Highly Dispersive Palladium Loading on ZnO by Galvanic Replacements with Improved Methane Sensing Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
2.3. Gas Sensor Fabrication and Gas Sensing Tests
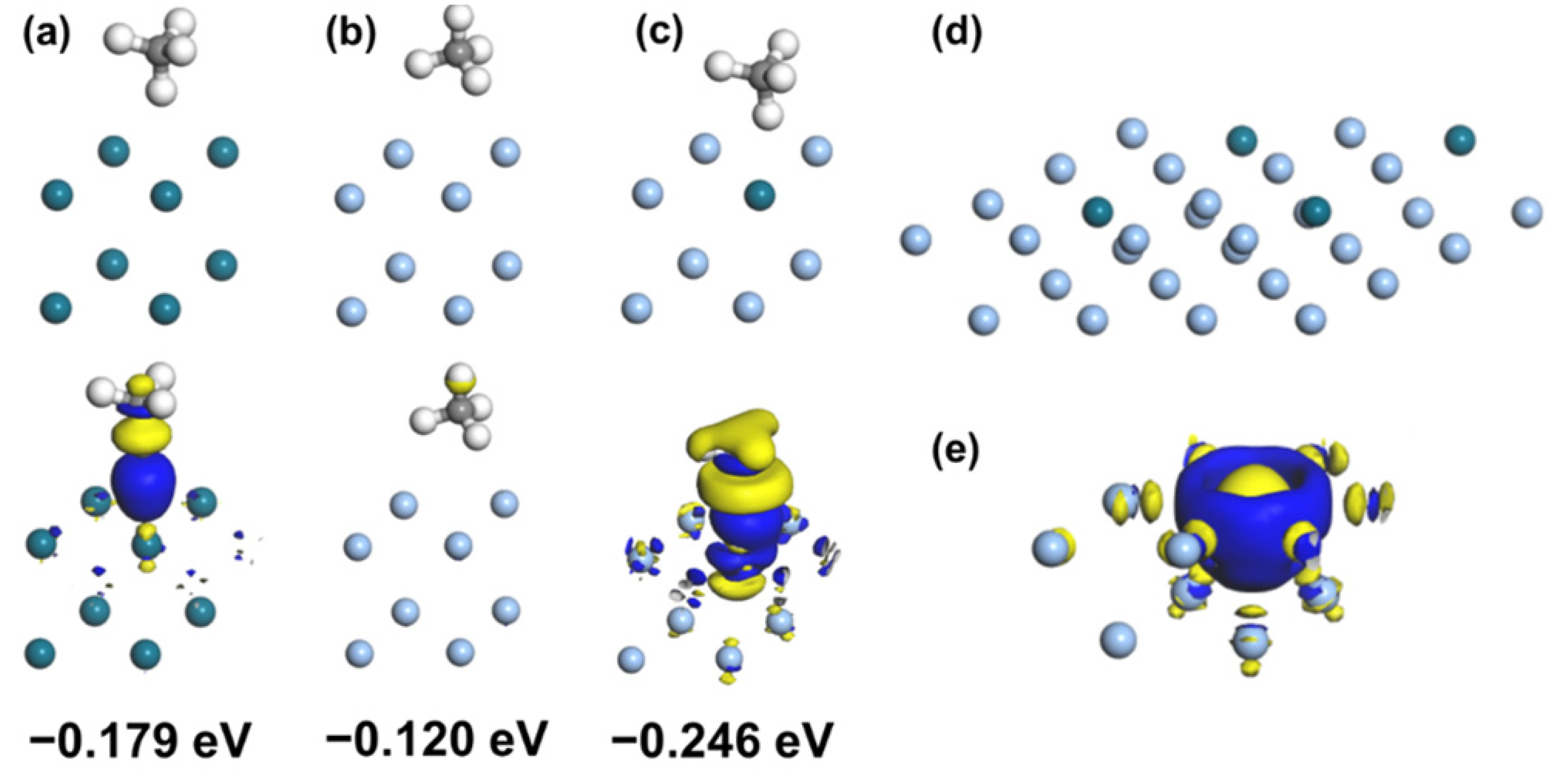
2.4. Computational Methodology
3. Results and Discussion
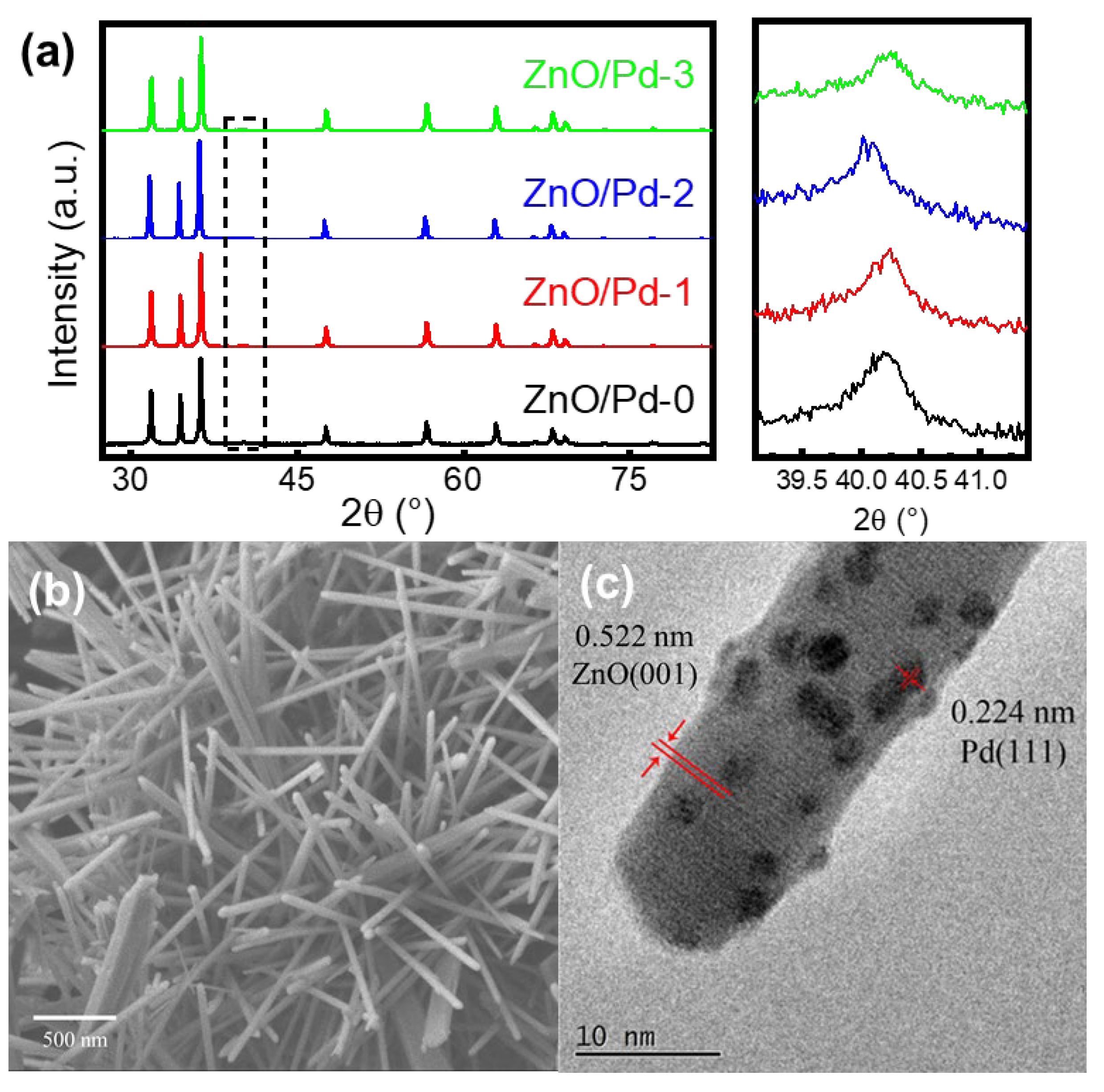
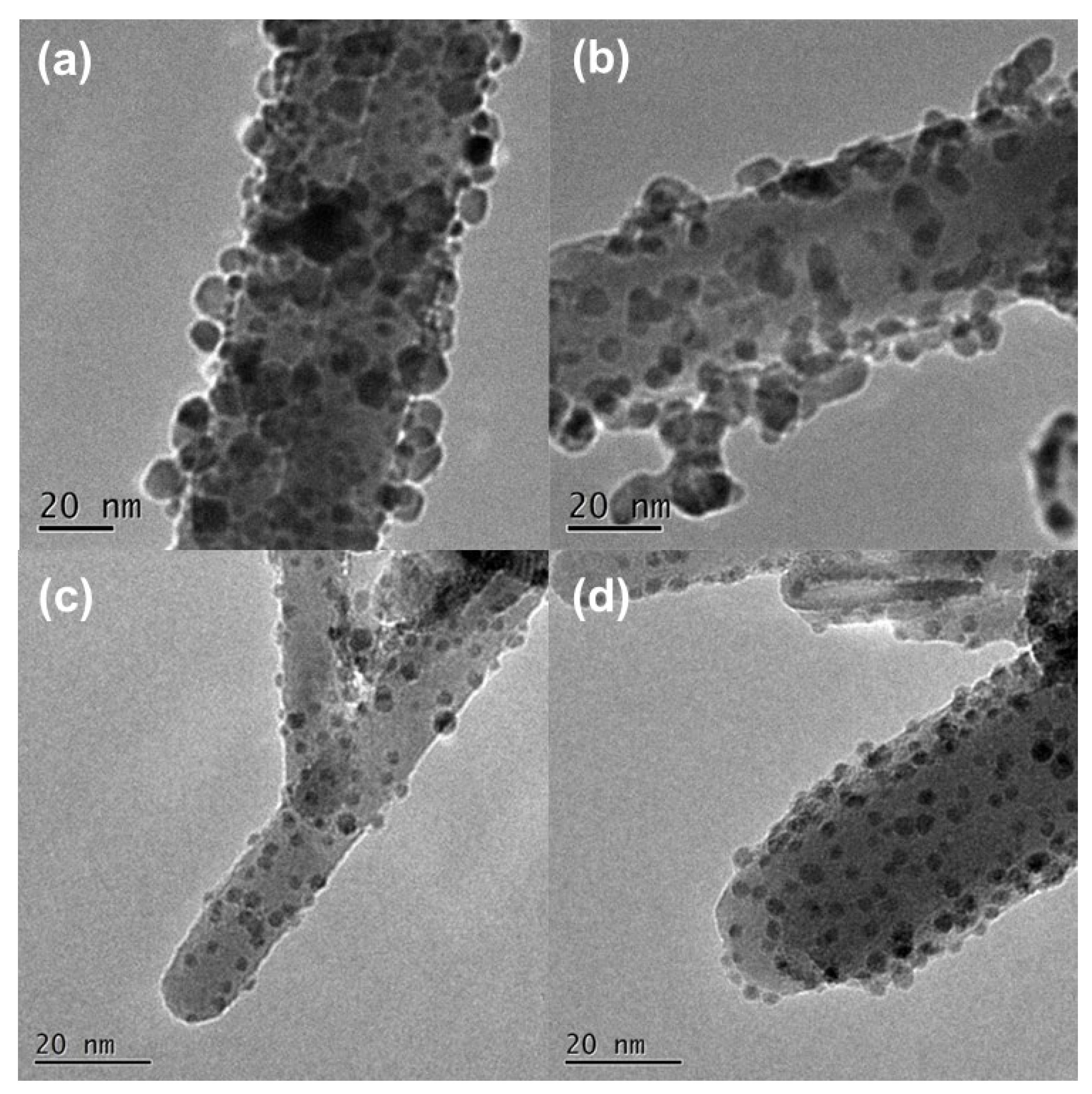
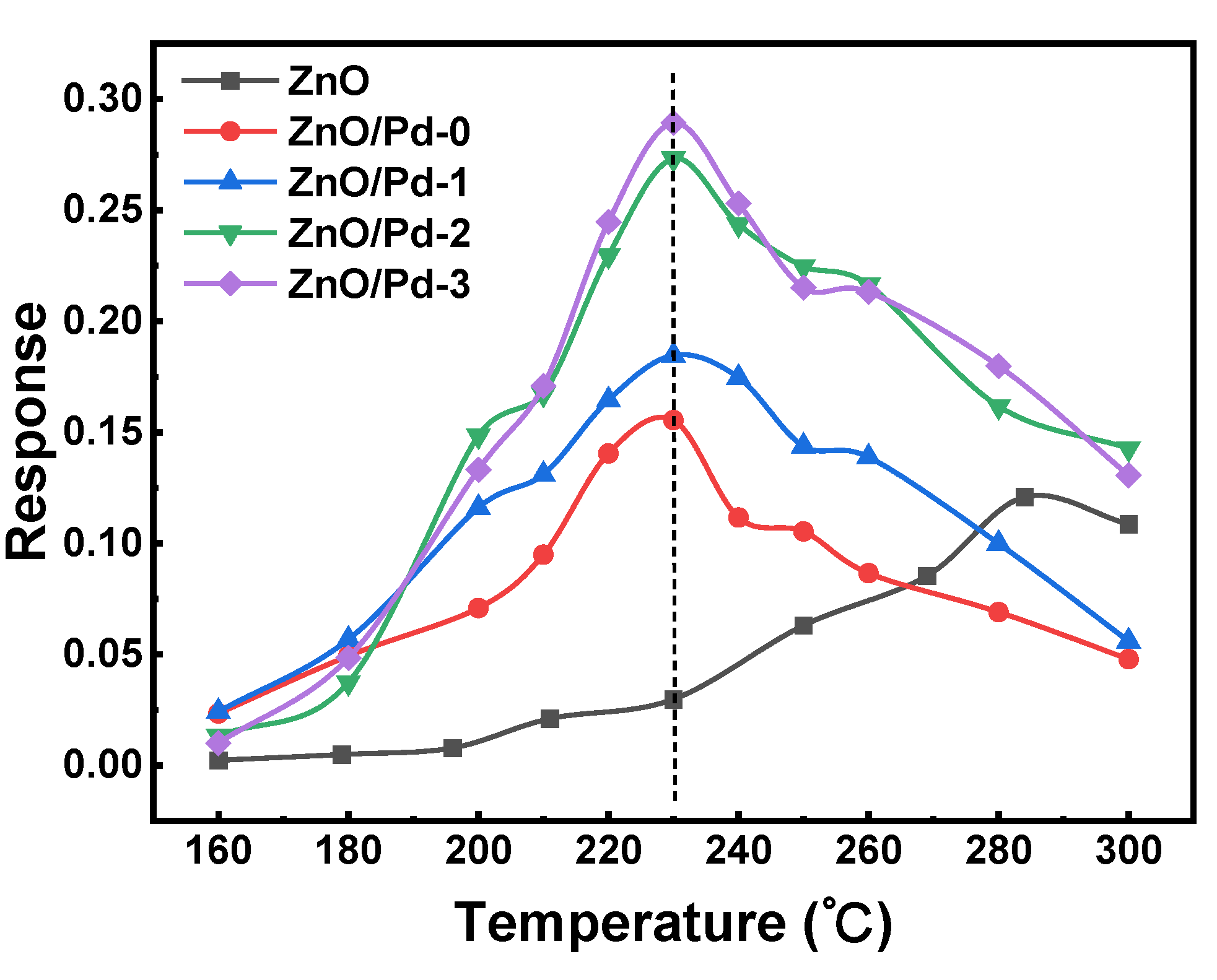
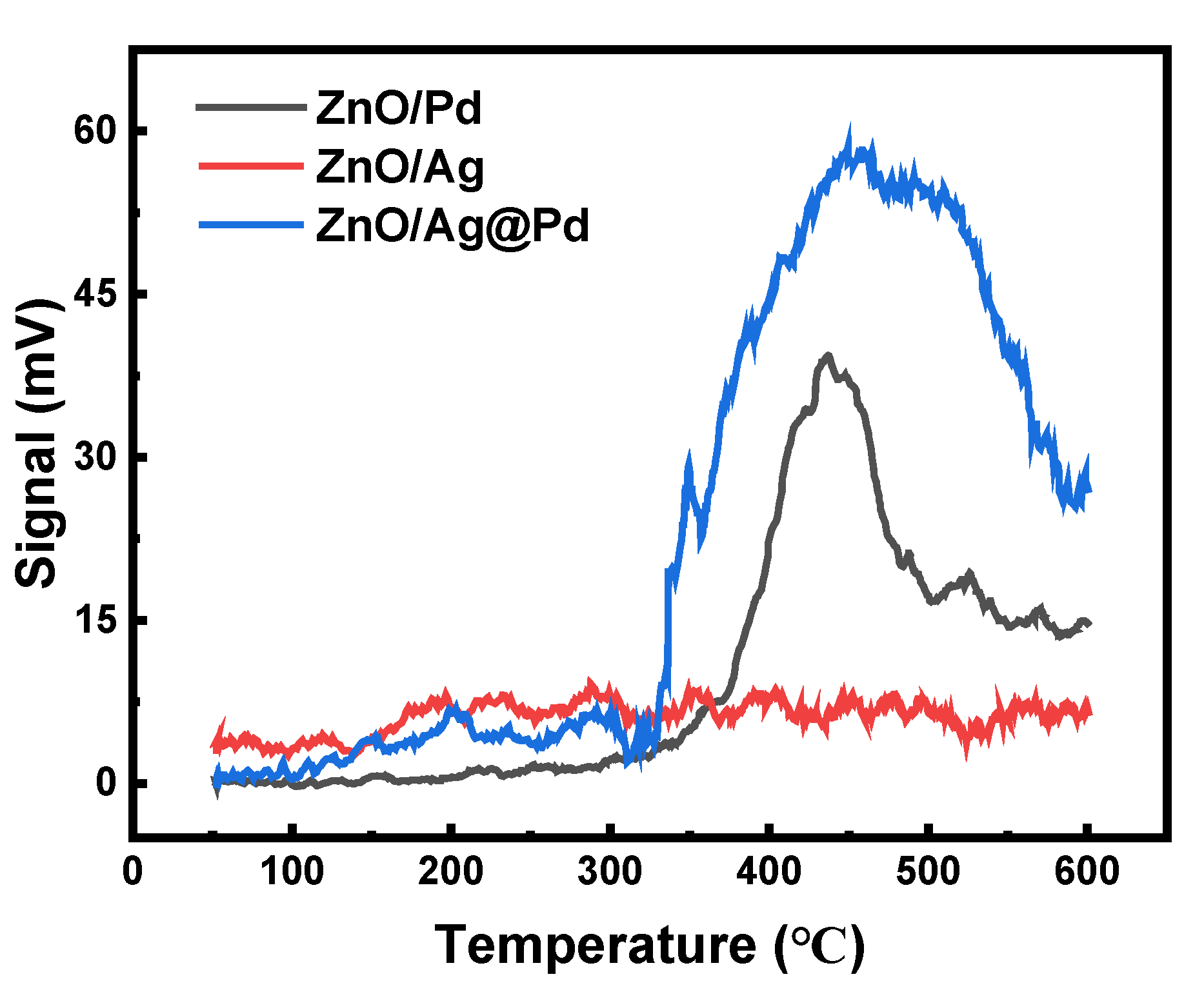
3.1. Effect of Pd Dispersion on the CH4 Sensing Performance
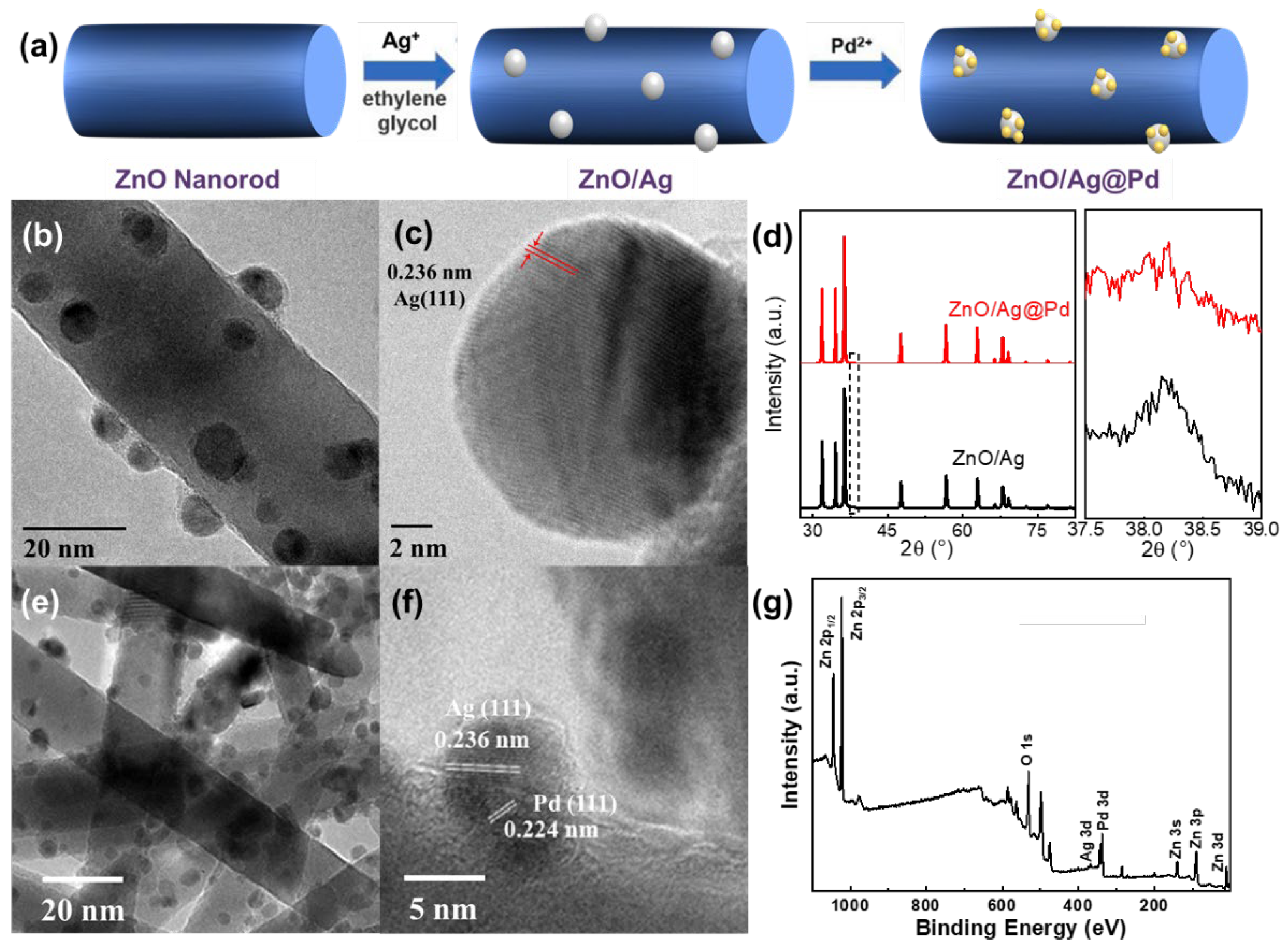
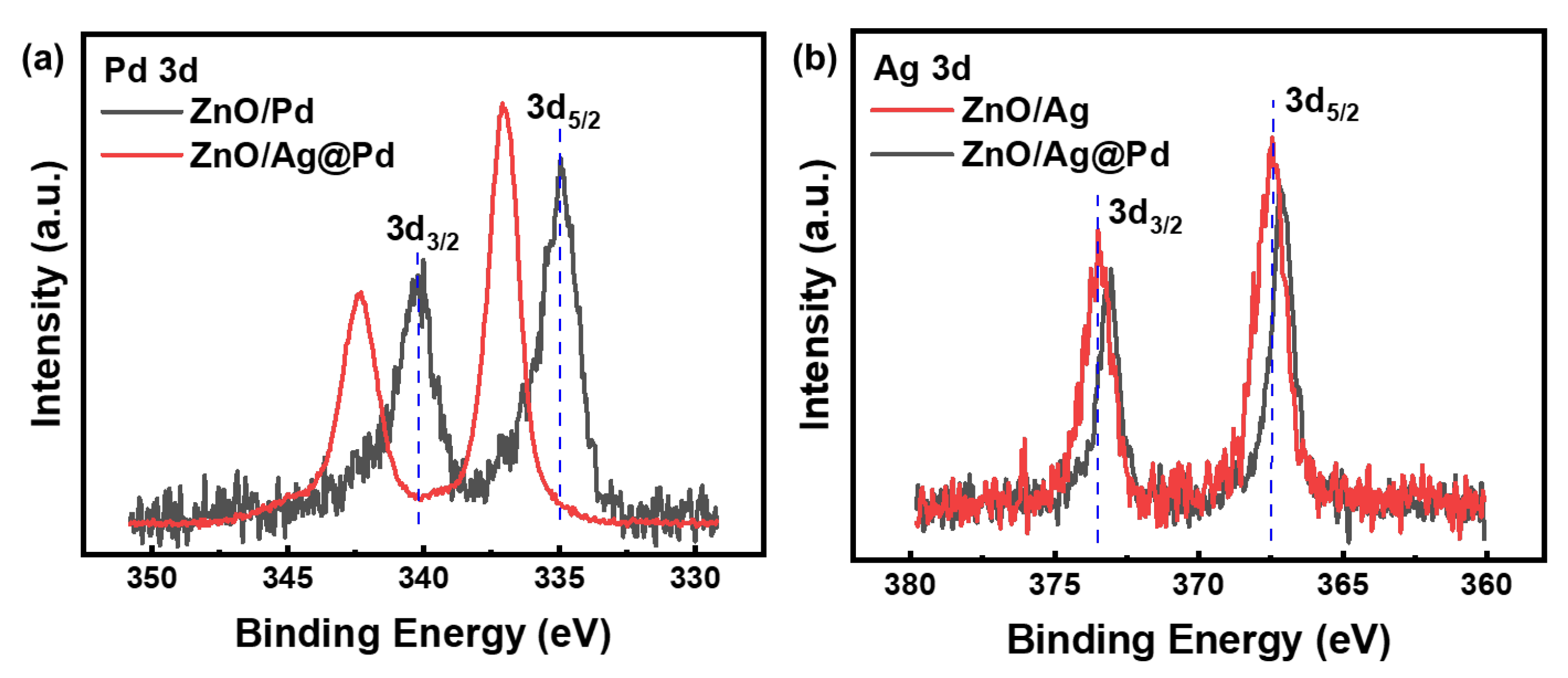
3.2. Highly Dispersive Pd with Enhanced CH4 Sensing Performance via Galvanic Replacement
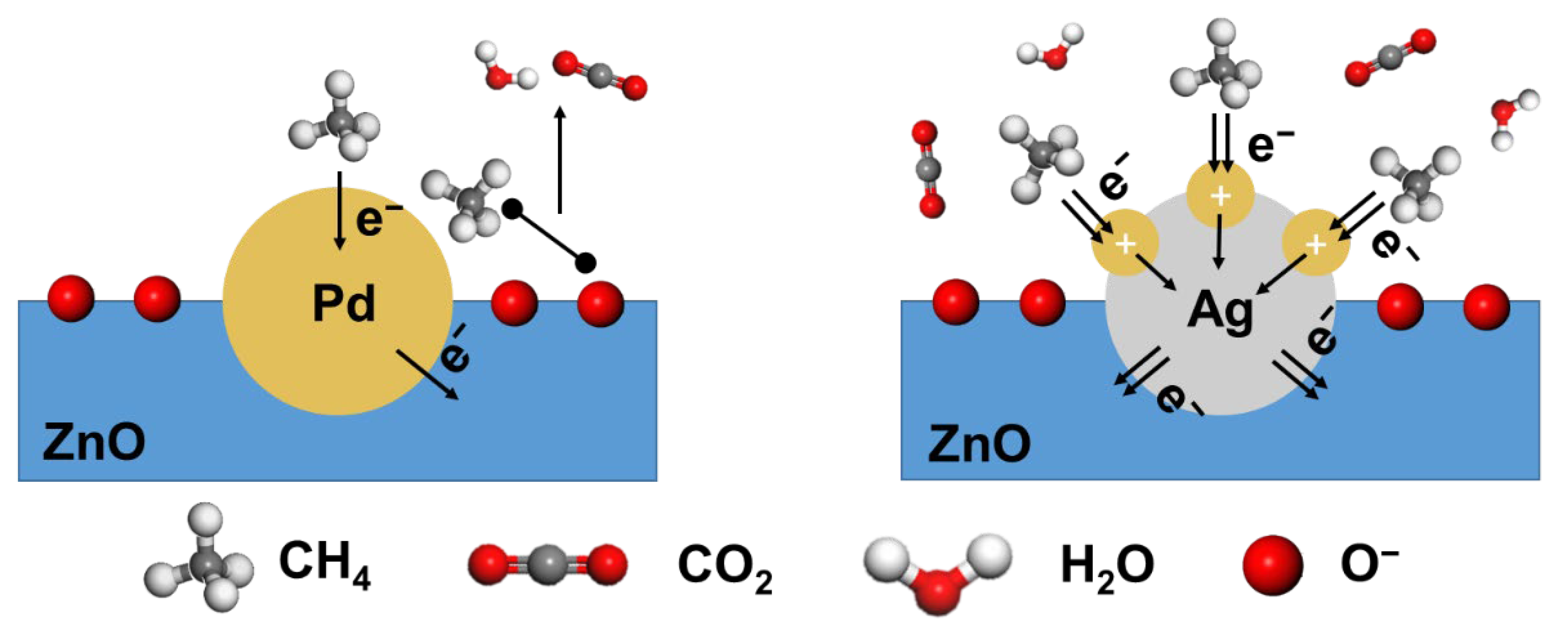
3.3. Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, T.; Zhou, Y.; Luo, Z.; Wen, H.; Zhao, J.; Su, B.; Cheng, F.; Deng, J. Flammability limit behavior of methane with