, J.; Locquet, J.-P. Antiferromagnetic LaFeO3 thin films and their effect on exchange bias. J. Phys. Condens. Matter 2008, 20, 264014. [Google Scholar] [CrossRef]
- Smolin, S.Y.; Choquette, A.K.; Wilks, R.G.; Gauquelin, N.; Félix, R.; Gerlach, D.; Ueda, S.; Krick, A.L.; Verbeeck, J.; Bär, M.; et al. Energy Level Alignment and Cation Charge States at the LaFeO3/LaMnO3 (001) Heterointerface. Adv. Mater. Interfaces 2017, 4, 1700183. [Google Scholar] [CrossRef]
- Hong, J.-P.; Park, A.-Y.; Lee, S.; Kang, J.; Shin, N.; Yoon, D.Y. Tuning of Ag work functions by self assembled monolayers of aromatic thiols for an efficient hole injection for solution processed triisopropylsilylethynyl pentacene organic thin film transistors. Appl. Phys. Lett. 2008, 92, 143311. [Google Scholar] [CrossRef]
- Magari, Y.; Makino, H.; Hashimoto, S.; Furuta, M. Origin of work function engineering of silver oxide for an In–Ga–Zn–O Schottky diode. Appl. Surf. Sci. 2020, 512, 144519. [Google Scholar] [CrossRef]
- Jaeckel, R.; Wagner, B. Photo-electric measurement of the work function of metals and its alteration after gas adsorption. Vacuum 1963, 13, 509–511. [Google Scholar] [CrossRef]
- OSHA. Occupational Chemical Database. Available online: https://www.osha.gov/chemicaldata/462 (accessed on 9 December 2022).
- Toan, N.; Saukko, S.; Lantto, V. Gas sensing with semiconducting perovskite oxide LaFeO3. Phys. B Condens. Mater. 2003, 327, 279–282. [Google Scholar] [CrossRef]
