Chip-Based and Wearable Tools for Isothermal Amplification and Electrochemical Analysis of Nucleic Acids
Abstract
:1. Introduction
2. Behind the Scenes: Classification of Isothermal Amplification Techniques and Basic Concepts of Electrochemical Methods
2.1. Nucleic Acid Sequence–Based Amplification (NASBA)
2.2. Strand Displacement Amplification (SDA)
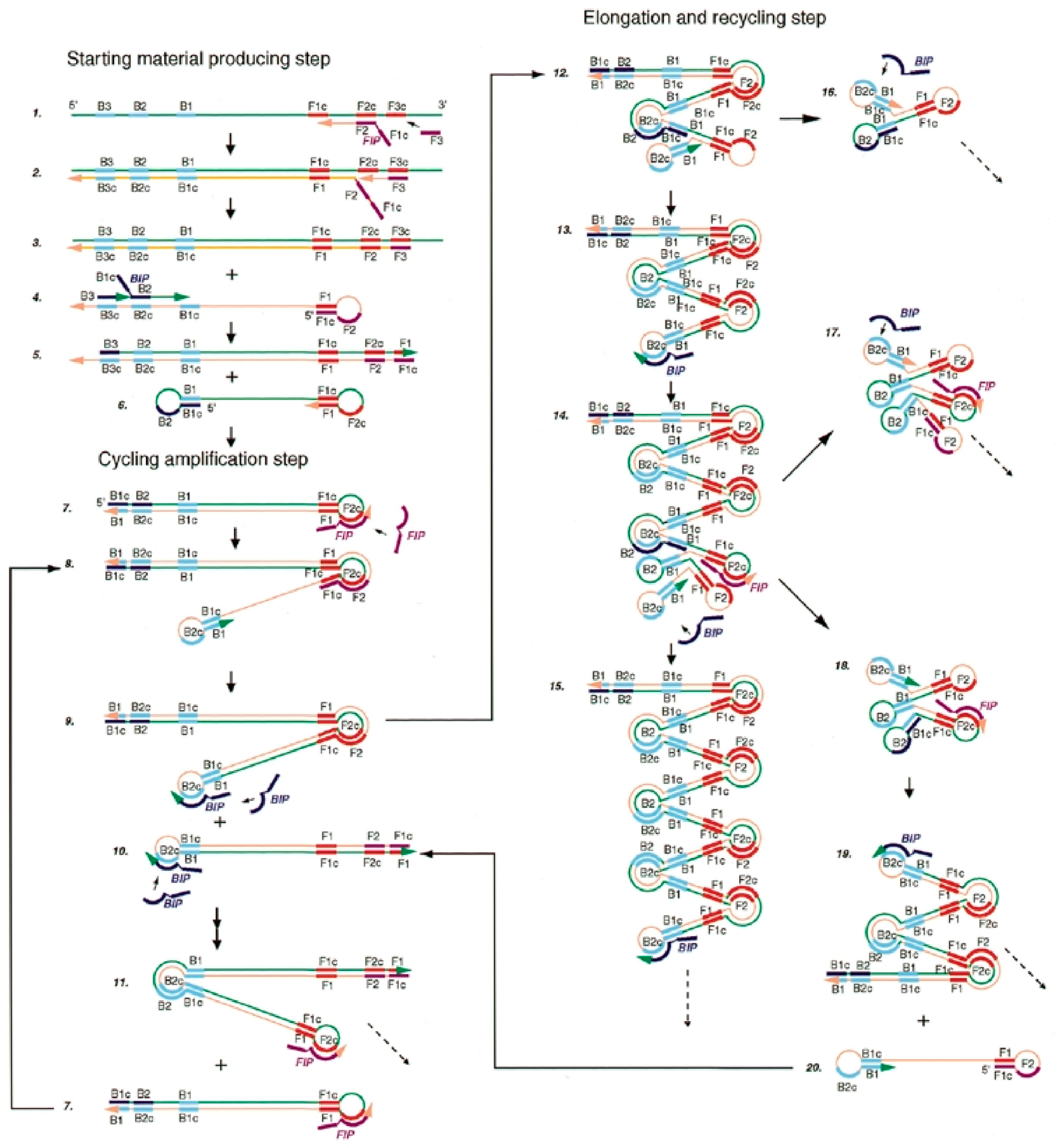
2.3. Loop-Mediated Isothermal Amplification (LAMP)
2.4. Helicase-Dependent Amplification (HDA)
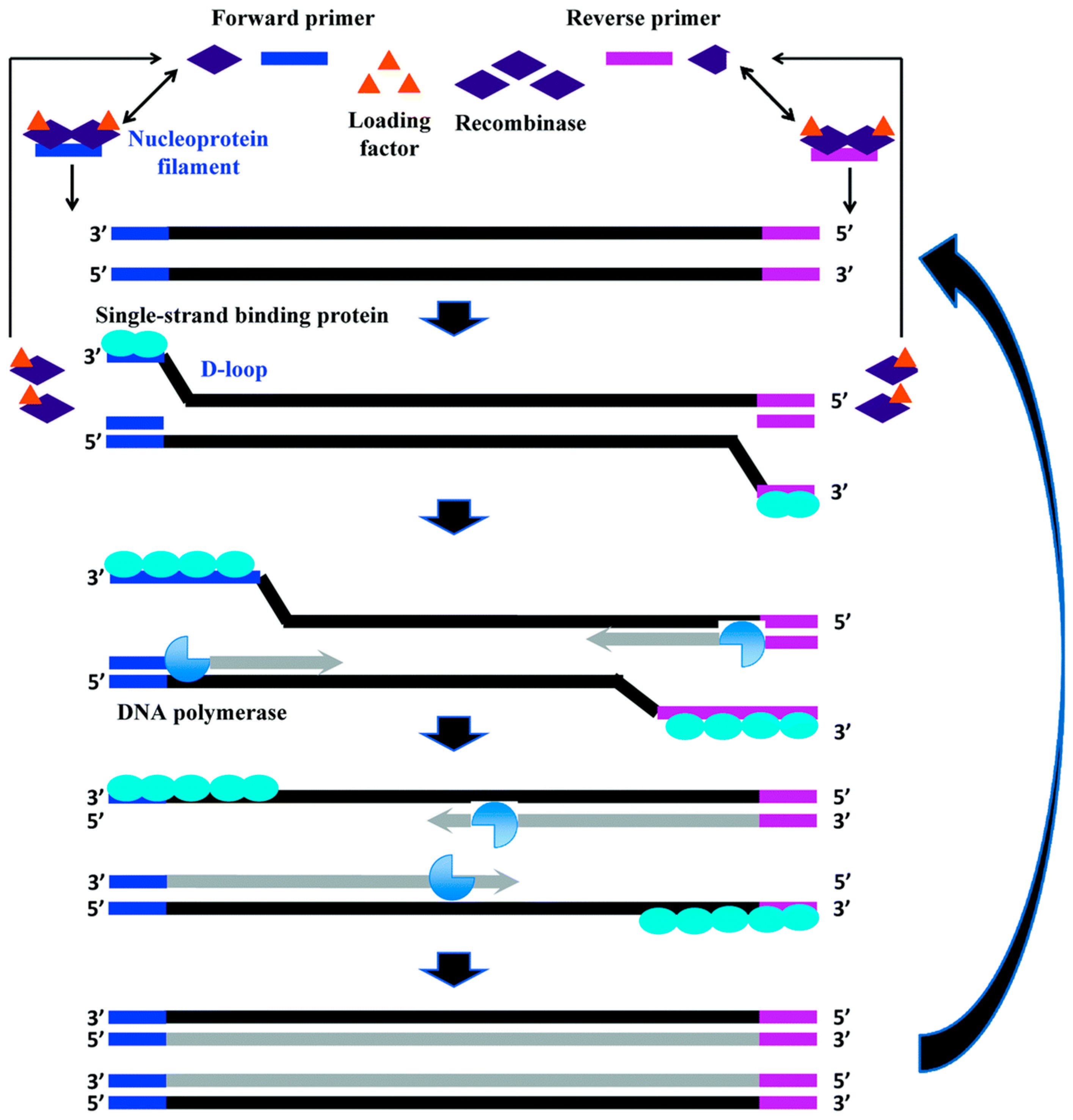
2.5. Recombinase Polymerase Amplification (RPA)
| Method | Temp (°C) | Reaction Time (min) | Target | Pros and Cons vs. PCR | Reference |
|---|---|---|---|---|---|
| NASBA | 41 | 90–120 | RNA | Power-saving (41 °C); ideal for RNA target; not ideal for DNA target | [17] |
| SDA | 37–49 | 20–120 | ssDNA RNA | Power-saving (37–50 °C); suitable for miRNA; sample preparation required | [18] |
| LAMP | 60–65 | 40–60 | ssDNA | Highly specific; commercial kits available; not suitable for small targets; complex primers design | [21] |
| HDA | 37–60 | 60–120 | dsDNA | Use of helicase enzyme to melt the dsDNA template; simplicity | [28] |
| RPA | 25–42 | 5–20 | dsDNA ssDNA RNA | Lower temperature, 37 °C; fast amplification (20 min); commercial kits available; stringent reaction conditions | [33] |
2.6. Electrochemical Detection of Nucleic Acids
3. Isothermal Amplification Techniques Coupled with Electrochemical Detection of Microbial Agents for Medical, Food Safety, and Environmental Applications
4. Isothermal Amplification Techniques Coupled to Electrochemical Detection of Cancer Biomarkers
5. Patents Involving Isothermal Amplification in Electrochemical Chip-Based Platforms
