Specific and Sensitive Determination of Folic Acid by Label-Free Chemosensors with Microscope Glass Slips as Single-Use Consumables
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
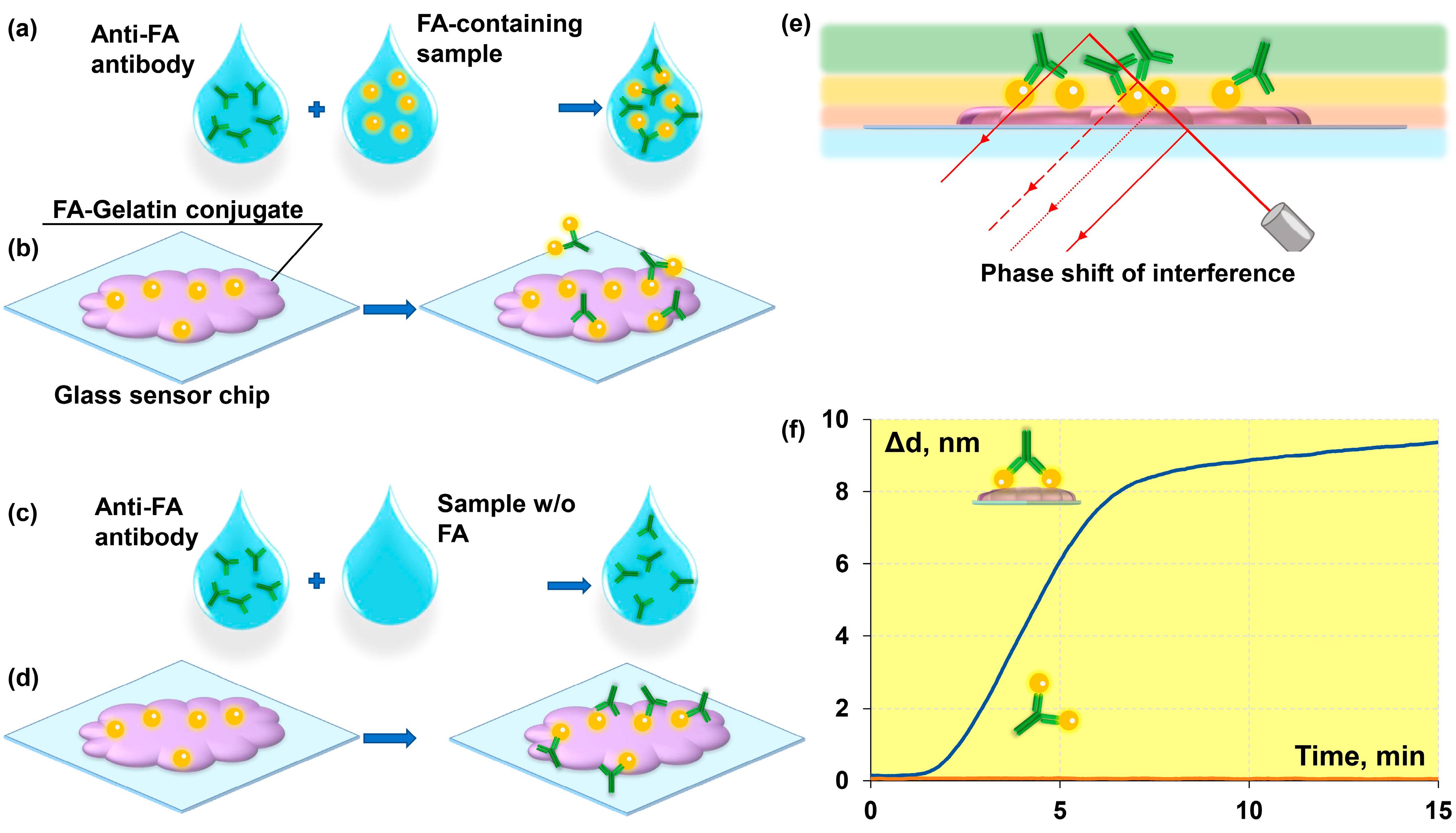
2.2. Instrument Based on the Spectral Correlation Interferometry
2.3. Preparation of “Folic Acid–Gelatin” Conjugate
2.4. Chemical Modifications of the Sensor Chip Surface
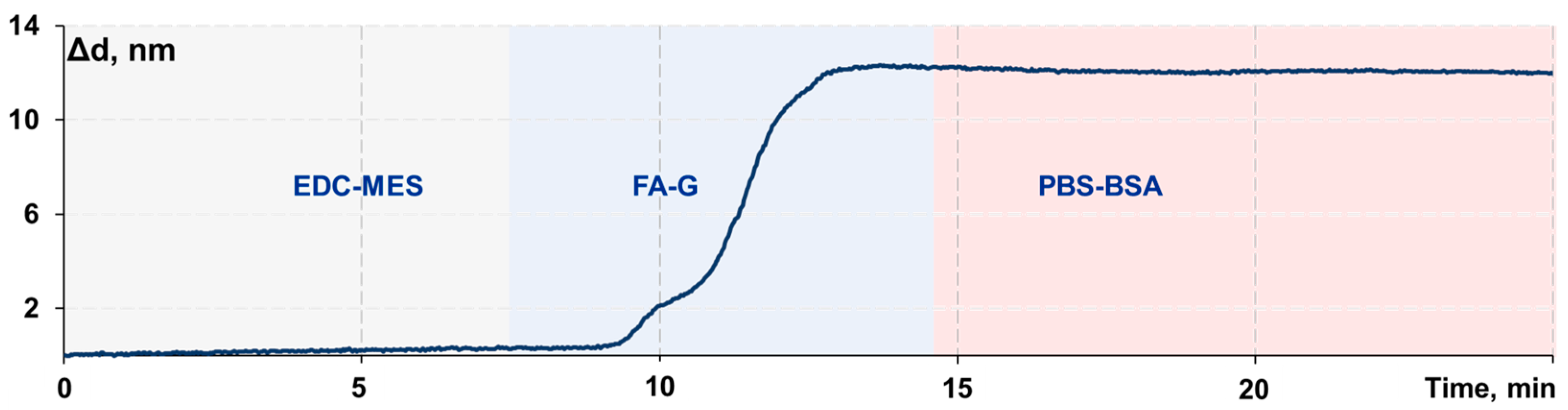
2.5. Immobilization of the “Folic Acid–Gelatin” Conjugate onto the Sensor Chip Surface
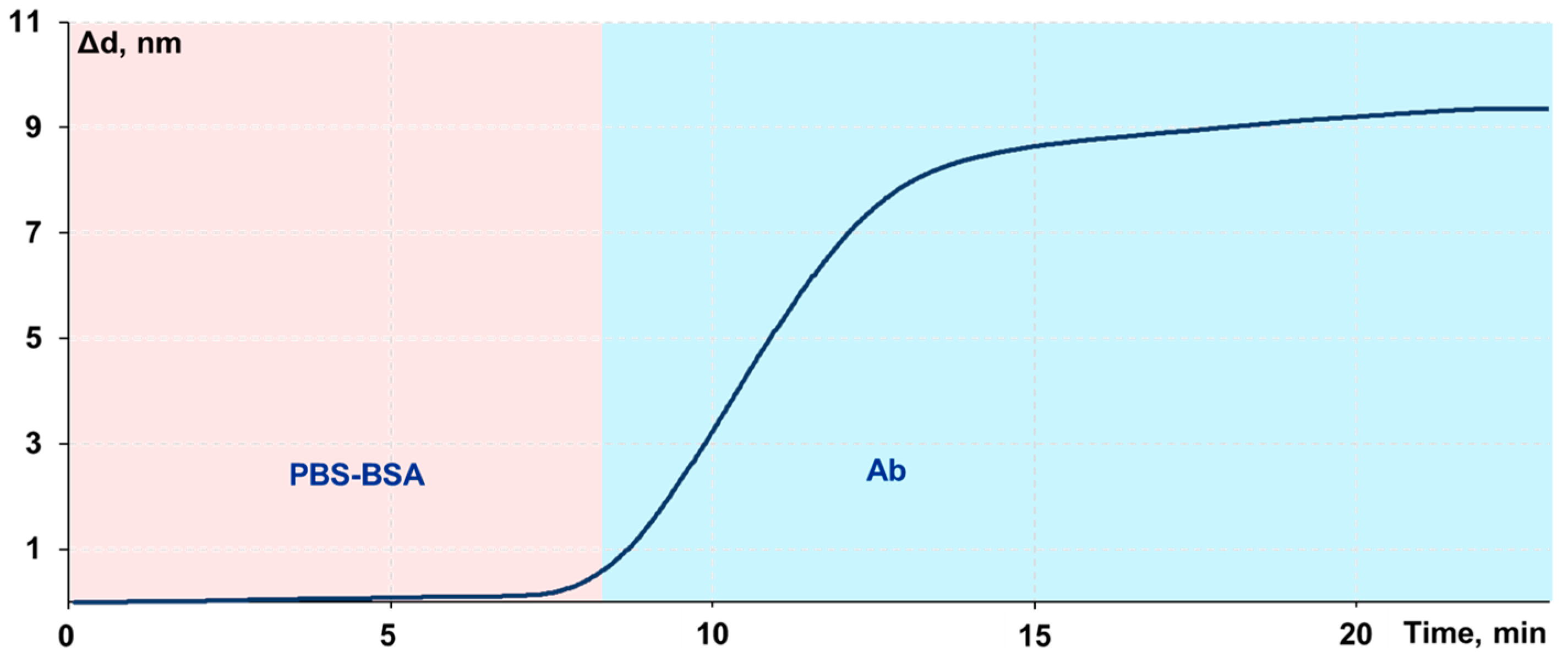
2.6. Assay Procedure
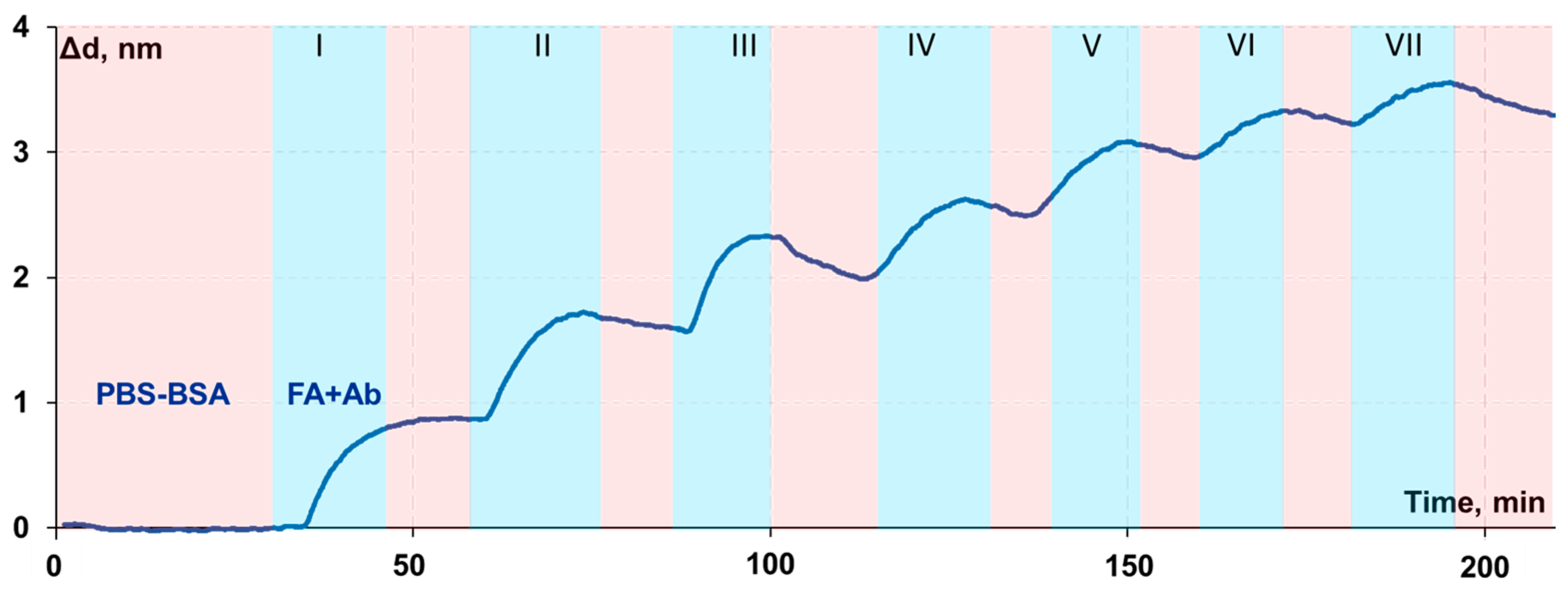
2.7. Investigation of the FA Antibody Biolayer Regeneration
3. Results and Discussion
3.1. Adjustment of the Protocol of a Biolayer Assembly on the Sensor Chip Surface
3.2. Dependence of the Biolayer Parameters on FA Antibody Concentration
3.3. Determination of the Detection Limit and Demonstration of Long-Term Chemosensor Operation
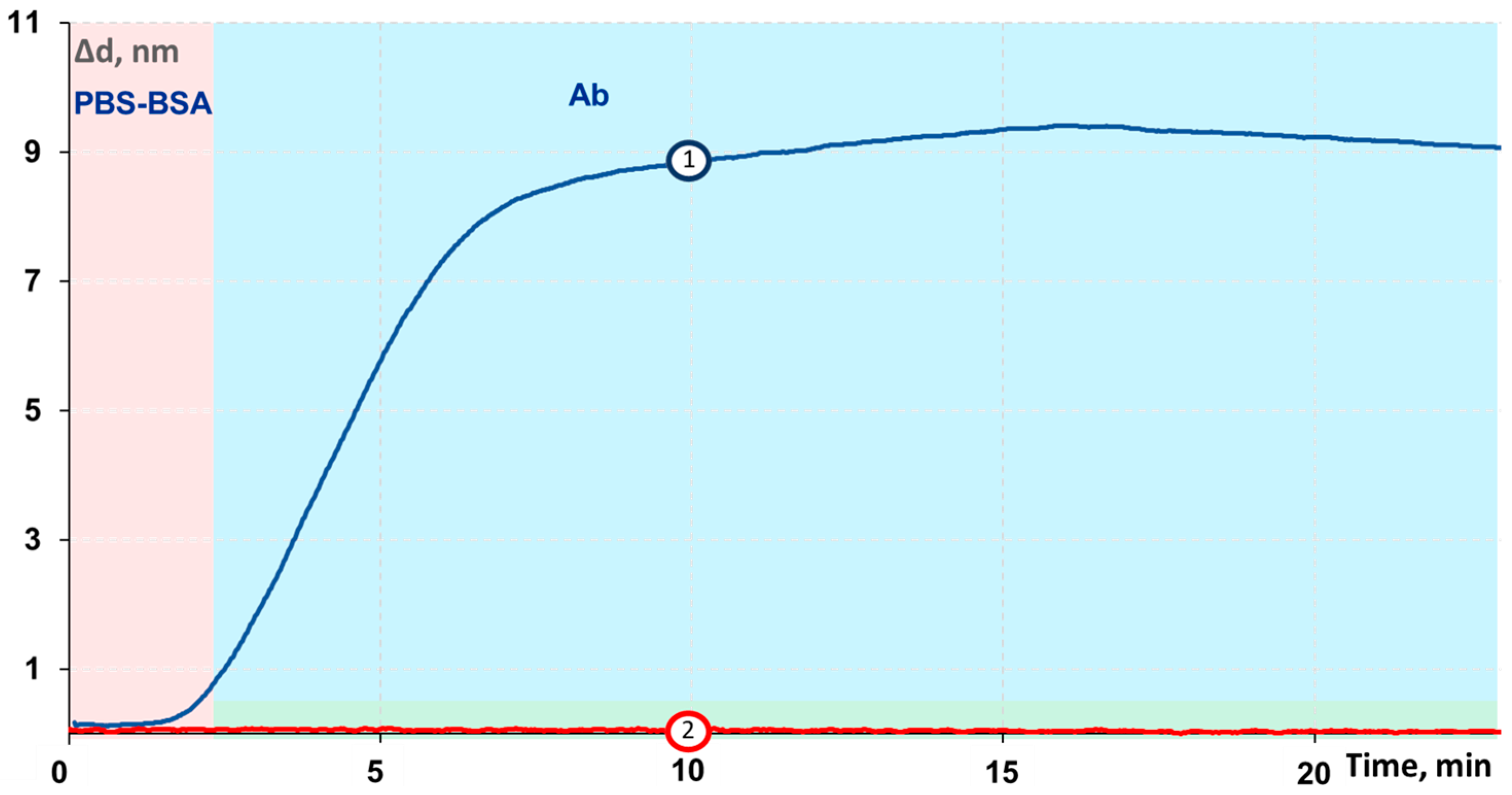
3.4. Investigation of Binding Specificity and Control Experiments
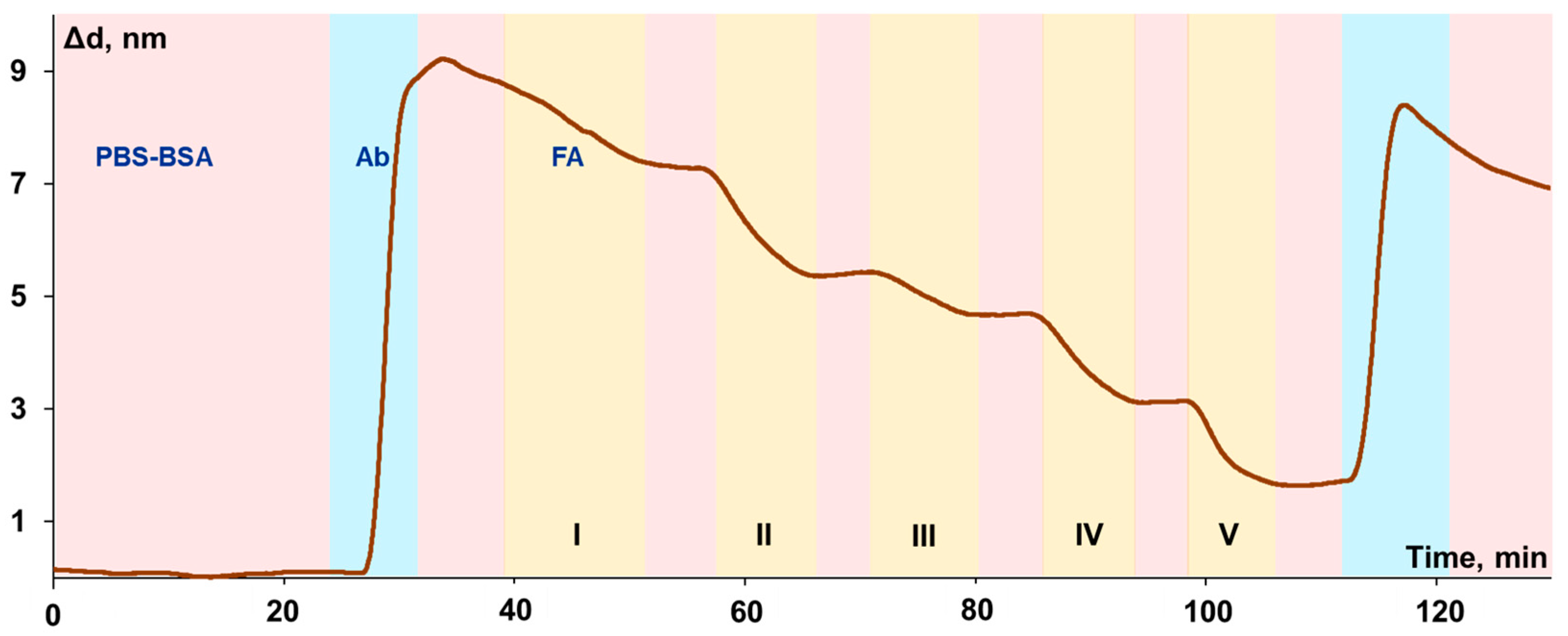
3.5. Investigation of the Biolayer Reconstruction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobczyńska-Malefora, A.; Harrington, D.J. Laboratory Assessment of Folate (Vitamin B 9) Status. J. Clin. Pathol. 2018, 71, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.; Bellevue, R.; Carmel, R. Pernicious Anemia with Neuropsychiatric Dysfunction in a Patient with Sickle Cell Anemia Treated with Folate Supplementation. N. Engl. J. Med. 2003, 348, 2204–2207. [Google Scholar] [CrossRef] [PubMed]
- di Tinno, A.; Cancelliere, R.; Micheli, L. Determination of Folic Acid Using Biosensors—A Short Review of Recent Progress. Sensors 2021, 21, 3360. [Google Scholar] [CrossRef] [PubMed]
