Abstract
Flexible wearable sensors show great potential for applications in wearable devices, remote health monitoring, artificial intelligence, soft robotics, and artificial skin due to their stretchability, bendability, thinness and portability, and excellent electrical properties. Hydrogels have tunable mechanical properties, excellent biocompatibility, and flexibility, making them attractive candidates for wearable flexible sensors. Among them, tremendous efforts have focused on the advancement of chitosan-based hydrogels (CS-Gels) to realize multifunctional wearable sensing by modifying hydrogel networks with additives/nanofillers/functional groups. Recently, remarkable progress has been made in flexible wearable sensors. Herein, this review summarizes recent advances in CS-Gels wearable sensors for applications such as human motion monitoring, health monitoring, human-machine interface and soft robotics. Representative synthesis methods and strategies for CS-Gels are briefly described, the problems and deficiencies of CS-Gels for wearable sensors are discussed. Finally, the possible opportunities and challenges for the future development of CS-Gels flexible wearable devices are proposed.
1. Introduction
Flexible wearable sensors are receiving increasing attention for their great potential in personalized health monitoring, human-machine interfaces, clinical diagnosis, soft robotics, and electronic skin [1,2,3]. Wearable sensors can convert external mechanical stimuli into easily detectable electrical signals with portability, high flexibility, fast response, and good compliance. Thin, lightweight, and portable flexible sensors facilitate the monitoring of health and exercise information, which is conducive to the timely detection, prevention, or rehabilitation of diseases. Currently, flexible wearable sensors have made remarkable progress and have been widely used for the detection of pulse, motion, temperature and biochemical parameters [4,5,6]. However, there are still many problems to be solved in the development of flexible wearable sensors. The conductive functional materials for flexible sensors mainly include carbon-based nanomaterials, liquid metals, and conductive polymers. Most of the conductive functional materials have complicated preparation processes, which increase the manufacturing cost of flexible sensors. In recent decades, many efforts have been made in designing high-performance electronic sensing materials [7,8,9,10]. In this regard, hydrogel-based flexible wearable sensing materials are ideal candidates for the fabrication of wearable sensors by easily tuning the mechanical properties, electrical conductivity, network structure, and biochemical properties of hydrogels.
Chitosan (CS) is the only alkaline polysaccharide present in nature, which is deacetylated from chitin, and has attracted great interest in the biomedical field due to biocompatibility, non-toxicity, biodegradability, antimicrobial ability and safety. Notably, the large number of hydroxyl and amino groups in the molecular structure of chitosan can lead to the development of physical/chemical crosslinked CS-Gels in different fields. The main physical interactions that form CS-Gels mainly include molecular entanglement, van der Walls forces, electrostatic forces, hydrogen bonds, interpolymer complexes, ionic and hydrophobic linkages [11,12]. However, physically crosslinked CS-Gels also pose some problems, such as lack of elastic modulus, tensile strength, and toughness. Moreover, a variety of chemical interactions can occur between the -OH and -NH2 groups on CS and the function groups in the chemical crosslinkers. Among them, crosslinking agents are molecules with at least two reactive groups that allow for the formation of bridges between polymer chains. Currently, the most common chemical crosslinkers for the manufacture of CS-Gels are dialdehydes (e.g., glutaraldehyde, acetaldehyde) and epoxides (e.g., epichlorohydrin, ethylene glycol diglycidyl ether, genipin) [13,14,15]. The physicochemical structure and mechanical properties of physically crosslinked CS-Gels can be easily tuned by modifying parameters such as the type and concentration of CS crosslinking agents. In addition, CS-Gels also exhibit unique porous structures, high capacitance and flexibility and are considered as promising materials for developing flexible wearable sensors. Over the past few years, many reinforcing substances, such as metal nanomaterials, carbon-based materials, and polymers, have been incorporated into CS, and the resulting CS-Gels have significantly improved in flexible wearable sensors [16,17,18].
Recently, with the increasing popularity of CS-based materials, many excellent reviews have been published on the subject. However, most of them focus on the fields of biomedicine, adsorbents, environmental remediation and food engineering [19,20,21,22]. Thus, a comprehensive and undated review of CS-Gels based flexible wearable sensors is still lacking. Herein, this article reviews the research on the application of CS-Gels in flexible wearable sensors. Firstly, the chemical structure and physicochemical properties of CS-Gels are introduced, and the synthetic strategies and design principles of CS-Gels for wearable sensors are summarized. The research and application of flexible wearable sensors based on CS-Gels in different fields are mainly discussed. Finally, the challenges and development directions of CS-Gels based flexible wearable sensors are summarized and prospected.
2. Structure and Properties of CS
Chitosan is the product of N-deacetylation of chitin, which is the most basic and important derivative of chitin. Chitin, also called β-(1,4)-2-acetamido-2-deoxy-D-glucose, widely exists in the shells of crustaceans such as shrimps, carbs and insects, as well as in the cell walls of fungi and algae. Chitin and chitosan have been intensively used in many fields including biomedical materials, food additives, environmental protection, agriculture, cosmetics, medical treatment and drug development due to their biodegradability, biocompatibility and antibacterial abilities [23,24,25,26]. The annual biosynthesis of chitin on earth amounts to billions of tons, and it is a natural polymer compound whose output is second only to cellulose in production. Chitin, chitosan and cellulose have similar chemical structures, with fiber being hydroxyl at the C2 position, and chitin and chitosan are replaced by an acetylamino and amino group at the C2, respectively [27,28]. They are all polymers of six-carbon sugars with a molecular weight of more than one million. The sources and chemical structures of chitin and chitosan are shown in Figure 1.
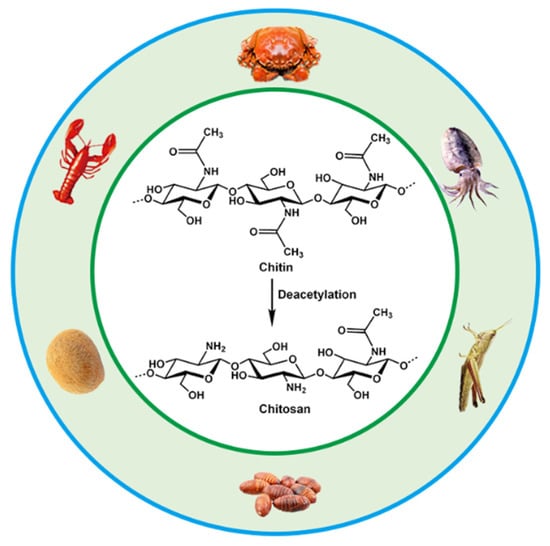
Figure 1.
Sources and chemical structures of chitin and chitosan.
The amino group in the molecular structure of CS is more reactive than the acetylamino group in the chitin molecule, which gives CS unique properties and enables chemical modification. CS exhibits a double helix structure with a helical pitch of 0.515 nm, and six sugar residues form a helical plane [27]. The amino groups, hydroxyl groups and N-acetylamino groups on the molecular chain of CS involved in the formation of intramolecular and intermolecular hydrogen bonds, forming the secondary structure of CS macromolecules. CS molecules are more likely to form crystalline regions because of the large number of hydrogen bonds, thus having high crystallinity and good physical and mechanical properties such as adsorption, film-forming, fiber-forming and moisturizing [29,30,31,32].
3. Design and Preparation of CS Hydrogels
Polymer hydrogels are highly water-swellable three-dimensional (3D) crosslinked networks that can rapidly swell in water and retain a large volume of water without dissolving in this swollen state [33,34]. The amount of water absorbed is closely related to the crosslinking density, the higher the degree of crosslinking, the lower the water absorption. This property of polymer hydrogels is similar to biological soft tissues, showing good physicochemical and biological properties, has wide application prospects in the controlled release of drugs, bioadhesion and biodegradable materials [35,36]. Chitosan acidic aqueous solution will immediately form a hydrogel when it encounters an alkaline environment, which is greatly affected by environmental pH. Therefore, the conventional preparation methods of CS hydrogels include physical crosslinking, chemical crosslinking and radiation crosslinking. In addition, the electrochemical deposition method developed in recent years for the preparation of CS gels has the characteristics of simple, rapid, non-polluting, reversible and controllable process, and is an economical and green new method for preparing chitosan gel [37,38,39,40].
3.1. Physically Crosslinked CS Hydrogels
Physically crosslinked hydrogels are mainly formed by crosslinking 3D network structures by physical interactions such as intermolecular entanglement, hydrogen bonds, hydrophobic interaction, van der Waals forces, coordination bonds, and ionic bonds [41,42,43,44]. Physically crosslinked hydrogels can be regarded as reversible hydrogels, and changes in physical states, such ionic strength, pH, temperature, stress, and solutes, could disrupt the structure of gels. Smart hydrogels with good biocompatibility and environmental responsiveness could be obtained under mild experimental conditions, which has become one of the current research hotspots [45,46,47].
Physically crosslinked hydrogels are generally prepared by the following methods: (1) Freeze-thaw cycles: hydrogels are prepared by microcrystals formed by repeated freeze-thaw processes [48,49]. (2) Ionic action: the principle of which is to use the electrostatic action of ions to form hydrogels. This method aggregates polyvalent ions with opposite charges in a polyelectrolyte solution to form a hydrogel [50,51]. The polycationic effect of CS and polyanionic polymers such as polyacrylic acid and alginate form hydrogels through electrostatic attraction between anions and cations. (3) Hydrogen bonding: the most common crosslinking method in physically crosslinked hydrogels [52,53,54]. The formed hydrogels are usually reversible, and hydrogen bonds are easily destroyed in salt solutions. (4) Hydrophobic interaction: when the polarity of the polymer solution changes, it will affect the hydrophobic interaction between molecular chains [55,56]. When this physical interaction increased, it can promote the self-assembly of molecules to form a gel. The solvent has a great dependence on hydrophobic interactions and affect the degree of crosslinking of polymer chains. Wearable strain sensors were prepared by dynamic physical crosslinking of polyacrylic acid, CS, and graphene oxide in a mixed solvent of water and glycerol [57], as shown in Figure 2a. The physical crosslinked CS gel had high stretchability (over 1000%), anti-freeze (use temperature range −20~70 °C), and excellent sensing performance (response time 40 ms).
