Metal–Organic Frameworks-Based Analytical Devices for Chiral Sensing and Separations: A Review (2012–2022)
Abstract
1. Chirality
2. Principles of Chiral Analysis
3. The Attraction of Functional Materials in Chirality
4. Metal–Organic Frameworks in Chiral Analysis
5. Metal–Organic Frameworks for Designing Enantioselective Sensors
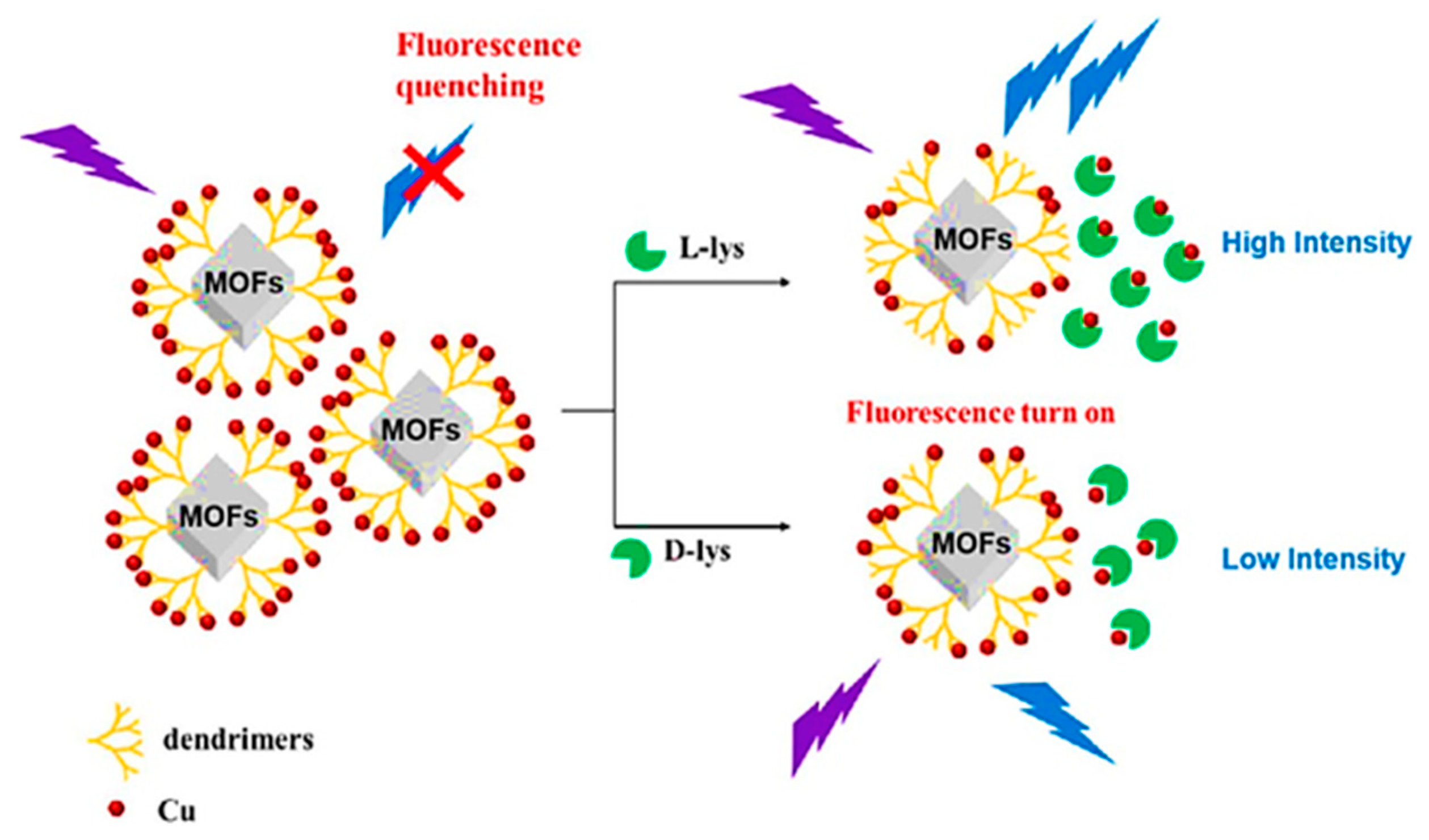
5.1. Fluorescence Enantioselective (Bio)Sensor
5.2. Circular Dichroism Enantioselective (Bio)Sensor
5.3. Quartz Crystal Microbalance Enantioselective (Bio)Sensor
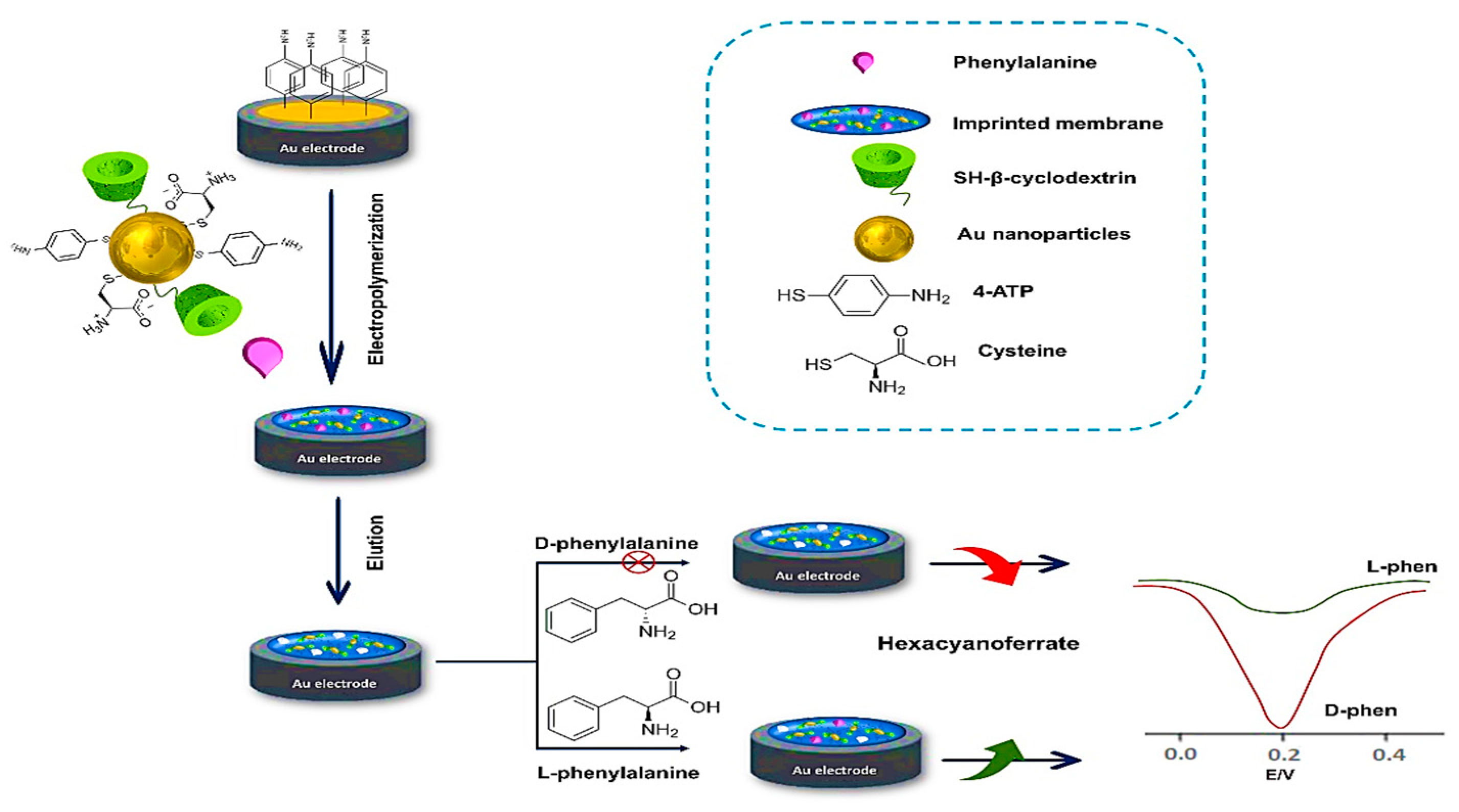
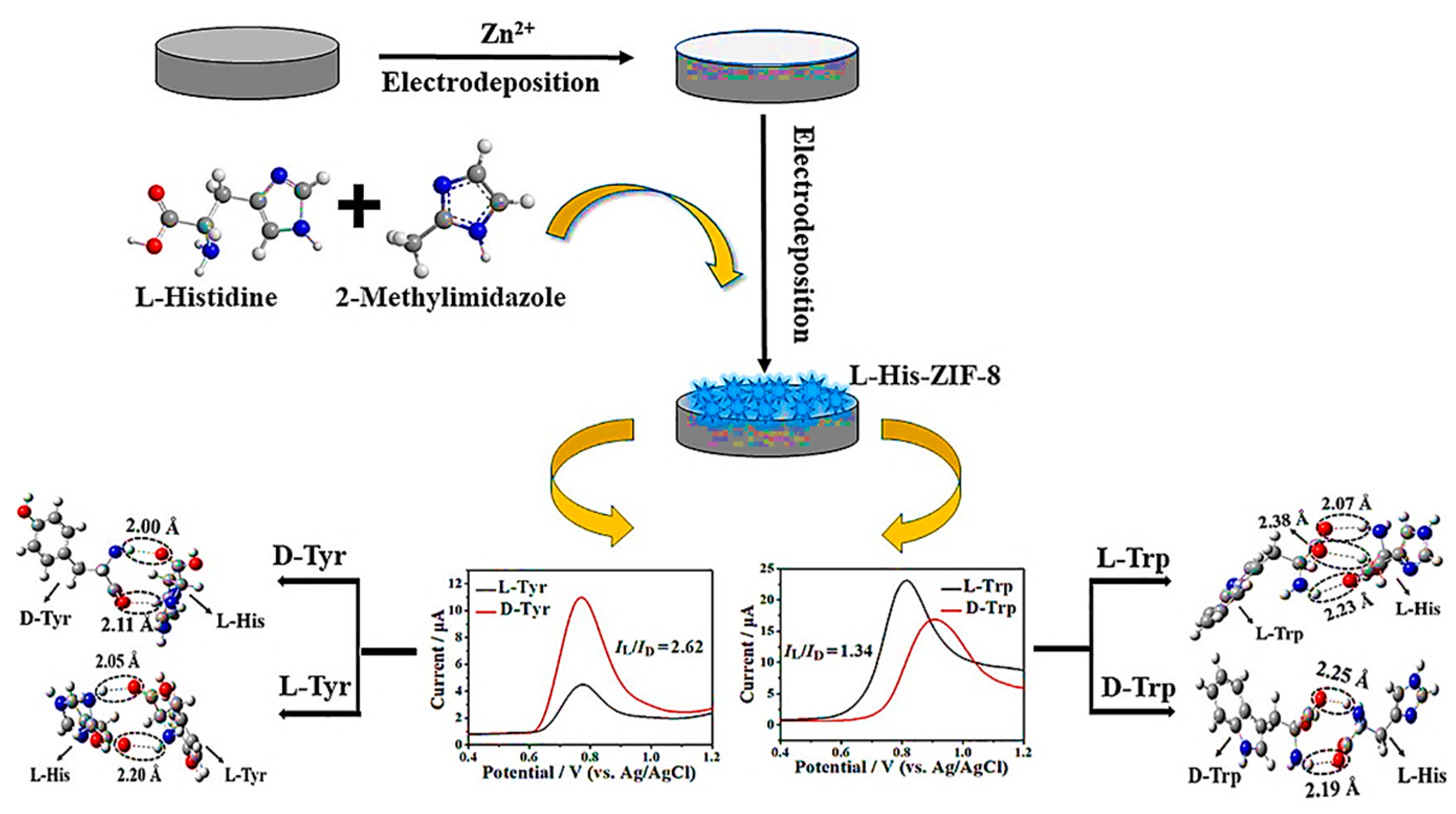
5.4. Electrochemical Enantioselective (Bio)Sensor
6. Metal–Organic Frameworks as Chiral Stationary Phases for Chromatographic Enantioseparation
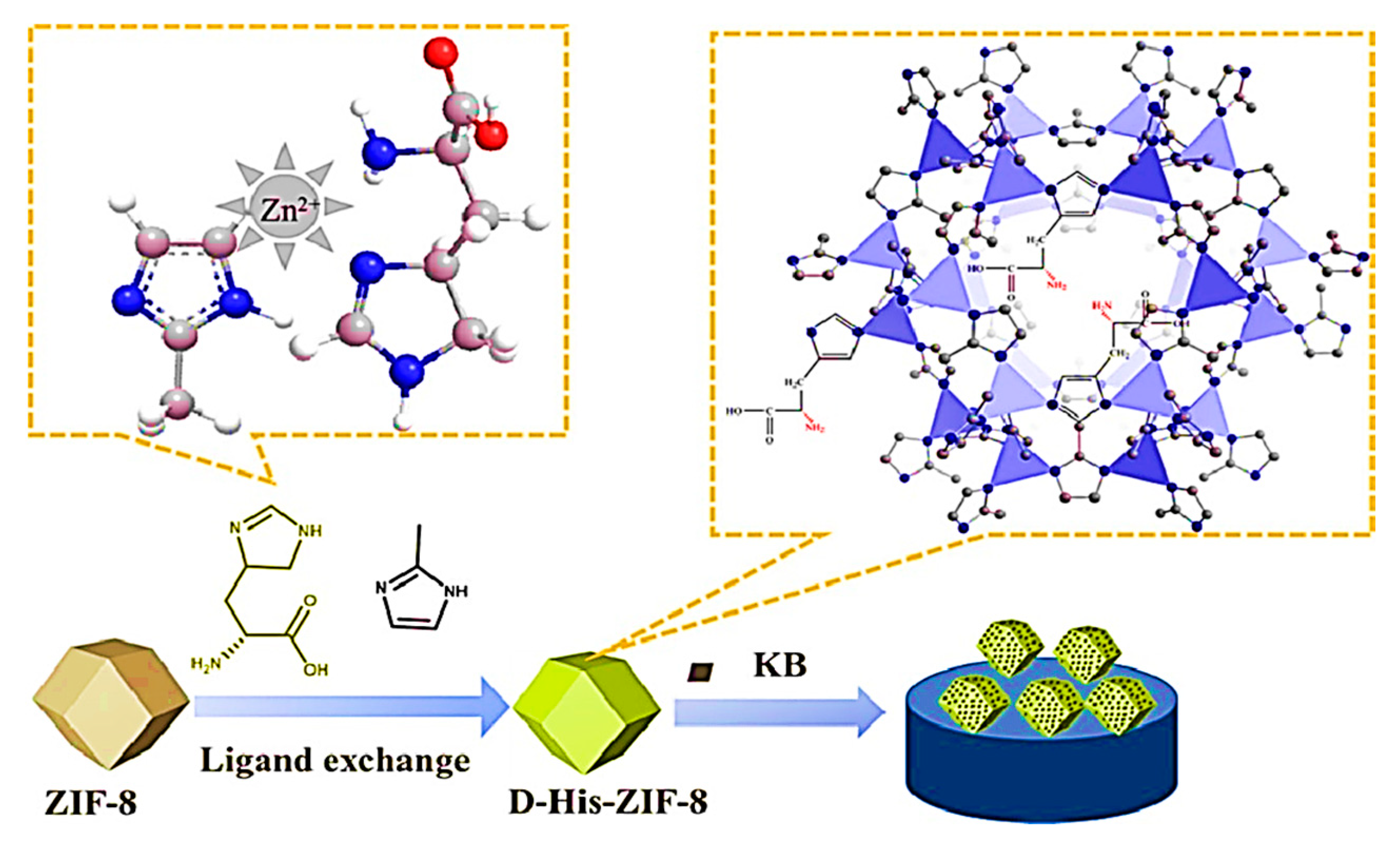
6.1. High-Performance Liquid Chromatography Chiral Separation
6.2. Gas Chromatography Enantioseparation
6.3. Capillary Electrochromatography Racemate Resolution
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milton, F.P.; Govan, J.; Mukhina, M.V.; Gun’ko, Y.K. The chiral nano-world: Chiroptically active quantum nanostructures. Nanoscale Horiz. 2016, 1, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Stalcup, A.M. Chiral separations. Annu. Rev. Anal. Chem. 2010, 3, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Andrushko, V.; Andrushko, N. Principles, concepts, and strategies of stereoselective synthesis. Stereoselective Synth. Drugs Nat. Prod. 2013, 3–44. [Google Scholar] [CrossRef]
- Purcell-Milton, F.; McKenna, R.; Brennan, L.J.; Cullen, C.P.; Guillemeney, L.; Tepliakov, N.V.; Baimuratov, A.S.; Rukhlenko, I.D.; Perova, T.S.; Duesberg, G.S. Induction of chirality in two-dimensional nanomaterials: Chiral 2D MoS2 nanostructures. ACS Nano 2018, 12, 954–964. [Google Scholar] [CrossRef] [PubMed]
- Duerinck, T.; Denayer, J. Metal–organic frameworks as stationary phases for chiral chromatographic and membrane separations. Chem. Eng. Sci. 2015, 124, 179–187. [Google Scholar] [CrossRef]
- Song, L.; Pan, M.; Zhao, R.; Deng, J.; Wu, Y. Recent advances, challenges and perspectives in enantioselective release. J. Control. Release 2020, 324, 156–171. [Google Scholar] [CrossRef]
- Trojanowicz, M. Enantioselective electrochemical sensors and biosensors: A mini-review. Electrochem. Commun. 2014, 38, 47–52. [Google Scholar] [CrossRef]
- D’Orazio, G.; Fanali, C.; Asensio-Ramos, M.; Fanali, S. Chiral separations in food analysis. TrAC Trends Anal. Chem. 2017, 96, 151–171. [Google Scholar] [CrossRef]
- Rocco, A.; Aturki, Z.; Fanali, S. Chiral separations in food analysis. TrAC Trends Anal. Chem. 2013, 52, 206–225. [Google Scholar] [CrossRef]
- Davankov, V.A. The nature of chiral recognition: Is it a three-point interaction? Chirality 1997, 9, 99–102. [Google Scholar] [CrossRef]
- Chen, S.; Ward, T. Comparison of the chiral separation of amino-acid derivatives by a teicoplanin and RN-β-CD CSPs using waterless mobile phases: Factors that enhance resolution. Chirality Pharmacol. Biol. Chem. Conseq. Mol. Asymmetry 2004, 16, 318–330. [Google Scholar]
- Nakano, Y.; Konya, Y.; Taniguchi, M.; Fukusaki, E. Development of a liquid chromatography-tandem mass spectrometry method for quantitative analysis of trace d-amino acids. J. Biosci. Bioeng. 2017, 123, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zuo, C.; Li, W.; Shi, W.; Zhou, X.; Wang, H.; Chen, S.; Du, J.; Chen, G.; Zhai, W. A novel D-peptide identified by mirror-image phage display blocks TIGIT/PVR for cancer immunotherapy. Angew. Chem. Int. Ed. 2020, 59, 15114–15118. [Google Scholar] [CrossRef] [PubMed]
- Fanali, S.; Chankvetadze, B. History, advancement, bottlenecks, and future of chiral capillary electrochromatography. J. Chromatogr. A 2021, 1637, 461832. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Lv, S.; Zhang, K.; Tang, D. Optical transformation of a CdTe quantum dot-based paper sensor for a visual fluorescence immunoassay induced by dissolved silver ions. J. Mater. Chem. B 2017, 5, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Pätzold, R.; Brückner, H. Gas chromatographic determination and mechanism of formation of D-amino acids occurring in fermented and roasted cocoa beans, cocoa powder, chocolate and cocoa shell. Amino Acids 2006, 31, 63–72. [Google Scholar] [CrossRef]
- West, C. Recent trends in chiral supercritical fluid chromatography. TrAC Trends Anal. Chem. 2019, 120, 115648. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Pei, D.; Liu, J.-F.; Di, D.-L. A review on chiral separation by counter-current chromatography: Development, applications and future outlook. J. Chromatogr. A 2018, 1531, 1–12. [Google Scholar] [CrossRef]
- Liang, Y.-D.; Song, J.-F. Flow-injection chemiluminescence determination of tryptophan through its peroxidation and epoxidation by peroxynitrous acid. J. Pharm. Biomed. Anal. 2005, 38, 100–106. [Google Scholar] [CrossRef]
- Vashistha, V.K.; Sethi, S.; Kumar, A.; Ahmed, S.; Das, D.K. Application of NMR Spectroscopy in Chiral Recognition of Drugs. Appl. NMR Spectrosc. 2021, 9, 131. [Google Scholar]
- Han, D.-Q.; Yao, Z.-P. Chiral mass spectrometry: An overview. TrAC Trends Anal. Chem. 2020, 123, 115763. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, N.; Cao, Q.; Zhang, L.; Wang, S.; Yao, W.; Chao, F. Immunochip for the detection of five kinds of chemicals: Atrazine, nonylphenol, 17-beta estradiol, paraverine and chloramphenicol. Biosens. Bioelectron. 2009, 24, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Huang, Y.; Yang, J.; Guo, Y.; Zeng, X.; Zhou, S.; Cheng, J.; Zhang, Y. An aptamer-based fluorescence bio-sensor for chiral recognition of arginine enantiomers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xue, S.; Li, A.; Ren, S. Functional materials in chiral capillary electrophoresis. Coord. Chem. Rev. 2021, 445, 214108. [Google Scholar] [CrossRef]
- Rutkowska, M.; Płotka-Wasylka, J.; Morrison, C.; Wieczorek, P.P.; Namieśnik, J.; Marć, M. Application of molecularly imprinted polymers in analytical chiral separations and analysis. TrAC Trends Anal. Chem. 2018, 102, 91–102. [Google Scholar] [CrossRef]
- Wu, Q.; Lv, H.; Zhao, L. Applications of carbon nanomaterials in chiral separation. TrAC Trends Anal. Chem. 2020, 129, 115941. [Google Scholar] [CrossRef]
- Tiwari, M.P.; Prasad, A. Molecularly imprinted polymer based enantioselective sensing devices: A review. Anal. Chim. Acta 2015, 853, 1–18. [Google Scholar] [CrossRef]
