Emerging Role of Biosensors and Chemical Indicators to Monitor the Quality and Safety of Meat and Meat Products
Abstract
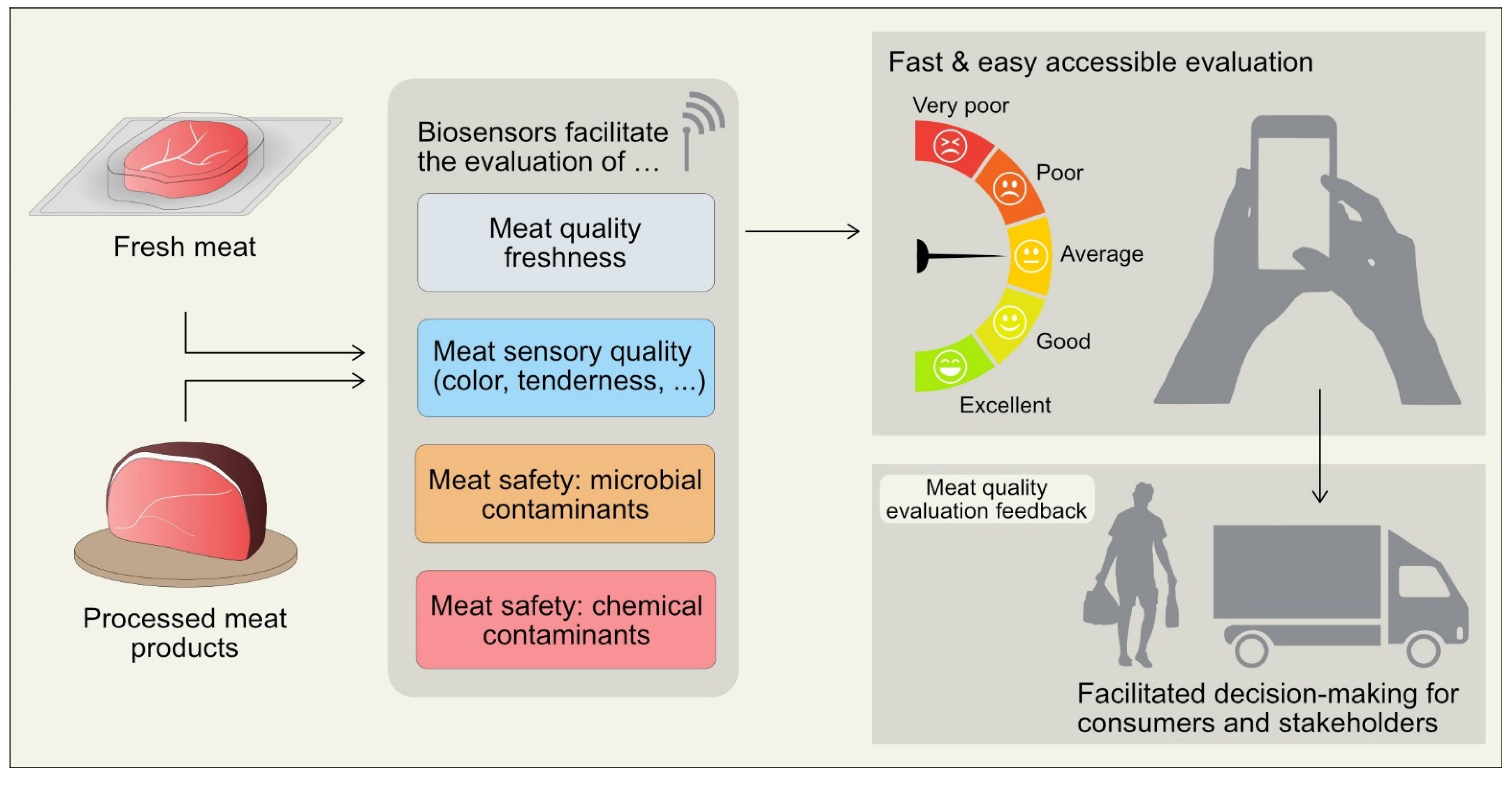
1. Introduction
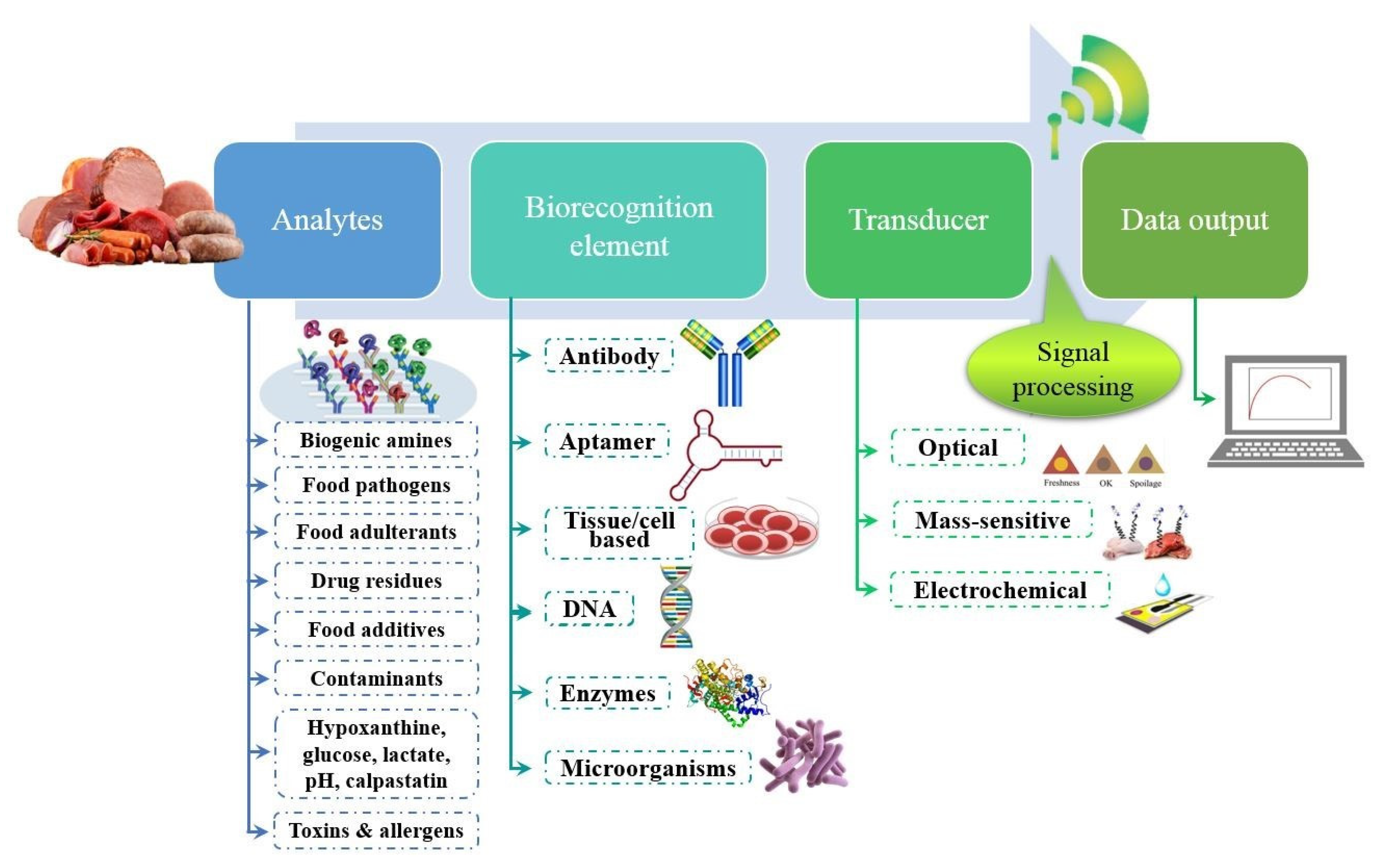
2. The Potential Use of Biosensors for Muscle Foods
3. Application of Biosensors in Assessing Meat Quality
3.1. Biosensors to Assess Meat Freshness
3.2. Biosensors to Evaluate Meat Tenderness
3.3. Biosensors to Detect Microbial Contaminants in Meat
3.4. Biosensors to Detect Contaminants, Antibiotics, and Drug Residues in Meat and Meat Products
4. Sensors and Indicators for Smart Packaging of Meat and Meat Products
5. Factors Influencing the Analytical Performance of Biosensors
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nastasijevic, I.; Mitrovic, R.; Jankovic, S. Biosensors for animal health and meat safety monitoring: Farm-to-slaughterhouse continuum. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Jakarta, Indonesia, 25–26 September 2021; IOP Publishing Ltd.: London, UK, 2021; Volume 854. [Google Scholar]
- Ahmed, I.; Lin, H.; Zou, L.; Li, Z.; Brody, A.L.; Qazi, I.M.; Lv, L.; Pavase, T.R.; Khan, M.U.; Khan, S.; et al. An overview of smart packaging technologies for monitoring safety and quality of meat and meat products. Packag. Technol. Sci. 2018, 31, 449–471. [Google Scholar] [CrossRef]
- Park, Y.W.; Kim, S.M.; Lee, J.Y.; Jang, W. Application of biosensors in smart packaging. Mol. Cell. Toxicol. 2015, 11, 277–285. [Google Scholar] [CrossRef]
- Couletm, P.M.; Blum, L.J. Biosensor Principles and Applications; Marcel Dekker: New York, NY, USA, 1999; Volume 15. [Google Scholar]
- Lim, S.A.; Ahmed, M.U. CHAPTER 1: Introduction to Food Biosensors. In Food Chemistry, Function and Analysis; RSC Publishing: London, UK, 2017; Volume 2017, pp. 1–21. ISBN 9781782623618. [Google Scholar]
- Sionek, B.; Przybylski, W.; Tambor, K. Biosensors in Evaluation of Quality of Meat and Meat Products—A Review. Ann. Anim. Sci. 2020, 20, 1151–1168. [Google Scholar] [CrossRef]
- Sionek, B.; Przybylski, W.; Bańska, A.; Florowski, T. Applications of biosensors for meat quality evaluations. Sensors 2021, 21, 7430. [Google Scholar] [CrossRef]
- Sharifi, S.; Vahed, S.Z.; Ahmadian, E.; Dizaj, S.M.; Eftekhari, A.; Khalilov, R.; Ahmadi, M.; Hamidi-Asl, E.; Labib, M. Detection of pathogenic bacteria via nanomaterials-modified aptasensors. Biosens. Bioelectron. 2020, 150, 111933. [Google Scholar] [CrossRef]
- Biswas, O.; Kandasamy, P.; Patnaik, S.; Lorenzo, J.M.; Das, A.K. Effect of phytochemicals on quality and safety aspects of meat and meat products. Indian J. Anim. Health 2021, 60, 97–108. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Bose, U.; Broadbent, J.A.; Hughes, J.; Sikes, A.; Anderson, A.; Caron, K.; Schmoelzl, S.; Colgrave, M.L. Biomarkers and biosensors for the diagnosis of noncompliant pH, dark cutting beef predisposition, and welfare in cattle. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2391–2432. [Google Scholar] [CrossRef]
- Ali, A.A.; Altemimi, A.B.; Alhelfi, N.; Ibrahim, S.A. Application of biosensors for detection of pathogenic food bacteria: A review. Biosensors 2020, 10, 58. [Google Scholar] [CrossRef]
- Kara, P.; Kılıçkaya, O.; Özsöz, M. Electrochemical DNA Biosensors in Food Safety; CRC Press: Boca Raton, FL, USA, 2010; pp. 123–134. [Google Scholar]
- Mungroo, N.A.; Neethirajan, S. Biosensors for the detection of antibiotics in poultry industry—A Review. Biosensors 2014, 4, 472–493. [Google Scholar] [CrossRef]
- Poghossian, A.; Geissler, H.; Schöning, M.J. Rapid methods and sensors for milk quality monitoring and spoilage detection. Biosens. Bioelectron. 2019, 140, 111272. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A review on biosensors and recent development of nanostructured materials-enabled biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review—Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 167, 037555. [Google Scholar] [CrossRef]
- Ivnitski, D.; Atanassov, P. Biosensors Based on Direct Bioelectrocatalysis for Environmental Monitoring. Biosens. Bioelectron. 1999, 14, 599–624. [Google Scholar] [CrossRef]
- Curulli, A. Electrochemical biosensors in food safety: Challenges and perspectives. Molecules 2021, 26, 2940. [Google Scholar] [CrossRef]
- Aynalem, B.; Muleta, D. Microbial Biosensors as Pesticide Detector: An Overview. J. Sens. 2021, 2021, 9. [Google Scholar] [CrossRef]
- Weng, X.; Luan, X.; Kong, C.; Chang, Z.; Li, Y.; Zhang, S.; Al-Majeed, S.; Xiao, Y. A Comprehensive Method for Assessing Meat Freshness Using Fusing Electronic Nose, Computer Vision, and Artificial Tactile Technologies. J. Sens. 2020, 2020, 14. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Stratakos, A.C. Application of Modified Atmosphere Packaging and Active/Smart Technologies to Red Meat and Poultry: A Review. Food Bioprocess Technol. 2012, 5, 1423–1446. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Coelho, L.C.C.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; de Almeida, J.M.M.M. Biosensors for biogenic amines: A review. Biosensors 2021, 11, 82. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Saraiva, C.; de Almeida, J.M.M.M. Evaluation of the Spoilage of Raw Chicken Breast Fillets Using Fourier Transform Infrared Spectroscopy in Tandem with Chemometrics. Food Bioprocess Technol. 2014, 7, 2330–2341. [Google Scholar] [CrossRef]
- Albelda, J.A.V.; Uzunoglu, A.; Santos, G.N.C.; Stanciu, L.A. Graphene-titanium dioxide nanocomposite based hypoxanthine sensor for assessment of meat freshness. Biosens. Bioelectron. 2017, 89, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Fooladi, E.; Malekaneh, M. A nanocomposite/crude extract enzyme-based xanthine biosensor. Anal. Biochem. 2014, 464, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Cázares, A.S.; Aristoy, M.C.; Toldrá, F. Hypoxanthine-based enzymatic sensor for determination of pork meat freshness. Food Chem. 2010, 123, 949–954. [Google Scholar] [CrossRef]
- Mooltongchun, M.; Teepoo, S. A Simple and Cost-effective Microfluidic Paper-Based Biosensor Analytical Device and its Application for Hypoxanthine Detection in Meat Samples. Food Anal. Methods 2019, 12, 2690–2698. [Google Scholar] [CrossRef]
- Garg, D.; Verma, N. Fibre-optic biosensor for the detection of xanthine for the evaluation of meat freshness. J. Phys. Conf. Ser. 2020, 1531, 12098. [Google Scholar] [CrossRef]
- Omanovic-Miklicanin, E.; Valzacchi, S. Development of new chemiluminescence biosensors for determination of biogenic amines in meat. Food Chem. 2017, 235, 98–103. [Google Scholar] [CrossRef]