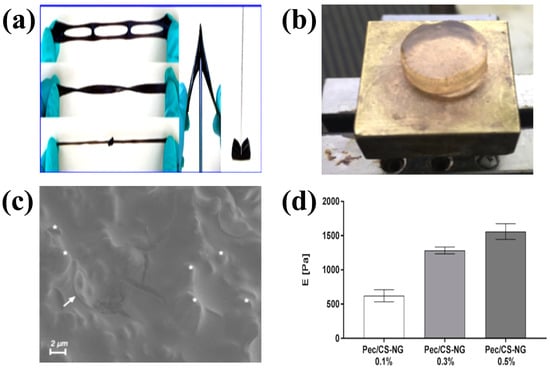
Figure 2.
Physically crosslinked CS. (a) Photographs of wearable CS hydrogels prepared by dynamic physical crosslinking; (b) Polysaccharide-based self-healing hydrogels; (c) Cryo-SEM images of CS nanogels; (d) Mechanical properties of CS nanogels. (a) Reproduced with permission from ref. [57]. Copyright Royal Society of Chemistry. (b–d) Adapted with permission from ref. [58]. Copyright 2019 Elsevier.
Self-healing hydrogels can be obtained by using the physical interaction between two polysaccharide chains of soluble pectin and CS (Figure 2b) [58]. Cryo-SEM revealed the presence of nanogels in the crosslinked matrix (Figure 2c). Due to the dynamic interaction between the pectin chains and the CS nanogels, the formed networks dissociated under the applied shear, allowing the hydrogel to flow. When the applied shear was removed, the storage modulus of the hydrogel can be quickly and fully recovered. The Young’s modulus of the hydrogels increased with increasing CS gel content (Figure 2d) indicating that higher crosslinking led to higher strength of the hydrogel.
3.2. Chemically Crosslinked CS Hydrogels
The chemical crosslinking method refers to the CS molecular chains involved in network through covalent bonds in the presence of crosslinking agents. Usually, glutaraldehyde (GA) [59,60,61], epichlorohydrin [62,63], formaldehyde [64,65], and genipin [14,66,67,68], are used as chemical crosslinking agents to form new covalent bonds at the crosslinking sites, so the chemical crosslinking process is generally irreversible and has good stability. Chemical crosslinking is an important method for preparing CS gels because it enhances the physical and mechanical properties of CS. The CS molecular chain contains a large number of hydrophilic groups, especially the amino group at the C2 position is often used as a crosslinking point, which can chemically interact with the functional groups on the crosslinking agent to form a crosslinking network [69,70]. To date, dialdehyde, especially glutaraldehyde (GA), are the most studied and prevalent crosslinkers for chemically modified chitosan. Dialdehyde formyl groups react with amino groups in CS to form covalent imine bonds. The semi-interpenetrating polymer network (IPN) hydrogel was prepared by crosslinking chitosan-polyvinylpyrrolidone with GA, and the gel showed good pH responsiveness [71]. In the presence of GA, crosslinked chitosan gels grafted with aminopropylsilane graphene oxide (GO) were prepared by sol-gel in acidic medium. The gel has high surface active sites and low swelling properties [72]. Khapre et al. modified chitosan with aniline in the presence of formaldehyde, and then further crosslinked chitosan with alginate using GA to obtain biocomposite gels [59]. Bilal et al. used 2.0% (v/v) GA to functionalize for 3 h in order to produce a fungal laccase-chitosan biocatalyst. This study demonstrates the efficient binding of laccase on the biopolymer network of glutaraldehyde-crosslinked chitosan, thereby enhancing the storage stability and substrate oxidation potential of the material [15].
The chemical reaction conditions using aldehyde groups and chitosan amino groups are mild and do not require the introduction of other auxiliary molecules such as reducing agents. However, the drawback of dialdehydes (GA, glyoxal, etc.) as crosslinkers is their high level of cytotoxicity and carcinogenic effects. The natural biological crosslinking agent genipin is an iridoid compound because its biological toxicity is much lower than other small organic molecules (formaldehyde, GA, etc.), and it is often used as a chemical crosslinking agent instead of aldehydes [73,74]. The biotoxicity of chitosan hydrogels can be greatly reduced. As a water-soluble bifunctional crosslinker, it reacts rapidly with chitosan to generate blue, fluorescent hydrogels. Under acidic and neutral conditions, genipin and chitosan are linked by amides and tertiary amines to form a crosslinked structure [75], as shown in Figure 3a. Delmar et al. studied genipin-crosslinked chitosan hydrogels and found that pH and crosslinking time significantly affected the properties of chitosan hydrogels (Figure 3b) [76]. Changing the pH in the range of 4.00–5.50 significantly affected the reaction, resulting in different appearance and properties of the hydrogel (Figure 3c). Increasing the pH by 1.5 units resulted in a fourfold reduction in gel time and a more than tenfold equilibrium swelling. The swelling ability of the hydrogel was significantly pH dependent, which was attributed to the degree of protonation of CS and the inability of protonated CS to react with genipin. Tavares et al. investigated the effect of deacetylation degrees (DD, 83, 94 and 96%) on the properties of chitosan-genipin crosslinked gels. Using dynamic rheological tests, they confirmed that CS gel strength depends on frequency and temperature. The higher DD of CS, the lower the gelation temperature and the stronger the gel network structure. In addition, the high DD of CS is easier to crosslink with genipin, which can significantly improve the mechanical properties of the gel [66]. Nasrabadi et al. modeled two chitosan polymer sequences and six monomer units crosslinked by genipin [77]. The formation mechanism of genipin-crosslinked chitosan (GSC) was studied by calculating activation enthalpy and activation Gibbs free energy. The results suggested that H2O molecules were involved in the formation of the gel by substituting secondary amide bonds for the ester functional group of genipin through a tetrahedral intermediate (SN2 mechanism). Comparing the GCS model with a simple model of one polymer chain showed that the GCS model had more negative binding energies and stronger hydrogen bonds than the simple model. Muhammad Ubaid et al. prepared and optimized chitosan hydrogel membranes containing metformin using different concentrations of genipin as a crosslinker [14]. The gel membranes exhibited significant pH-sensitive behavior. The presence of hydrogen and ionic bonds between chitosan and genipin ensures that the drug is intact in the matrix system. The obtained hydrogels can be used for drug delivery. The ninhydrin assay allows for the measurement of the cross-linkage of the genipin crosslinked chitosan network and determination of the appropriate crosslinker concentration for gels used for swelling and thermomechanical analysis. Using a combined analysis of the modified Arrhenius and William Landel Ferry theories, Whitehead et al. determined the glass transition temperature range of −68 to −8 °C for genipin-crosslinked chitosan networks (40~60%, w/w solids), providing important guidance for the design and control of targeted delivery systems for biologically active compounds [78].
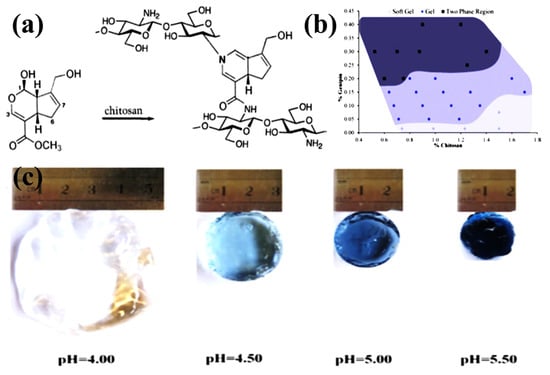
Figure 3.
Chemically crosslinked CS. (a) Crosslinking reaction between genipin and CS; (b) Phase diagram of genipin crosslinked CS hydrogel; (c) The swelling appearance of genipin-crosslinked CS hydrogels at different pH values. (a) Reprinted with permission from ref. [75]. Copyright 2009 Elsevier. (b,c) Reprinted with permission from ref. [76]. Copyright 2015 Elsevier.
3.3. Irradiation-Crosslinked CS Hydrogels
The radiation crosslinking method refers to the gelation of CS by the interaction between molecular chains to form a crosslinked network structure under the action of high-energy light source such as ultraviolet rays, electron beams, γ-rays, etc. Irradiation can cause crosslinking and gel formation when the polysaccharide solution at the high concentration. Huh et al., introduced methacrylate groups on the CS molecular chain to obtain methacrylated hexanoyl glycol chitosan (M-HGC), and irradiated them with UV light at 220–260 nm [79]. It was found that there was a significant change at 15 min under UV irradiation and the signal of double-bonded peaks almost disappeared after 30 min and formed a gel, indicating that the crosslinking of C=C occurred under light irradiation. N-(2-hydroxyethyl)prop-2-enamide (HEPE) was grafted onto CS by reversible addition–fragmentation chain transfer (RAFT) radical polymerization under γ-ray irradiation, and the amino group need not be protected during the reaction. The drug was attached to the polymer by generating a Shiff base with the amino group of CS, and the product can self-assemble to form nanomicelles with pH and temperature sensitivity (Figure 4a) [80]. Nasef et al. crosslinked polyvinyl alcohol (PVA) and chitosan under γ-ray irradiation. The study showed that the dissolution rate of the composite hydrogel film decreased significantly with increasing radiation dose, which can be used as an elastic biomaterial for artificial skin [81]. Chan et al. prepared chitosan (CS)/corncob (CC) biocomposite gels by electron beam irradiation membrane [82]. When radiation was exposed to the biocomposite membrane, the free radicals generated by the radiolysis of water may attack CC to form CC radicals. These CC radicals may then attack long CS chains to form new CS-CC bonds (Figure 4b). The induced crosslinking of CS/CC increased after electron beam irradiation. Compared with the unirradiated biocomposite films, the irradiated CS/CC biocomposite films showed better thermal stability and biodegradability.