- Fanali, C. Enantiomers separation by capillary electrochromatography. TrAC Trends Anal. Chem. 2019, 120, 115640. [Google Scholar] [CrossRef]
- Zhang, Q. Ionic liquids in capillary electrophoresis for enantioseparation. TrAC Trends Anal. Chem. 2018, 100, 145–154. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Lei, J.; Qian, R.; Ling, P.; Cui, L.; Ju, H. Design and sensing applications of metal–organic framework composites. TrAC Trends Anal. Chem. 2014, 58, 71–78. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Khan, M.I.; Li, X.; Zhu, Q.-L.; Wu, X.-T. Recent progress in asymmetric catalysis and chromatographic separation by chiral metal–organic frameworks. Catalysts 2018, 8, 120. [Google Scholar] [CrossRef]
- Liu, K.-G.; Sharifzadeh, Z.; Rouhani, F.; Ghorbanloo, M.; Morsali, A. Metal-organic framework composites as green/sustainable catalysts. Coord. Chem. Rev. 2021, 436, 213827. [Google Scholar] [CrossRef]
- Berthod, A. Chiral recognition mechanisms in enantiomers separations: A general view. In Chiral Recognition in Separation Methods; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–32. [Google Scholar]
- Yan, Z.-H.; Li, D.; Yin, X.-B. Review for chiral-at-metal complexes and metal-organic framework enantiomorphs. Sci. Bull. 2017, 62, 1344–1354. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-organic frameworks in green analytical chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef]
- Gong, W.; Chen, Z.; Dong, J.; Liu, Y.; Cui, Y. Chiral Metal–Organic Frameworks. Chem. Rev. 2022, 122, 9078–9144. [Google Scholar] [CrossRef]
- Yuan, S.; Deng, Y.-K.; Xuan, W.-M.; Wang, X.-P.; Wang, S.-N.; Dou, J.-M.; Sun, D. Spontaneous chiral resolution of a 3D (3, 12)-connected MOF with an unprecedented ttt topology consisting of cubic [Cd 4 (μ 3-OH) 4] clusters and propeller-like ligands. CrystEngComm 2014, 16, 3829–3833. [Google Scholar] [CrossRef]
- Sharifzadeh, Z.; Berijani, K.; Morsali, A. Chiral metal–organic frameworks based on asymmetric synthetic strategies and applications. Coord. Chem. Rev. 2021, 445, 214083. [Google Scholar] [CrossRef]
- Berijani, K.; Chang, L.-M.; Gu, Z.-G. Chiral templated synthesis of homochiral metal-organic frameworks. Coord. Chem. Rev. 2023, 474, 214852. [Google Scholar] [CrossRef]
- Yutkin, M.; Zavakhina, M.; Samsonenko, D.; Dybtsev, D.; Fedin, V. Porous homo-and heterochiral cobalt (II) aspartates with high thermal stability of the metal-organic framework. Russ. Chem. Bull. 2010, 59, 733–740. [Google Scholar] [CrossRef]
- Burchell, T.J.; Puddephatt, R.J. Homochiral and heterochiral coordination polymers and networks of silver (I). Inorg. Chem. 2006, 45, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Dai, Q.; Zheng, H.; Chen, M.; Dai, L. Novel MOF-derived Co@ N-C bifunctional catalysts for highly efficient Zn–air batteries and water splitting. Adv. Mater. 2018, 30, 1705431. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.; Velazquez, E.R.; Leijenaar, R.T.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Corrigendum: Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4644. [Google Scholar] [CrossRef]
- Newsam, J.; Treacy, M.M.; Koetsier, W.; Gruyter, C.D. Structural characterization of zeolite beta. Proc. R. Soc. London. A. Math. Phys. Sci. 1988, 420, 375–405. [Google Scholar]
- Peng, Y.; Gong, T.; Zhang, K.; Lin, X.; Liu, Y.; Jiang, J.; Cui, Y. Engineering chiral porous metal-organic frameworks for enantioselective adsorption and separation. Nat. Commun. 2014, 5, 4406. [Google Scholar] [CrossRef]
- Wang, X.; Lamprou, A.; Svec, F.; Bai, Y.; Liu, H. Polymer-based monolithic column with incorporated chiral metal–organic framework for enantioseparation of methyl phenyl sulfoxide using nano-liquid chromatography. J. Sep. Sci. 2016, 39, 4544–4548. [Google Scholar] [CrossRef]
- Zor, E.; Bingol, H.; Ersoz, M. Chiral sensors. TrAC Trends Anal. Chem. 2019, 121, 115662. [Google Scholar] [CrossRef]
- Huang, H.; Bian, G.; Zong, H.; Wang, Y.; Yang, S.; Yue, H.; Song, L.; Fan, H. Chiral sensor for enantiodiscrimination of varied acids. Org. Lett. 2016, 18, 2524–2527. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Li, H.; Liu, J.; Liu, Z.; Wang, K. Application of chiral materials in electrochemical sensors. Microchim. Acta 2020, 187, 676. [Google Scholar] [CrossRef]
- Liu, D.; Lu, K.; Poon, C.; Lin, W. Metal–organic frameworks as sensory materials and imaging agents. Inorg. Chem. 2014, 53, 1916–1924. [Google Scholar] [CrossRef]
- Saadati, A.; Hassanpour, S.; de la Guardia, M.; Mosafer, J.; Hashemzaei, M.; Mokhtarzadeh, A.; Baradaran, B. Recent advances on application of peptide nucleic acids as a bioreceptor in biosensors development. TrAC Trends Anal. Chem. 2019, 114, 56–68. [Google Scholar] [CrossRef]
- Kholafazad Kordasht, H.; Hassanpour, S.; Baradaran, B.; Nosrati, R.; Hashemzaei, M.; Mokhtarzadeh, A.; de la Guardia, M. Biosensing of microcystins in water samples; recent advances. Biosens. Bioelectron. 2020, 165, 112403. [Google Scholar] [CrossRef] [PubMed]
- Pugh, V.J.; Hu, Q.S.; Pu, L. The first dendrimer-based enantioselective fluorescent sensor for the recognition of chiral amino alcohols. Angew. Chem. 2000, 112, 3784–3787. [Google Scholar] [CrossRef]
- Pu, L. Enantioselective fluorescent sensors: A tale of BINOL. Acc. Chem. Res. 2012, 45, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Wanderley, M.M.; Wang, C.; Wu, C.-D.; Lin, W. A chiral porous metal–organic framework for highly sensitive and enantioselective fluorescence sensing of amino alcohols. J. Am. Chem. Soc. 2012, 134, 9050–9053. [Google Scholar] [CrossRef]
- Chandrasekhar, P.; Mukhopadhyay, A.; Savitha, G.; Moorthy, J.N. Remarkably selective and enantiodifferentiating sensing of histidine by a fluorescent homochiral Zn-MOF based on pyrene-tetralactic acid. Chem. Sci. 2016, 7, 3085–3091. [Google Scholar] [CrossRef]
- Zhao, X.; Nguyen, E.T.; Hong, A.N.; Feng, P.; Bu, X. Chiral isocamphoric acid: Founding a large family of homochiral porous materials. Angew. Chem. Int. Ed. 2018, 57, 7101–7105. [Google Scholar] [CrossRef]