- Troen, A.M.; Mitchell, B.; Sorensen, B.; Wener, M.H.; Johnston, A.; Wood, B.; Selhub, J.; McTiernan, A.; Yasui, Y.; Oral, E.; et al. Unmetabolized Folic Acid in Plasma Is Associated with Reduced Natural Killer Cell Cytotoxicity among Postmenopausal Women. J. Nutr. 2006, 136, 189–194. [Google Scholar] [CrossRef]
- Protiva, P.; Mason, J.B.; Liu, Z.; Hopkins, M.E.; Nelson, C.; Marshall, J.R.; Lambrecht, R.W.; Pendyala, S.; Kopelovich, L.; Kim, M.; et al. Altered Folate Availability Modifies the Molecular Environment of the Human Colorectum: Implications for Colorectal Carcinogenesis. Cancer Prev. Res. 2011, 4, 530–543. [Google Scholar] [CrossRef]
- Batra, B.; Narwal, V.; Kalra, V.; Sharma, M.; Rana, J. Folic Acid Biosensors: A Review. Process Biochem. 2020, 92, 343–354. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z.; Liu, T.; Yu, F.; Zeng, J.; Zhang, Y.; Yin, L.; Liu, X.; Jiang, H.; Wang, X. Folic Acid-Modified Cerium-Doped Carbon Dots as Photoluminescence Sensors for Cancer Cells Identification and Fe(III) Detection. Chemosensors 2022, 10, 219. [Google Scholar] [CrossRef]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting Folate Receptor Alpha for Cancer Treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef]
- Bryant, C. Nelson The Expanding Role of Mass Spectrometry in Folate Research. Curr. Anal. Chem. 2007, 3, 219–231. [Google Scholar] [CrossRef]
- Devendiran, R.M.; kumar Chinnaiyan, S.; Yadav, N.K.; Moorthy, G.K.; Ramanathan, G.; Singaravelu, S.; Sivagnanam, U.T.; Perumal, P.T. Green Synthesis of Folic Acid-Conjugated Gold Nanoparticles with Pectin as Reducing/Stabilizing Agent for Cancer Theranostics. RSC Adv. 2016, 6, 29757–29768. [Google Scholar] [CrossRef]