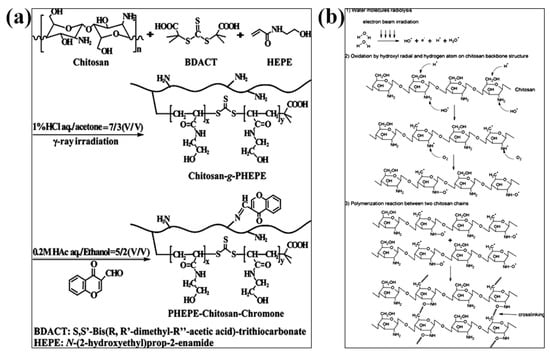
Figure 4.
Radiation-crosslinked CS hydrogels. (a) Schematic diagram of crosslinking CS reaction by γ-irradiation. Reprinted with permission from ref. [80]. Copyright 2013 American Chemical Society; (b) crosslinking reaction between CS and corncob irradiated by electron beam. Reprinted with permission from ref. [82]. Copyright 2018 Society of Plastics Engineers.
3.4. Electrodeposited CS Hydrogels
CS is a weak electrolyte with specific pH responsiveness, and it is also the only natural cationic polymer that can be deposited to form hydrogels by electric field-induced deposition [83,84,85]. When the pH is less than its pKa (about 6.3), the protonation of the chitosan amino group leads to the dissolution of CS in cationic form; when the pH is close to or greater than its pKa, the amino group is deprotonated and precipitated. After applying an electric field, the H+ in the solution undergoes a reduction reaction at the cathode, causing the pH of the cathode surface to rise, inducing deprotonation of CS molecules and precipitation from the solution to form a CS gel. The CS gel film can be easily washed out by acid and could also be preserved after being crosslinked by a crosslinking agent. Compared with physical and chemical crosslinking methods, the preparation of CS hydrogels by electrodeposition shows unique advantages, such as simple operation, mild reaction, no need to add other chemical reagents, and can be carried out in the aqueous phase [86,87,88]. Recently, Yan et al. fabricated a vascular-like structured CS hydrogel with a diameter of about 0.4 mm by a templated electrodeposition process stimulated with an oscillating electrical signal (Figure 5) [89]. The method spatially and temporally controls the internal multilayer structure of the hydrogel by using pulsed electrical signals (ON-OFF model), with short interruptions (OFF steps) forming tight boundaries between each individual layer. The work provides a very promising self-assembly technique for constructing hydrogel coatings and artificial blood vessel regeneration. Yang et al. used electrodeposition-induced covalent crosslinking of CS and epichlorohydrin to obtain CS-based hydrogel contact lenses [90]. The electrodeposited hydrogel exhibits favorable optical properties, mechanical properties, and biocompatibility. The geometry of CS hydrogel could be simply tailored by electrode templates, the properties can be tuned by electrical signal and electrochemical crosslinking. In addition, the use of electrodeposition to print 3D CS hydrogels has received considerable attention for biomedical applications. Noriko Taira et al. performed 3D chitosan/gelatin hydrogel bioprinting by electrodeposition with a needle-shaped device [86]. This 3D design approach allows us to rapidly electrodeposit large hydrogels into several shapes, which holds promise for future tissue engineering, drug delivery, and on-chip applications.
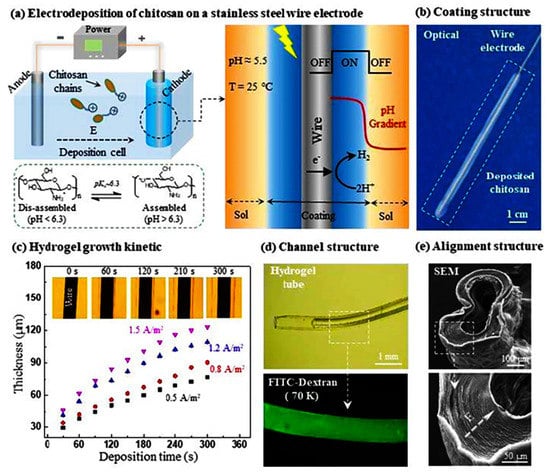
Figure 5.
Wire templated electrodeposition of vessel-like structured CS hydrogel. Reproduced with permission from ref. [89]. Copyright Royal Society of Chemistry.
4. Synthesis and Modification of CS-Based Hydrogels for Flexile Wearable Sensors
CS is highly permeable to water and anions, easily adheres to negatively charged surfaces, aggregates with polyanionic compounds, and chelated metal cations, and has been widely used as a biosensor [91]. CS-Gels can be prepared by chemically or physically crosslinking to form composites. Chemical crosslinking provides excellent gel properties, stabilities and mechanical properties through covalent bonding with the addition of crosslinkers. The amino and hydroxyl groups of CS molecular chains could be chemically modified to immobilize sensing compounds such as enzymes, conductive polymers, or stimulus-responsive polymers for sensing applications. Physically crosslinked hydrogels are achieved through non-covalent bonding (e.g., electrostatic interactions, hydrogen bonds, ionic hydrogelation, and hydrophobic interactions) without the addition of crosslinking agents. The non-covalent bonds are easily broken under physiological conditions, thus providing good biodegradability for the hydrogels [92]. In recent years, CS-Gels sensors have been cleverly designed and widely used for real-time and efficient monitoring of physiological signals or target biomolecules, which demonstrates their potential applications in the field of flexible wearable sensors [93,94,95]. In this context, a large number of CS-based composite hydrogel sensors have been established, including pressure and strain sensors. The synthesis and application of CS-based hydrogels for flexible wearable sensors are shown in Figure 6, and the comparison of CS-based hydrogel materials, synthesis strategies and applications reported in related work is summarized in Table 1.
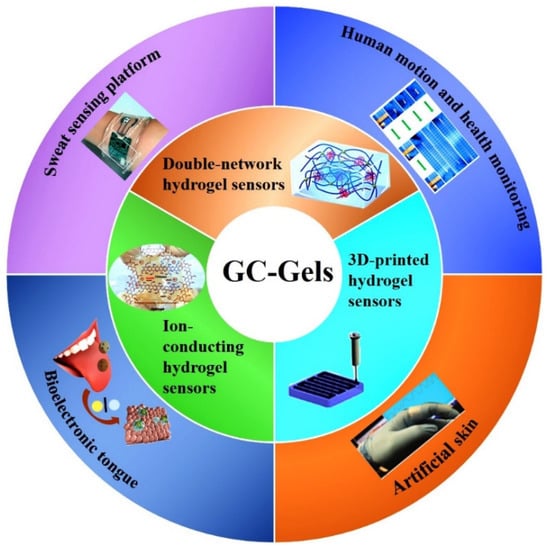
Figure 6.
Types and applications of CS-Gels wearable sensors.

Table 1.
Summary of synthesis strategies and applications of CS-Gels wearable sensors.
4.1. CS/Carbon-Based Nanomaterial Composite Hydrogel Sensors
Carbon-based nanomaterials, such as carbon nanotubes (CNTs), graphene, graphene oxide, fullerenes, carbon nanoparticles, play an important role in building 3D conducting networks in polymer matrices due to their high electrical conductivity, high stability, and excellent surface-to-volume ratio. By introducing carbon-based nanomaterials into hydrogel matrices, not only electron transport pathways through π-conjugated structures are established, but also the mechanical properties of hydrogels are significantly improved owing to their abundant surface functionalization and hydrophobic associations [102]. Pirahmadi et al. prepared polyvinyl alcohol/chitosan (PVA/CS) hydrogel networks (water content 90 wt%) with physical crosslinking by the freeze-thaw method and used to mechanically enhance the mechanical properties of the composite hydrogels by introducing high modulus CNTs [103]. The PVA/CS-Gels containing 0.25 wt% CNT showed electrical conductivity greater than 9 mS/cm and 260% improved shape memory behavior of the hydrogels for potential use in soft smart robots, flexible wearable electronics, and smart sensors and actuators. Figure 7a demonstrated the use of glucose oxidase/CS/CNTs hydrogel extraction and analysis for cystic fibrosis and blood glucose monitoring [104]. The CS/CNTs hydrogel wearable sensing platform incorporates an electrochemically enhanced iontophoretic interface (Figure 7b). This interface can extract sufficient sweat volume and deliver it to the sensor surface for robust analysis. Furthermore, the use of programming the interface to periodically induce sweating of various secretions can be used for cystic fibrosis diagnosis and real-time monitoring of blood/sweat glucose (Figure 7c,e).
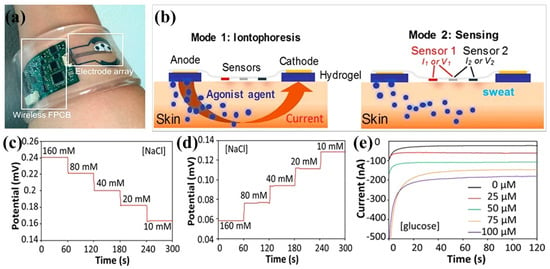
Figure 7.