- Yano, Y.; Kataho, N.; Watanabe, M.; Nakamura, T.; Asano, Y. Evaluation of beef aging by determination of hypoxanthine and xanthine contents: Application of a xanthine sensor. Food Chem. 1995, 52, 439–445. [Google Scholar] [CrossRef]
- Li, H.; Chen, Q.; Zhao, J.; Wu, M. Nondestructive detection of total volatile basic nitrogen (TVB-N) content in pork meat by integrating hyperspectral imaging and colorimetric sensor combined with a nonlinear data fusion. LWT-Food Sci. Technol. 2015, 63, 268–274. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Shi, J.; Zhang, F.; Zhou, X.; Li, Y.; Holmes, M.; Zhang, W.; Zou, X. Titanium dioxide-polyaniline/silk fibroin microfiber sensor for pork freshness evaluation. Sens. Actuators B Chem. 2018, 260, 465–474. [Google Scholar] [CrossRef]
- Daszczuk, A.; Dessalegne, Y.; Drenth, I.; Hendriks, E.; Jo, E.; Van Lente, T.; Oldebesten, A.; Parrish, J.; Poljakova, W.; Purwanto, A.A.; et al. Bacillus subtilis biosensor engineered to assess meat spoilage. ACS Synth. Biol. 2014, 3, 999–1002. [Google Scholar] [CrossRef]
- Dervisevic, M.; Dervisevic, E.; Azak, H.; Çevik, E.; Şenel, M.; Yildiz, H.B. Novel amperometric xanthine biosensor based on xanthine oxidase immobilized on electrochemically polymerized 10-[4H-dithieno(3,2-b:2′,3′-d)pyrrole-4-yl]decane-1-amine film. Sens. Actuators B Chem. 2016, 225, 181–187. [Google Scholar] [CrossRef]
- Zór, K.; Castellarnau, M.; Pascual, D.; Pich, S.; Plasencia, C.; Bardsley, R.; Nistor, M. Design, development and application of a bioelectrochemical detection system for meat tenderness prediction. Biosens. Bioelectron. 2011, 26, 4283–4288. [Google Scholar] [CrossRef]
- Costa, C.A.B.; Grazhdan, D.; Fiutowski, J.; Nebling, E.; Blohm, L.; Lofink, F.; Rubahn, H.G.; de Oliveira Hansen, R. Meat and fish freshness evaluation by functionalized cantilever-based biosensors. Microsyst. Technol. 2020, 26, 867–871. [Google Scholar] [CrossRef]
- Wang, G.; Sun, J.; Yao, Y.; An, X.; Zhang, H.; Chu, G.; Jiang, S.; Guo, Y.; Sun, X.; Liu, Y. Detection of Inosine Monophosphate (IMP) in Meat Using Double-Enzyme Sensor. Food Anal. Methods 2020, 13, 420–432. [Google Scholar] [CrossRef]
- Geesink, G.H.; Van Der Palen, J.G.P.; Kent, M.P.; Veiseth, E.; Hemke, G.; Koohmaraie, M. Quantification of calpastatin using an optical surface plasmon resonance biosensor. Meat Sci. 2005, 71, 537–541. [Google Scholar] [CrossRef][Green Version]
- Bratcher, C.L.; Grant, S.A.; Vassalli, J.T.; Lorenzen, C.L. Enhanced efficiency of a capillary-based biosensor over an optical fiber biosensor for detecting calpastatin. Biosens. Bioelectron. 2008, 23, 1674–1679. [Google Scholar] [CrossRef]
- Uwimbabazi, E.; Mukasekuru, M.R.; Sun, X. Glucose Biosensor Based on a Glassy Carbon Electrode Modified with Multi-Walled Carbon Nanotubes-Chitosan for the Determination of Beef Freshness. Food Anal. Methods 2017, 10, 2667–2676. [Google Scholar] [CrossRef]
- Gagaoua, M.; Bonnet, M.; De Koning, L.; Picard, B. Reverse Phase Protein array for the quantification and validation of protein biomarkers of beef qualities: The case of meat color from Charolais breed. Meat Sci. 2018, 145, 308–319. [Google Scholar] [CrossRef]
- Gagaoua, M.; Duffy, G.; Alvarez, C.; Burgess, C.M.; Hamill, R.; Crofton, E.; Botinestean, C.; Ferragina, A.; Cafferky, J.; Mullen, A.M.; et al. Current research and emerging tools to improve fresh red meat quality. Ir. J. Agric. Food Res. 2022. [Google Scholar] [CrossRef]
- Listrat, A.; Gagaoua, M.; Andueza, D.; Gruffat, D.; Normand, J.; Mairesse, G.; Picard, B.; Hocquette, J.F. What are the drivers of beef sensory quality using metadata of intramuscular connective tissue, fatty acids and muscle fiber characteristics? Livest. Sci. 2020, 240, 104209. [Google Scholar] [CrossRef]
- Gagaoua, M.; Picard, B.; Soulat, J.; Monteils, V. Clustering of sensory eating qualities of beef: Consistencies and differences within carcass, muscle, animal characteristics and rearing factors. Livest. Sci. 2018, 214, 245–258. [Google Scholar] [CrossRef]
- Gagaoua, M.; Terlouw, E.M.C.; Mullen, A.M.; Franco, D.; Warner, R.D.; Lorenzo, J.M.; Purslow, P.P.; Gerrard, D.; Hopkins, D.L.; Troy, D.; et al. Molecular signatures of beef tenderness: Underlying mechanisms based on integromics of protein biomarkers from multi-platform proteomics studies. Meat Sci. 2021, 172, 108311. [Google Scholar] [CrossRef]
- Purslow, P.P.; Gagaoua, M.; Warner, R.D. Insights on meat quality from combining traditional studies and proteomics. Meat Sci. 2021, 174, 108423. [Google Scholar] [CrossRef]
- Gagaoua, M.; Hafid, K.; Boudida, Y.; Becila, S.; Ouali, A.; Picard, B.; Boudjellal, A.; Sentandreu, M.A. Caspases and Thrombin Activity Regulation by Specific Serpin Inhibitors in Bovine Skeletal Muscle. Appl. Biochem. Biotechnol. 2015, 177, 279–303. [Google Scholar] [CrossRef]
- Hopkins, G.B.; Geesink, G. Protein degradation post mortem and tenderisation. In Applied Muscle Biology and Meat Science; Du, M., McCormick, R., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Milton Park, UK, 2009. [Google Scholar]
- Ouali, A.; Gagaoua, M.; Boudida, Y.; Becila, S.; Boudjellal, A.; Herrera-Mendez, C.H.; Sentandreu, M.A. Biomarkers of meat tenderness: Present knowledge and perspectives in regards to our current understanding of the mechanisms involved. Meat Sci. 2013, 95, 854–870. [Google Scholar] [CrossRef] [PubMed]
- Hyatt, H.W.; Powers, S.K. The Role of Calpains in Skeletal Muscle Remodeling with Exercise and Inactivity-induced Atrophy. Int. J. Sports Med. 2020, 41, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Gagaoua, M.; Troy, D.; Mullen, A.M. The Extent and Rate of the Appearance of the Major 110 and 30 kDa Proteolytic Fragments during Post-Mortem Aging of Beef Depend on the Glycolysing Rate of the Muscle and Aging Time: An LC-MS/MS Approach to Decipher Their Proteome and Associated Pathways. J. Agric. Food Chem. 2021, 69, 602–614. [Google Scholar] [CrossRef]
- Gagaoua, M.; Warner, R.D.; Purslow, P.; Ramanathan, R.; Mullen, A.M.; López-Pedrouso, M.; Franco, D.; Lorenzo, J.M.; Tomasevic, I.; Picard, B.; et al. Dark-cutting beef: A brief review and an integromics meta-analysis at the proteome level to decipher the underlying pathways. Meat Sci. 2021, 181, 108611. [Google Scholar] [CrossRef]
- Claudia Terlouw, E.M.; Picard, B.; Deiss, V.; Berri, C.; Hocquette, J.F.; Lebret, B.; Lefèvre, F.; Hamill, R.; Gagaoua, M. Understanding the determination of meat quality using biochemical characteristics of the muscle: Stress at slaughter and other missing keys. Foods 2021, 10, 84. [Google Scholar] [CrossRef]