- Han, Z.; Wang, K.; Guo, Y.; Chen, W.; Zhang, J.; Zhang, X.; Siligardi, G.; Yang, S.; Zhou, Z.; Sun, P. Cation-induced chirality in a bifunctional metal-organic framework for quantitative enantioselective recognition. Nat. Commun. 2019, 10, 5117. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Yan, B. Zirconium Metal Organic Framework-Based Hybrid Sensors with Chiral and Luminescent Centers Fabricated by Postsynthetic Modification for the Detection and Recognition of Tryptophan Enantiomers. Inorg. Chem. 2022, 61, 9615–9622. [Google Scholar] [CrossRef]
- Guo, D.; Zhou, X.; Huang, S.; Zhu, Y. Enantioselective fluorescent detection of lysine enantiomers by functionalized achiral metal organic frameworks. Microchem. J. 2022, 181, 107666. [Google Scholar] [CrossRef]
- Freedman, T.B.; Cao, X.; Dukor, R.K.; Nafie, L.A. Absolute configuration determination of chiral molecules in the solution state using vibrational circular dichroism. Chirality 2003, 15, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yin, J.; Yoon, J. Recent advances in development of chiral fluorescent and colorimetric sensors. Chem. Rev. 2014, 114, 4918–4959. [Google Scholar] [CrossRef] [PubMed]
- Neidig, M.L.; Wecksler, A.T.; Schenk, G.; Holman, T.R.; Solomon, E.I. Kinetic and spectroscopic studies of N694C lipoxygenase: A probe of the substrate activation mechanism of a nonheme ferric enzyme. J. Am. Chem. Soc. 2007, 129, 7531–7537. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.; Luong, N.; Fan, Z.; Kuzyk, A.; Nickels, P.C.; Zhang, T.; Smith, D.M.; Yurke, B.; Kuang, W.; Govorov, A.O. Chiral plasmonic DNA nanostructures with switchable circular dichroism. Nat. Commun. 2013, 4, 2948. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; Di Bari, L.; Pescitelli, G. Application of electronic circular dichroism in configurational and conformational analysis of organic compounds. Chem. Soc. Rev. 2007, 36, 914–931. [Google Scholar] [CrossRef]
- Wang, F.; Tang, Y.-H.; Zhang, J. Achievement of bulky homochirality in zeolitic imidazolate-related frameworks. Inorg. Chem. 2015, 54, 11064–11066. [Google Scholar] [CrossRef]
- Li, M.-Y.; Wang, F.; Gu, Z.-G.; Zhang, J. Synthesis of homochiral zeolitic metal–organic frameworks with amino acid and tetrazolates for chiral recognition. RSC Adv. 2017, 7, 4872–4875. [Google Scholar] [CrossRef]
- Zhao, Y.-W.; Wang, Y.; Zhang, X.-M. Homochiral MOF as circular dichroism sensor for enantioselective recognition on nature and chirality of unmodified amino acids. ACS Appl. Mater. Interfaces 2017, 9, 20991–20999. [Google Scholar] [CrossRef]
- Yao, R.-X.; Fu, H.-H.; Yu, B.; Zhang, X.-M. Chiral metal–organic frameworks constructed from four-fold helical chain SBUs for enantioselective recognition of α-hydroxy/amino acids. Inorg. Chem. Front. 2018, 5, 153–159. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, Y.; Zhang, X.; Sun, Z.; Jiao, C. Homochiral MOF as Chiroptical Sensor for Determination of Absolute Configuration and Enantiomeric Ratio of Chiral Tryptophan. Adv. Opt. Mater. 2021, 9, 2001889. [Google Scholar] [CrossRef]
- Duan, H.-J.; Yang, C.-X.; Yan, X.-P. Chiral metal–organic framework coated quartz crystal microbalance for chiral discrimination. RSC Adv. 2015, 5, 30577–30582. [Google Scholar] [CrossRef]
- Liu, B.; Shekhah, O.; Arslan, H.K.; Liu, J.; Wöll, C.; Fischer, R.A. Enantiopure metal–organic framework thin films: Oriented SURMOF growth and enantioselective adsorption. Angew. Chem. Int. Ed. 2012, 51, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-H.; Gu, Z.-G.; Zhang, J. Surface-coordinated metal–organic framework thin films (SURMOFs) for electrocatalytic applications. Nanoscale 2020, 12, 12712–12730. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-M.; Chang, L.-M.; Yang, X.-K.; Luo, T.; Xu, H.; Gu, Z.-G.; Zhang, J. Liquid-Phase Epitaxial Growth of Azapyrene-Based Chiral Metal–Organic Framework Thin Films for Circularly Polarized Luminescence. ACS Appl. Mater. Interfaces 2019, 11, 31421–31426. [Google Scholar] [CrossRef] [PubMed]
- Cakici, M.; Gu, Z.-G.; Nieger, M.; Bürck, J.; Heinke, L.; Bräse, S. Planar-chiral building blocks for metal–organic frameworks. Chem. Commun. 2015, 51, 4796–4798. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-L.; Zang, R.-B.; Shao, R.; Guan, R.-F.; Xie, M.-H. Chiral UiO-MOFs based QCM sensors for enantioselective discrimination of hazardous biomolecule. J. Hazard. Mater. 2021, 413, 125467. [Google Scholar] [CrossRef]
- Okur, S.; Qin, P.; Chandresh, A.; Li, C.; Zhang, Z.; Lemmer, U.; Heinke, L. An enantioselective e-nose: An array of nanoporous homochiral MOF films for stereospecific sensing of chiral odors. Angew. Chem. Int. Ed. 2021, 60, 3566–3571. [Google Scholar] [CrossRef]
- Hassanpour, S.; Baradaran, B.; Hejazi, M.; Hasanzadeh, M.; Mokhtarzadeh, A.; de la Guardia, M. Recent trends in rapid detection of influenza infections by bio and nanobiosensor. TrAC Trends Anal. Chem. 2018, 98, 201–215. [Google Scholar] [CrossRef]
- Zhu, G.; Kingsford, O.J.; Yi, Y.; Wong, K.-y. Recent advances in electrochemical chiral recognition. J. Electrochem. Soc. 2019, 166, H205. [Google Scholar] [CrossRef]
- Liu, W.; Yin, X.-B. Metal–organic frameworks for electrochemical applications. TrAC Trends Anal. Chem. 2016, 75, 86–96. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal–organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Sun, T.; Geng, F.; Chen, P.; Gao, Y. Metal-organic frameworks-based electrochemical sensors and biosensors. Int. J. Electrochem. Sci. 2019, 14, 5287–5304. [Google Scholar] [CrossRef]
- Chuang, C.H.; Kung, C.W. Metal—Organic frameworks toward electrochemical sensors: Challenges and opportunities. Electroanalysis 2020, 32, 1885–1895. [Google Scholar] [CrossRef]
- Kuang, R.; Zheng, L.; Chi, Y.; Shi, J.; Chen, X.; Zhang, C. Highly efficient electrochemical recognition and quantification of amine enantiomers based on a guest-free homochiral MOF. RSC Adv. 2017, 7, 11701–11706. [Google Scholar] [CrossRef]
- Wu, T.; Wei, X.; Ma, X.; Li, J. Amperometric sensing of L-phenylalanine using a gold electrode modified with a metal organic framework, a molecularly imprinted polymer, and β-cyclodextrin-functionalized gold nanoparticles. Microchim. Acta 2017, 184, 2901–2907. [Google Scholar] [CrossRef]
- Wei, X.; Li, L.; Lian, H.; Cao, X.; Liu, B. Grain-like chiral metal-organic framework/multi-walled carbon nanotube composited electrosensing interface for enantiorecognition of Tryptophan. J. Electroanal. Chem. 2021, 886, 115108. [Google Scholar] [CrossRef]