- Murade, P.A.; Sangawar, V.S.; Chaudhari, G.N.; Kapse, V.D.; Bajpeyee, A.U. Acetone gas-sensing performance of Sr-doped nanostructured LaFeO3 semiconductor prepared by citrate sol-gel route. Curr. Appl. Phys. 2011, 11, 451–456. [Google Scholar] [CrossRef]
- Liu, H.; Sun, H.; Xie, R.; Zhang, X.; Zheng, K.; Peng, T.; Wuc, X.; Zhang, Y. Substrate-dependent structural and CO sensing properties of LaCoO3 epitaxial films. Appl. Surf. Sci. 2018, 442, 742–749. [Google Scholar] [CrossRef]
- Marikutsa, A.; Yang, L.; Rumyantseva, M.; Batuk, M.; Hadermann, J.; Gaskov, A. Sensitivity of nanocrystalline tungsten oxide to CO and ammonia gas determined by surface catalysts. Sens. Actuators B Chem. 2018, 277, 336–346. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Gaskov, A. Specific Interaction of PdOx- and RuOy-Modified Tin Dioxide with CO and NH3 Gases: Kelvin Probe and DRIFT Studies. J. Phys. Chem. C 2015, 119, 24342–24350. [Google Scholar] [CrossRef]
- Moltved, K.A.; Kepp, K.P. The Chemical Bond between Transition Metals and Oxygen: Electronegativity, d-Orbital Effects, and Oxophilicity as Descriptors of Metal−Oxygen Interactions. J. Phys. Chem. C 2019, 123, 18432–18444. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Mahajan, S.; Jagtap, S. Metal-oxide semiconductors for carbon monoxide (CO) gas sensing: A review. Appl. Mater. Today 2020, 18, 100483. [Google Scholar] [CrossRef]