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miranda-Castro, R.; Palchetti, I.; de-Los-Santos-Álvarez, N. The Translational Potential of Electrochemical DNA-Based Liquid Biopsy. Front. Chem. 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Pillozzi, S.; Bernini, A.; Palchetti, I.; Crociani, O.; Antonuzzo, L.; Campanacci, D.; Scoccianti, G. Soft Tissue Sarcoma: An Insight on Biomarkers at Molecular, Metabolic and Cellular Level. Cancers 2021, 13, 3044. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Dominguez, M.V.; Zottel, A.; Šamec, N.; Jovčevska, I.; Dincer, C.; Kahlert, U.D.; Nickel, A.-C. Current Technologies for RNA-Directed Liquid Diagnostics. Cancers 2021, 13, 5060. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.; Uthicke, S. Sensitive Environmental DNA Detection via Lateral Flow Assay (Dipstick)—A Case Study on Corallivorous Crown-of-Thorns Sea Star (Acanthaster cf. solaris) Detection. Environ. DNA 2021, 3, 323–342. [Google Scholar] [CrossRef]
- Ma, B.; Fang, J.; Lin, W.; Yu, X.; Sun, C.; Zhang, M. A Simple and Efficient Method for Potential Point-of-Care Diagnosis of Human Papillomavirus Genotypes: Combination of Isothermal Recombinase Polymerase Amplification with Lateral Flow Dipstick and Reverse Dot Blot. Anal. Bioanal. Chem. 2019, 411, 7451–7460. [Google Scholar] [CrossRef] [PubMed]
- Anik, Ü. 12-Electrochemical Medical Biosensors for POC Applications. In Medical Biosensors for Point of Care (POC) Applications; Narayan, R.J., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 275–292. ISBN 978-0-08-100072-4. [Google Scholar]
- Wang, J. Electrochemical Biosensors: Towards Point-of-Care Cancer Diagnostics. Biosens. Bioelectron. 2006, 21, 1887–1892. [Google Scholar] [CrossRef]