- Hou, Y.; Liang, J.; Kuang, X.; Kuang, R. Simultaneous electrochemical recognition of tryptophan and penicillamine enantiomers based on MOF-modified β-CD. Carbohydr. Polym. 2022, 290, 119474. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Yan, S.; Chen, J.; Zhang, M.; Zhao, R.; Niu, X.; Wang, K. Fusiform-like metal-organic framework for enantioselective discrimination of tryptophan enantiomers. Electrochim. Acta 2022, 419, 140409. [Google Scholar] [CrossRef]
- Niu, X.; Yan, S.; Chen, J.; Li, H.; Wang, K. Enantioselective recognition of L/D-amino acids in the chiral nanochannels of a metal-organic framework. Electrochim. Acta 2022, 405, 139809. [Google Scholar] [CrossRef]
- Liu, N.; Yang, B.; Yin, Z.-Z.; Cai, W.; Li, J.; Kong, Y. A chiral sensing platform based on chiral metal-organic framework for enantiodiscrimination of the isomers of tyrosine and tryptophan. J. Electroanal. Chem. 2022, 918, 116445. [Google Scholar] [CrossRef]
- Long, Z.; Jia, J.; Wang, S.; Kou, L.; Hou, X.; Sepaniak, M.J. Visual enantioselective probe based on metal organic framework incorporating quantum dots. Microchem. J. 2013, 110, 764–769. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, M.; Kong, F.; Yang, Y. A water-stable homochiral luminescent MOF constructed from an achiral acylamide-containing dicarboxylate ligand for enantioselective sensing of penicillamine. Chem. Commun. 2018, 54, 10901–10904. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, K.; Min, H.; Xu, J.; Shi, W.; Cheng, P. Bifunctionalized Metal–Organic Frameworks for Pore-Size-Dependent Enantioselective Sensing. Angew. Chem. Int. Ed. 2022, 61, e202204066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Chen, X.; Liu, Y.; Han, Y.; Cui, Y. Single-crystalline ultrathin 2D porous nanosheets of chiral metal–organic frameworks. J. Am. Chem. Soc. 2021, 143, 3509–3518. [Google Scholar] [CrossRef]
- Tan, C.; Yang, K.; Dong, J.; Liu, Y.; Liu, Y.; Jiang, J.; Cui, Y. Boosting enantioselectivity of chiral organocatalysts with ultrathin two-dimensional metal–organic framework nanosheets. J. Am. Chem. Soc. 2019, 141, 17685–17695. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, X.; Xu, X.; Tian, F.; Liu, Z. Fabrication of a “turn-on”-type enantioselective fluorescence sensor via a modified achiral MOF: Applications for synchronous detection of phenylalaninol enantiomers. Analyst 2021, 146, 937–942. [Google Scholar] [CrossRef]
- Kuang, X.; Ye, S.; Li, X.; Ma, Y.; Zhang, C.; Tang, B. A new type of surface-enhanced Raman scattering sensor for the enantioselective recognition of d/l-cysteine and d/l-asparagine based on a helically arranged Ag NPs@ homochiral MOF. Chem. Commun. 2016, 52, 5432–5435. [Google Scholar] [CrossRef]
- Zhao, Y.-W.; Guo, L.-E.; Zhang, F.-Q.; Yao, J.; Zhang, X.-M. Turn-On Fluorescence Enantioselective Sensing of Hydroxyl Carboxylic Enantiomers by Metal–Organic Framework Nanosheets with a Homochiral Tetracarboxylate of Cyclohexane Diamide. ACS Appl. Mater. Interfaces 2021, 13, 20821–20829. [Google Scholar] [CrossRef]
- Kuang, X.; Ma, Y.; Zhang, C.; Su, H.; Zhang, J.; Tang, B. A new synthesis strategy for chiral CdS nanotubes based on a homochiral MOF template. Chem. Commun. 2015, 51, 5955–5958. [Google Scholar] [CrossRef][Green Version]
- Shili, Q.; Yangyang, S.; Xudong, H.; Hongtao, C.; Lidi, G.; Zhongyu, H.; Dongsheng, Z.; Xinyao, L.; Sibing, Z. Chiral fluorescence recognition of glutamine enantiomers by a modified Zr-based MOF based on solvent-assisted ligand incorporation. RSC Adv. 2021, 11, 37584–37594. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, X.; Ran, P.; Xia, Q.; Yang, C.; Ma, J.; Fu, Y. A novel luminescence-functionalized metal-organic framework nanoflowers electrochemiluminesence sensor via “on-off” system. Biosens. Bioelectron. 2017, 91, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-H.; Li, T.; Chen, J.-H.; Ma, J.-G.; Cheng, P. The electrochemical discrimination of pinene enantiomers by a cyclodextrin metal–organic framework. Dalton Trans. 2017, 46, 6830–6834. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Liu, Z.; Tong, L.; Zhao, L.; Kuang, X.; Kuang, R.; Ju, H. One-step electrodeposition of the MOF@ CCQDs/NiF electrode for chiral recognition of tyrosine isomers. Dalton Trans. 2020, 49, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Kuang, X.; Kuang, R.; Tong, L.; Liu, Z.; Hou, Y.; Sun, X.; Wang, Z.; Wei, Q. MOF-based supramolecule helical nanomaterials: Toward highly enantioselective electrochemical recognition of penicillamine. ACS Appl. Mater. Interfaces 2019, 12, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Guo, J.; Dai, Z.; Liu, C.; Zhao, J.; Gao, Z.; Song, Y.-Y. Engineering homochiral MOFs in TiO2 nanotubes as enantioselective photoelectrochemical electrode for chiral recognition. Anal. Chem. 2021, 93, 12067–12074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qiao, C.; Cai, X.; Xia, Z.; Han, J.; Yang, Q.; Zhou, C.; Chen, S.; Gao, S. Microcalorimetry-guided pore-microenvironment optimization to improve sensitivity of Ni-MOF electrochemical biosensor for chiral galantamine. Chem. Eng. J. 2021, 426, 130730. [Google Scholar] [CrossRef]
- Liu, Z.; Kuang, X.; Sun, X.; Zhang, Y.; Wei, Q. Electrochemical enantioselective recognition penicillamine isomers based on chiral C-dots/MOF hybrid arrays. J. Electroanal. Chem. 2019, 846, 113151. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Y.; He, S.; Lian, H.; Cao, X.; Liu, B. L-histidine-regulated zeolitic imidazolate framework modified electrochemical interface for enantioselective determination of L-glutamate. Electrochim. Acta 2021, 400, 139464. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Feng, P.-Q.; Guo, Z.; Wei, X. Water-sTable 1D double-chain Cu metal–organic framework-based electrochemical biosensor for detecting l-tyrosine. Langmuir 2020, 36, 14123–14129. [Google Scholar] [CrossRef]
- Tistaert, C.; Dejaegher, B.; Vander Heyden, Y. Chromatographic separation techniques and data handling methods for herbal fingerprints: A review. Anal. Chim. Acta 2011, 690, 148–161. [Google Scholar] [CrossRef]
- Li, X.; Chang, C.; Wang, X.; Bai, Y.; Liu, H. Applications of homochiral metal-organic frameworks in enantioselective adsorption and chromatography separation. Electrophoresis 2014, 35, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-Y.; Yang, C.-X.; Chang, N.; Yan, X.-P. Metal–organic frameworks for analytical chemistry: From sample collection to chromatographic separation. Acc. Chem. Res. 2012, 45, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Corella-Ochoa, M.N.; Tapia, J.B.; Rubin, H.N.; Lillo, V.; González-Cobos, J.; Núñez-Rico, J.L.; Balestra, S.R.; Almora-Barrios, N.; Lledós, M.; Güell-Bara, A. Homochiral metal–organic frameworks for enantioselective separations in liquid chromatography. J. Am. Chem. Soc. 2019, 141, 14306–14316. [Google Scholar] [CrossRef] [PubMed]