CS/carbon-based nanomaterial composite hydrogel. (a) Image of glucose oxidase/CS/CNTs hydrogel autonomous sweat extraction and sensing platform; (b) Schematic diagram of the ionophoresis therapy and sensing model of the CS/CNTs hydrogel wearable sensing platform; Open circuit potential response of sodium (c) and chloride (d) sensors in NaCl solution; (e) Chronoamperometric responses of the CS/CNTs hydrogel sensor to glucose solutions. Reprinted with permission from ref. [104]. Freely available online through the PNAS open access option.
4.2. CS-Based Double-Network Hydrogel Sensors
Double-network (DN) hydrogels are two interpenetrating crosslinked polymer networks prepared based on physical or chemical crosslinking, and the mechanical properties of hydrogels can be improved by the DN structure. DN hydrogels are mainly composed of a rigid network and a flexible network, where the rigid network is used to dissipate energy during deformation and increase the strength of the gel, while the flexible network is used to enhance the tensile properties of the gel [105,106]. The synergistic effect of the two networks effectively enhances the pressure conduction and dispersion and endows the hydrogel with excellent mechanical properties. Recently, a novel DN chitosan hydrogel has been developed by introducing dynamic crosslinked networks into the chitosan network. Figure 8a shows the flexible polyacrylamide (PAAm) network composed of dynamically crosslinked chitosan (CS) and doped polyaniline (PANI) via hydrogen bonding between hydroxyl, amide and aniline groups to form DN [18]. The obtained hydrogels exhibited a dense network interpenetrating structure, which facilitates the mechanical properties and conductivity improvement (Figure 8b). At a strain of 600%, the hysteresis loop of the PANI/CS DN hydrogel was significantly enlarged with increasing CS content (Figure 8c). It is suggested that CS doping acts as a “sacrificial bond” that contributes to energy dissipation. As the strain continues to increase, the dynamic crosslinking in the PANI/CS DN hydrogel was broken to release mechanical energy. When the dissipation energy was increased to 0.99 MJ/m3, the dissipation coefficient of the hydrogel reached 52.94% (Figure 8d). The hydrogel sensor has good stability and reproducibility under different strains (Figure 8e). This DN-structured CS hydrogel exhibits excellent mechanical properties, amazing electrical conductivity, and good freezing resistance, and shows excellent sensitivity in detecting stretching and bending as well as human activities. Zhao et al. designed and prepared non-expandable DN hydrogels by adding CS to poly(acrylic acid-2-methoxyethyl acrylate)-Fe3+ [P(AA-MEA)-Fe] network (Figure 8f) [107]. Due to the non-covalent physical crosslinking of CS, the prepared hydrogels showed excellent mechanical flexibility and mechanical strength (Figure 8g) with tensile, toughness, and recovery capacities of 1199%, 2.01 MJ/m3, and 97.22%, respectively. Due to the introduction of CS, the hydrogel exhibited excellent non-swelling properties in water (pH = 1, 4 and 7), saline, seawater and organic solvents (dodecane, n-hexane and chloroform) (Figure 8h). After immersion in deionized water for 7 days, the hydrogel still achieved a mechanical strength of 0.467 MPa and an elongation of 1072%. The strain sensitivity of the hydrogel sensor increases rapidly with increasing tensile stain, showing the remarkable strain sensitivity of the hydrogel (Figure 8i). Such hydrogel-based flexible sensors can satisfy the periodic real-time signals of human motion monitoring underwater (e.g., joint movements and electronic skin contact). Sahoo et al. constructed DN hydrogels by the synergistic interaction between CS, tetraethylene glycol (TEG) and polyacrylic acid (PAA). The hydrogels possess abundant hydroxyl functional groups and can self-adhere to various materials with up to 90–95% self-healing properties. Owing to the dynamic crosslinking nature of the polymer, the obtained DN hydrogels exhibit tunable mechanical properties and have potential applications in real-time soft human motion sensors [108].
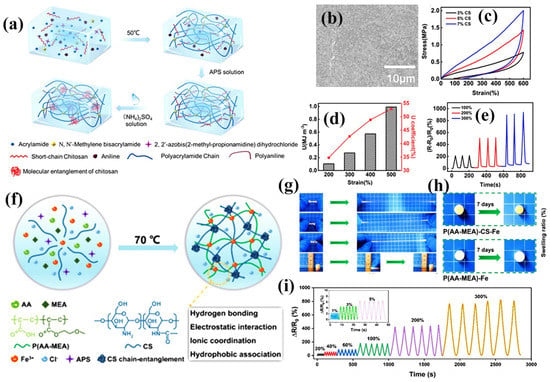
Figure 8.
Construction of CS DN composite hydrogel sensors. (a) Schematic diagram of the synthesis of the interpenetrating PANI/CS-PAAm DN hydrogel; (b) SEM image of PANI/CS-PAAm hydrogel; (c) Comparison of hysteresis loop for different CS content at 600% strain; (d) Corresponding dissipated energy and energy dissipation rate per cycle; (e) Time-dependent relative resistance change in the sensor while monitoring multiscale strain; (f) Schematic diagram of the fabrication process and group interactions of the non-swelling P(AA-MEA)-CS-Fe hydrogel; (g) Photographs of the excellent mechanical hydrogels; (h) Image of hydrogels after immersion in water for 7 days; (i) Tensile strain sensing performance of non-swollen P(AA-MEA)-CS-Fe hydrogel. (a–e) Reprinted with permission from ref. [18]. Copyright 2021 American Chemical Society. (f–i) Reprinted with permission from ref. [107]. Copyright 2022 Elsevier B.V.
4.3. CS-Based Ion-Conducting Hydrogel Sensors
Ion conduction is a key process for achieving physiological signal transmission in biological systems [109]. Under the electric field, the free ions in the ion-conducting hydrogels are directionally transported, which is similar to the signaling principle in living organisms, allowing for the exchange of information with the surrounding environmental tissues through diffusion. Ion-conducting hydrogels avoid the conversion between electron and ion signals, and have low interface impedance with tissues, which have low impedance at the interface with tissue and great biocompatibility, making them ideal materials for wearable and implantable flexible devices [110,111]. Recently, Li et al., inspired by the adhesion of natural mussels, in situ polymerized acrylamide (AM) in a CS matrix to prepare a hydrogel (PAM@CS), and then introduced plant polyphenol tannins acid (TA) into the system with Fe3+ as a redox agent for ultrafast polymerization, and prepared ion-conductive composite hydrogels PAM@CS/TA-Fe within 1 min at 60 °C (Figure 9a) [100]. PAM@CS hydrogel is ionically conductive and show good sensing properties in detecting joint motion and subtle physiological signals such as articulation and alcohol consumption. Inspired by the human tongue sensing mechanism, Khan et al. synthesized supramolecular ionic hydrogels by first copolymerizing acrylic acid (AA) and acrylamide (AM) with CS in the presence of NaCl electrolyte to form CS/poly(AM-co-AA) 3D network structure [112]. Then, the secretory protein mucin was added, N,N,N′,N′-tetramethylethylenediamine (TEMED) was used as a promoter and ammonium persulfate (APS) as an initiator to form an artificial bioelectronic tongue (E-tongue) (Figure 9b) for the detection of astringent and bitter substances. Figure 9c shows a schematic diagram of the sensing mechanism of E-tongue in contact with astringent or bitter substances. In this case, mucin plays a crucial role in the detection of target analytes. The electronic tongue, which contains mucin as secreted protein and NaCl as ion transport electrolyte, exhibited excellent sensitivity of 0.2 and 0.12 wt%−1, and a wide detection range of 29.3 mM-0.59 μM and 63.8 mM-6.38 μM, respectively. In addition, the sensitivity of the electronic tongue is not affected at −5 °C, which is suitable for subzero temperature environments (Figure 9d). This work provides a promising approach for the development of flexible, curable artificial bioelectronic tongues with potential applications in food taste detection and humanoid robotics.
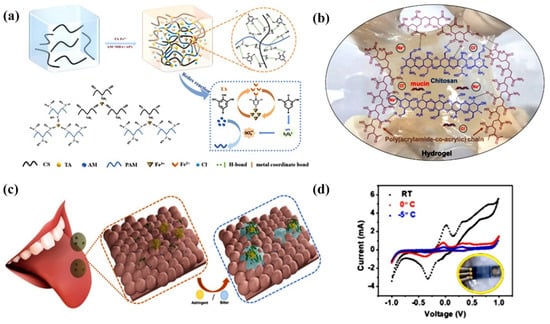
Figure 9.
CS-based ion-conducting hydrogel sensors. (a) Schematic diagram of PAM@CS/TA-Fe hydrogel synthesis process; (b) Chemical structure of the conductive self-healing hydrogel; (c) Schematic diagram of electronic tongue sensing mechanism; (d) Performance of the electron tongue at different temperatures (RT, 0 and −5 °C). (a) Reprinted with permission from ref. [100]. Copyright 2022 Elsevier B.V. (b–d) Reprinted with permission from ref. [112]. Copyright 2021 Elsevier B.V.