- Advani, P.G.; Schonfeld, S.J.; Curtis, R.E.; Dores, G.M.; Linet, M.S.; Sigel, B.S.; Lam, C.J.K.; Tucker, M.A.; Chanock, S.J.; Morton, L.M. Risk of Therapy-Related Myelodysplastic Syndrome/Acute Myeloid Leukemia after Childhood Cancer: A Population-Based Study. Leukemia 2019, 33, 2947–2978. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Progress of Folic Acid-Folate Receptor as Drug Carriers in Targeted Drug Delivery System. SHS Web Conf. 2022, 144, 1002. [Google Scholar] [CrossRef]
- Baker, H.; Herbert, V.; Frank, O.; Pasher, I.; Hutner, S.H.; Wasserman, L.R.; Sobotka, H. A Microbiologic Method for Detecting Folic Acid Deficiency in Man. Clin. Chem. 1959, 5, 275–280. [Google Scholar] [CrossRef]
- Chanarin, I.; Elmes, P.C.; Mollin, D.L. Folic-Acid Studies in Megaloblastic Anaemia Due to Primidone. BMJ 1958, 2, 80–82. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sobczyńska-Malefora, A. Methods for Assessment of Folate (Vitamin B9). In Laboratory Assessment of Vitamin Status; Harrington, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 219–264. [Google Scholar] [CrossRef]
- Waxman, S.; Schreiber, C.; Herbert, V. Radioisotopic Assay for Measurement of Serum Folate Levels. Blood 1971, 38, 219–228. [Google Scholar] [CrossRef]
- Deconinck, E.; Crevits, S.; Baten, P.; Courselle, P.; de Beer, J. A Validated Ultra High Pressure Liquid Chromatographic Method for Qualification and Quantification of Folic Acid in Pharmaceutical Preparations. J. Pharm. Biomed. Anal. 2011, 54, 995–1000. [Google Scholar] [CrossRef] [PubMed]
- Bagley, P.J.; Selhub, J. Analysis of Folate Form Distribution by Affinity Followed by Reversed-Phase Chromatography with Electrochemical Detection. Clin. Chem. 2000, 46, 404–411. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Gregory, J.F., 3rd. Enzymatic Deconjugation of Erythrocyte Polyglutamyl Folates during Preparation for Folate Assay: Investigation with Reversed-Phase Liquid Chromatography. Clin. Chem. 1996, 42, 1847–1854. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, J.; Hayakawa, T.; Tsuge, H. Assays of Methylenetetrahydrofolate Reductase and Methionine Synthase Activities by Monitoring 5-Methyltetrahydrofolate and Tetrahydrofolate Using High-Performance Liquid Chromatography with Fluorescence Detection. Anal. Biochem. 2001, 299, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Santhosh-Kumar, C.R.; Kolhouse, J.F. Molar Quantitation of Folates by Gas Chromatography-Mass Spectrometry. Vitam. Coenzymes Part K 1997, 281, 26–38. [Google Scholar] [CrossRef]
- Pfeiffer, C.M.; Fazili, Z.; McCoy, L.; Zhang, M.; Gunter, E.W. Determination of Folate Vitamers in Human Serum by Stable-Isotope-Dilution Tandem Mass Spectrometry and Comparison with Radioassay and Microbiologic Assay. Clin. Chem. 2004, 50, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Kanjilal, G.; Mahajan, S.N.; Rao, G.R. Colorimetric Determination of Folic Acid in Pharmaceutical Preparations. Analyst 1975, 100, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wang, L. Chemiluminescence Inhibition Assay for Folic Acid Using Flow Injection Analysis. Phytochem. Anal. 2003, 14, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Hla-Pe, U. Aung-Than-Batu A Fluorometric Method for Urinary Folic Acid Determination in Folic Acid Absorption and Load Tests. Clin. Chim. Acta 1969, 24, 381–387. [Google Scholar] [CrossRef]
- Cruces Blanco, C.; Segura Carretero, A.; Fernández Gutierrez, A.; Román Ceba, M. Micellar-Enhanced Synchronous-Derivative Fluorescence Determination of Derivatized Folic Acid in Pharmaceutical Preparations. J. Pharm. Biomed. Anal. 1995, 13, 1019–1025. [Google Scholar] [CrossRef]
- Indyk, H.E. The Determination of Folic Acid in Milk and Paediatric Formulae by Optical Biosensor Assay Utilising Folate Binding Protein. Int. Dairy J. 2010, 20, 106–112. [Google Scholar] [CrossRef]