- Firooz, S.K.; Armstrong, D.W. Metal-organic frameworks in separations: A review. Anal. Chim. Acta 2022, 1234, 340208. [Google Scholar] [CrossRef]
- Si, T.; Lu, X.; Zhang, H.; Wang, S.; Liang, X.; Guo, Y. Metal-organic framework-based core-shell composites for chromatographic stationary phases. TrAC Trends Anal. Chem. 2022, 149, 116545. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Dybtsev, D.N.; Bryliakov, K.P.; Talsi, E.P.; Fedin, V.P. Enantioselective chromatographic resolution and one-pot synthesis of enantiomerically pure sulfoxides over a homochiral Zn- organic framework. J. Am. Chem. Soc. 2007, 129, 12958–12959. [Google Scholar] [CrossRef]
- Tanaka, K.; Muraoka, T.; Hirayama, D.; Ohnish, A. Highly efficient chromatographic resolution of sulfoxides using a new homochiral MOF–silica composite. Chem. Commun. 2012, 48, 8577–8579. [Google Scholar] [CrossRef]
- Kuang, X.; Ma, Y.; Su, H.; Zhang, J.; Dong, Y.-B.; Tang, B. High-performance liquid chromatographic enantioseparation of racemic drugs based on homochiral metal–organic framework. Anal. Chem. 2014, 86, 1277–1281. [Google Scholar] [CrossRef]
- Hartlieb, K.J.; Holcroft, J.M.; Moghadam, P.Z.; Vermeulen, N.A.; Algaradah, M.M.; Nassar, M.S.; Botros, Y.Y.; Snurr, R.Q.; Stoddart, J.F. CD-MOF: A versatile separation medium. J. Am. Chem. Soc. 2016, 138, 2292–2301. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Li, X.; Yu, A.; Zhang, S. Controlled fabrication of core-shell silica@ chiral metal-organic framework for significant improvement chromatographic separation of enantiomers. Talanta 2020, 218, 121155. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, N.; Zhang, J.; Wang, B.; Xie, S.; Yuan, L. Chiral metal–organic framework d-His-ZIF-8@ SiO2 core–shell microspheres used for HPLC enantioseparations. ACS Appl. Mater. Interfaces 2020, 12, 16903–16911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, K.; Zhao, X.; Zhang, W.; Liu, Y.; Jiang, J.; Cui, Y. Highly stable Zr (IV)-based metal–organic frameworks for chiral separation in reversed-phase liquid chromatography. J. Am. Chem. Soc. 2020, 143, 390–398. [Google Scholar] [CrossRef]
- Chen, J.-K.; Yu, Y.-Y.; Xu, N.-Y.; Guo, P.; Zhang, J.-H.; Wang, B.-J.; Xie, S.-M.; Yuan, L.-M. Chiral polyaniline modified Metal-Organic framework Core-Shell composite MIL-101@ c-PANI for HPLC enantioseparation. Microchem. J. 2021, 169, 106576. [Google Scholar] [CrossRef]
- Ma, X.; Guo, Y.; Zhang, L.; Wang, K.; Yu, A.; Zhang, S.; Ouyang, G. Crystal morphology tuning and green post-synthetic modification of metal organic framework for HPLC enantioseparation. Talanta 2022, 239, 123143. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Muraoka, T.; Otubo, Y.; Takahashi, H.; Ohnishi, A. HPLC enantioseparation on a homochiral MOF–silica composite as a novel chiral stationary phase. RSC Adv. 2016, 6, 21293–21301. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Zhang, J.; Kong, J.; Yuan, L. A 3D Homochiral MOF [Cd2 (d-cam) 3]• 2Hdma• 4dma for HPLC Chromatographic Enantioseparation. Chirality 2016, 28, 340–346. [Google Scholar] [CrossRef]
- Yuan, B.; Li, L.; Yu, Y.; Xu, N.; Fu, N.; Zhang, J.; Zhang, M.; Wang, B.; Xie, S.; Yuan, L. Chiral metal-organic framework [Co2 (D-cam) 2 (TMDPy)]@ SiO2 core-shell microspheres for HPLC separation. Microchem. J. 2021, 161, 105815. [Google Scholar] [CrossRef]
- Zhang, M.; Pu, Z.-J.; Chen, X.-L.; Gong, X.-L.; Zhu, A.-X.; Yuan, L.-M. Chiral recognition of a 3D chiral nanoporous metal–organic framework. Chem. Commun. 2013, 49, 5201–5203. [Google Scholar] [CrossRef]
- Tanaka, K.; Hotta, N.; Nagase, S.; Yoza, K. Efficient HPLC enantiomer separation using a pillared homochiral metal–organic framework as a novel chiral stationary phase. New J. Chem. 2016, 40, 4891–4894. [Google Scholar] [CrossRef]
- Zhang, M.; Xue, X.-D.; Zhang, J.-H.; Xie, S.-M.; Zhang, Y.; Yuan, L.-M. Enantioselective chromatographic resolution using a homochiral metal–organic framework in HPLC. Anal. Methods 2014, 6, 341–346. [Google Scholar] [CrossRef]
- Hailili, R.; Wang, L.; Qv, J.; Yao, R.; Zhang, X.-M.; Liu, H. Planar Mn4O cluster homochiral metal–organic framework for HPLC separation of pharmaceutically important (±)-ibuprofen racemate. Inorg. Chem. 2015, 54, 3713–3715. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Hu, C.; Li, L.; Zhang, J.; Fu, N.; Wang, B.; Yuan, L. Homochiral metal-organic framework for HPLC separation of enantiomers. Microchem. J. 2018, 139, 487–491. [Google Scholar] [CrossRef]
- Yang, C.-X.; Zheng, Y.-Z.; Yan, X.-P. γ-Cyclodextrin metal–organic framework for efficient separation of chiral aromatic alcohols. RSC Adv. 2017, 7, 36297–36301. [Google Scholar] [CrossRef]
- Zhang, J.H.; Nong, R.Y.; Xie, S.M.; Wang, B.J.; Ai, P.; Yuan, L.M. Homochiral metal-organic frameworks based on amino acid ligands for HPLC separation of enantiomers. Electrophoresis 2017, 38, 2513–2520. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.-H.; Zhang, Y.; Wang, B.-J.; Xie, S.-M.; Yuan, L.-M. Chromatographic study on the high performance separation ability of a homochiral [Cu2 (d-Cam) 2 (4, 4′-bpy)] n based-column by using racemates and positional isomers as test probes. J. Chromatogr. A 2014, 1325, 163–170. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Zhang, J.-H.; He, Y.-J.; Li, Q.; Luo, L.; Zhang, M.; Yuan, L.-M. Homochiral metal-organic framework immobilized on silica gel by the interfacial polymerization for HPLC enantioseparations. J. Liq. Chromatogr. Relat. Technol. 2018, 41, 903–909. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, B.; Hu, C.; Fu, N.; Xu, N.; Zhang, J.; Wang, B.; Xie, S.; Yuan, L. Homochiral Metal–Organic Framework [Co (L)(bpe) 2 (H2O) 2]· H2O Used for Separation of Racemates in High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2021, 59, 355–360. [Google Scholar] [CrossRef]
- Chen, J.K.; Xu, N.Y.; Guo, P.; Wang, B.J.; Zhang, J.H.; Xie, S.M.; Yuan, L.M. A chiral metal-organic framework core-shell microspheres composite for high-performance liquid chromatography enantioseparation. J. Sep. Sci. 2021, 44, 3976–3985. [Google Scholar] [CrossRef]