- Van Eenennaam, A.L.; Li, J.; Thallman, R.M.; Quaas, R.L.; Dikeman, M.E.; Gill, C.A.; Franke, D.E.; Thomas, M.G. Validation of commercial DNA tests for quantitative beef quality traits. J. Anim. Sci. 2007, 85, 891–900. [Google Scholar] [CrossRef]
- Wheeler, T.L.; Shackelford, S.D.; Koohmaraie, M. Technical note: Sampling methodology for relating sarcomere length, collagen concentration, and the extent of postmortem proteolysis to beef and pork longissimus tenderness. J. Anim. Sci. 2002, 80, 982–987. [Google Scholar] [CrossRef]
- Boccard, R.; Buchter, L.; Casteels, E.; Cosentino, E.; Dransfield, E.; Hood, D.E.; Joseph, R.L.; MacDougall, D.B.; Rhodes, D.N.; Schön, I.; et al. Procedures for measuring meat quality characteristics in beef production experiments. Report of a working group in the commission of the European communities’ (CEC) beef production research programme. Livest. Prod. Sci. 1981, 8, 385–397. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Das, A.; Biswas, S. Hazards and safety issues of meat and meat products. Food Saf. Hum. Heal. 2019, 145–168. [Google Scholar] [CrossRef]
- Pradhan, S.R.; Patra, G.; Nanda, P.K.; Dandapat, P.; Bandyopadhyay, S.; Das, A.K. Comparative Microbial Load Assessment of Meat, Contact Surfaces and Water Samples in Retail Chevon meat Shops and Abattoirs of Kolkata, W.B, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 158–164. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, C.W.; Wang, J.; Oh, D.H. Advances in rapid detection methods for foodborne pathogens. J. Microbiol. Biotechnol. 2014, 24, 297–312. [Google Scholar] [CrossRef]
- Rajapaksha, P.; Elbourne, A.; Gangadoo, S.; Brown, R.; Cozzolino, D.; Chapman, J. A review of methods for the detection of pathogenic microorganisms. Analyst 2019, 144, 396–411. [Google Scholar] [CrossRef]
- Batani, G.; Bayer, K.; Böge, J.; Hentschel, U.; Thomas, T. Fluorescence in situ hybridization (FISH) and cell sorting of living bacteria. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Weng, X.; Neethirajan, S. Ensuring food safety: Quality monitoring using microfluidics. Trends Food Sci. Technol. 2017, 65, 10–22. [Google Scholar] [CrossRef]
- Singh, A.; Poshtiban, S.; Evoy, S. Recent advances in bacteriophage based biosensors for food-borne pathogen detection. Sensors 2013, 13, 1763–1786. [Google Scholar] [CrossRef]
- Alamer, S.; Eissa, S.; Chinnappan, R.; Herron, P.; Zourob, M. Rapid colorimetric lactoferrin-based sandwich immunoassay on cotton swabs for the detection of foodborne pathogenic bacteria. Talanta 2018, 185, 275–280. [Google Scholar] [CrossRef]
- Zhang, G. Foodborne Pathogenic Bacteria Detection: An Evaluation of Current and Developing Methods. McMaster Univ. Undergrad. Health Sci. J. 2013, 1, 27–30. [Google Scholar]
- Xiang, C.; Li, R.; Adhikari, B.; She, Z.; Li, Y.; Kraatz, H.B. Sensitive electrochemical detection of Salmonella with chitosan-gold nanoparticles composite film. Talanta 2015, 140, 122–127. [Google Scholar] [CrossRef]
- Morant-Miñana, M.C.; Elizalde, J. Microscale electrodes integrated on COP for real sample Campylobacter spp. detection. Biosens. Bioelectron. 2015, 70, 491–497. [Google Scholar] [CrossRef]
- Che, Y.; Li, Y.; Slavik, M. Detection of Campylobacter jejuni in poultry samples using an enzyme-linked immunoassay coupled with an enzyme electrode. Biosens. Bioelectron. 2001, 16, 791–797. [Google Scholar] [CrossRef]
- Masdor, N.A.; Altintas, Z.; Tothill, I.E. Sensitive detection of Campylobacter jejuni using nanoparticles enhanced QCM sensor. Biosens. Bioelectron. 2016, 78, 328–336. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Hu, Q.; Wang, R.; Li, Y.; Kidd, M. Rapid and sensitive detection of campylobacter jejuni in poultry products using a nanoparticle-Based piezoelectric immunosensor integrated with magnetic immunoseparation. J. Food Prot. 2018, 81, 1321–1330. [Google Scholar] [CrossRef]
- Fulgione, A.; Cimafonte, M.; Della Ventura, B.; Iannaccone, M.; Ambrosino, C.; Capuano, F.; Proroga, Y.T.R.; Velotta, R.; Capparelli, R. QCM-based immunosensor for rapid detection of Salmonella Typhimurium in food. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Jasim, I.; Shen, Z.; Zhao, L.; Dweik, M.; Zhang, S.; Almasri, M. A microfluidic based biosensor for rapid detection of Salmonella in food products. PLoS ONE 2019, 14, e0216873. [Google Scholar] [CrossRef]
- Liang, P.S.; Park, T.S.; Yoon, J.Y. Rapid and reagentless detection of microbial contamination within meat utilizing a smartphone-based biosensor. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Shama, G.; Malik, D.J. The uses and abuses of rapid bioluminescence-based ATP assays. Int. J. Hyg. Environ. Health 2013, 216, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lomakina, G.Y.; Modestova, Y.A.; Ugarova, N.N. Bioluminescence assay for cell viability. Biochemistry 2015, 80, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, G.R.; Cutter, C.N.; Willett, J.L. Incorporation of bacteriocin in plastic retains activity and inhibits surface growth of bacteria on meat. Food Microbiol. 1999, 16, 229–235. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Huang, J.; Li, K.; Zhang, W.; Xian, Y.; Jin, L. Combining biofunctional magnetic nanoparticles and ATP bioluminescence for rapid detection of Escherichia coli. Talanta 2009, 77, 1332–1336. [Google Scholar] [CrossRef]
- Vizzini, P.; Manzano, M.; Farre, C.; Meylheuc, T.; Chaix, C.; Ramarao, N.; Vidic, J. Highly sensitive detection of Campylobacter spp. In chicken meat using a silica nanoparticle enhanced dot blot DNA biosensor. Biosens. Bioelectron. 2021, 171, 1–8. [Google Scholar] [CrossRef]
- Ohk, S.H.; Bhunia, A.K. Multiplex fiber optic biosensor for detection of Listeria monocytogenes, Escherichia coli O157: H7 and Salmonella enterica from ready-to-eat meat samples. Food Microbiol. 2013, 33, 166–171. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, X.; Liu, Y.; Duan, N.; Wu, S.; Wang, Z.; Xu, B. Gold nanoparticles enhanced SERS aptasensor for the simultaneous detection of Salmonella typhimurium and Staphylococcus aureus. Biosens. Bioelectron. 2015, 74, 872–877. [Google Scholar] [CrossRef]
- Ohk, S.H.; Koo, O.K.; Sen, T.; Yamamoto, C.M.; Bhunia, A.K. Antibody-aptamer functionalized fibre-optic biosensor for specific detection of Listeria monocytogenes from food. J. Appl. Microbiol. 2010, 109, 808–817. [Google Scholar] [CrossRef]