the addition of gaseous fuel at various relative humidities. Process Saf. Environ. Prot. 2020, 140, 178–189. [Google Scholar] [CrossRef]
- Muller, S.; Zimina, A.; Steininger, R.; Flessau, S.; Osswald, J.; Grunwaldt, J.D. High Stability of Rh Oxide-Based Thermoresistive Catalytic Combustion Sensors Proven by Operando X-ray Absorption Spectroscopy and X-ray Diffraction. ACS Sens. 2020, 5, 2486–2496. [Google Scholar] [CrossRef]
- Simion, C.E.; Florea, O.G.; Florea, M.; Neaţu, F.; Neaţu, Ş.; Trandafir, M.M.; Stănoiu, A. CeO2:Mn3O4 Catalytic Micro-Converters Tuned for CH4 Detection Based on Catalytic Combustion under Real Operating Conditions. Materials 2020, 13, 2196. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, M.M.; Zhang, D.; Gao, Z. Improving the Performance of Catalytic Combustion Type Methane Gas Sensors Using Nanostructure Elements Doped with Rare Earth Cocatalysts. Sensors 2011, 11, 19–31. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Ji, H.; Zeng, W.; Li, Y. Gas sensing mechanisms of metal oxide semiconductors: A focus review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef] [PubMed]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, Z.; Tian, C.; Yuan, W.; Hua, Z.; Fan, S.; Wu, Y.; Tian, X. Selective detection of methane by HZSM-5 zeolite/Pd-SnO2 gas sensors. Sens. Actuators B Chem. 2020, 321, 128567. [Google Scholar] [CrossRef]
- Chen, R.; Wang, J.; Luo, S.; Xiang, L.; Li, W.; Xie, D. Unraveling photoexcited electron transfer pathway of oxygen vacancy-enriched ZnO/Pd hybrid toward visible light-enhanced methane detection at a relatively low temperature. Appl. Catal. B Environ. 2020, 264, 118554. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Swart, H.C.; Motaung, D.E. Fabrication of TiO2 nanofibers based sensors for enhanced CH4 performance induced by notable surface area and acid treatment. Vacuum 2021, 187, 110102. [Google Scholar] [CrossRef]