- Xu, N.; Yuan, B.; Hu, C.; Yu, Y.; Fu, N.; Zhang, J.; Xie, S.; Yuan, L. Homochiral Metal–Organic Framework [Ni (S-mal)(bpy)] n Used for the Separation of Racemic Compounds by High Performance Liquid Chromatography. J. Anal. Chem. 2021, 76, 749–754. [Google Scholar] [CrossRef]
- Menezes, T.; Santos, K.; Franceschi, E.; Borges, G.; Dariva, C.; Egues, S.; De Conto, J.; Santana, C. Synthesis of the chiral stationary phase based on functionalized ZIF-8 with amylose carbamate. J. Mater. Res. 2020, 35, 2936–2949. [Google Scholar] [CrossRef]
- Yohannes, A.; Feng, X.; Yao, S. Dispersive solid-phase extraction of racemic drugs using chiral ionic liquid-metal-organic framework composite sorbent. J. Chromatogr. A 2020, 1627, 461395. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Yu, A.; Zhang, S.; Hu, G.; Ouyang, G. Experimentally probing the chiral recognition mechanism of 1, 1′-bi-2-naphthol on a nitrogen enriched chiral metal-organic framework. Microchem. J. 2022, 174, 107092. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, X.; Yu, A.; Zhao, W.; Zhang, W.; Zhang, S.; Wei, L.; Cook, D.J.; Roy, A. Functionalized metal-organic framework nanocomposites for dispersive solid phase extraction and enantioselective capture of chiral drug intermediates. J. Chromatogr. A 2018, 1537, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.-J.; Koh, D.-Y. Homochiral Metal-Organic Framework Based Mixed Matrix Membrane for Chiral Resolution. Membranes 2022, 12, 357. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.-M.; Zhang, Z.-J.; Wang, Z.-Y.; Yuan, L.-M. Chiral metal–organic frameworks for high-resolution gas chromatographic separations. J. Am. Chem. Soc. 2011, 133, 11892–11895. [Google Scholar] [CrossRef]
- Tang, W.Q.; Xu, J.Y.; Gu, Z.Y. Metal–organic-framework-based gas chromatographic separation. Chem.–Asian J. 2019, 14, 3462–3473. [Google Scholar] [CrossRef]
- Xie, S.-m.; Zhang, X.-h.; Wang, B.-j.; Zhang, M.; Zhang, J.-h.; Yuan, L.-m. 3D Chiral nanoporous metal–organic framework for chromatographic separation in GC. Chromatographia 2014, 77, 1359–1365. [Google Scholar] [CrossRef]
- Yang, J.-r.; Xie, S.-m.; Zhang, J.-h.; Chen, L.; Nong, R.-y.; Yuan, L.-m. Metal–organic framework [Cd (LTP) 2] n for improved enantioseparations on a chiral cyclodextrin stationary phase in GC. J. Chromatogr. Sci. 2017, 54, 1467–1474. [Google Scholar] [CrossRef]
- Xie, S.-M.; Zhang, X.-H.; Zhang, Z.-J.; Zhang, M.; Jia, J.; Yuan, L.-M. A 3-D open-framework material with intrinsic chiral topology used as a stationary phase in gas chromatography. Anal. Bioanal. Chem. 2013, 405, 3407–3412. [Google Scholar] [CrossRef]
- Xie, S.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, M.; Yuan, L. Chiral 3D open-framework material Ni (D-cam)(H2O) 2 used as GC stationary phase. Chirality 2014, 26, 27–32. [Google Scholar] [CrossRef]
- Xie, S.-M.; Zhang, X.-H.; Zhang, Z.-J.; Yuan, L.-M. Porous chiral metal-organic framework InH (D-C10H14O4) 2 with anionic-type diamond network for high-resolution gas chromatographic enantioseparations. Anal. Lett. 2013, 46, 753–763. [Google Scholar] [CrossRef]
- Zhang, X.-h.; Xie, S.-m.; Duan, A.-h.; Wang, B.-j.; Yuan, L.-M. Separation performance of MOFs Zn (ISN) 2· 2H2O as stationary phase for high-resolution GC. Chromatographia 2013, 76, 831–836. [Google Scholar] [CrossRef]
- Yang, J.-r.; Xie, S.-m.; Liu, H.; Zhang, J.-h.; Yuan, L.-m. Metal–Organic Framework InH (d-C10H14O4) 2 for Improved Enantioseparations on a Chiral Cyclodextrin Stationary Phase in GC. Chromatographia 2015, 78, 557–564. [Google Scholar] [CrossRef]
- Liu, H.; Xie, S.M.; Ai, P.; Zhang, J.H.; Zhang, M.; Yuan, L.M. Metal–Organic Framework Co (d-Cam) 1/2 (bdc) 1/2 (tmdpy) for Improved Enantioseparations on a Chiral Cyclodextrin Stationary Phase in Gas Chromatography. ChemPlusChem 2014, 79, 1103–1108. [Google Scholar] [CrossRef]
- Kou, W.-T.; Yang, C.-X.; Yan, X.-P. Post-synthetic modification of metal–organic frameworks for chiral gas chromatography. J. Mater. Chem. A 2018, 6, 17861–17866. [Google Scholar] [CrossRef]
- Fanali, S.; Catarcini, P.; Blaschke, G.; Chankvetadze, B. Enantioseparations by capillary electrochromatography. Electrophoresis 2001, 22, 3131–3151. [Google Scholar] [CrossRef]
- Fei, Z.-X.; Zhang, M.; Zhang, J.-H.; Yuan, L.-M. Chiral metal–organic framework used as stationary phases for capillary electrochromatography. Anal. Chim. Acta 2014, 830, 49–55. [Google Scholar] [CrossRef]
- Ye, N.; Ma, J.; An, J.; Li, J.; Cai, Z.; Zong, H. Separation of amino acid enantiomers by a capillary modified with a metal–organic framework. RSC Adv. 2016, 6, 41587–41593. [Google Scholar] [CrossRef]
- Ding, W.; Yu, T.; Du, Y.; Sun, X.; Feng, Z.; Zhao, S.; Ma, X.; Ma, M.; Chen, C. A metal organic framework-functionalized monolithic column for enantioseparation of six basic chiral drugs by capillary electrochromatography. Microchim. Acta 2020, 187, 51. [Google Scholar] [CrossRef]
- Sun, X.; Niu, B.; Zhang, Q.; Chen, Q. MIL-53-based homochiral metal–organic framework as a stationary phase for open-tubular capillary electrochromatography. J. Pharm. Anal. 2022, 12, 509–516. [Google Scholar] [CrossRef]
- Lidi, G.; Xingfang, H.; Shili, Q.; Hongtao, C.; Xuan, Z.; Bingbing, W. l-Cysteine modified metal–organic framework as a chiral stationary phase for enantioseparation by capillary electrochromatography. RSC Adv. 2022, 12, 6063–6075. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhu, D.; Zhang, J.; Du, Y. Homochiral iron-based γ-cyclodextrin metal-organic framework for stereoisomer separation in the open tubular capillary electrochromatography. J. Pharm. Biomed. Anal. 2022, 215, 114777. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, C.; Ma, M.; Feng, Z.; Du, Y. In-situ grown metal organic framework synergistic system for the enantioseparation of three drugs in open tubular capillary electrochromatography. J. Sep. Sci. 2022, 45, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Zhang, L.; Zhang, J.; Ma, M.; Du, Y.; Gan, J.; Yang, J. Metal organic framework-modified monolithic column immobilized with pepsin for enantioseparation in capillary electrochromatography. J. Chromatogr. B 2022, 1203, 123306. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Hu, X.; Qin, S.; Chu, H.; Tang, Y.; Li, X.; Wang, B. One-pot synthesis of a novel chiral Zr-based metal-organic framework for capillary electrochromatographic enantioseparation. Electrophoresis 2022, 43, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Fei, Z.X.; Zhang, M.; Xie, S.M.; Yuan, L.M. Capillary electrochromatographic fast enantioseparation based on a chiral metal–organic framework. Electrophoresis 2014, 35, 3541–3548. [Google Scholar] [CrossRef]