- Muniandy, S.; Dinshaw, I.J.; Teh, S.J.; Lai, C.W.; Ibrahim, F.; Thong, K.L.; Leo, B.F. Graphene-based label-free electrochemical aptasensor for rapid and sensitive detection of foodborne pathogen. Anal. Bioanal. Chem. 2017, 409, 6893–6905. [Google Scholar] [CrossRef]
- Chen, I.H.; Horikawa, S.; Bryant, K.; Riggs, R.; Chin, B.A.; Barbaree, J.M. Bacterial assessment of phage magnetoelastic sensors for Salmonella enterica Typhimurium detection in chicken meat. Food Control 2017, 71, 273–278. [Google Scholar] [CrossRef]
- Liu, G.; Chai, C.; Yao, B. Rapid Evaluation of Salmonella pullorum Contamination in Chicken Based on a Portable Amperometric Sensor. J. Biosens. Bioelectron. 2013, 4, 137–143. [Google Scholar] [CrossRef]
- Rasooly, A. Surface plasmon resonance analysis of staphylococcal enterotoxin B in food. J. Food Prot. 2001, 64, 37–43. [Google Scholar] [CrossRef]
- Helali, S.; Sawelem Eid Alatawi, A.; Abdelghani, A. Pathogenic Escherichia coli biosensor detection on chicken food samples. J. Food Saf. 2018, 38, e12510. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Peng, D.; Xie, S.; Chen, D.; Pan, Y.; Tao, Y.; Yuan, Z. Construction of electrochemical immunosensor based on gold-nanoparticles/carbon nanotubes/chitosan for sensitive determination of T-2 toxin in feed and swine meat. Int. J. Mol. Sci. 2018, 19, 3895. [Google Scholar] [CrossRef]
- Ma, X.; Jiang, Y.; Jia, F.; Yu, Y.; Chen, J.; Wang, Z. An aptamer-based electrochemical biosensor for the detection of Salmonella. J. Microbiol. Methods 2014, 98, 94–98. [Google Scholar] [CrossRef]
- Oh, S.Y.; Heo, N.S.; Shukla, S.; Cho, H.J.; Vilian, A.T.E.; Kim, J.; Lee, S.Y.; Han, Y.K.; Yoo, S.M.; Huh, Y.S. Development of gold nanoparticle-aptamer-based LSPR sensing chips for the rapid detection of Salmonella typhimurium in pork meat. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Fei, J.; Dou, W.; Zhao, G. A sandwich electrochemical immunosensor for Salmonella pullorum and Salmonella gallinarum based on a screen-printed carbon electrode modified with an ionic liquid and electrodeposited gold nanoparticles. Microchim. Acta 2015, 182, 2267–2275. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Li, Y. Rapid detection of Escherichia coli O157:H7 and Salmonella Typhimurium in foods using an electrochemical immunosensor based on screen-printed interdigitated microelectrode and immunomagnetic separation. Talanta 2016, 148, 200–208. [Google Scholar] [CrossRef]
- Wei, D.; Oyarzabal, O.A.; Huang, T.S.; Balasubramanian, S.; Sista, S.; Simonian, A.L. Development of a surface plasmon resonance biosensor for the identification of Campylobacter jejuni. J. Microbiol. Methods 2007, 69, 78–85. [Google Scholar] [CrossRef]
- Yang, G.J.; Huang, J.L.; Meng, W.J.; Shen, M.; Jiao, X.A. A reusable capacitive immunosensor for detection of Salmonella spp. based on grafted ethylene diamine and self-assembled gold nanoparticle monolayers. Anal. Chim. Acta 2009, 647, 159–166. [Google Scholar] [CrossRef]
- Banerjee, P.; Bhunia, A.K. Cell-based biosensor for rapid screening of pathogens and toxins. Biosens. Bioelectron. 2010, 26, 99–106. [Google Scholar] [CrossRef]
- Abdalhai, M.H.; Fernandes, A.M.; Xia, X.; Musa, A.; Ji, J.; Sun, X. Electrochemical Genosensor to Detect Pathogenic Bacteria (Escherichia coli O157:H7) As Applied in Real Food Samples (Fresh Beef) to Improve Food Safety and Quality Control. J. Agric. Food Chem. 2015, 63, 5017–5025. [Google Scholar] [CrossRef]
- Alhogail, S.; Suaifan, G.A.R.Y.; Zourob, M. Rapid colorimetric sensing platform for the detection of Listeria monocytogenes foodborne pathogen. Biosens. Bioelectron. 2016, 86, 1061–1066. [Google Scholar] [CrossRef]
- Ma, X.; Xu, X.; Xia, Y.; Wang, Z. SERS aptasensor for Salmonella typhimurium detection based on spiny gold nanoparticles. Food Control 2018, 84, 232–237. [Google Scholar] [CrossRef]
- Thakur, M.S.; Ragavan, K.V. Biosensors in food processing. J. Food Sci. Technol. 2013, 50, 625–641. [Google Scholar] [CrossRef]
- Mansouri, M.; Fathi, F.; Jalili, R.; Shoeibie, S.; Dastmalchi, S.; Khataee, A.; Rashidi, M.R. SPR enhanced DNA biosensor for sensitive detection of donkey meat adulteration. Food Chem. 2020, 331, 127163. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Suryani, A.A.; Agustina, M.; Gaffar, S.; Anggraeni, A. A Gold Nanoparticle–DNA Bioconjugate–Based Electrochemical Biosensor for Detection of Sus scrofa mtDNA in Raw and Processed Meat. Food Anal. Methods 2019, 12, 2591–2600. [Google Scholar] [CrossRef]
- Ruiz-Valdepeñas Montiel, V.; Gutiérrez, M.L.; Torrente-Rodríguez, R.M.; Povedano, E.; Vargas, E.; Reviejo, J.; Linacero, R.; Gallego, F.J.; Campuzano, S.; Pingarrón, J.M. Disposable Amperometric Polymerase Chain Reaction-Free Biosensor for Direct Detection of Adulteration with Horsemeat in Raw Lysates Targeting Mitochondrial DNA. Anal. Chem. 2017, 89, 9474–9482. [Google Scholar] [CrossRef]
- Dinçkaya, E.; Akyilmaz, E.; Sezgintürk, M.K.; Ertaş, F.N. Sensitive nitrate determination in water and meat samples by amperometric biosensor. Prep. Biochem. Biotechnol. 2010, 40, 119–128. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, X.; Teng, S.; Xu, X.; Zhou, G. Development and validation of a surface plasmon resonance biosensor for specific detection of porcine serum albumin in food. J. AOAC Int. 2018, 101, 1868–1872. [Google Scholar] [CrossRef]
- Swaidan, A.; Barras, A.; Addad, A.; Tahon, J.F.; Toufaily, J.; Hamieh, T.; Szunerits, S.; Boukherroub, R. Colorimetric sensing of dopamine in beef meat using copper sulfide encapsulated within bovine serum albumin functionalized with copper phosphate (CuS-BSA-Cu3(PO4)2) nanoparticles. J. Colloid Interface Sci. 2021, 582, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhu, Y.; Chen, Y.; Xu, X.; Zhou, G. Rapid visual detection of eight meat species using optical thin-film biosensor chips. J. AOAC Int. 2015, 98, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Pikkemaat, M.G.; Rapallini, M.L.B.A.; Karp, M.T.; Elferink, J.W.A. Application of a luminescent bacterial biosensor for the detection of tetracyclines in routine analysis of poultry muscle samples. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2010, 27, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.; Baxter, A.; Young, P.; Kennedy, G.; Elliott, C.; Weigel, S.; Gatermann, R.; Ashwin, H.; Stead, S.; Sharman, M. Detection of chloramphenicol and chloramphenicol glucuronide residues in poultry muscle, honey, prawn and milk using a surface plasmon resonance biosensor and Qflex® kit chloramphenicol. Anal. Chim. Acta 2005, 529, 109–113. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Huang, P.; Wu, F.Y. A label-free colorimetric aptasensor based on controllable aggregation of AuNPs for the detection of multiplex antibiotics. Food Chem. 2020, 304, 125377. [Google Scholar] [CrossRef]