4.4. Three-Dimensional Printed CS Hydrogel Sensors
Three-dimensional (3D) printing technology is based on digital models to create 3D functional devices with complex geometries in a later-by-layer fashion. Moreover, 3D printing has changed the concept of free-form construction and end-user customization and is considered as a next-generation tool for wearable sensors due to its easy-to-use, rapid manufacturing capabilities. This additive manufacturing technique allows for complex geometries to be printed precisely into desired microstructures, enabling on-demand customization of 3D wearable biomedical electronics. Currently, 3D printing-based technologies for the development of wearable sensors such as glucose sensors, artificial skin, sweat sensors, tactile sensors, microfluidic biosensors, strain sensors, pressure sensors, wearable oximeters are attracting great interest in the field of healthcare [113,114,115,116]. Hao et al. used CS prepared under alkaline conditions for rapid 3D printing of composite multifunctional hydrogels [117]. Figure 10a shows a schematic diagram of 3D printing using CS hydrogel ink. The CS hydrogel can not only print uninterruptedly at room temperature without clogging the nozzle, but also has a stable shape retention during printing. Some graphics (e.g., squares) can be designed by CAD and then rendered by a 3D printer (Figure 10b). The 3D printed-CS hydrogel exhibited high sensitivity, stability and repeatability when used for temperature detection (Figure 10c–d). Darabi et al. developed a conductive self-healing CS hydrogel with pressure-sensitive properties, and the 3D printing process is shown in Figure 10e [118]. The dynamic ionic interaction between the carboxyl group of poly(acrylic acid) and iron ions contributes to the self-healing abilities. The application of CS-Gels in human motion detection (e.g., finger bending and relaxation resistance changes) and their good 3D printing properties reveal their practical potential (Figure 10f).
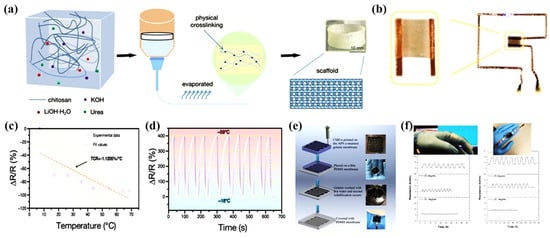
Figure 10.
3D-printed CS hydrogel sensors. (a) Schematic diagram of 3D printing of CS hydrogel ink; (b) Physical photo of the flexible electronic sensor; (c) Correlation resistance at different temperatures; (d) Relationship for the resistance change rates; (e) 3D-printed wearable sensor fabricated by conductive self-healing CS hydrogel; (f) 3D-printed conductive self-healing CS hydrogel for monitoring the resistance change in finger bending and relaxation.(a–d) Reprinted with permission from ref. [117]. Copyright 2022 Wiley-VCH GmbH. (e–f) Reprinted with permission from ref. [118]. Copyright 2017 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
It should be noted that 3D printing technology still has many limitations, such as the lack of fine processing conditions, high requirements for raw materials, post-processing compatibility, anisotropic mechanical strength, lack of corresponding standards and complete product technology chain. However, with the development of 3D printing technology, miniaturization/integration, customization, higher resolution, and lower cost biomedical sensors will continue to innovate for the foreseeable future.
5. Problems and Challenges
CS hydrogel sensors have great prospects in the field of flexible sensing due to their excellent flexibility, stretchability, and biocompatibility. Flexible sensors designed with CS hydrogels as sensitive materials show great application potential in biomedicine, wearable electronics, health monitoring, etc. Although CS hydrogel-based sensors have many advantages, they still have shortcomings, such as insufficient stability in harsh environments, water evaporation in high-temperature and dry environments, and moisture condensation in low-temperature environments, which make the use of CS hydrogel-based sensors more stringent [119,120,121]. At present, the commonly used solution is to use glutaraldehyde, epichlorohydrin, genipin, CaCl2 aqueous solution, etc. to crosslink the hydrogel to effectively prevent the evaporation of water molecules. In addition, combined water is more difficult to form crystals than free water, and its average molecular motion ability is lower than that of free water molecules, so it is difficult to transfer freely at low temperatures, thus hindering the growth of ice crystals [59,122]. The CaCl2 aqueous solution firmly fixes the water molecules in the polymer network through the strong coordination bonds between H2O-Ca2+, thus endowing the hydrogel with anti-freeze and moisturizing properties.
The operating range is an important parameter that needs to be considered in the practical application of CS hydrogel sensors. When the CS hydrogel sensor undergoes a large degree of deformation, the response baseline drift usually occurs, which seriously affects the accuracy of the CS hydrogel sensor. Therefore, addressing the sensitivity to a single variable is the key to improving the performance of flexible CS hydrogel sensors. The large operating range puts higher requirements on the tensile properties and fatigue resistance of CS hydrogels, which need to be optimized according to actual needs. Electrode corrosion also often affects the performance of CS hydrogel sensors. Due to the long-term application of voltage on the electrodes, the surface of the sensor is prone to corrosion and accumulation of reaction products, which seriously affects the performance and service life of the CS hydrogel sensor. In addition, insufficient air permeability is also a major problem for CS hydrogel sensors. Despite the good biocompatibility of CS hydrogel materials, long-term adhesion to the skin surface can prevent the skin from breathing, resulting in inflammation. Therefore, the realization of CS hydrogels as flexible wearable devices is still full of opportunities and challenges in the fields of human motion monitoring, human-machine interface, and health monitoring.
6. Conclusions and Outlook
In recent years, the application prospects of flexible sensors in fields including wearable devices, medical electronics, implantable devices, and electronic skin have attracted the extensive attention of researchers. Hydrogel materials have mechanical properties that are similar to biological tissues and that are biocompatible, making them one of the ideal materials for application in flexible sensing devices. Many CS-Gels have been developed for flexible wearable sensing devices in different working environments. This review provides a comprehensive summary of the development of CS-Gels flexible wearable sensors in recent years. In terms of materials, the development of various carbon nanomaterials and metal nanomaterials has laid the foundation for the fabrication of flexible CS-Gels sensors with advanced performance. From the perspective of preparation technology, various printing processes are becoming more and more mature, especially the development of 3D printing, which has shown great vitality in advanced manufacturing. Although great progress has been made in the application of CS-Gels in flexible wearable devices, there are still huge opportunities and challenges for their preparation, functionality and practical applications. The influence of CS-Gels network structure on sensing performance still needs to be studied in depth. It is necessary to develop new methods for the synthesis and processing of CS-Gels and to precisely construct the hydrogel network structure. By revealing the relationship between the hydrogel structure and performance, the effect of improving the sensing performance of CS-Gels is achieved. At present, the detection function of CS-Gels sensors is relatively simple and realizing multi-dimensional signal monitoring can effectively promote the practical application of CS-Gels sensors. In addition to stress-strain signals, changes in physiological parameters such as temperature, humidity, and pH within the human body can also effectively reflect changes in human health status. Therefore, the development of CS-Gels sensors with fast response rate, high sensitivity, and wide detection range for signals such temperature, humidity, and pH has important application prospects in the field of smart wearable and implantable devices.
Author Contributions
Conceptualization, C.X. and Y.Z.; investigation, W.S.; resources, H.L.; data curation, C.X.; writing—original draft preparation, S.W. and C.X.; writing—review and editing, S.W. and P.Q; visualization, J.C. and F.D.; supervision, S.W. and P.Q.; project administration, S.W.; funding acquisition, S.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (51808263) and the Youth Talent Cultivation Program of Jiangsu University (2018).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Xu, T.; Zhang, X. Multifunctional conductive hydrogel-based flexible wearable sensors. TrAC Trends Anal. Chem. 2021, 134, 116130. [Google Scholar] [CrossRef]