- Sun, X.; Tao, Y.; Du, Y.; Ding, W.; Chen, C.; Ma, X. Metal organic framework HKUST-1 modified with carboxymethyl-β-cyclodextrin for use in improved open tubular capillary electrochromatographic enantioseparation of five basic drugs. Microchim. Acta 2019, 186, 462. [Google Scholar] [CrossRef]
- Pan, C.; Lv, W.; Niu, X.; Wang, G.; Chen, H.; Chen, X. Homochiral zeolite-like metal-organic framework with DNA like double-helicity structure as stationary phase for capillary electrochromatography enantioseparation. J. Chromatogr. A 2018, 1541, 31–38. [Google Scholar] [CrossRef]
- Ding, W.; Ma, M.; Du, Y.; Chen, C.; Ma, X. Metal organic framework ZIF-90 modified with lactobionic acid for use in improved open tubular capillary electrochromatographic enantioseparation of five basic drugs. Microchim. Acta 2020, 187, 651. [Google Scholar] [CrossRef]
- Ma, J.; Ye, N.; Li, J. Covalent bonding of homochiral metal-organic framework in capillaries for stereoisomer separation by capillary electrochromatography. Electrophoresis 2016, 37, 601–608. [Google Scholar] [CrossRef]
- Pan, C.; Wang, W.; Zhang, H.; Xu, L.; Chen, X. In situ synthesis of homochiral metal–organic framework in capillary column for capillary electrochromatography enantioseparation. J. Chromatogr. A 2015, 1388, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Mao, Z.; Zhou, W.; Chen, Z. γ-Cyclodextrin metal-organic framework supported by polydopamine as stationary phases for electrochromatographic enantioseparation. Talanta 2020, 218, 121160. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Y.; Zhang, Y.; Cheng, Y.; Ye, J.; Chu, Q.; Cheng, G. Rapid preparation and evaluation of chiral open-tubular columns supported with bovine serum album and zeolite imidazolate framework-8 for mini-capillary electrochromatography. J. Chromatogr. A 2020, 1625, 461284. [Google Scholar] [CrossRef] [PubMed]
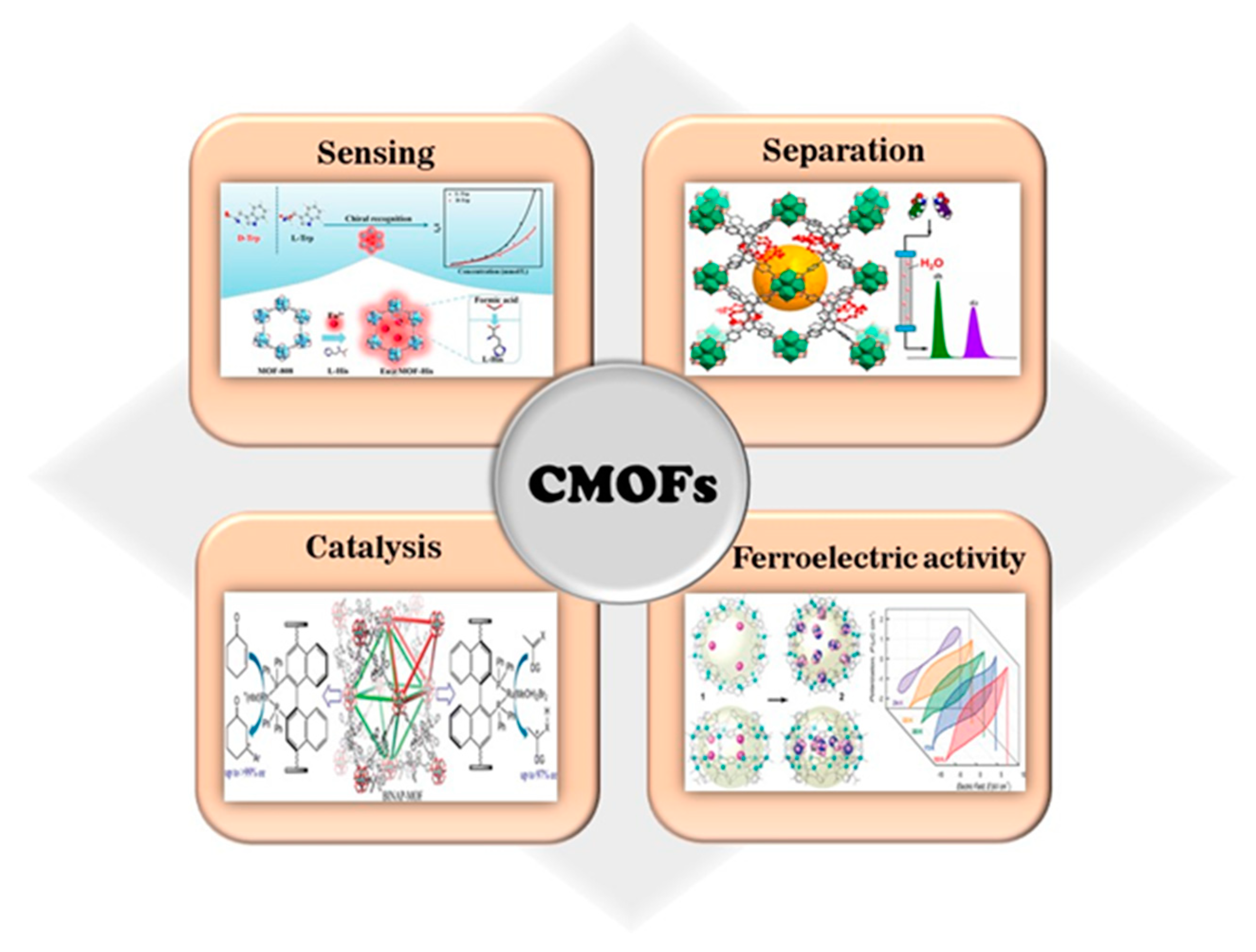
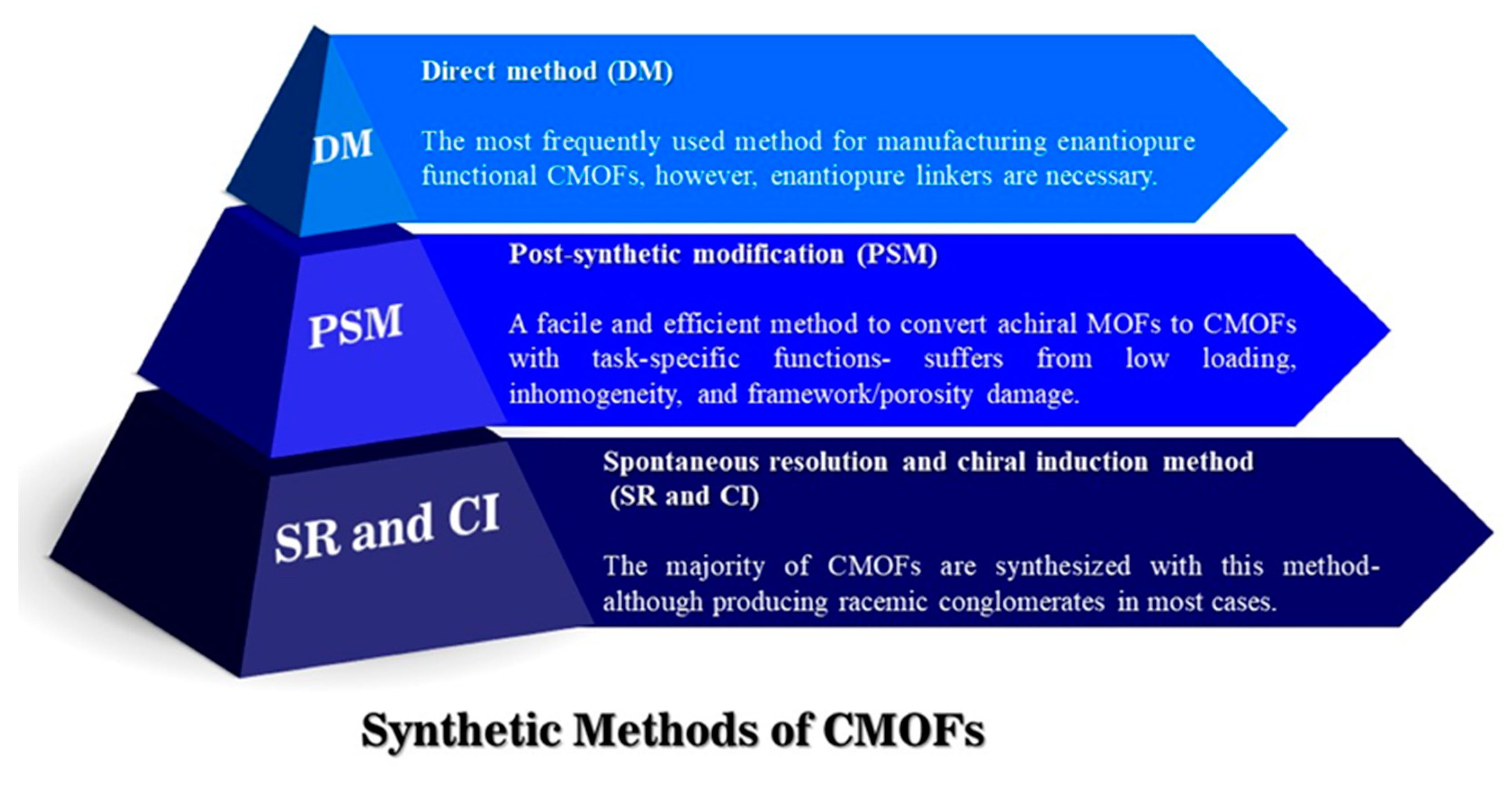
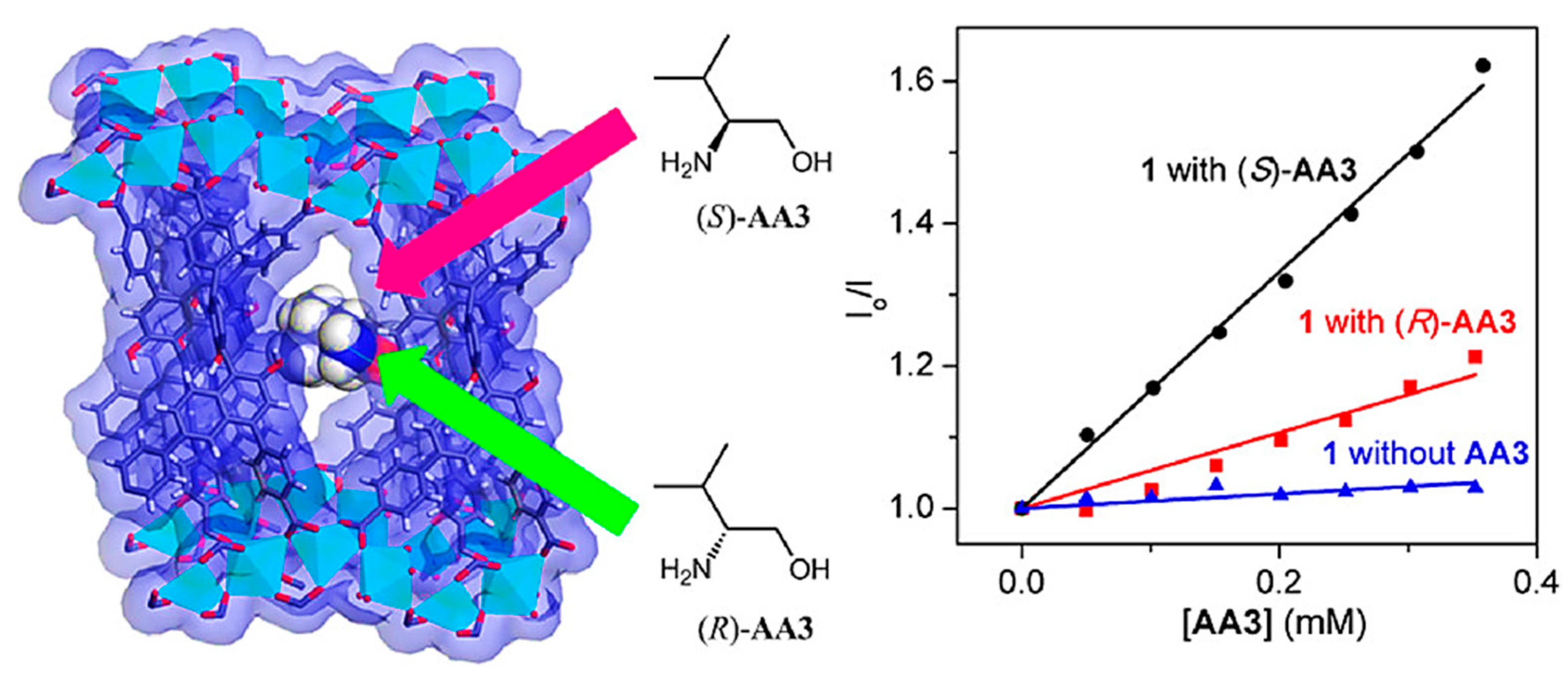
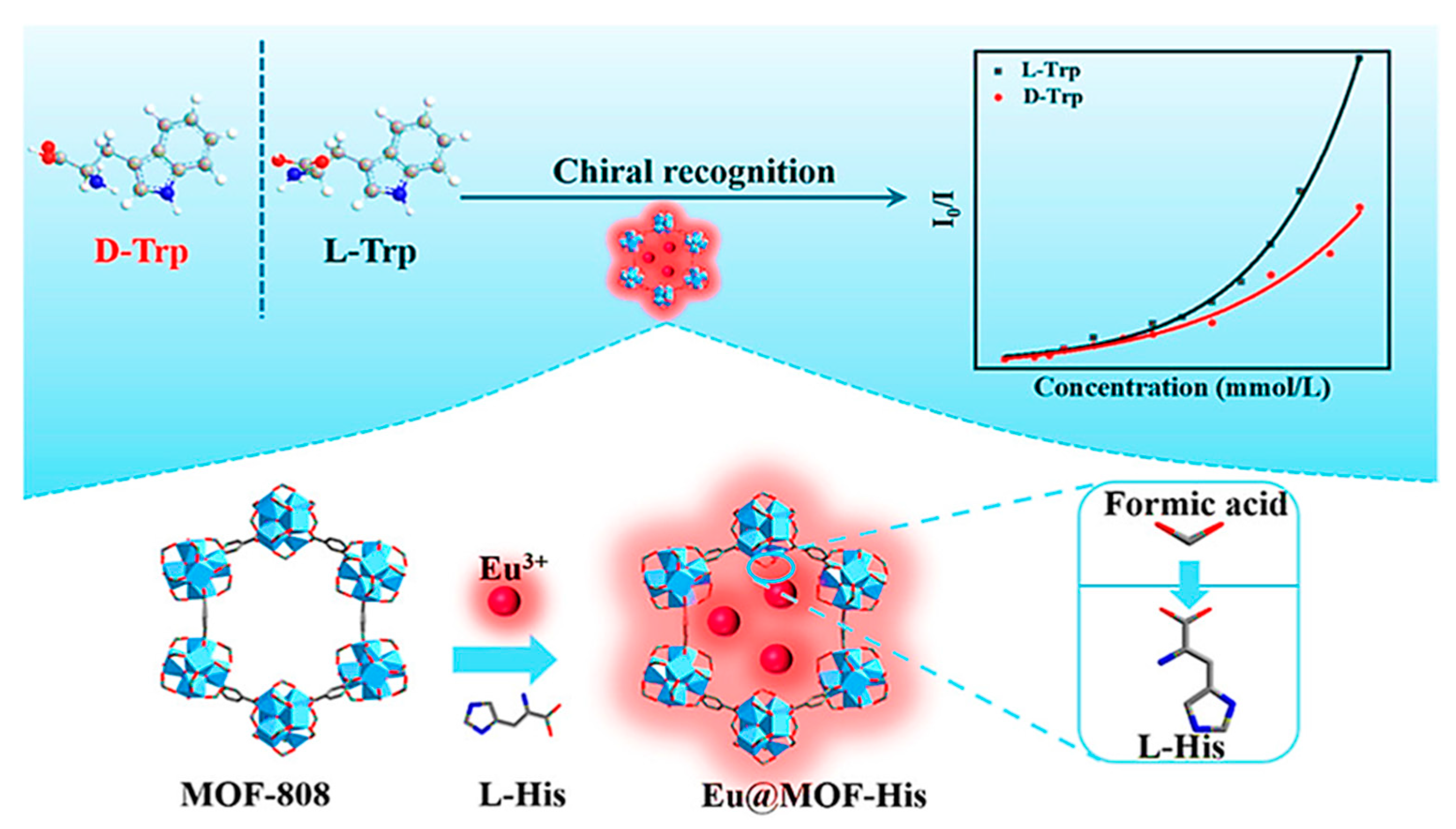



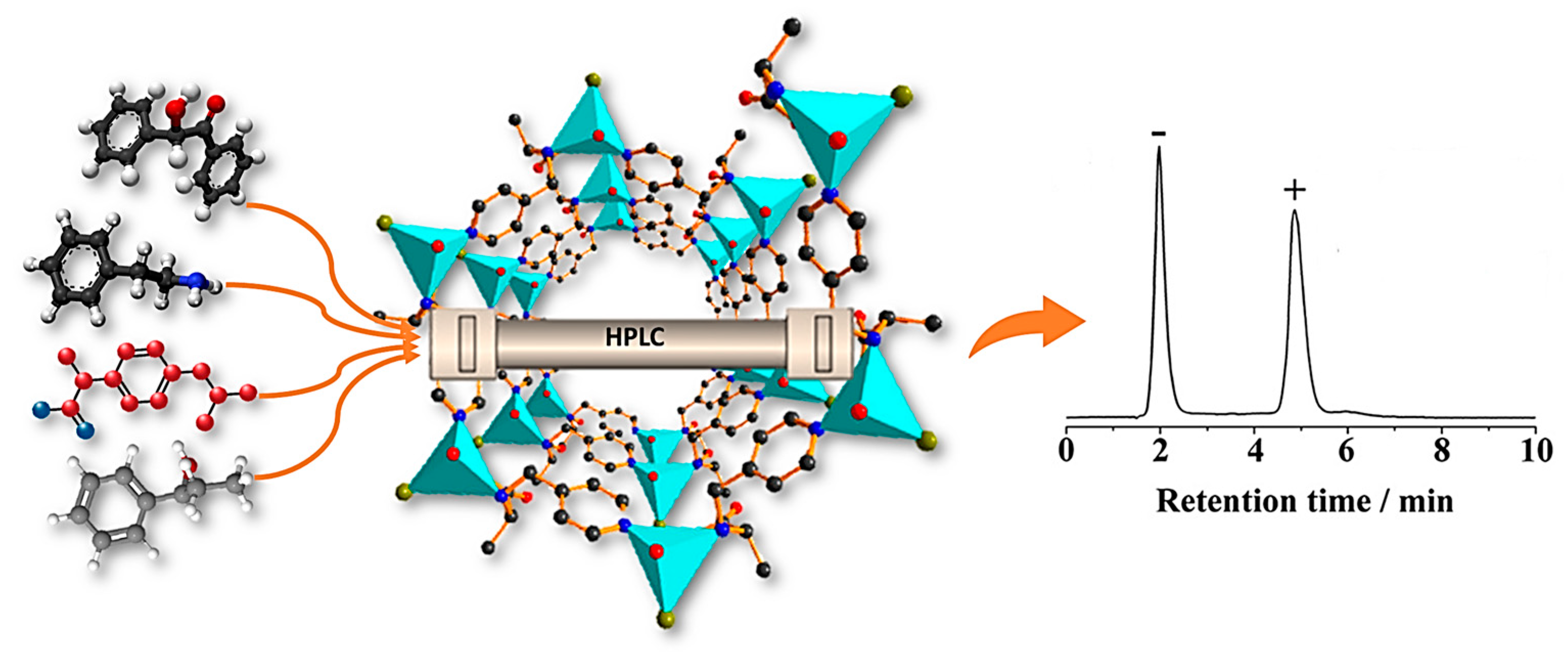
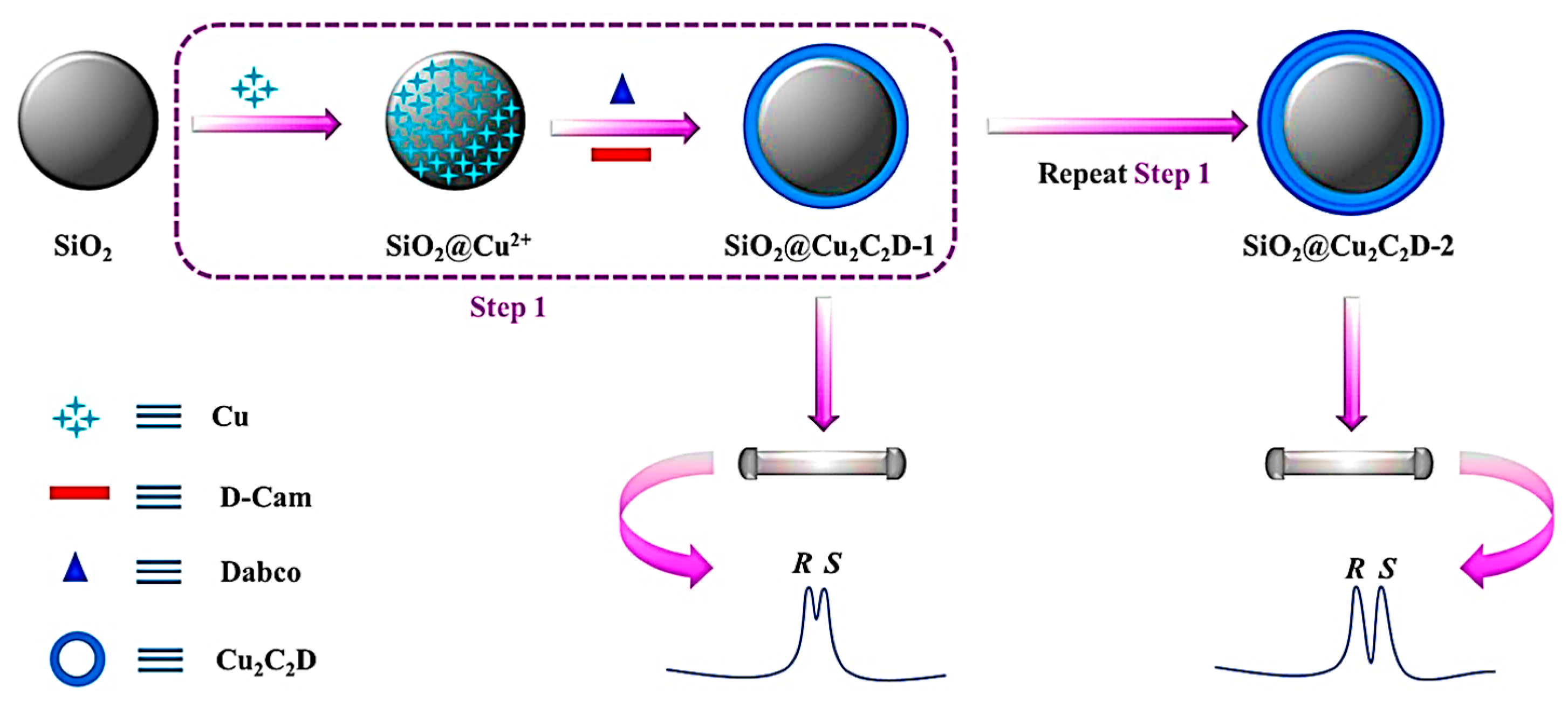
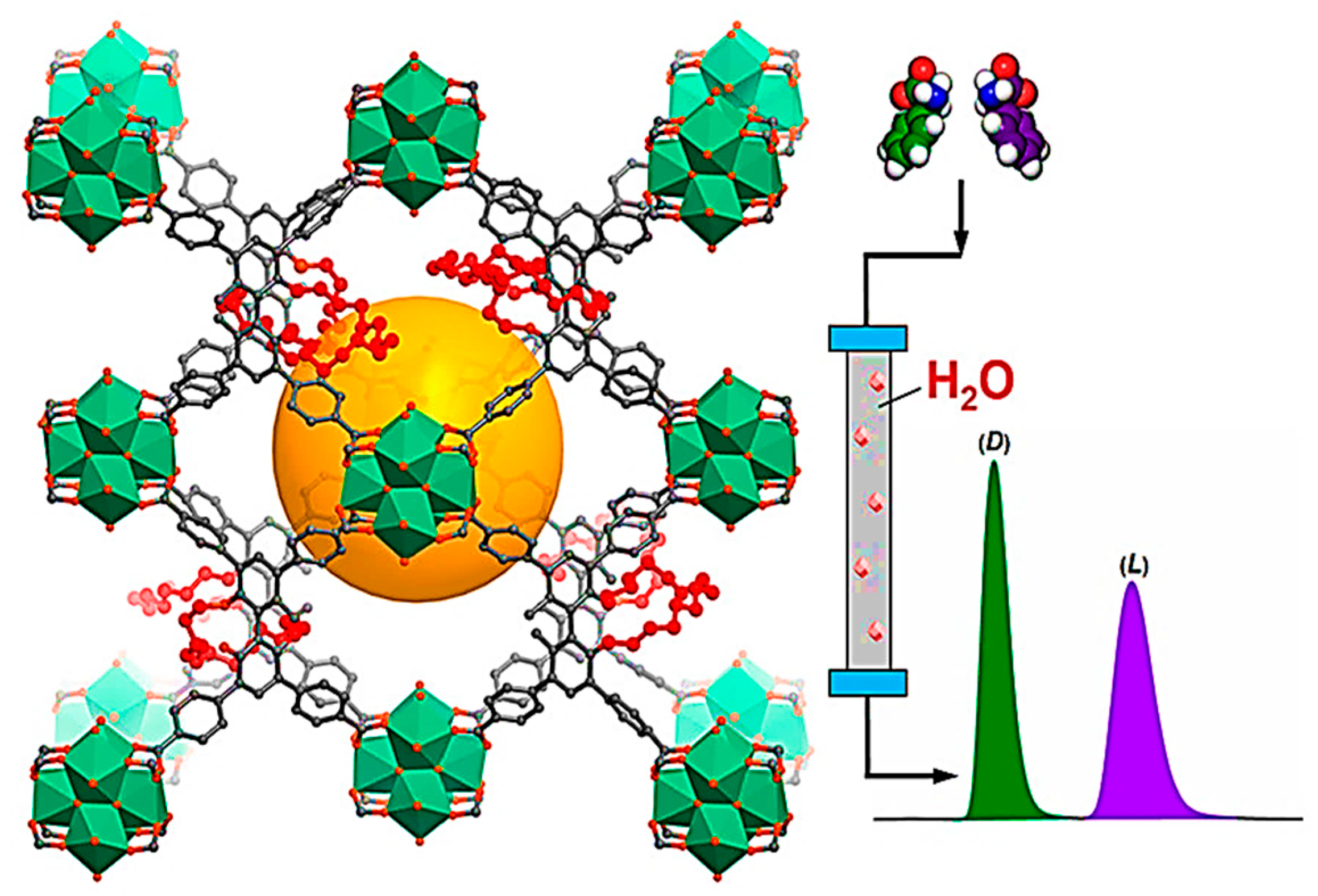
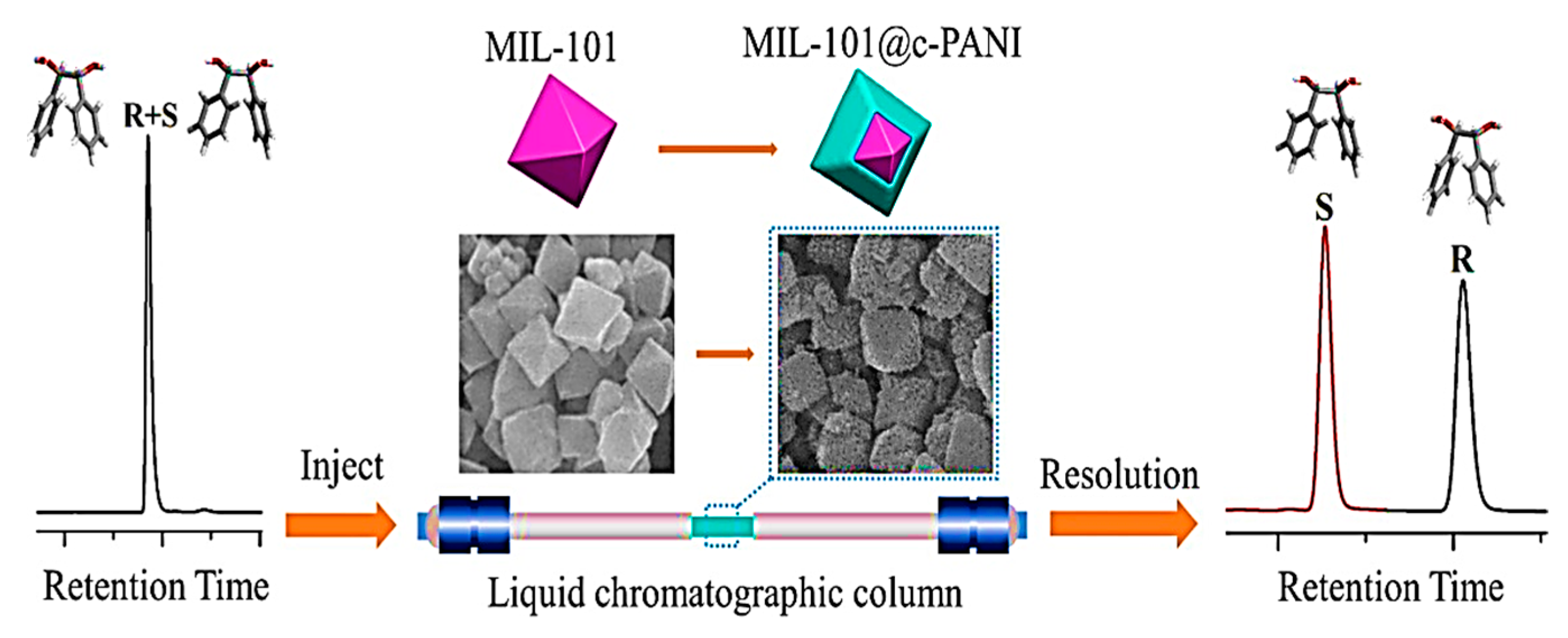
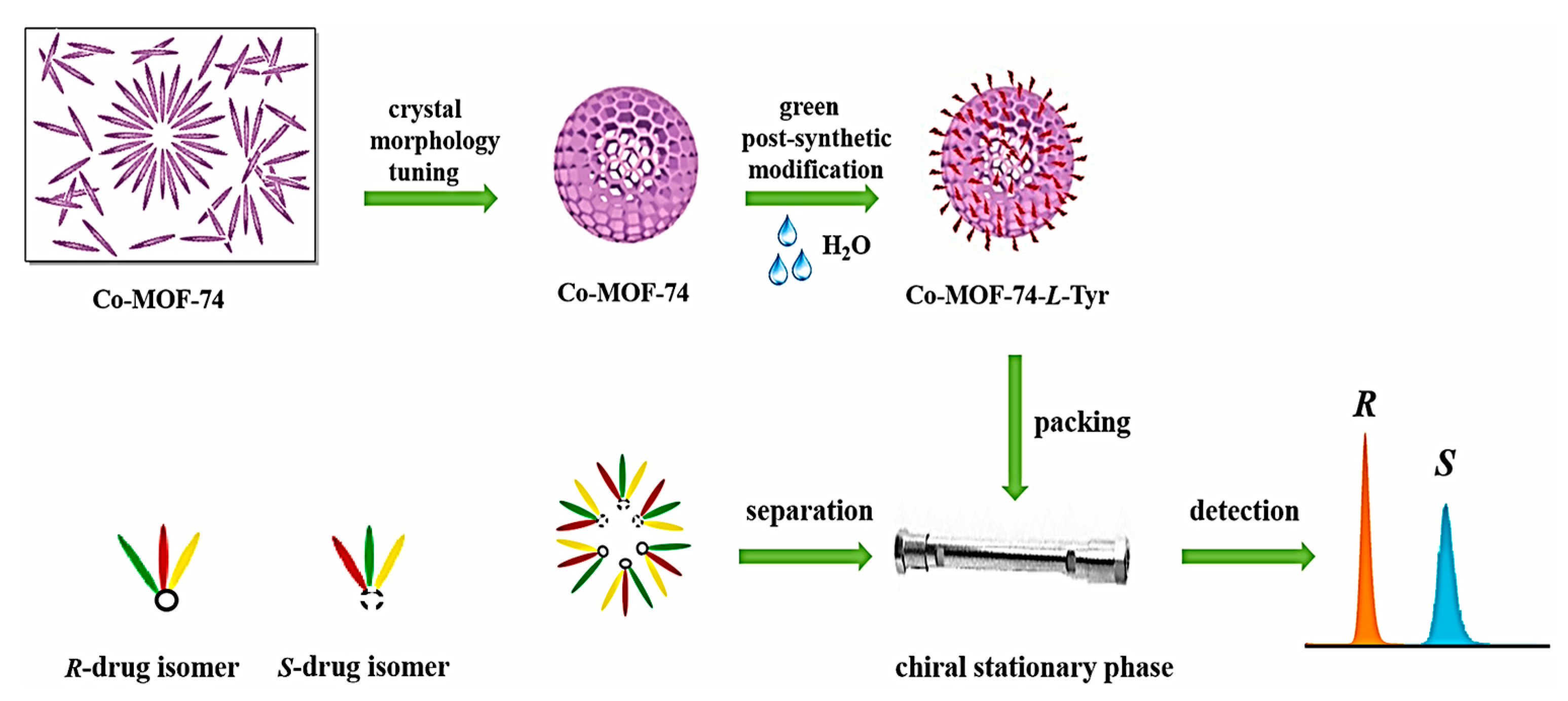
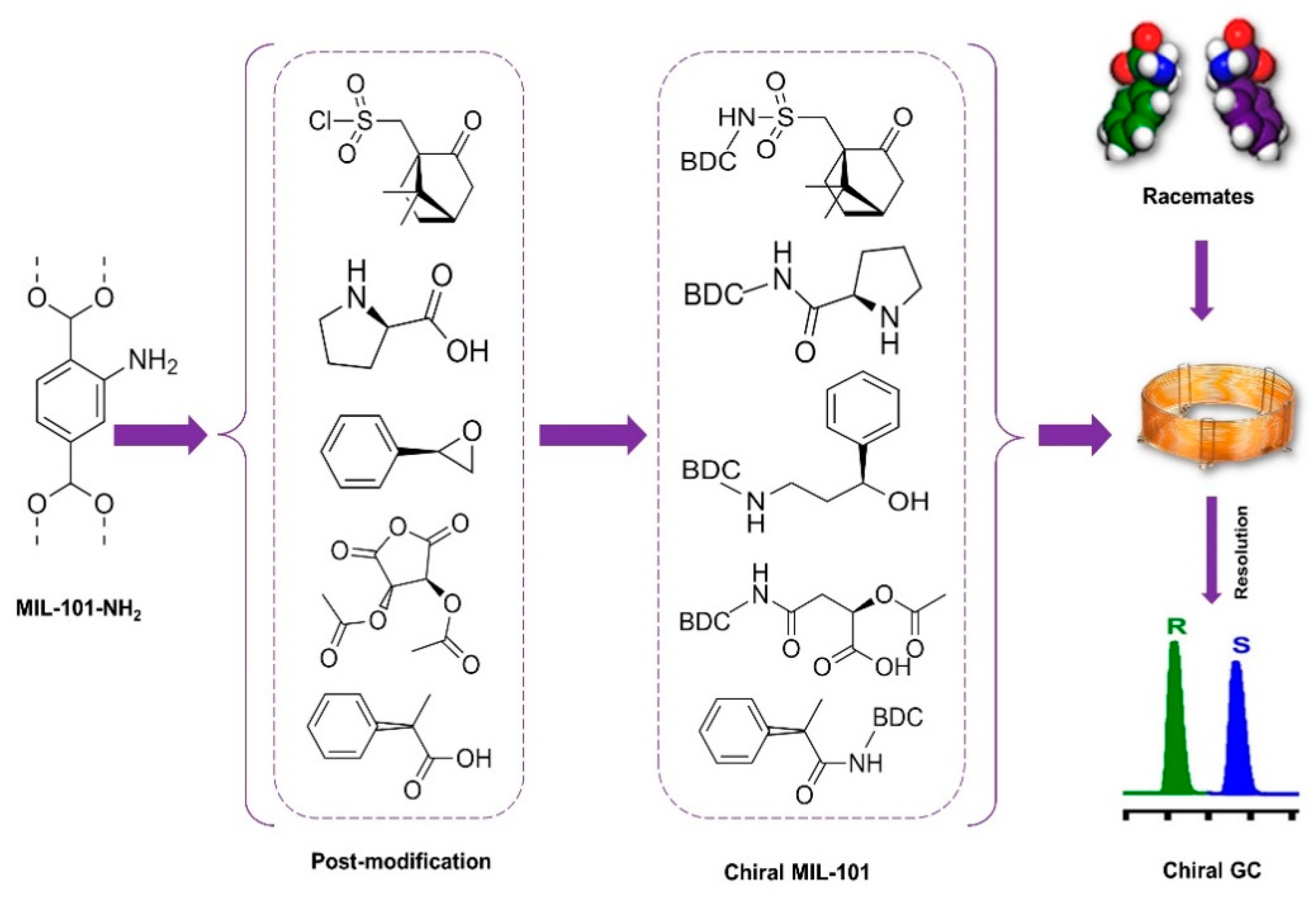
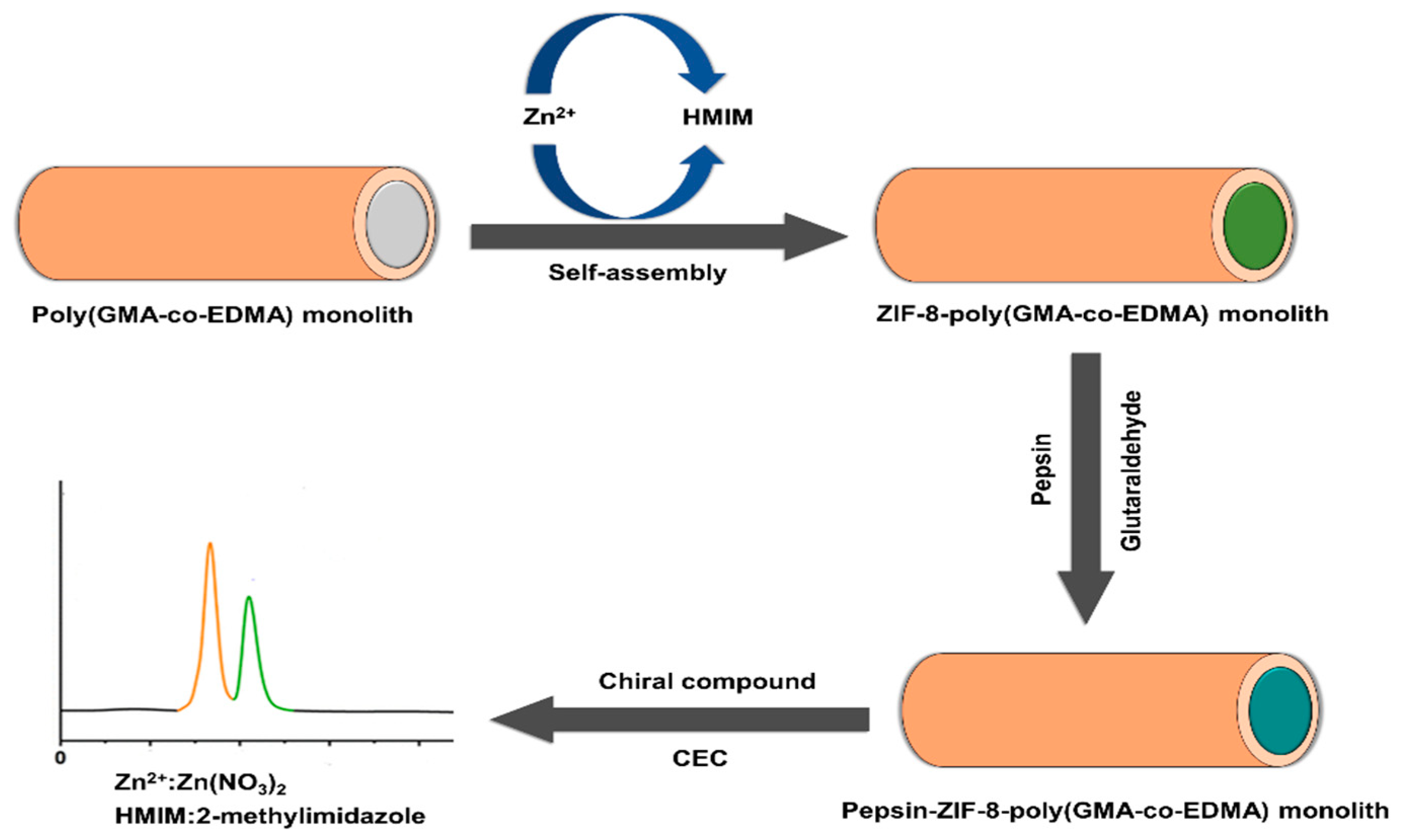

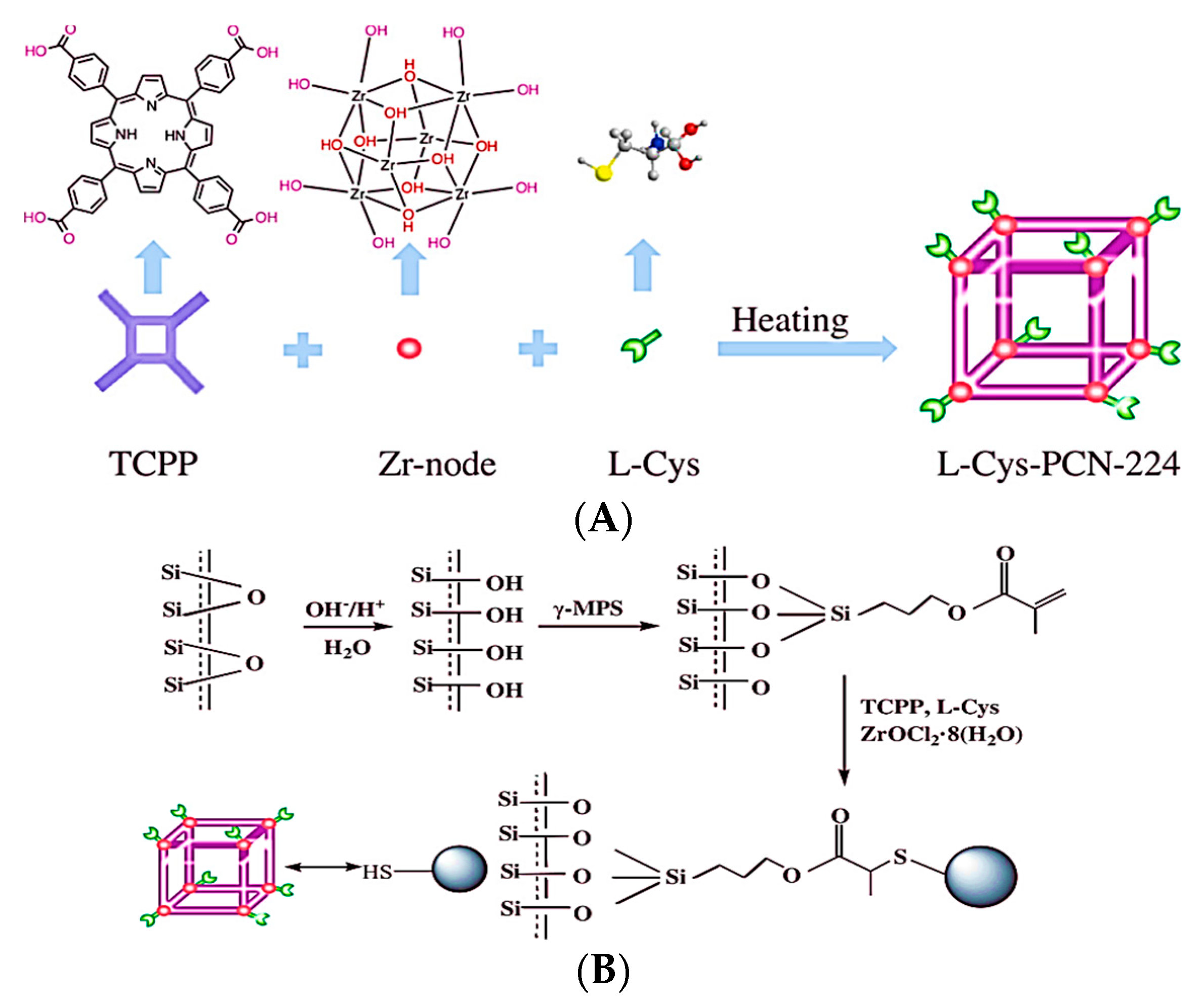
| MOF Formula | Analytes | Transduction Type | LOD | Linear Range | Ref. |
|---|---|---|---|---|---|
| MPA 1-capped CdTe QD 2 @MOF | d/l-tartaric acids, d/l-dimethyl tartrates, d/l-mandelic acids | Fluorescence | - | 30–500 µg/mL | [92] |
| {[Cd(l)(4,4′-bipy)]·DMA·5H2O}n | d/l-penicillamine | Fluorescence | 0.9 μM (D-Pen) 2.1 μM (L-Pen) | 20–167 μM | [93] |
| IRMOF 3-74-I/II-Mg-C-Tb | Phenylethanol, phenethylamine, cinchonine and cinchonidine, and N-benzylcinchoninium chloride and N-benzylcinchonidinium chloride | Fluorescence | - | 0.01–17 mM | [94] |
| Single-crystalline 2D MONs 4 | α-pinene, limonene, β-pinene, valencene, and isolongifolene | Fluorescence | - | 0–175 μM | [95] |
| 3D layered porous MOF | Alaninol, leucinol, phenylalaminol, and phenylglycinol | Fluorescence | - | 30–150 μL | [96] |
| S-1 (l-AP@UiO-66-(COOH)2) and R-1 (d-AP@UiO-66-(COOH)2) | l/d-amino propanol | Fluorescence | - | 10−4–10−2 M | [97] |
| Helical-Ag NPs @ MOF | d/l-cysteine and d/l-asparagine | Fluorescence | - | 1.0 μM | [98] |
| 2D HMOF-3 nanosheets (HMOF-3-NS) | R/S-mandelic acid, d/l-tartaric acid, d/l-lactic acid, d/l-alanine, and d/l-tryptophan | Fluorescence | - | 0–50 μM | [99] |
| nanorod-shaped homochiral Cd-MOF | d/l-aspartic acid | Fluorescence | - | 1 nM | [100] |
| Zr-MOF | d/l-glutamine (Gln) | Fluorescence | d-Gln 6.6 × 10−4 M and l-Gln 3.3 × 10−4 M | 10–100 μM | [101] |
| [Zn(L)(2,2′-bipy)]·H2O (LNNU-1) | Tryptophan | Fluorescence | 1.94 µM (L-Trp), 2.59 µM (D-Trp), 1.91 µM (DL-Trp) | 0–0.125 mM | [71] |
| Ru (ruthenium)-MOF | Tryptophan | Electrochemical | 0.33 nM | 1.0 nM–1.0 mM | [102] |
| CD (cyclodextrin)-MOF | α/β-Pinene | Electrochemical | - | 0.5–5.0 mM | [103] |
| MOF@CCQDs/NiF 5 | d/l-Tyrosine | Electrochemical | 6.12 × 10−6 M (D-Tyr) 9.85 × 10−7 M (L-Tyr) | 0.2–1.2 mM | [104] |
| h-HDGA@ZIF-67 | Penicillamine (Pen) | Electrochemical | 0.022 μM (L-Pen) 0.015 μM (D-Pen) | 3.25–19.50 mM | [105] |
| TiO2/MIL-125-NH2 NTs | 3,4-dihydroxyphenylalanine (L/D-DOPA) enantiomers | Electrochemical | 0.24 μM | 1.0–10.0 μM | [106] |
| AChE 6/L-Ni-BPY/DpAu/GCE | Galantamine hydrobromide | Electrochemical | 0.31 pM | 1 × 10−12~1 × 10−6 M | [107] |
| C-dots@MOF/CuF | Penicillamine | Electrochemical | - | 100–600 μM | [108] |
| l-His-ZIF | d/l-Glutamate | Electrochemical | 0.06 nM | 0.1–50 nM | [109] |
| Cu-MOF: {[Cu(fdc)(bpe)(H2O)(DMF)]·0.5H2O}n | L-Tryptophan | Electrochemical | 5.822 μM | 0.01 to 0.09 mM | [110] |
| Type of MOF | Analyte | Mobile Phase | Form | Repeatability (RSD%) | Ref. |
|---|---|---|---|---|---|
| (R)-CuMOF-1 and (R)-ZnMOF-1 | Sulfoxides, sec-alcohols, β-lactams, benzoins, flavanones, and epoxides | Hexane/EtOH (50/50) | Packing (10 cm length × 4.6 mm i.d.) | - | [126] |
| [Cd2(d-cam)3]·2Hdma·4dma (Cd-MOF) | (±)-1-(1-naphthyl)ethanol | Hexane/dichloromethane (1/1) | Packing (25 cm long × 2.0 mm i.d.) | 2.1% | [127] |
| MOF [Co2(d-cam)2(TMDPy)]@SiO2 core-shell composites | Positional isomers (trans-stilbene oxide and phenylenediamine) | n-Hexane/isopropanol (9/1) | Packing (25 cm length × 2.1 mm i.d.) | 0.86% and 0.78% | [128] |
| [(CH3)2NH2][Cd(bpdc)1.5]·2DMA | 1,1′-Bi-2-naphthol, 1,2-diphenyl-1,2-ethanediol, 1-(4-chlorophenyl)ethanol, furoin, benzoin, flavanone, Troger’s base, 3-benzyloxy-1,2-propanediol, 3,5-dinitro-N-(1-phenylethyl)benzamide, and warfarin sodium | Hexane/dichloromethane (2/1) | Packing (25 cm long × 2.0 mm i.d.) | - | [129] |
| (R)-MOF-4-silica and (R)-MOF-5-silica composites | Sulfoxides, sec-alcohols, and flavanones | Hexane/2-PrOH (90/10) | Packing (10 cm long × 4.6 mm i.d.) | - | [130] |