- Sun, Y.; He, J.; Waterhouse, G.I.N.; Xu, L.; Zhang, H.; Qiao, X.; Xu, Z. A selective molecularly imprinted electrochemical sensor with GO@COF signal amplification for the simultaneous determination of sulfadiazine and acetaminophen. Sens. Actuators B Chem. 2019, 300, 126993. [Google Scholar] [CrossRef]
- Yu, S.; Wei, Q.; Du, B.; Wu, D.; Li, H.; Yan, L.; Ma, H.; Zhang, Y. Label-free immunosensor for the detection of kanamycin using Ag@Fe3O4 nanoparticles and thionine mixed graphene sheet. Biosens. Bioelectron. 2013, 48, 224–229. [Google Scholar] [CrossRef]
- Zhang, N.; Xiao, F.; Bai, J.; Lai, Y.; Hou, J.; Xian, Y.; Jin, L. Label-free immunoassay for chloramphenicol based on hollow gold nanospheres/chitosan composite. Talanta 2011, 87, 100–105. [Google Scholar] [CrossRef]
- Wei, Q.; Zhao, Y.; Du, B.; Wu, D.; Li, H.; Yang, M. Ultrasensitive detection of kanamycin in animal derived foods by label-free electrochemical immunosensor. Food Chem. 2012, 134, 1601–1606. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, G.; Liu, G.; Pan, M.; Wang, X.; Kong, L.; He, X.; Wang, S. Electrochemical sensor based on molecularly imprinted polymer film via sol-gel technology and multi-walled carbon nanotubes-chitosan functional layer for sensitive determination of quinoxaline-2-carboxylic acid. Biosens. Bioelectron. 2013, 47, 475–481. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, H.; Li, X.Q.; Yuan, X.X.; Li, H.; Deng, L.G.; Zhang, H.; Wang, W.Z.; Yang, G.S.; Meng, M.; et al. Detection of ractopamine residues in pork by surface plasmon resonance-based biosensor inhibition immunoassay. Food Chem. 2012, 130, 1061–1065. [Google Scholar] [CrossRef]
- Cai, Y.; He, X.; Cui, P.L.; Liu, J.; Li, Z.B.; Jia, B.J.; Zhang, T.; Wang, J.P.; Yuan, W.Z. Preparation of a chemiluminescence sensor for multi-detection of benzimidazoles in meat based on molecularly imprinted polymer. Food Chem. 2019, 280, 103–109. [Google Scholar] [CrossRef]
- Erofeeva, V.; Zakirova, Y.; Yablochnikov, S.; Prys, E.; Prys, I. The use of antibiotics in food technology: The case study of products from Moscow stores. Proc. E3S Web Conf. 2021, 311, 10005. [Google Scholar] [CrossRef]
- Gelband, H.; Miller-Petrie, M.; Suraj, P.; Sumanth, G.; Levinson, J.; Barter, D.; White, A.; Laxminarayan, R. The state of the world’s antibiotics 2015. Wound Health South. Afr. 2015, 8, 30–34. [Google Scholar]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic use in agriculture and its consequential resistance in environmental sources: Potential public health implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Babapour, A.; Azami, L.; Fartashmehr, J. Overview of antibiotic residues in beef and mutton in Ardebil, North West of Iran. World Appl. Sci. J. 2012, 19, 1495–1500. [Google Scholar] [CrossRef]
- Oyedeji, A.O.; Msagati, T.A.M.; Williams, A.B.; Benson, N.U. Detection and quantification of multiclass antibiotic residues in poultry products using solid-phase extraction and high-performance liquid chromatography with diode array detection. Heliyon 2021, 7, 100312. [Google Scholar] [CrossRef]
- Vishnuraj, M.R.; Kandeepan, G.; Rao, K.H.; Chand, S.; Kumbhar, V. Occurrence, public health hazards and detection methods of antibiotic residues in foods of animal origin: A comprehensive review. Cogent Food Agric. 2016, 2. [Google Scholar] [CrossRef]
- Yuan, J.; Oliver, R.; Aguilar, M.I.; Wu, Y. Surface plasmon resonance assay for chloramphenicol. Anal. Chem. 2008, 80, 8329–8333. [Google Scholar] [CrossRef]
- McGrath, T.; Baxter, A.; Ferguson, J.; Haughey, S.; Bjurling, P. Multi sulfonamide screening in porcine muscle using a surface plasmon resonance biosensor. Anal. Chim. Acta 2005, 529, 123–127. [Google Scholar] [CrossRef]
- Gao, D.; Guan, C.; Wen, Y.; Zhong, X.; Yuan, L. Multi-hole fiber based surface plasmon resonance sensor operated at near-infrared wavelengths. Opt. Commun. 2014, 313, 94–98. [Google Scholar] [CrossRef]
- Mohammad-Razdari, A.; Ghasemi-Varnamkhasti, M.; Izadi, Z.; Rostami, S.; Ensafi, A.A.; Siadat, M.; Losson, E. Detection of sulfadimethoxine in meat samples using a novel electrochemical biosensor as a rapid analysis method. J. Food Compos. Anal. 2019, 82, 103252. [Google Scholar] [CrossRef]
- Stevenson, H.S.; Shetty, S.S.; Thomas, N.J.; Dhamu, V.N.; Bhide, A.; Prasad, S. Ultrasensitive and Rapid-Response Sensor for the Electrochemical Detection of Antibiotic Residues within Meat Samples. ACS Omega 2019, 4, 6324–6330. [Google Scholar] [CrossRef]
- Tang, D.; Tang, J.; Su, B.; Chen, G. Ultrasensitive electrochemical immunoassay of staphylococcal enterotoxin B in food using enzyme-nanosilica-doped carbon nanotubes for signal amplification. J. Agric. Food Chem. 2010, 58, 10824–10830. [Google Scholar] [CrossRef]
- Rout, M.; Panigrahi, S.; Routray, A.; Swain, K. Methods for detection of adulteration in meat. In Recent Research Trends in Veterinary Sciences and Animal Husbandry; AkiNik Publications: Rohini, India, 2018; Volume 3, pp. 1–10. [Google Scholar]
- Lee, E.J.; Shin, H.S. Development of a freshness indicator for monitoring the quality of beef during storage. Food Sci. Biotechnol. 2019, 28, 1899–1906. [Google Scholar] [CrossRef]
- Sohail, M.; Sun, D.W.; Zhu, Z. Recent developments in intelligent packaging for enhancing food quality and safety. Crit. Rev. Food Sci. Nutr. 2018, 58, 2650–2662. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Shao, P.; Liu, L.; Yu, J.; Lin, Y.; Gao, H.; Chen, H.; Sun, P. An overview of intelligent freshness indicator packaging for food quality and safety monitoring. Trends Food Sci. Technol. 2021, 118, 285–296. [Google Scholar] [CrossRef]
- Balbinot-Alfaro, E.; Craveiro, D.V.; Lima, K.O.; Costa, H.L.G.; Lopes, D.R.; Prentice, C. Intelligent Packaging with pH Indicator Potential. Food Eng. Rev. 2019, 11, 235–244. [Google Scholar] [CrossRef]
- Rodrigues, C.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Bio-based sensors for smart food packaging—current applications and future trends. Sensors 2021, 21, 2148. [Google Scholar] [CrossRef]
- Shukla, V.; Kandeepan, G.; Vishnuraj, M.R. Development of On-Package Indicator Sensor for Real-Time Monitoring of Buffalo Meat Quality During Refrigeration Storage. Food Anal. Methods 2015, 8, 1591–1597. [Google Scholar] [CrossRef]