- Schaad, N.W.; Frederick, R.D. Real-Time PCR and Its Application for Rapid Plant Disease Diagnostics. Can. J. Plant Pathol. 2002, 24, 250–258. [Google Scholar] [CrossRef]
- Diehl, F.; Li, M.; He, Y.; Kinzler, K.W.; Vogelstein, B.; Dressman, D. BEAMing: Single-Molecule PCR on Microparticles in Water-in-Oil Emulsions. Nat. Methods 2006, 3, 551–559. [Google Scholar] [CrossRef]
- Ferapontova, E.E. Basic Concepts and Recent Advances in Electrochemical Analysis of Nucleic Acids. Curr. Opin. Electrochem. 2017, 5, 218–225. [Google Scholar] [CrossRef]
- Labuda, J.; Brett, A.M.O.; Evtugyn, G.; Fojta, M.; Mascini, M.; Ozsoz, M.; Palchetti, I.; Paleček, E.; Wang, J. Electrochemical nucleic acid-based biosensors: Concepts, terms, and methodology (IUPAC Technical Report). Pure Appl. Chem. 2010, 82, 1161–1187. [Google Scholar] [CrossRef]
- Sfragano, P.S.; Pillozzi, S.; Palchetti, I. Electrochemical and PEC Platforms for MiRNA and Other Epigenetic Markers of Cancer Diseases: Recent Updates. Electrochem. Commun. 2021, 124, 106929. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Li, Q.; Wang, L.; Fan, C. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J. Advances in Isothermal Amplification: Novel Strategies Inspired by Biological Processes. Biosens. Bioelectron. 2015, 64, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, M.C.; Spoto, G. Integration of Isothermal Amplification Methods in Microfluidic Devices: Recent Advances. Biosens. Bioelectron. 2017, 90, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.N.D.; Lee, N.Y. Advances in Nucleic Acid Amplification-Based Microfluidic Devices for Clinical Microbial Detection. Chemosensors 2022, 10, 123. [Google Scholar] [CrossRef]
- Compton, J. Nucleic Acid Sequence-Based Amplification. Nature 1991, 350, 91–92. [Google Scholar] [CrossRef]