- Degler, D.; Wicker, S.; Weimar, U.; Barsan, N. Identifying the Active Oxygen Species in SnO2 Based Gas Sensing Materials: An Operando IR Spectrsocopy Study. J. Phys. Chem. C 2015, 119, 11792–11799. [Google Scholar] [CrossRef]
- Staerz, A.; Weimar, U.; Barsan, N. Current state of knowledge on the metal oxide based gas sensing mechanism. Sens. Actuators B Chem. 2022, 358, 131531. [Google Scholar] [CrossRef]
- Yang, C.; Grimaud, A. Factors Controlling the Redox Activity of Oxygen in Perovskites: From Theory to Application for Catalytic Reactions. Catalysts 2017, 7, 149. [Google Scholar] [CrossRef]
- Sadovskaya, E.M.; Ivanova, Y.A.; Pinaeva, L.G.; Grasso, G.; Kuznetsova, T.G.; van Veen, A.; Sadykov, V.A.; Mirodatos, C. Kinetics of Oxygen Exchange over CeO2-ZrO2 Fluorite-Based Catalysts. J. Phys. Chem. A 2007, 111, 4498–4505. [Google Scholar] [CrossRef]
- Martin, D.; Duprez, D. Mobility of Surface Species on Oxides. 1. Isotopic Exchange of 18O2 with 16O of SiO2, Al2O3, ZrO2, MgO, CeO2, and CeO2-Al2O3. Activation by Noble Metals. Correlation with Oxide Basicity. J. Phys. Chem. 1996, 100, 9429–9438. [Google Scholar] [CrossRef]
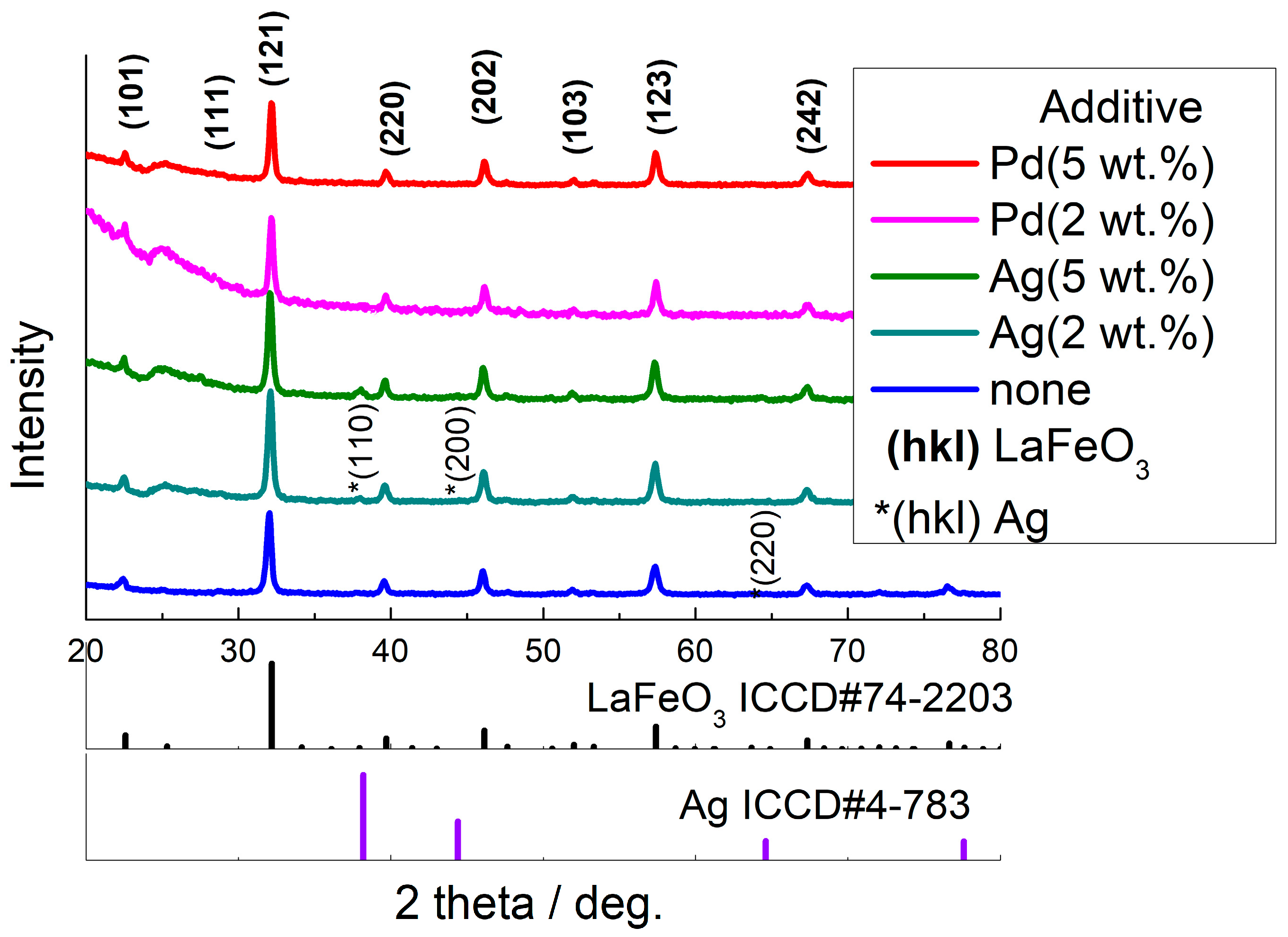

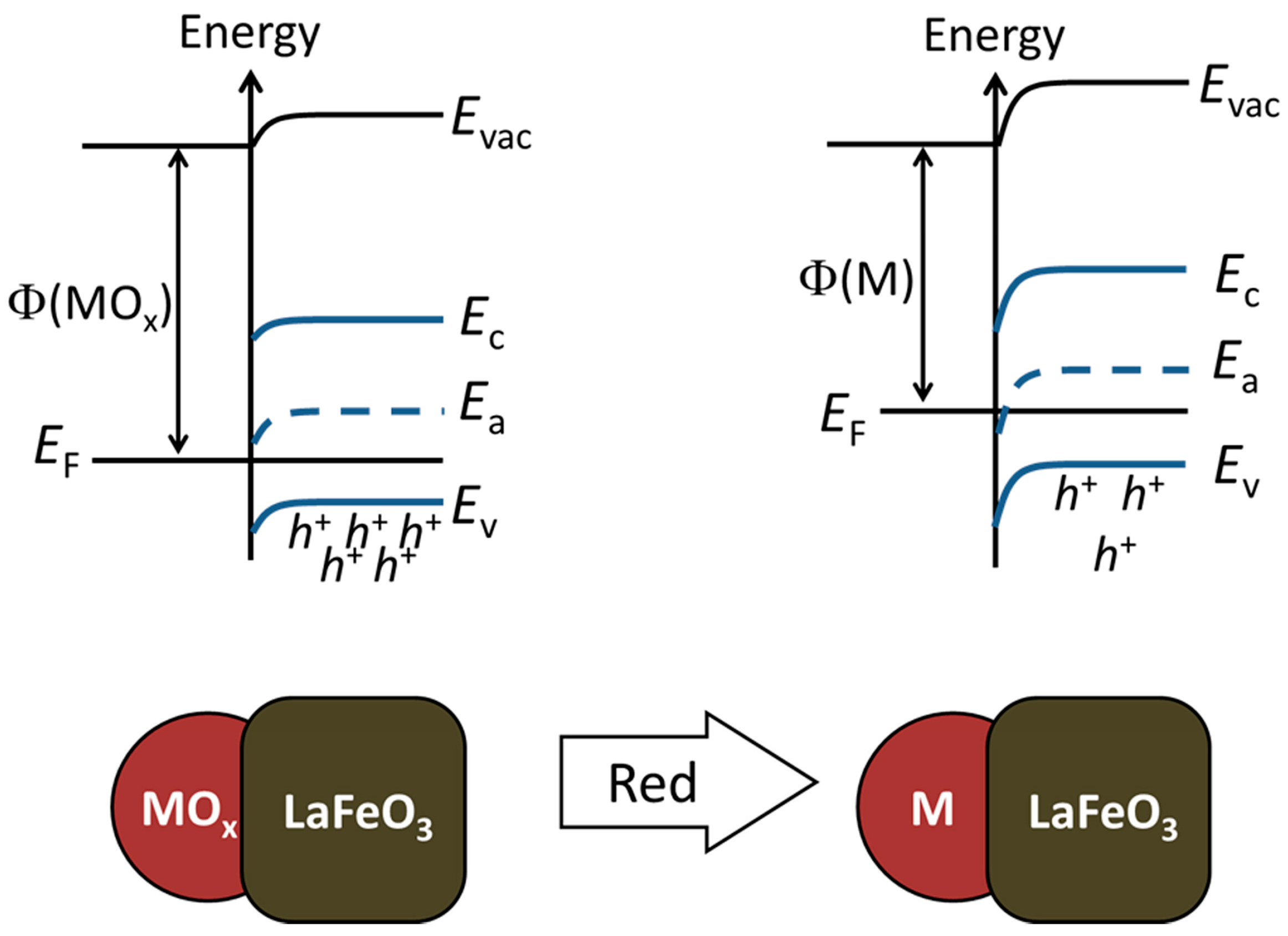
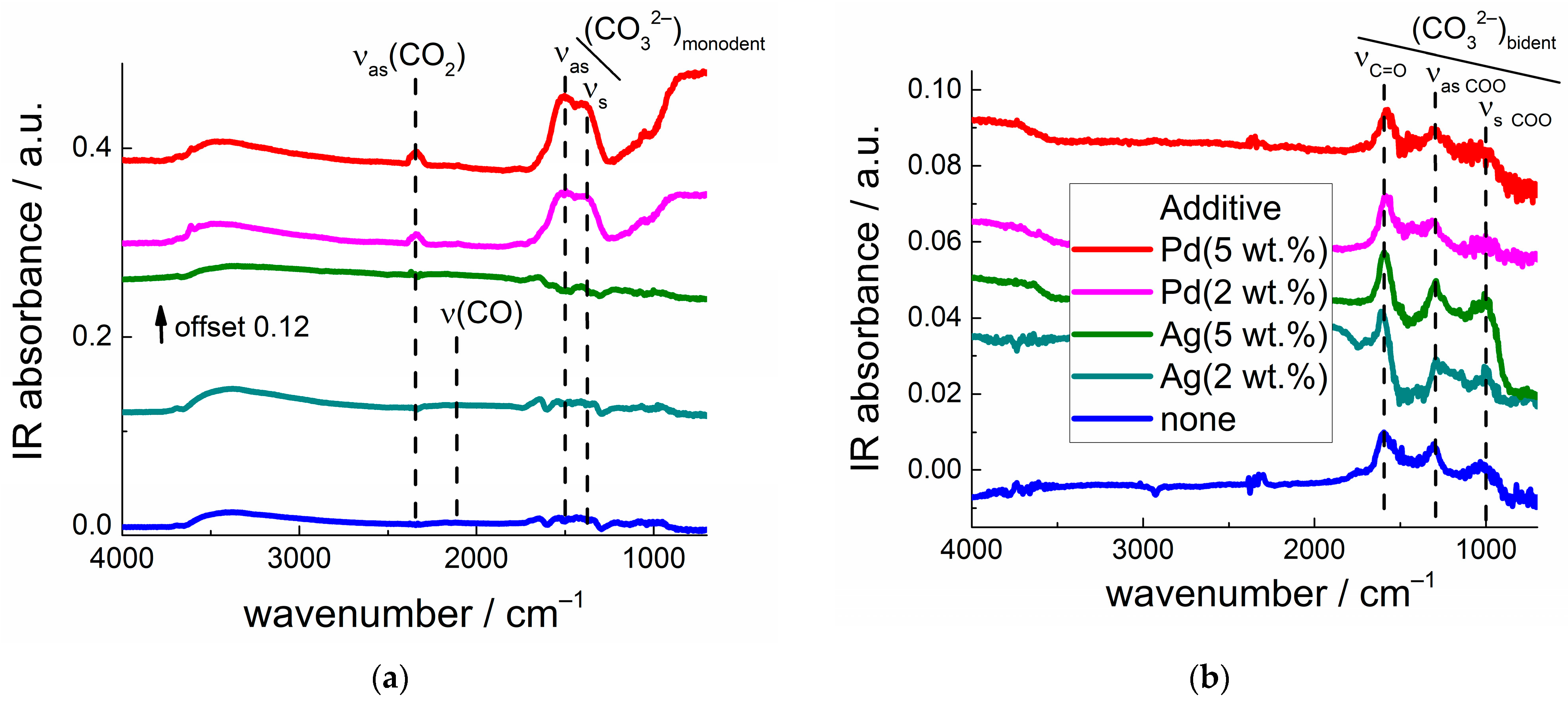
| Sample | Percentage of Additive, wt.% | Crystallite Size (dXRD), nm | BET Area, m2/g |