- Kuswandi, B.; Damayanti, F.; Jayus, J.; Abdullah, A.; Heng, L.Y. Simple and low-cost on-package sticker sensor based on litmus paper for real-time monitoring of beef freshness. J. Math. Fundam. Sci. 2015, 47, 236–251. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Bhandari, B.; Guo, Z. Applicability of a colorimetric indicator label for monitoring freshness of fresh-cut green bell pepper. Postharvest Biol. Technol. 2018, 140, 85–92. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Adhikari, B.; Devahastin, S.; Wang, H. Double-layer indicator films aided by BP-ANN-enabled freshness detection on packaged meat products. Food Packag. Shelf Life 2022, 31, 100808. [Google Scholar] [CrossRef]
- Liang, T.; Sun, G.; Cao, L.; Li, J.; Wang, L. A pH and NH3 sensing intelligent film based on Artemisia sphaerocephala Krasch. gum and red cabbage anthocyanins anchored by carboxymethyl cellulose sodium added as a host complex. Food Hydrocoll. 2019, 87, 858–868. [Google Scholar] [CrossRef]
- Shukla, V.; Kandeepan, G.; Vishnuraj, M.R.; Soni, A. Anthocyanins Based Indicator Sensor for Intelligent Packaging Application. Agric. Res. 2016, 5, 205–209. [Google Scholar] [CrossRef]
- Golasz, L.B.; da Silva, J.; da Silva, S.B. Film with anthocyanins as an indicator of chilled pork deterioration. Food Sci. Technol. 2013, 33, 155–162. [Google Scholar] [CrossRef]
- Choi, I.; Lee, J.Y.; Lacroix, M.; Han, J. Intelligent pH indicator film composed of agar/potato starch and anthocyanin extracts from purple sweet potato. Food Chem. 2017, 218, 122–128. [Google Scholar] [CrossRef]
- Zhang, P.; Li, Y.; Chong, S.; Yan, S.; Yu, R.; Chen, R.; Si, J.; Zhang, X. Identification and quantitative analysis of anthocyanins composition and their stability from different strains of Hibiscus syriacus L. flowers. Ind. Crops Prod. 2022, 177, 114457. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.W.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Kandeepan, G. Biodegradable Nanocomposite Packaging Films for Meat and Meat Products: A Review. J. Packag. Technol. Res. 2021, 5, 143–166. [Google Scholar] [CrossRef]
- Sharma, R.; Jafari, S.M.; Sharma, S. Antimicrobial bio-nanocomposites and their potential applications in food packaging. Food Control 2020, 112, 107086. [Google Scholar] [CrossRef]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Tavassoli, M.; McClements, D.J.; Hamishehkar, H. Multifunctional halochromic packaging materials: Saffron petal anthocyanin loaded-chitosan nanofiber/methyl cellulose matrices. Food Hydrocoll. 2021, 111, 106237. [Google Scholar] [CrossRef]
- Vedove, T.M.A.R.D.; Maniglia, B.C.; Tadini, C.C. Production of sustainable smart packaging based on cassava starch and anthocyanin by an extrusion process. J. Food Eng. 2021, 289, 110274. [Google Scholar] [CrossRef]
- Dudnyk, I.; Janeček, E.-R.; Vaucher-Joset, J.; Stellacci, F. Edible sensors for meat and seafood freshness. Sens. Actuators B Chem. 2018, 259, 1108–1112. [Google Scholar] [CrossRef]
- Zhai, X.; Zou, X.; Shi, J.; Huang, X.; Sun, Z.; Li, Z.; Sun, Y.; Li, Y.; Wang, X.; Holmes, M.; et al. Amine-responsive bilayer films with improved illumination stability and electrochemical writing property for visual monitoring of meat spoilage. Sens. Actuators B Chem. 2020, 302, 127130. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, X.; Xie, F.; Fan, Y.; Xu, X.; Qi, J.; Xiong, G.; Gao, X.; Zhang, F. pH-responsive double-layer indicator films based on konjac glucomannan/camellia oil and carrageenan/anthocyanin/curcumin for monitoring meat freshness. Food Hydrocoll. 2021, 118, 106695. [Google Scholar] [CrossRef]
- Niu, X.; Wang, W.; Kitamura, Y.; Wang, J.; Sun, J.; Ma, Q. Design and characterization of bio-amine responsive films enriched with colored potato (Black King Kong) anthocyanin for visual detecting pork freshness in cold storage. J. Food Meas. Charact. 2021, 15, 4659–4668. [Google Scholar] [CrossRef]
- Chayavanich, K.; Thiraphibundet, P.; Imyim, A. Biocompatible film sensors containing red radish extract for meat spoilage observation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 226, 117601. [Google Scholar] [CrossRef] [PubMed]
- Kuntzler, S.G.; Costa, J.A.V.; Brizio, A.P.D.R.; Morais, M.G. de Development of a colorimetric pH indicator using nanofibers containing Spirulina sp. LEB 18. Food Chem. 2020, 328, 126768. [Google Scholar] [CrossRef]
- Yildiz, E.; Sumnu, G.; Kahyaoglu, L.N. Monitoring freshness of chicken breast by using natural halochromic curcumin loaded chitosan/PEO nanofibers as an intelligent package. Int. J. Biol. Macromol. 2021, 170, 437–446. [Google Scholar] [CrossRef]
- Kanatt, S.R. Development of active/intelligent food packaging film containing Amaranthus leaf extract for shelf life extension of chicken/fish during chilled storage. Food Packag. Shelf Life 2020, 24, 100506. [Google Scholar] [CrossRef]
- Lamri, M.; Bhattacharya, T.; Boukid, F.; Chentir, A.; Dib, A.L.; Das, D.; Djenane, D.; Gagaoua, M. Nanotechnology as a Processing and Packaging Tool to Improve Meat Quality and Safety. Foods 2021, 10, 2633. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Rocha-Santos, T.A.P.; Cardoso, S.; Duarte, A.C.; Cardosa, S. Strategies for enhancing the analytical performance of nanomaterial-based sensors. TrAC Trends Anal. Chem. 2013, 47, 27–36. [Google Scholar] [CrossRef]
| Biosensor Type | Advantages | Disadvantages |
|---|---|---|
| Electrochemical |
|
|
| Optical |
|
|
| Mass-based |
|
|
| Analyte | Biorecognition Element | Electrode Used | Immobilization Technique and Detection | Meat and Meat Products | Detection Limit/Sensitivity or Correlation | Refs |
|---|---|---|---|---|---|---|
| Hypoxanthine | Xanthine oxidase | Amperometric hypoxanthine sensor; Graphene/titanium dioxide nanocomposite | Electro catalytic activity | Pork | LOD:9.5 μM, Sensitivity: 4.1 nA/μM | [25] |
| Glucose, triglycerides, and lactic acid | Glucose, lactic acid, etc., in drip loss | Strip method; Accutrend Plus apparatus | - | Longissimus muscle of pork | Accuracy level 86.54% (Rc = 0.93, p < 0.01) | [7] |
| Xanthine | Xanthine oxidase | Pencil graphite electrode | Immobilizing by glutaralde-hyde | Chicken meat | LOD: 0.074 M Sensitivity: 124 mA m−1 | [35] |
| Calpastatin | Specific antibody for calpastatin | Tendercheck system; portable electrochemical device | Immunoreaction | Beef meat | Correlation (R2 = 0.62) between calpastatin and WBSF values | [36] |
| Hypoxanthine | Xanthine oxidase | Paper-based colorimetric biosensor | Dienzyme catalytic reaction | Fresh and processed meat samples | LOD: 1.8 mg L−1 Quantitative limit: 6.1 mg L−1 | [28] |