- Indyk, H.E.; Woollard, D.C. Single Laboratory Validation of an Optical Biosensor Method for the Determination of Folate in Foods. J. Food Compos. Anal. 2013, 29, 87–93. [Google Scholar] [CrossRef]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening Surface Plasmon Resonance Effect of Metal Nanoparticles for Practical Spectroscopic Application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar] [CrossRef]
- Olaru, A.; Bala, C.; Jaffrezic-Renault, N.; Aboul-Enein, H.Y. Surface Plasmon Resonance (SPR) Biosensors in Pharmaceutical Analysis. Crit. Rev. Anal. Chem. 2015, 45, 97–105. [Google Scholar] [CrossRef]
- Scherbahn, V.; Nizamov, S.; Mirsky, V.M. Toward Ultrasensitive Surface Plasmon Resonance Sensors. In Label-Free Biosensing: Advanced Materials, Devices and Applications; Schöning, M.J., Poghossian, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 409–448. [Google Scholar] [CrossRef]
- Altug, H.; Oh, S.-H.; Maier, S.A.; Homola, J. Advances and Applications of Nanophotonic Biosensors. Nat. Nanotechnol. 2022, 17, 5–16. [Google Scholar] [CrossRef]
- Dudak, F.C.; Boyaci, İ.H. Peptide-Based Surface Plasmon Resonance Biosensor for Detection of Staphylococcal Enterotoxin B. Food Anal. Methods 2014, 7, 506–511. [Google Scholar] [CrossRef]
- Wright, J.D.; Oliver, J.V.; Nolte, R.J.M.; Holder, S.J.; Sommerdijk, N.A.J.M.; Nikitin, P.I. The Detection of Phenols in Water Using a Surface Plasmon Resonance System with Specific Receptors. Sens. Actuators B Chem. 1998, 51, 305–310. [Google Scholar] [CrossRef]
- Chang, C.-C. Recent Advancements in Aptamer-Based Surface Plasmon Resonance Biosensing Strategies. Biosensors 2021, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Kochergin, V.E.; Beloglazov, A.A.; Valeiko, M.V.; Nikitin, P.I. Phase Properties of a Surface-Plasmon Resonance from the Viewpoint of Sensor Applications. Quantum Electron. 1998, 28, 444–448. [Google Scholar] [CrossRef]