- Tan, Y.; Lei, Y. Atomic layer deposition of Rh nanoparticles on WO3 thin film for CH4 gas sensing with enhanced detection characteristics. Ceram. Int. 2020, 46, 9936–9942. [Google Scholar] [CrossRef]
- Rong, Q.; Xiao, B.; Zeng, J.; Yu, R.; Zi, B.; Zhang, G.; Zhu, Z.; Zhang, J.; Wu, J.; Liu, Q. Pt Single Atom-Induced Activation Energy and Adsorption Enhancement for an Ultrasensitive ppb-Level Methanol Gas Sensor. ACS Sens. 2022, 7, 199–206. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Y.; Meng, X.; Zhang, Z.; Cao, J. A gas sensor based on Ag-modified ZnO flower-like microspheres: Temperature-modulated dual selectivity to CO and CH4. Surf. Interfaces 2021, 24, 101110. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Yao, M.; Sun, G.; Zhang, Z. Enhanced CH4 sensing properties of Pd modified ZnO nanosheets. Ceram. Int. 2019, 45, 13150–13157. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, H.; Chen, C.; Lin, C. Investigation of humidity sensor based on Au modified ZnO nanosheets via hydrothermal method and first principle. Sens. Actuators B Chem. 2019, 287, 526–534. [Google Scholar] [CrossRef]
- Luo, S.; Zeng, Z.; Zeng, G.; Liu, Z.; Xiao, R.; Chen, M.; Tang, L.; Tang, W.; Lai, C.; Cheng, M.; et al. Metal Organic Frameworks as Robust Host of Palladium Nanoparticles in Heterogeneous Catalysis: Synthesis, Application, and Prospect. ACS Appl. Mater. Interfaces 2019, 11, 32579–32598. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Fu, H.; Wan, H.; Wan, Y.; Qu, X.; Xu, Z.; Yin, D.; Zheng, S. Enhanced catalytic hydrogenation reduction of bromate on Pd catalyst supported on CeO2 modified SBA-15 prepared by strong electrostatic adsorption. Appl. Catal. B Environ. 2018, 229, 32–40. [Google Scholar] [CrossRef]