| Cadaverine | Cyclam (1,4,8,11-tetraazacyclotetradecane) | Titanium adhesion layer (3 nm); silicon nitride cantilevers containing piezoelectric layers | Silicon; gold | Beef, chicken, or pork | - | [37] |
| Xanthine | Guanine deaminase; Xanthine oxidase | Circular plastic discs as biosensor; Fiber optic probe (Ocean Optics) | Enzyme immobilization by hydro sol-gel method; Co-immobilization of xanthine oxidase and adsorptive dye phenol red | Chicken meat | LOD: 0.5 μM Linear range: 0.5 μM–150 μM | [29] |
| IMP | 5′-nucleotidase and xanthine oxidase | Three-electrode system including a modified GCE electrode, an Ag/AgCl electrode, a platinum wire as an auxiliary electrode. A multilayer film of GCE/Ti3C2TX-Au@Pt nanoflowers/5′-nucle-otidase-xanthine oxidase/BSA | GCE/Ti3C2TX-Au@Pt nanoflowers | Chicken, pork, beef, lamb | LOD: 2.73 ng mL−1 Linear range: 0.04~17 g L−1 Correlation coefficient: 0.9964 | [38] |
| Calpastatin | Muscle sample, monoclonal anti-calpastatin antibody | The sensor chip | Carboxymethylated dextran layer with N-hydroxysuccinimidemediated by N-ethyl-N-(3-diethylaminopropyl) carbodiimide | Beef meat | Correlation coefficients: 0.51–0.99 Mean inter-assay CV: 5.8% | [39] |
| Volatiles in spoiled meat | Bacteria specific genetic material; BioBrick compatible integration plasmid | Promoter PsboA | Transcriptome analysis | Pork/cow minced meat (Ratio: 70%/30%) | - | [34] |
| Calpastatin | Anti calpastatin antibody | Capillary tube optic biosensor | Silanization covalent immobilization | Longissimus muscle from beef | Calpastatin activity (R2 = 0.6058) | [40] |
| Xanthine | Xanthine oxidase | Hybrid nanocomposite film; Carbon paste electrode | Covalent immobilization by glutaraldehyde | Chicken meat | LOD: 0.1 µM Linear working concentration: 0.2–36.0 µM (R2 = 0.997) | [26] |
| Putrescine | 4-aminobutyraldehyde with putrescine oxidase or diamine oxidase as catalysts | Luminometer microplates with different enzymes | Self-adhesive reinforcement ring | Beef, pork, chicken, turkey meat samples | LOD: 0.8 mg/L–1.3 mg/L Linearity range: 1–2 mg/L | [30] |
| Glucose | Glucose oxidase | Glassy carbon electrode modified with multi-walled carbon nanotubes and chitosan | Cross-linking with enzyme through glutaraldehyde with BSA | Beef meat | LOD: 0.05 mM Linear range: 0.2–1.2 mmol L−1 linearity (R2 = 0.9902) | [41] |
| TVB-N | Pork meat (derivatives of biogenic total volatile basic nitrogen) | Hyperspectral imaging and colorimetric sensors | Adaptive boosting algorithm for data fusion and modeling | Pork meat | Correlation coefficient (R2 = 0.932) | [32] |
| Analyte | Biorecognition Element | Electrode Used | Immobilization Technique and Detection | Meat and Meat Products | Limit of Detection/Sensitivity or Correlation | Refs. |
|---|---|---|---|---|---|---|
| Bacteria (Campylobacter spp.) | Genomic Campylobacter DNA | Paper membrane | Biotinylated silica-nanoparticles | Chicken meat | LOD: 3 pg/μL of DNA | [80] |
| Salmonella enterica, Listeria monocytogenes, and Escherichia coli O157:H7 | Alexa Fluor 647-labeled monoclonal antibodies | Streptavidin coated optical waveguides | Biotinylated polyclonal antibodies | Ready-to-eat beef, chicken and turkey breast meat | LOD: 103 CFU/mL | [81] |
| Salmonella Typhimurium and Staphyloccocus aureus | Aptamer | The Raman signal probe and the capture probe; gold nanoparticles modified with Raman molecules (Mercaptobenzoic acid and 5,5′-Dithiobis (2-nitrobenzoic acid) | Fe3O4 magnetic gold nanoparticles | Pork paste | LOD: 35 CFU/mL for S. aureus and 15 CFU/mL for S. Typhimurium Recovery rate: 94.12%–108.33% | [82] |
| L. monocytogenes | Aptamer, a single-stranded oligonucleotide ligand | Fiber-optic sensor | Streptavidin-coated optical waveguide surface; Alexa Fluor 647-conju-gated A8 | Ready-to-eat such as sliced beef, chicken, and turkey | LOD: 102 CFU 25 g−1 | [83] |
| Whole cell of S. enterica serovar Typhimurium | DNA bases of aptamer | Reduced graphene oxide -azophloxine nanocomposite aptasensor | Azophloxine | Chicken meat | LOD: 1 CFU/mL Linear range (detection): 1–8 log CFU/mL | [84] |
| S. enterica serovar Typhimurium | Phage peptide | Magnetoelastic biosensor | Gold-coated sensor layer | Chicken fillets (boneless and skinless) | LOD: 7.86 × 103 CFU/mm2 Analysis time: 2–10 min | [85] |
| Salmonella pullorum | Anti-Salmonella polyclonal antibodies | Screen printed electrode modified with multi-wall carbon nanotubes-chitosan-peroxidase was connected to a portable self-made amperometric sensor | Cellulose nitrate membrane | Chicken sample | LOD: 100 CFU/mL Detection time: 1.5 to 2 h | [86] |
| Staphylococcal enterotoxin B | Antibody | Electro-optical biosensor | Carboxymethyl-dextran | Potted meat (Hormel) | Sensitivity: 1–10 ng/mL | [87] |
| E coli K-12 | Anti-E.coli antibody | Electrochemical impedance spectroscopy and SPR imaging techniques | Gold surface | Frozen chicken meat | LOD: 3 log CFU/mL | [88] |
| Trichothecene T-2 toxin | Anti-T-2 (toxin) and T-2-bovine serum albumen | Electrochemical immunosensor (GCE) | Gold nanoparticles/carboxylic group-functionalized single-walled carbon nanotubes/chitosan composite | Swine meat | LOD: 0.14 µg/L Recovery: 91.42%–100.80% | [89] |
| Microbial contamination (Meat spoilage) | - | Gyro sensor and the digital camera of a smartphone | - | Ground beef | LOD: 1 log CFU/mL at 45° and 2 log CFU/mL at 30° and 60° | [75] |
| Salmonella serotypes (B, and D) | Aptamer ssDNA | GCE | Graphene oxide and gold nanoparticles | Ready-to-eat turkey samples | LOD: 300 cells/mL Detection time: <1 h | [74] |
| Salmonella | Aptamer ssDNA | GCE | Graphene oxide and gold nanoparticles | Pork samples | LOD: 3 CFU/mL | [90] |
| S. Typhimurium | Aptamer | Plasmonic sensor | Gold nanoparticles | Pork meat sample | LOD: 4 logCFU/mL | [91] |
| S. Typhimurium | Antibodies | Quartz-crystal microbalance | Functionalized nanoparticles | Chicken meat samples | LOD: 1 CFU/mL | [73] |
| Salmonella gallinarum and S. pullorum | Antibodies | Screen-printed carbon electrode | Gold nanoparticles | Chicken meat | LOD: 3 log CFU/mL | [92] |
| E. coli O157:H7 | Antibody | Integration of bifunctional glucose oxidase–polydopamine based polymeric nanocomposites and Prussian blue modified screen-printed interdigitated microelectrodes | Gold nanoparticles | Ground beef | LOD: 2.05 × 103 CFU/g | [93] |
| Campylobacter jejuni | Antibodies | Optical biosensor based on SPR | Immunoaffinity | Broiler samples | Good sensitivity: 103 CFU/mL against C. jejuni | [94] |