- Thormählen, I.; Straub, J.; Grigull, U. Refractive Index of Water and Its Dependence on Wavelength, Temperature, and Density. J. Phys. Chem. Ref. Data 1985, 14, 933. [Google Scholar] [CrossRef]
- Orlov, A.V.; Pushkarev, A.V.; Znoyko, S.L.; Novichikhin, D.O.; Bragina, V.A.; Gorshkov, B.G.; Nikitin, P.I. Multiplex Label-Free Biosensor for Detection of Autoantibodies in Human Serum: Tool for New Kinetics-Based Diagnostics of Autoimmune Diseases. Biosens. Bioelectron. 2020, 159, 112187. [Google Scholar] [CrossRef]
- Nikitin, P.I.; Gorshkov, B.G.; Nikitin, E.P.; Ksenevich, T.I. Picoscope, a New Label-Free Biosensor. Sens. Actuators B Chem. 2005, 111–112, 500–504. [Google Scholar] [CrossRef]
- Galukande, M.; Jombwe, J.; Fualal, F.; Baingana, R.; Gakwaya, A. Reference Values for Serum Levels of Folic Acid and Vitamin B12 in a Young Adult Ugandan Population. Afr. Health Sci. 2011, 11, 240–243. [Google Scholar] [CrossRef]
- Wahlin, Å.; Bäckman, L.; Hultdin, J.; Adolfsson, R.; Nilsson, L.-G. Reference Values for Serum Levels of Vitamin B 12 and Folic Acid in a Population-Based Sample of Adults between 35 and 80 Years of Age. Public Health Nutr. 2002, 5, 505–511. [Google Scholar] [CrossRef]
- Snow, C.F. Laboratory Diagnosis of Vitamin B12 and Folate Deficiency: A Guide for the Primary Care Physician. Arch. Intern. Med. 1999, 159, 1289–1298. [Google Scholar] [CrossRef]
- Tomasiuk, R.; Zubrzycki, I.Z.; Wiacek, M. Age-Dependent Changes in Fat- and Water-Soluble Vitamins—National Health and Nutrition Examination Surveys Study. Front. Med. 2022, 9, 907067. [Google Scholar] [CrossRef] [PubMed]