|---|---|---|---|
| LaFeO3 | - | 23 ± 4 | 8 |
| LaFeO3/Ag(2 wt.%) | 2.1 ± 0.2 Ag | 24 ± 4 LaFeO3; 37 ± 6 Ag | 6 |
| LaFeO3/Ag(5 wt.%) | 4.9 ± 0.3 Ag | 23 ± 4 LaFeO3; 32 ± 5 Ag | 6 |
| LaFeO3/Pd(2 wt.%) | 2.2 ± 0.2 Pd | 24 ± 5 LaFeO3 | 7 |
| LaFeO3/Pd(5 wt.%) | 5.1 ± 0.4 Pd | 23 ± 4 LaFeO3 | 6 |
| Sample | Fraction from Total Content of the Element, at.% | ||||
|---|---|---|---|---|---|
| Mn+ | M0 | O(I) | O(II) | O(III) | |
| LaFeO3 | - | - | 18 | 52 | 30 |
| LaFeO3/Ag(2 wt.%) | 24 (Ag+) | 76 (Ag0) | 17 | 57 | 26 |
| LaFeO3/Ag(5 wt.%) | 32 (Ag+) | 68 (Ag0) | 12 | 60 | 29 |
| LaFeO3/Pd(2 wt.%) | 63 (Pd2+) | 37 (Pd0) | 15 | 43 | 42 |
| LaFeO3/Pd(5 wt.%) | 66 (Pd2+) | 34 (Pd0) | 16 | 38 | 46 |
| Sample | Activation Energy of Conduction, eV | |
|---|---|---|
| Lower Temperature | Higher Temperature | |
| LaFeO3 | 0.55 ± 0.02 | 0.13 ± 0.02 |
| LaFeO3/Ag(2 wt.%) | 0.61 ± 0.02 | |
| LaFeO3/Ag(5 wt.%) | 0.66 ± 0.03 | |
| LaFeO3/Pd(2 wt.%) | 0.61 ± 0.02 | |
| LaFeO3/Pd(5 wt.%) | 0.63 ± 0.02 | |
| Material | Synthesis Method | Morphology | CO Concentration, ppm | Operating T, °C | Sensor Signal, % | Ref. |
|---|---|---|---|---|---|---|
| LaFeO3 | Sol–gel | Nanowires | 50 | 250–270 | 25 | [36] |
| rGO/LaFeO3 | Hydrothermal | Microspheres | 5 | 250 | 17 | [27] |
| La1-xMgxFeO3 | Sol–gel | Nanofibers | 2500 | 350 | 4000 | [16] |
| La1-xSrxFeO3 | Microspheres | 50 | 400 | 2.5 | [37] | |