- Chen, S.; Qi, J.; Fan, S.; Qiao, Z.; Yeo, J.C.; Lim, C.T. Flexible wearable sensors for cardiovascular health monitoring. Adv. Healthc. Mater. 2021, 10, 2100116. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Fu, Q.; Meng, L.; Hao, S.; Dai, R.; Yang, J. Recent progress in natural biopolymers conductive hydrogels for flexible wearable sensors and energy devices: Materials, structures, and performance. ACS Appl. Bio Mater. 2020, 4, 85–121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liu, J.; He, S.; Liu, A.; Zhang, J.; Xu, H.; Shao, W. Flexible wearable sensors based on lignin doped organohydrogels with multi-functionalities. Chem. Eng. J. 2022, 430, 132653. [Google Scholar] [CrossRef]
- Anwer, A.H.; Khan, N.; Ansari, M.Z.; Baek, S.-S.; Yi, H.; Kim, S.; Noh, S.M.; Jeong, C. Recent advances in touch sensors for flexible wearable devices. Sensors 2022, 22, 4460. [Google Scholar] [CrossRef]
- del Bosque, A.; Sánchez-Romate, X.F.; Sánchez, M.; Ureña, A. Flexible wearable sensors based in carbon nanotubes reinforced poly (Ethylene Glycol) Diglycidyl ether (PEGDGE): Analysis of strain sensitivity and proof of concept. Chemosensors 2021, 9, 158. [Google Scholar] [CrossRef]
- Fu, X.; Wang, L.; Zhao, L.; Yuan, Z.; Zhang, Y.; Wang, D.; Wang, D.; Li, J.; Li, D.; Shulga, V. Controlled Assembly of MXene Nanosheets as an Electrode and Active Layer for High-Performance Electronic Skin. Adv. Funct. Mater. 2021, 31, 2010533. [Google Scholar] [CrossRef]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.-H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Zhao, Q.; Wang, S.; Huang, Q.; Yuan, Z.; Zhang, Y.; Jiang, Y.; Tai, H. Halloysite nanotubes: Natural, environmental-friendly and low-cost nanomaterials for high-performance humidity sensor. Sens. Actuators B Chem. 2020, 317, 128204. [Google Scholar] [CrossRef]
- Mohamed, M.E.B.; Attia, N.F.; Elashery, S.E. Greener and facile synthesis of hybrid nanocomposite for ultrasensitive iron (II) detection using carbon sensor. Microporous Mesoporous Mater. 2021, 313, 110832. [Google Scholar] [CrossRef]
- Pita-López, M.L.; Fletes-Vargas, G.; Espinosa-Andrews, H.; Rodriguez-Rodriguez, R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: A state-of-the-art review. Eur. Polym. J. 2021, 145, 110176. [Google Scholar] [CrossRef]
- Crini, G.; Torri, G.; Lichtfouse, E.; Kyzas, G.Z.; Wilson, L.D.; Morin-Crini, N. Dye removal by biosorption using cross-linked chitosan-based hydrogels. Environ. Chem. Lett. 2019, 17, 1645–1666. [Google Scholar] [CrossRef]
- Iglesias, N.; Galbis, E.; Valencia, C.; Díaz-Blanco, M.J.; Lacroix, B.; de-Paz, M.-V. Biodegradable double cross-linked chitosan hydrogels for drug delivery: Impact of chemistry on rheological and pharmacological performance. Int. J. Biol. Macromol. 2020, 165, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Ubaid, M.; Murtaza, G. Fabrication and characterization of genipin cross-linked chitosan/gelatin hydrogel for pH-sensitive, oral delivery of metformin with an application of response surface methodology. Int. J. Biol. Macromol. 2018, 114, 1174–1185. [Google Scholar] [CrossRef]
- Bilal, M.; Jing, Z.; Zhao, Y.; Iqbal, H.M. Immobilization of fungal laccase on glutaraldehyde cross-linked chitosan beads and its bio-catalytic potential to degrade bisphenol A. Biocatal. Agric. Biotechnol. 2019, 19, 101174. [Google Scholar] [CrossRef]
- Cui, C.; Shao, C.; Meng, L.; Yang, J. High-strength, self-adhesive, and strain-sensitive chitosan/poly (acrylic acid) double-network nanocomposite hydrogels fabricated by salt-soaking strategy for flexible sensors. ACS Appl. Mater. Interfaces 2019, 11, 39228–39237. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Xu, J.; Duan, L.; Gao, G. Chitosan-driven skin-attachable hydrogel sensors toward human motion and physiological signal monitoring. Carbohydr. Polym. 2021, 268, 118240. [Google Scholar] [CrossRef]
- Cong, J.; Fan, Z.; Pan, S.; Tian, J.; Lian, W.; Li, S.; Wang, S.; Zheng, D.; Miao, C.; Ding, W. Polyacrylamide/chitosan-based conductive double network hydrogels with outstanding electrical and mechanical performance at low temperatures. ACS Appl. Mater. Interfaces 2021, 13, 34942–34953. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Saheed, I.O.; Da Oh, W.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants—A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of chitosan in environmental remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current advancements in chitosan-based film production for food technology; A review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef] [PubMed]
- El Knidri, H.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Ganesan, A.R.; Muralisankar, T.; Jayakumar, R.; Sathishkumar, P.; Uthayakumar, V.; Chandirasekar, R.; Revathi, N. Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci. Technol. 2020, 105, 17–42. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.; Maia, P.C.; Lima, M.A.B.d.; Franco, L.d.O.; Campos-Takaki, G.M.d. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef]
- Li, B.; Elango, J.; Wu, W. Recent advancement of molecular structure and biomaterial function of chitosan from marine organisms for pharmaceutical and nutraceutical application. Appl. Sci. 2020, 10, 4719. [Google Scholar] [CrossRef]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Franca, E.F.; Freitas, L.C.; Lins, R.D. Chitosan molecular structure as a function of N-acetylation. Biopolymers 2011, 95, 448–460. [Google Scholar] [CrossRef]
- Calixto, G.M.F.; Victorelli, F.D.; Dovigo, L.N.; Chorilli, M. Polyethyleneimine and chitosan polymer-based mucoadhesive liquid crystalline systems intended for buccal drug delivery. AAPS PharmSciTech 2018, 19, 820–836. [Google Scholar] [CrossRef]
- Wang, T.; Chen, L.; Shen, T.; Wu, D. Preparation and properties of a novel thermo-sensitive hydrogel based on chitosan/hydroxypropyl methylcellulose/glycerol. Int. J. Biol. Macromol. 2016, 93, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, K.; Shi, W.; Cai, J. Preparation and performance evaluation of chitosan/polyvinylpyrrolidone/polyvinyl alcohol electrospun nanofiber membrane for heavy metal ions and organic pollutants removal. Int. J. Biol. Macromol. 2022, 210, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Win, P.; Lin, C.-G.; Long, Y.; Chen, W.; Chen, G.; Song, Y.-F. Covalently cross-linked layered double hydroxide nanocomposite hydrogels with ultrahigh water content and excellent mechanical properties. Chem. Eng. J. 2018, 335, 409–415. [Google Scholar] [CrossRef]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Feksa, L.R.; Troian, E.A.; Muller, C.D.; Viegas, F.; Machado, A.B.; Rech, V.C. Hydrogels for biomedical applications. In Nanostructures for the Engineering of Cells, Tissues and Organs; Elsevier: Amsterdam, The Netherlands, 2018; pp. 403–438. [Google Scholar]
- Li, Y.; Yang, J.; Yu, X.; Sun, X.; Chen, F.; Tang, Z.; Zhu, L.; Qin, G.; Chen, Q. Controlled shape deformation of bilayer films with tough adhesion between nanocomposite hydrogels and polymer substrates. J. Mater. Chem. B 2018, 6, 6629–6636. [Google Scholar] [CrossRef]
- Wang, B.; Hua, J.; You, R.; Yan, K.; Ma, L. Electrochemically deposition of catechol-chitosan hydrogel coating on coronary stent with robust copper ions immobilization capability and improved interfacial biological activity. Int. J. Biol. Macromol. 2021, 181, 435–443. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Y.; Xiao, L.; Deng, H.; Du, Y.; Shi, X. Electrochemical deposition to construct a nature inspired multilayer chitosan/layered double hydroxides hybrid gel for stimuli responsive release of protein. J. Mater. Chem. B 2015, 3, 7577–7584. [Google Scholar] [CrossRef]
- Da Silva, A.C.; Wang, J.; Minev, I.R. Electro-assisted printing of soft hydrogels via controlled electrochemical reactions. Nat. Commun. 2022, 13, 1353. [Google Scholar] [CrossRef]
- Helú, M.A.B.; Liu, L. Rational shaping of hydrogel by electrodeposition under fluid mechanics for electrochemical writing on complex shaped surfaces at microscale. Chem. Eng. J. 2021, 416, 129029. [Google Scholar] [CrossRef]
- Hu, Y.; Du, Z.; Deng, X.; Wang, T.; Yang, Z.; Zhou, W.; Wang, C. Dual physically cross-linked hydrogels with high stretchability, toughness, and good self-recoverability. Macromolecules 2016, 49, 5660–5668. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Yu, X.; Sun, X.; Zhu, L.; Qin, G.; Dai, Y.; Chen, Q. Tough and conductive dual physically cross-linked hydrogels for wearable sensors. Ind. Eng. Chem. Res. 2019, 58, 17001–17009. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, G.; Zeng, X.; Li, J.; Li, G.; Huang, W.; Sun, R.; Wong, C. High-strength, tough, fatigue resistant, and self-healing hydrogel based on dual physically cross-linked network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, X.; Yang, B.; Lai, L.; Chen, N.; Hu, J.; Lu, Q. Dual physically cross-linked hydrogels incorporating hydrophobic interactions with promising repairability and ultrahigh elongation. Adv. Funct. Mater. 2021, 31, 2008187. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Farsangi, Z.J.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Y.; Zhang, Y.; Zheng, J.; Wu, H.; Chen, Y.; Xu, S.; Yang, J.; Liu, C.; Zhang, Y. Dual cross-linked ion-based temperature-responsive conductive hydrogels with multiple sensors and steady electrocardiogram monitoring. Chem. Mater. 2020, 32, 7670–7678. [Google Scholar] [CrossRef]