- da Silva, A.G.M.; Rodrigues, T.S.; Haigh, S.J.; Camargo, P.H.C. Galvanic replacement reaction: Recent developments for engineering metal nanostructures towards catalytic applications. Chem. Commun. (Camb.) 2017, 53, 7135–7148. [Google Scholar] [CrossRef]
- Richard-Daniel, J.; Boudreau, D. Enhancing Galvanic Replacement in Plasmonic Hollow Nanoparticles: Understanding the Role of the Speciation of Metal Ion Precursors. ChemNanoMat 2020, 6, 907–915. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, G.; Han, Z.-K.; Zhang, S.; Sarker, D.; Xu, W.W.; Pan, X.; Li, G.; Baiker, A. Synergistic Effects of Ternary PdO–CeO2–OMS-2 Catalyst Afford High Catalytic Performance and Stability in the Reduction of NO with CO. ACS Appl. Mater. Interfaces 2021, 13, 622–630. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Zhou, Y.; Li, G.; Li, Z.; Liu, C.; Bao, M.; Shen, W. Selective hydrogenation of the C=C bond in α,β-unsaturated aldehydes and ketones over ultra-small Pd–Au clusters. Nanoscale 2016, 8, 18626–18629. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Chen, R.; Wang, J.; Xie, D.; Xiang, L. Designed synthesis of ZnO/Pd@ZIF-8 hybrid structure for highly sensitive and selective detection of methane in the presence of NO2. Sens. Actuators B Chem. 2021, 344, 130220. [Google Scholar] [CrossRef]
- Chen, R.S.; Wang, J.; Xia, Y.; Xiang, L. Near infrared light enhanced room-temperature NO2 gas sensing by hierarchical ZnO nanorods functionalized with PbS quantum dots. Sens. Actuators B Chem. 2018, 255, 2538–2545. [Google Scholar] [CrossRef]
- Delley, B. An All-Electron Numerical-Method for Solving the Local Density Functional for Polyatomic-Molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Luo, S.; Xie, D.; Yu, Y.; Xiang, L. Highly Dispersive Palladium Loading on ZnO by Galvanic Replacements with Improved Methane Sensing Performance. Chemosensors 2022, 10, 329. https://doi.org/10.3390/chemosensors10080329
Chen R, Luo S, Xie D, Yu Y, Xiang L. Highly Dispersive Palladium Loading on ZnO by Galvanic Replacements with Improved Methane Sensing Performance. Chemosensors. 2022; 10(8):329. https://doi.org/10.3390/chemosensors10080329
Chicago/Turabian StyleChen, Renjie, Shirui Luo, Dan Xie, Yangxin Yu, and Lan Xiang. 2022. "Highly Dispersive Palladium Loading on ZnO by Galvanic Replacements with Improved Methane Sensing Performance" Chemosensors 10, no. 8: 329. https://doi.org/10.3390/chemosensors10080329
APA StyleChen, R., Luo, S., Xie, D., Yu, Y., & Xiang, L. (2022). Highly Dispersive Palladium Loading on ZnO by Galvanic Replacements with Improved Methane Sensing Performance. Chemosensors, 10(8), 329. https://doi.org/10.3390/chemosensors10080329