| Salmonella spp. | Salmonella spp. monoclonal antibodies | GCE | Grafted ethylene diamine and self-assembled gold nanoparticle monolayer | Pork meat | LOD: 2 log CFU/mL Dection time: 40 min | [95] |
| L. monocytogenes and Bacillus cereus | B-Lymphocyte Ped-2E9 cell-line | Cell based biosensor | Collagen matrix | Ready-to-eat hotdog and salami | LOD for pathogens: 3 log LOD for toxins: 10–40 ng | [96] |
| E. coli O157:H7 | Complementary DNA | Gold electrode surface | Multiwalled carbon nanotubes | Fresh beef | LOD: 1.97 × 10–14 M correlation coefficient: 0.989 | [97] |
| Listeria | Protease | Gold sensor surface | D-amino acid substrate | Meat | LOD: 2 log CFU/g | [98] |
| S. Typhimurium | Thiolated S. Typhimurium aptamer; biotinylated aptamer | Surface-enhanced Raman scattering nano probes | Spiny gold nanoparticles | Pork samples | LOD: 4 CFU/mL | [99] |
| Analyte | Biorecognition Element | Electrode Used | Immodilization Techniques and Detection | Meat and Meat Products | Detection Limit/Sensitivity or Correlation | Refs. |
|---|---|---|---|---|---|---|
| DNA (Donkey meat) | DNA | Multi-parameter SPR device with gold chips | A boiling solution of NH3 (30%), H2O2 (30%), and Milli-Q water in ratio 1:1:5 for 10 min at 95 °C, drying by N2 stream; incubated in thiolated capture probe | Beef sausages | LOD: 1.0 nM | [101] |
| DNA (Pork meat) | DNA probe with gold nanoparticles bioconjugate | Gold-modified screen-printed carbon electrode | MPA (3 -me rcaptop ropionic acid), EDC (1-ethyl-3-(3-dimethylamino propyl) carbodiimide), and NHS (N-Hydroxysuccinimide) | Food products | LOD: 0.58 μg/mL Recovery rate: 101.74% | [102] |
| DNA (Horse meat) | Antibody specific to RNA/DNA duplexes and a bacterial protein conjugated with a horseradish peroxidase homopolymer | Disposable screen-printed carbon electrodes | Immobilization at magnetic microcarriers | Beef containing horse meat | Can detect beef meat adulterated with even 0.5% (w/w) of horse meat within 1 h | [103] |
| Nitrate (Contaminants) | Nitrate reductase | Ag/AgCl reference electrode, platinum auxiliary electrode, GCE | Bloom gelatin | Meat samples | LOD: 2.2 × 10–9 M LOQ: 5.0–90.0 × 10−9 M response time: 10 s | [104] |
| Porcine serum albumin | Anti-T-2 (toxin) and T-2-bovine serum albumen | GCE | Gold nanoparticles/carboxylic group-functionalized single-walled carbon nanotubes/chitosan composite | Pork and its products | LOD: 19.81 ng/mL Linear range: 1.0–450 ng/mL | [105] |
| Dopamine Adulterant | Anti-dopamine substance | Colorimetric sensor | CuS-BSA-Cu3 (PO4)2 nanoparticles-copper sulfide encapsulated within bovine serum albumin functionalized with copper phosphate | Beef meat | LOD: 0.13 mM Linear range: 0.05–100 mM | [106] |
| DNA | Enzymes | Optical thin-film biosensor chip | Silicon | Meat from deer, rabbit, duck, beef, horse, sheep, and pork | LOD: 0.5 pg | [107] |
| Tetracyclines | Lyophilized reconstituted sensor cells | Cell-biosensor | Solution based | Poultry muscle samples | Sensitivity: 10 µg/kg | [108] |
| Chloramphenicol (CAP) | CAP derivative and antibody | Immunochemical screening assays using SPR | Regeneration solution | Poultry muscle | Detection capabilities: 0.02 µg/kg | [109] |
| CAP and tetracycline (TET) | ss-DNA fragment coordinately controlling gold nanoparticles aggregation | Colorimetric aptasensor | Gold nanoparticles | Chicken | LOD: 32.9 nM (TET) and 7.0 nM (CAP) Linear range: 0.05–3.0 µM | [110] |
| Sulfadiazine (SDZ) and acetaminophen (AP) | Molecularly imprinted polymer | Electrochemical sensor; GCE | A graphene oxide@covalent organic framework nanocomposite for signal amplification. | Pork and chicken samples | LOD: 0.160 μM (SDZ) and 0.032 μM (AP) | [111] |
| Kanamycin | Antibody of kanamycin | Thionine mixed graphene sheet | Silver hybridized mesoporous ferro-ferric oxide nanoparticles | Pork meat sample | LOD: 15 pg/mL, Linear range: 0.05–16 ng/mL Recovery: 96.7–102% | [112] |
| Chloramphenicol (CAP) | Monoclonal antibody to CAP (anti-CAP) | Electrochemical immunosensor; Electrochemical impedance spectroscopy technique | Entrapped into gold nanospheres/chitosan composite modified on a GCE | Beef and pork meat samples | LOD: 0.06 ng/mL Linear range: 0.1–1000 ng/mL | [113] |
| Kanamycin | Anti-kanamycin antibody | Amperometric immunosensor based on graphene sheet -nafion/thionine/platinum nanoparticles | Electrostatic adsorption | Chicken liver | LOD: (5.74 pg/mL) Linear range: 0.01–12.0 ng/mL | [114] |
| Quinoxaline-2-carboxylic acid | Molecularly imprinted polymer | Modified GCE and differential pulse voltammetry | Multi-walled carbon nanotubes-chitosan functional composite | Pork products | LOD: 4.4 × 10−7 mol/L Linear range: 2.0 × 10−6–1.0 × 10−3 mol/L | [115] |
| Ractopamine | Ractopamine derivative | SPR biosensor inhibition immunoassay | SPR-2004 biosensor chip | Pork | LOD: 0.6 μg/kg pork sample | [116] |
| Benzimidazoles | Molecularly imprinted polymer | Chemiluminescence sensor on 96 -well microplate | Horseradish peroxidase-labeled hapten as binding agent | Beef and mutton samples | LOD: 1.5–21 pg/mL Rate of recovery: 65.8%–91.2% | [117] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nanda, P.K.; Bhattacharya, D.; Das, J.K.; Bandyopadhyay, S.; Ekhlas, D.; Lorenzo, J.M.; Dandapat, P.; Alessandroni, L.; Das, A.K.; Gagaoua, M. Emerging Role of Biosensors and Chemical Indicators to Monitor the Quality and Safety of Meat and Meat Products. Chemosensors 2022, 10, 322. https://doi.org/10.3390/chemosensors10080322
Nanda PK, Bhattacharya D, Das JK, Bandyopadhyay S, Ekhlas D, Lorenzo JM, Dandapat P, Alessandroni L, Das AK, Gagaoua M. Emerging Role of Biosensors and Chemical Indicators to Monitor the Quality and Safety of Meat and Meat Products. Chemosensors. 2022; 10(8):322. https://doi.org/10.3390/chemosensors10080322
Chicago/Turabian StyleNanda, Pramod Kumar, Dipanwita Bhattacharya, Jyotishka Kumar Das, Samiran Bandyopadhyay, Daniel Ekhlas, Jose M. Lorenzo, Premanshu Dandapat, Laura Alessandroni, Arun K. Das, and Mohammed Gagaoua. 2022. "Emerging Role of Biosensors and Chemical Indicators to Monitor the Quality and Safety of Meat and Meat Products" Chemosensors 10, no. 8: 322. https://doi.org/10.3390/chemosensors10080322
APA StyleNanda, P. K., Bhattacharya, D., Das, J. K., Bandyopadhyay, S., Ekhlas, D., Lorenzo, J. M., Dandapat, P., Alessandroni, L., Das, A. K., & Gagaoua, M. (2022). Emerging Role of Biosensors and Chemical Indicators to Monitor the Quality and Safety of Meat and Meat Products. Chemosensors, 10(8), 322. https://doi.org/10.3390/chemosensors10080322