- Yang, J.; Kang, Q.; Zhang, B.; Fang, X.; Liu, S.; Qin, G.; Chen, Q. Strong, tough, anti-freezing, non-drying and sensitive ionic sensor based on fully physical cross-linked double network hydrogel. Mater. Sci. Eng. C 2021, 130, 112452. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Chiao, M.; Mahmoud, K.A.; Wu, L.; Wang, B. Preparation and characterization of PVA/PVP conductive hydrogels formed by freeze–thaw processes as a promising material for sensor applications. J. Mater. Sci. 2022, 57, 8029–8038. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.; Hyun, J. Free-form three-dimensional nanocellulose structure reinforced with poly (vinyl alcohol) using freeze-thaw process. Carbohydr. Polym. 2022, 298, 120055. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, R.; Pan, F.; Fu, Z. Polyvinyl Alcohol/Graphene Oxide Conductive Hydrogels via the Synergy of Freezing and Salting Out for Strain Sensors. Sensors 2022, 22, 3015. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; He, S.; Ma, S.; Zeng, A.; Chen, G.; Tang, X.; Sun, Y.; Xu, F.; Zeng, X.; Lin, L. Catechol-based all-wood hydrogels with anisotropic, tough, and flexible properties for highly sensitive pressure sensing. Chem. Eng. J. 2022, 427, 131896. [Google Scholar] [CrossRef]
- Du, C.; Zhang, X.N.; Sun, T.L.; Du, M.; Zheng, Q.; Wu, Z.L. Hydrogen-bond association-mediated dynamics and viscoelastic properties of tough supramolecular hydrogels. Macromolecules 2021, 54, 4313–4325. [Google Scholar] [CrossRef]
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.-N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly (vinyl alcohol)/poly (acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 6, 8105–8114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, Q.; Thundat, T.; Zeng, H. Stretchable, injectable, and self-healing conductive hydrogel enabled by multiple hydrogen bonding toward wearable electronics. Chem. Mater. 2019, 31, 4553–4563. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Li, X.; Liu, W.; Yu, X.; Yan, F.; Sun, J. Dynamic hydrophobic domains enable the fabrication of mechanically robust and highly elastic poly (vinyl alcohol)-based hydrogels with excellent self-healing ability. ACS Mater. Lett. 2020, 2, 764–770. [Google Scholar] [CrossRef]
- Qi, C.; Dong, Z.; Huang, Y.; Xu, J.; Lei, C. Tough, Anti-Swelling Supramolecular Hydrogels Mediated by Surfactant–Polymer Interactions for Underwater Sensors. ACS Appl. Mater. Interfaces 2022, 14, 30385–30397. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Song, S.; Li, Y.; Gao, G. Highly sensitive and wearable gel-based sensors with a dynamic physically cross-linked structure for strain-stimulus detection over a wide temperature range. J. Mater. Chem. C 2019, 7, 11303–11314. [Google Scholar] [CrossRef]
- Shitrit, Y.; Davidovich-Pinhas, M.; Bianco-Peled, H. Shear thinning pectin hydrogels physically cross-linked with chitosan nanogels. Carbohydr. Polym. 2019, 225, 115249. [Google Scholar] [CrossRef]
- Khapre, M.A.; Pandey, S.; Jugade, R.M. Glutaraldehyde-cross-linked chitosan–alginate composite for organic dyes removal from aqueous solutions. Int. J. Biol. Macromol. 2021, 190, 862–875. [Google Scholar] [CrossRef]
- Bui, T.H.; Lee, W.; Jeon, S.-B.; Kim, K.-W.; Lee, Y. Enhanced Gold (III) adsorption using glutaraldehyde-crosslinked chitosan beads: Effect of crosslinking degree on adsorption selectivity, capacity, and mechanism. Sep. Purif. Technol. 2020, 248, 116989. [Google Scholar] [CrossRef]
- Islam, N.; Wang, H.; Maqbool, F.; Ferro, V. In vitro enzymatic digestibility of glutaraldehyde-crosslinked chitosan nanoparticles in lysozyme solution and their applicability in pulmonary drug delivery. Molecules 2019, 24, 1271. [Google Scholar] [CrossRef]
- Marrakchi, F.; Hameed, B.; Hummadi, E. Mesoporous biohybrid epichlorohydrin crosslinked chitosan/carbon–clay adsorbent for effective cationic and anionic dyes adsorption. Int. J. Biol. Macromol. 2020, 163, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, H.; Xiao, N.; Ma, X.; Liu, C.; Zhong, L.; Xiao, G. Preparation and properties of epichlorohydrin-cross-linked chitosan/hydroxyethyl cellulose based CuO nanocomposite films. Cellulose 2022, 29, 4413–4426. [Google Scholar] [CrossRef]
- Medellín-Castillo, N.A.; Isaacs-Páez, E.D.; Rodríguez-Méndez, I.; González-García, R.; Labrada-Delgado, G.J.; Aragón-Piña, A.; García-Arreola, M.E. Formaldehyde and tripolyphosphate crosslinked chitosan hydrogels: Synthesis, characterization and modeling. Int. J. Biol. Macromol. 2021, 183, 2293–2304. [Google Scholar] [CrossRef] [PubMed]
- Atangana, E. Adsorption of Zn (II) and Pb (II) ions from aqueous solution using chitosan cross-linked formaldehyde adsorbent to protect the environment. J. Polym. Environ. 2019, 27, 2281–2291. [Google Scholar] [CrossRef]
- Tavares, L.; Flores, E.E.E.; Rodrigues, R.C.; Hertz, P.F.; Noreña, C.P.Z. Effect of deacetylation degree of chitosan on rheological properties and physical chemical characteristics of genipin-crosslinked chitosan beads. Food Hydrocoll. 2020, 106, 105876. [Google Scholar] [CrossRef]
- Vlasceanu, G.M.; Crica, L.E.; Pandele, A.M.; Ionita, M. Graphene oxide reinforcing genipin crosslinked chitosan-gelatin blend films. Coatings 2020, 10, 189. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, R.; Li, M.; Yu, F.; He, C. Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite. Carbohydr. Polym. 2019, 220, 141–148. [Google Scholar] [CrossRef]
- Kang, M.L.; Ko, J.-Y.; Kim, J.E.; Im, G.-I. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials 2014, 35, 9984–9994. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, L.; Zhang, H.; Luo, J.; Gao, X. Green Fabrication of Chitin/Chitosan Composite Hydrogels and Their Potential Applications. Macromol. Biosci. 2021, 21, 2000389. [Google Scholar] [CrossRef]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef]
- Sharma, P.; Singh, A.K.; Shahi, V.K. Selective adsorption of Pb (II) from aqueous medium by cross-linked chitosan-functionalized graphene oxide adsorbent. ACS Sustain. Chem. Eng. 2018, 7, 1427–1436. [Google Scholar] [CrossRef]
- Bryś, M.; Urbańska, K.; Olas, B. Novel Findings regarding the Bioactivity of the Natural Blue Pigment Genipin in Human Diseases. Int. J. Mol. Sci. 2022, 23, 902. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gong, X.; Bo, A.; Zhang, L.; Zhang, M.; Zang, E.; Zhang, C.; Li, M. Iridoids: Research advances in their phytochemistry, biological activities, and pharmacokinetics. Molecules 2020, 25, 287. [Google Scholar] [CrossRef] [PubMed]
- Muzzarelli, R.A. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohydr. Polym. 2009, 77, 1–9. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. The dramatic effect of small pH changes on the properties of chitosan hydrogels crosslinked with genipin. Carbohydr. Polym. 2015, 127, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, M.; Morsali, A.; Beyramabadi, S.A. An applied quantum-chemical model for genipin-crosslinked chitosan (GCS) nanocarrier. Int. J. Biol. Macromol. 2020, 165, 1229–1240. [Google Scholar] [CrossRef]
- Whitehead, F.A.; Young, S.A.; Kasapis, S. Swelling behaviour and glass transition in genipin-crosslinked chitosan systems. Int. J. Biol. Macromol. 2020, 164, 3075–3083. [Google Scholar] [CrossRef]
- Cho, I.S.; Cho, M.O.; Li, Z.; Nurunnabi, M.; Park, S.Y.; Kang, S.-W.; Huh, K.M. Synthesis and characterization of a new photo-crosslinkable glycol chitosan thermogel for biomedical applications. Carbohydr. Polym. 2016, 144, 59–67. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Zhang, S.; Huang, L.; Hua, D.; Zhu, X. A facile approach for controlled modification of chitosan under γ-ray irradiation for drug delivery. Macromolecules 2013, 46, 814–818. [Google Scholar] [CrossRef]
- Nasef, S.M.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. Int. J. Biol. Macromol. 2019, 137, 878–885. [Google Scholar] [CrossRef]
- Chan, M.Y.; Koay, S.C. Biodegradation and thermal properties of crosslinked chitosan/corn cob biocomposite films by electron beam irradiation. Polym. Eng. Sci. 2019, 59, E59–E68. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, X.; Guo, X.; Zhang, Z.; Chen, Y.; Wang, Y. Electrodeposition of chitosan based on coordination with metal ions in situ-generated by electrochemical oxidation. J. Mater. Chem. B 2016, 4, 3331–3338. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Xiong, Y.; Cheng, Y.; Wu, H.-C.; Liu, Y.; Morrow, B.H.; Ben-Yoav, H.; Ghodssi, R.; Rubloff, G.W.; Shen, J. Chitosan to connect biology to electronics: Fabricating the bio-device interface and communicating across this interface. Polymers 2014, 7, 1–46. [Google Scholar] [CrossRef]