| LaFeO3 | Coprecipitation | 3D porous | 1000 | 155 | 10 | [14] |
| LaCoO3 | PAD deposition | Thin films | 100 | 150 | 55–80 | [38] |
| LaCoO3/Pd | Electrospinning | Nanowires | 100 | 250 | 113 | [21] |
| LaFeO3/Ag | Sol–gel | Nanoparticles | 20 | 200 | 130 | This work |
| LaFeO3/Pd | 150 | 210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chumakova, V.; Marikutsa, A.; Platonov, V.; Khmelevsky, N.; Rumyantseva, M. Distinct Roles of Additives in the Improved Sensitivity to CO of Ag- and Pd-Modified Nanosized LaFeO3. Chemosensors 2023, 11, 60. https://doi.org/10.3390/chemosensors11010060
Chumakova V, Marikutsa A, Platonov V, Khmelevsky N, Rumyantseva M. Distinct Roles of Additives in the Improved Sensitivity to CO of Ag- and Pd-Modified Nanosized LaFeO3. Chemosensors. 2023; 11(1):60. https://doi.org/10.3390/chemosensors11010060
Chicago/Turabian StyleChumakova, Valentina, Artem Marikutsa, Vadim Platonov, Nikolay Khmelevsky, and Marina Rumyantseva. 2023. "Distinct Roles of Additives in the Improved Sensitivity to CO of Ag- and Pd-Modified Nanosized LaFeO3" Chemosensors 11, no. 1: 60. https://doi.org/10.3390/chemosensors11010060
APA StyleChumakova, V., Marikutsa, A., Platonov, V., Khmelevsky, N., & Rumyantseva, M. (2023). Distinct Roles of Additives in the Improved Sensitivity to CO of Ag- and Pd-Modified Nanosized LaFeO3. Chemosensors, 11(1), 60. https://doi.org/10.3390/chemosensors11010060