| Helical MOF [Zn2(D-Cam)2(4,4′-bpy)]n (1) | (±)-1-(1-Naphthyl)ethanol, Tröger’s base | Hexane and hexane/dichloromethane (95/5) | Packing (25 cm long × 4.6 mm i.d.) | - | [131] |
| (Me2NH2)2[Mn4O(D-cam)4]·(H2O)5 | (±)-ibuprofen and (±)-1-phenyl-1,2-ethadiol | Hexane/isopropyl alcohol (96/4) | Packing (10 cm length × 4.6 mm i.d.) | - | [132] |
| Homochiral MOF [Cu(S-mal)(bpe)]n | Benzion, propranolol hydrochloride, ketoprofen, DNB-leucine, amlodipine, hydrobenzoin, chlorpheniramine maleate, p-hydroxyphenylglycine, naproxen, furoin, mandelic acid, 1-(9-anthryl)-2,2,2-trifluoroethanol, 1,1′-bi-2-naphthol, praziquantel, ibuprofen, and 1-(1-naphthyl)ethanol | Different ratios of hexane/isopropanol (v/v) | Packing (25 cm long × 2.0 mm i.d.) | 1.1% | [133] |
| γ-CD (cyclodextrin) MOF | Aromatic alcohols | Different ratios of dichloromethane: MeOH and hexane: Dichloromethane (v/v) | Packing (25 cm long × 4.6 mm i.d.) | 0.3–0.4%, 1.5–2.1%, and 1.1–1.9% | [134] |
| [Zn(L-tyr)]n(L-tyrZn), [Zn4(btc)2(Hbtc)(L-His)2(H2O)4]·1.5H2O, {[Zn2(L-trp)2(bpe)2(H2O)2]·2H2O·2NO3}n, [Co2(L-Trp)(INT)2(H2O)2(ClO4)], [Co2(sdba)((L-Trp)2] and [Co(L-Glu)(H2O)·H2O]∞ | Alcohols, amines, ketones, ethers, organic acids | Different ratios of hexane/isopropanol or hexane/dichloromethane (v/v) | Packing (25 cm long × 2.0 mm i.d.) | - | [135] |
| [Cu2(d-Cam)2(4,4′-bpy)]n | Positional isomers and 1-(9-anthryl)-2,2,2-trifluoroethanol, 1,1′-bi-2-naphthol, flavanone, 2-phenyl-1-propanol, 1-(1-naphthyl)ethanol, and 3-benzyloxy-1,2-propanediol | Hexane/isopropanol (98/2) and different ratios of hexane/dichloromethane (v/v) | Packing (25 cm long × 4.6 mm i.d.) | - | [136] |
| [Nd3(D-cam)8(H2O)4Cl]n | Ofloxacin, warfarin, naproxen, furoin, benzoin, phenylalanine, glutamic acid, threonine, tyrosine, alanine, cysteine, aspartic acid, valine, histidine, lencine, serine, isoleucine | n-Hexane/isopropanol (9/1), MeCN/H2O (6/4), MeOH/H2O (6/4), ethanol absolute | Packing (25 cm long × 2.0 mm i.d.) | - | [137] |
| [Co(L)(bpe)2(H2O)2]·H2O | 1,1′-Bi-2-naphthol, mandelic acid, atenolol, 1,2-diphenylethylene glycol, 1,2-diphenylethanone, 3,5-dinitro-N-(1-phenylethyl) benzamide, DNB-leucine, chlorphenamine maleate, and ibuprofen | n-Hexane/isopropanol (99/1) | Packing (25 cm long × 4.6 mm i.d.) | 0.69% | [138] |
| [Cu2(d-Cam)2(4,4′-bpy)]n@SiO2 | 1-(9-Anthryl)-2,2,2-trifluoroethanol, salbutamol, 1-phenylethanol, 1-(4-chlorophenyl)ethanol, 3-benzyloxy-1,2-propanediol, alprenolol, praziquantel, flavanone, zopiclone, benzoin, furoin, trans-stilbene oxide, and Tröger’s base | Different ratios of hexane/isopropanol with (v/v) | Packing (25 cm long × 2.1 mm i.d.) | 1.0, 1.5, 3.0, and 2.0% | [139] |
| Homochiral MOF [Ni(S-mal)(bpy)]n | Amlodipine, ibuprofen, flurbiprofen, propranolol, maleate chlorphenamine maleate and 1-p-chlorophenyl-ethanol | n-Hexane-isopropanol (8/2, 9/1, 95/5, 99/1) | Packing (25 cm long × 2.1 mm i.d.) | - | [140] |
| ZIF-8-PEI-CA | Tröger’s base | MeOH/2-propanol (10/1) | Packing (6 cm length × 4.6 mm i.d.) | - | [141] |
| Chiral ionic liquid@MOF | Atenolol, propranolol, metoprolol, racecadotril, and raceanisodamine | MeOH/water (20/80) | Packing (25 cm length × 4.6 mm i.d.) | 5% | [142] |
| TAMOF-1 (triazole acid MOF) | (±)-Ibuprofen and (±)-thalidomide | Acetonitrile | Packing (100 mm × 4.6 mm i.d.) | - | [114] |
| CMOF D-His-ZIF-L (DHZL) | S-1,1′-Bi-2-naphthol (S-BINOL) | n-Hexane/isopropanol (55/45) | Packing (25 cm length × 4.6 mm i.d.) | - | [143] |
| Magnetic graphene oxide- MOF [Zn2(d-Cam)2(4,4′-bpy)]n (MGO-ZnCB) | 1, 1′-Bi-2-naphthol (BN) and 2, 2′-furoin (Furoin) | Hexane/isopropanol (55/45) | Packing (25 cm length × 4.6 mm i.d.) | - | [144] |
| L-His-ZIF-8/Torlon® | 1-Phenylethanol | 0.1% Trifluoro acetic acid in n-hexane/EtOH (95/5) | Packing (25 cm length × 4.6 mm i.d.) | - | [145] |
| Separation Mode | Type of MOF | Chiral Selector | Analyte | Column Preparation Strategy | RSD% (Run-to-Run, Day-to-Day, and Column-to-Column) | Ref. |
|---|---|---|---|---|---|---|
| Packed CEC | [In3O(obb)3(HCO2)(H2O)]·solvents | - | (±)-hydrobenzoin, (±)-1-phenyl-1,2-ethanediol, and clenbuterol | Pressurized packing | 1.51–3.63, 1.83–3.98, and 3.42–5.66% | [167] |
| OT-CEC | HKUST-1 of type Cu3(BTC)2 (or MOF-199) | carboxymethyl-β-cyclodextrin | propranolol, esmolol, metoprolol, amlodipine and sotalol | 5.00, 6.60, and 7.5% | [168] | |
| OT-CEC | zeolite-like MOF JLU-Liu23 | - | epinephrine, isoprenaline, and synephrine and their analog terbutaline | Dynamic coating | 0.3–0.6%, 0.8–2.2% and 3.5–6.5% | [169] |
| OT-CEC | ZIF-90 | lactobionic acid | propranolol, metoprolol, atenolol, bisoprolol, and sotalol | In situ synthesis | 1.8, 2.7, and 7.4% | [170] |
| OT-CEC | [Cu(mal)(bpy)]⋅H2O | - | ephedrine, pseudoephedrine, penicillamine, l-penicillamine, d-phenylalanine, l-phenylalanine | Covalent bonding | 0.38–0.41, 0.41–0.42, and 2.22–2.65% | [171] |
| OT-CEC | AlaZnCl | - | isoprenaline, synephrine, norepinephrine, epinephrine, terbutaline, and carvedilol | In situ synthesis | 0.56–0.55, 1.20–0.93, 4.01–3.81% | [172] |
| OT-CEC | PDA-γ-CD-MOF(Cu-SD) | γ-cyclodextrin | dansyl (Dns)-DL-phenylalanine, Dns-DL-leucine, Dns-DL-valine, Dns-DL-threonine, and Dns-DL-serine | Static coating | 0.27, 1.45 and 4.88% | [173] |
| OT-CEC | BSA@ZIF-8 | - | methylparaben, ethylparaben, propylparaben, butylparaben, position isomers, Ephedrine and pseudoephedrine | Dynamic coating | 4.1–5.3, 5.5–6.7, and 7.4–8.7% | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanpour, S.; Niaei, N.; Petr, J. Metal–Organic Frameworks-Based Analytical Devices for Chiral Sensing and Separations: A Review (2012–2022). Chemosensors 2023, 11, 29. https://doi.org/10.3390/chemosensors11010029
Hassanpour S, Niaei N, Petr J. Metal–Organic Frameworks-Based Analytical Devices for Chiral Sensing and Separations: A Review (2012–2022). Chemosensors. 2023; 11(1):29. https://doi.org/10.3390/chemosensors11010029
Chicago/Turabian StyleHassanpour, Soodabeh, Navid Niaei, and Jan Petr. 2023. "Metal–Organic Frameworks-Based Analytical Devices for Chiral Sensing and Separations: A Review (2012–2022)" Chemosensors 11, no. 1: 29. https://doi.org/10.3390/chemosensors11010029
APA StyleHassanpour, S., Niaei, N., & Petr, J. (2023). Metal–Organic Frameworks-Based Analytical Devices for Chiral Sensing and Separations: A Review (2012–2022). Chemosensors, 11(1), 29. https://doi.org/10.3390/chemosensors11010029