- Nawrotek, K.; Tylman, M.; Adamus-Włodarczyk, A.; Rudnicka, K.; Gatkowska, J.; Wieczorek, M.; Wach, R. Influence of chitosan average molecular weight on degradation and stability of electrodeposited conduits. Carbohydr. Polym. 2020, 244, 116484. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Ino, K.; Ida, H.; Nashimoto, Y.; Shiku, H. Electrodeposition-based rapid bioprinting of 3D-designed hydrogels with a pin art device. Biofabrication 2019, 11, 035018. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Nawrotek, K.; Grams, J. Understanding electrodeposition of chitosan–hydroxyapatite structures for regeneration of tubular-shaped tissues and organs. Materials 2021, 14, 1288. [Google Scholar] [CrossRef]
- Yan, K.; Yang, C.; Zhong, W.; Lu, Z.; Li, X.; Shi, X.; Wang, D. Wire templated electrodeposition of vessel-like structured chitosan hydrogel by using a pulsed electrical signal. Soft Matter 2020, 16, 9471–9478. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.; Wang, W.; Liu, H.; Deng, H.; Du, Y.; Shi, X. Electrodeposition induced covalent cross-linking of chitosan for electrofabrication of hydrogel contact lenses. Carbohydr. Polym. 2022, 292, 119678. [Google Scholar] [CrossRef]
- Sun, X.; Agate, S.; Salem, K.S.; Lucia, L.; Pal, L. Hydrogel-based sensor networks: Compositions, properties, and applications—A review. ACS Appl. Bio Mater. 2020, 4, 140–162. [Google Scholar] [CrossRef]
- Yang, J.; Shen, M.; Luo, Y.; Wu, T.; Chen, X.; Wang, Y.; Xie, J. Advanced applications of chitosan-based hydrogels: From biosensors to intelligent food packaging system. Trends Food Sci. Technol. 2021, 110, 822–832. [Google Scholar] [CrossRef]
- Ou, Y.; Tian, M. Advances in multifunctional chitosan-based self-healing hydrogels for biomedical application. J. Mater. Chem. B 2021, 9, 7955–7971. [Google Scholar] [CrossRef]
- Han, F.; Wang, T.; Liu, G.; Liu, H.; Xie, X.; Wei, Z.; Li, J.; Jiang, C.; He, Y.; Xu, F. Materials with Tunable Optical Properties for Wearable Epidermal Sensing in Health Monitoring. Adv. Mater. 2022, 34, 2109055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ren, B.; Zhang, Y.; Xu, L.; Huang, Q.; He, Y.; Li, X.; Wu, J.; Yang, J.; Chen, Q. From design to applications of stimuli-responsive hydrogel strain sensors. J. Mater. Chem. B 2020, 8, 3171–3191. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, X.; Zhang, J.; Duan, L.; Gao, G. A highly conductive hydrogel driven by phytic acid towards a wearable sensor with freezing and dehydration resistance. J. Mater. Chem. A 2021, 9, 22615–22625. [Google Scholar] [CrossRef]
- Kim, J.-N.; Lee, J.; Lee, H.; Oh, I.-K. Stretchable and self-healable catechol-chitosan-diatom hydrogel for triboelectric generator and self-powered tremor sensor targeting at Parkinson disease. Nano Energy 2021, 82, 105705. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Shen, B.; Wang, Y.; Peng, P.; Tang, F.; Feng, J. Highly transparent, self-healing, injectable and self-adhesive chitosan/polyzwitterion-based double network hydrogel for potential 3D printing wearable strain sensor. Mater. Sci. Eng. C 2020, 117, 111298. [Google Scholar] [CrossRef]
- Chen, S.; Huang, J.; Zhou, Z.; Chen, Q.; Hong, M.; Yang, S.; Fu, H. Highly elastic anti-fatigue and anti-freezing conductive double network hydrogel for human body sensors. Ind. Eng. Chem. Res. 2021, 60, 6162–6172. [Google Scholar] [CrossRef]
- Li, J.; Yang, Z.; Jiang, Z.; Ni, M.; Xu, M. A self-healing and self-adhesive chitosan based ion-conducting hydrogel sensor by ultrafast polymerization. Int. J. Biol. Macromol. 2022, 209, 1975–1984. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, Y.; Sun, X.; Liang, H. Preparation of stretchable and self-healable dual ionically cross-linked hydrogel based on chitosan/polyacrylic acid with anti-freezing property for multi-model flexible sensing and detection. Int. J. Biol. Macromol. 2021, 193, 629–637. [Google Scholar] [CrossRef]
- Sun, X.; Qin, Z.; Ye, L.; Zhang, H.; Yu, Q.; Wu, X.; Li, J.; Yao, F. Carbon nanotubes reinforced hydrogel as flexible strain sensor with high stretchability and mechanically toughness. Chem. Eng. J. 2020, 382, 122832. [Google Scholar] [CrossRef]
- Pirahmadi, P.; Kokabi, M.; Alamdarnejad, G. Polyvinyl alcohol/chitosan/carbon nanotubes electroactive shape memory nanocomposite hydrogels. J. Appl. Polym. Sci. 2021, 138, 49995. [Google Scholar] [CrossRef]
- Emaminejad, S.; Gao, W.; Wu, E.; Davies, Z.A.; Yin Yin Nyein, H.; Challa, S.; Ryan, S.P.; Fahad, H.M.; Chen, K.; Shahpar, Z. Autonomous sweat extraction and analysis applied to cystic fibrosis and glucose monitoring using a fully integrated wearable platform. Proc. Natl. Acad. Sci. USA 2017, 114, 4625–4630. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, J.; Gu, J.; Peng, S.; Xiang, X.; Chen, K.; Yang, X.; Guan, L.; Jiang, X.; Hou, L. Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J. Mater. Chem. A 2020, 8, 6776–6784. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, M.; Han, D.; Deng, Z.; Cao, X.; Tian, J.; Ye, Q. A high-performance GelMA–GelMA homogeneous double-network hydrogel assisted by 3D printing. J. Mater. Chem. B 2022, 10, 3906–3915. [Google Scholar] [CrossRef]
- Zhao, Z.; Qin, X.; Cao, L.; Li, J.; Wei, Y. Chitosan-enhanced nonswelling hydrogel with stable mechanical properties for long-lasting underwater sensing. Int. J. Biol. Macromol. 2022, 212, 123–133. [Google Scholar] [CrossRef]
- Sahoo, S.D.; Vasudha, T.; Muthuvijayan, V.; Prasad, E. Chitosan-Based Self-Healable and Adhesive Hydrogels for Flexible Strain Sensor Application. ACS Appl. Polym. Mater. 2022, 4, 9176–9185. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Chen, Y.; Zheng, X.; Liu, H.; Li, H. Multiple-stimuli-responsive and cellulose conductive ionic hydrogel for smart wearable devices and thermal actuators. ACS Appl. Mater. Interfaces 2020, 13, 1353–1366. [Google Scholar] [CrossRef]
- Kong, W.; Wang, C.; Jia, C.; Kuang, Y.; Pastel, G.; Chen, C.; Chen, G.; He, S.; Huang, H.; Zhang, J. Muscle-inspired highly anisotropic, strong, ion-conductive hydrogels. Adv. Mater. 2018, 30, 1801934. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Zhang, B.; Wan, K.; Zhu, J.; Xu, J.; Zhang, C.; Liu, T. Hydrogen-bonded network enables semi-interpenetrating ionic conductive hydrogels with high stretchability and excellent fatigue resistance for capacitive/resistive bimodal sensors. Chem. Eng. J. 2021, 411, 128506. [Google Scholar] [CrossRef]
- Khan, A.; Ahmed, S.; Sun, B.-Y.; Chen, Y.-C.; Chuang, W.-T.; Chan, Y.-H.; Gupta, D.; Wu, P.-W.; Lin, H.-C. Self-healable and anti-freezing ion conducting hydrogel-based artificial bioelectronic tongue sensing toward astringent and bitter tastes. Biosens. Bioelectron. 2022, 198, 113811. [Google Scholar] [CrossRef] [PubMed]
- Emon, M.O.F.; Alkadi, F.; Philip, D.G.; Kim, D.-H.; Lee, K.-C.; Choi, J.-W. Multi-material 3D printing of a soft pressure sensor. Addit. Manuf. 2019, 28, 629–638. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, X.; Huang, H.; Wang, H.; Lin, W.; Peng, Z. Full 3D printing of stretchable piezoresistive sensor with hierarchical porosity and multimodulus architecture. Adv. Funct. Mater. 2019, 29, 1807569. [Google Scholar] [CrossRef]
- Agarwala, S.; Goh, G.L.; Yap, Y.L.; Goh, G.D.; Yu, H.; Yeong, W.Y.; Tran, T. Development of bendable strain sensor with embedded microchannels using 3D printing. Sens. Actuators A Phys. 2017, 263, 593–599. [Google Scholar] [CrossRef]
- Ragones, H.; Schreiber, D.; Inberg, A.; Berkh, O.; Kósa, G.; Freeman, A.; Shacham-Diamand, Y. Disposable electrochemical sensor prepared using 3D printing for cell and tissue diagnostics. Sens. Actuators B Chem. 2015, 216, 434–442. [Google Scholar] [CrossRef]
- Hao, F.; Maimaitiyiming, X. Fast 3D Printing with Chitosan/Polyvinyl alcohol/Graphene Oxide Multifunctional Hydrogel Ink that has UltraStretch Properity. ChemistrySelect 2022, 7, e202200201. [Google Scholar] [CrossRef]
- Darabi, M.A.; Khosrozadeh, A.; Mbeleck, R.; Liu, Y.; Chang, Q.; Jiang, J.; Cai, J.; Wang, Q.; Luo, G.; Xing, M. Skin-inspired multifunctional autonomic-intrinsic conductive self-healing hydrogels with pressure sensitivity, stretchability, and 3D printability. Adv. Mater. 2017, 29, 1700533. [Google Scholar] [CrossRef]
- Wei, X.; Ma, K.; Cheng, Y.; Sun, L.; Chen, D.; Zhao, X.; Lu, H.; Song, B.; Yang, K.; Jia, P. Adhesive, conductive, self-healing, and antibacterial hydrogel based on chitosan–polyoxometalate complexes for wearable strain sensor. ACS Appl. Polym. Mater. 2020, 2, 2541–2549. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, L.; Guo, M.; Sun, Z.; Chen, Y.; Wu, Y.; Li, Y.; Xiang, D.; Li, H.; Li, Z. Double-network hydrogel-based stretchable, adhesive, and conductive e-skin sensor coupled human skin-like biocompatible and protective properties. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129803. [Google Scholar] [CrossRef]
- Duan, J.; Liu, F.; Kong, Y.; Hao, M.; He, J.; Wang, J.; Wang, S.; Liu, H.; Sang, Y. Homogeneous chitosan/graphene oxide nanocomposite hydrogel-based actuator driven by efficient photothermally induced water gradients. ACS Appl. Nano Mater. 2020, 3, 1002–1009. [Google Scholar] [CrossRef]
- Heijmans, K.; Nab, S.; Holkenborg, B.K.; Pathak, A.D.; Gaastra-Nedea, S.; Smeulders, D. Development of a reactive force field for CaCl2· nH2O, and the application to thermochemical energy storage. Comput. Mater. Sci. 2021, 197, 110595. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).