- Orlov, A.V.; Nikitin, M.P.; Bragina, V.A.; Znoyko, S.L.; Zaikina, M.N.; Ksenevich, T.I.; Gorshkov, B.G.; Nikitin, P.I. A New Real-Time Method for Investigation of Affinity Properties and Binding Kinetics of Magnetic Nanoparticles. J. Magn. Magn. Mater. 2015, 380, 231–235. [Google Scholar] [CrossRef]
- Ivanov, A.E.; Solodukhina, N.; Wahlgren, M.; Nilsson, L.; Vikhrov, A.A.; Nikitin, M.P.; Orlov, A.V.; Nikitin, P.I.; Kuzimenkova, M.V.; Zubov, V.P. Reversible Conformational Transitions of a Polymer Brush Containing Boronic Acid and Its Interaction with Mucin Glycoprotein. Macromol. Biosci. 2011, 11, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Burenin, A.G.; Urusov, A.E.; Betin, A.V.; Orlov, A.V.; Nikitin, M.P.; Ksenevich, T.I.; Gorshkov, B.G.; Zherdev, A.V.; Dzantiev, B.B.; Nikitin, P.I. Direct Immunosensing by Spectral Correlation Interferometry: Assay Characteristics versus Antibody Immobilization Chemistry. Anal. Bioanal. Chem. 2015, 407, 3955–3964. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Vaughn, M.W. Morphology and Amine Accessibility of (3-Aminopropyl) Triethoxysilane Films on Glass Surfaces. Scanning 2008, 30, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Azadeh, M.; Gorovits, B.; Kamerud, J.; MacMannis, S.; Safavi, A.; Sailstad, J.; Sondag, P. Calibration Curves in Quantitative Ligand Binding Assays: Recommendations and Best Practices for Preparation, Design, and Editing of Calibration Curves. AAPS J. 2018, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Modupe, O.; Maurras, J.B.; Diosady, L.L. A Spectrophotometric Method for Determining the Amount of Folic Acid in Fortified Salt. J. Agric. Food Res. 2020, 2, 100060. [Google Scholar] [CrossRef]
- Aviva Systems Biology. Folic Acid ELISA Kit (OKEH02550) from Aviva Systems Biology. Available online: https://www.biocompare.com/25138-Assay-Kit/10144611-Folic-acid-ELISA-Kit-OKEH02550/?pda=9956%7C10144611_0_1%7C%7C4%7CFolate&dfp=true#productspecs (accessed on 19 December 2022).
- Lapa, R.A.S.; Lima, J.L.F.C.; Reis, B.F.; Santos, J.L.M.; Zagatto, E.A.G. Photochemical-Fluorimetric Determination of Folic Acid in a Multicommutated Flow System. Anal. Chim. Acta 1997, 351, 223–228. [Google Scholar] [CrossRef]
- Bertuzzi, T.; Rastelli, S.; Mulazzi, A.; Rossi, F. LC-MS/MS Determination of Mono-Glutamate Folates and Folic Acid in Beer. Food Anal. Methods 2019, 12, 722–728. [Google Scholar] [CrossRef]
- Guignard, L.; Nembrini, C.; Moulin, J.; Meisser, K.; Silva-Zolezzi, I.; Kratzsch, J.; Vogel, M.; Kiess, W.; Eckhardt, E. A Rapid Chemiluminescence Assay for Measurement of Folate in Small Volumes of Breast Milk. Molecules 2019, 24, 2730. [Google Scholar] [CrossRef]
- Roche Diagnostics International Ltd. Elecsys® Folate RBC Electrochemiluminescence Immunoassay (ECLIA) for the In Vitro Quantitative Determination of Folate in Erythrocytes (Red Blood Cells, RBC); Roche Diagnostics International Ltd.: Rotkreuz, Switzerland, 2014; Available online: https://diagnostics.roche.com/content/dam/diagnostics/ch/de/gesundheitsthemen/anaemia/Anemia_Factsheet_FolateRBC.pdf (accessed on 19 December 2022).



| Detection Method | Dynamic Range, nM | Assay Time | LOD, nM | Ref. |
|---|---|---|---|---|
| SCI | 0.0009–220 | 20–30 min | 0.0009 | this work |
| Spectrophotometry | 2265–56,600 | 25 min | 24.9 | [49] |
| ELISA | 0.71–45.4 | ~3 h | 0.272 | [50] |
| Photochemical-fluorimetric | 227–90,702 | 2–10 min | 227 | [51] |
| LC-MS/MS | 0.226–227 | 10–30 min | 0.226 | [52] |
| Chemiluminescence | 8.4–120 | 60 min | 1.1 | [53] |
| Electrochemiluminescence immunoassay | 272–1407 | 27 min | 45 | [54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novichikhin, D.O.; Orlov, A.V.; Antopolsky, M.L.; Znoyko, S.L.; Nikitin, P.I. Specific and Sensitive Determination of Folic Acid by Label-Free Chemosensors with Microscope Glass Slips as Single-Use Consumables. Chemosensors 2023, 11, 17. https://doi.org/10.3390/chemosensors11010017
Novichikhin DO, Orlov AV, Antopolsky ML, Znoyko SL, Nikitin PI. Specific and Sensitive Determination of Folic Acid by Label-Free Chemosensors with Microscope Glass Slips as Single-Use Consumables. Chemosensors. 2023; 11(1):17. https://doi.org/10.3390/chemosensors11010017
Chicago/Turabian StyleNovichikhin, Denis O., Alexey V. Orlov, Maxim L. Antopolsky, Sergey L. Znoyko, and Petr I. Nikitin. 2023. "Specific and Sensitive Determination of Folic Acid by Label-Free Chemosensors with Microscope Glass Slips as Single-Use Consumables" Chemosensors 11, no. 1: 17. https://doi.org/10.3390/chemosensors11010017
APA StyleNovichikhin, D. O., Orlov, A. V., Antopolsky, M. L., Znoyko, S. L., & Nikitin, P. I. (2023). Specific and Sensitive Determination of Folic Acid by Label-Free Chemosensors with Microscope Glass Slips as Single-Use Consumables. Chemosensors, 11(1), 17. https://doi.org/10.3390/chemosensors11010017