- Walker, G.T.; Fraiser, M.S.; Schram, J.L.; Little, M.C.; Nadeau, J.G.; Malinowski, D.P. Strand Displacement Amplification—An Isothermal, in Vitro DNA Amplification Technique. Nucleic Acids Res. 1992, 20, 1691–1696. [Google Scholar] [CrossRef] [Green Version]
- Spargo, C.; Dean, C.; Nycz, C.; Walker, G. SDA Target Amplification. In Nonradioactive Analysis of Biomolecules; Springer: Berlin/Heidelberg, Germany, 2000; pp. 356–366. ISBN 978-3-540-64601-3. [Google Scholar]
- Deiman, B.; van Aarle, P.; Sillekens, P. Characteristics and Applications of Nucleic Acid Sequence-Based Amplification (NASBA). Mol. Biotechnol. 2002, 20, 163–179. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [Green Version]
- Keikha, M. LAMP Method as One of the Best Candidates for Replacing with PCR Method. Malays. J. Med. Sci. MJMS 2018, 25, 121–123. [Google Scholar] [CrossRef]
- Francois, P.; Tangomo, M.; Hibbs, J.; Bonetti, E.-J.; Boehme, C.C.; Notomi, T.; Perkins, M.D.; Schrenzel, J. Robustness of a Loop-Mediated Isothermal Amplification Reaction for Diagnostic Applications. FEMS Immunol. Med. Microbiol. 2011, 62, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.H.; Park, B.H.; Oh, S.J.; Choi, G.; Kim, D.H.; Lee, E.Y.; Seo, T.S. Development of a High-Throughput Centrifugal Loop-Mediated Isothermal Amplification Microdevice for Multiplex Foodborne Pathogenic Bacteria Detection. Sens. Actuators B Chem. 2017, 246, 146–153. [Google Scholar] [CrossRef]
- Ali, Z.; Aman, R.; Mahas, A.; Rao, G.S.; Tehseen, M.; Marsic, T.; Salunke, R.; Subudhi, A.K.; Hala, S.M.; Hamdan, S.M.; et al. ISCAN: An RT-LAMP-Coupled CRISPR-Cas12 Module for Rapid, Sensitive Detection of SARS-CoV-2. Virus Res. 2020, 288, 198129. [Google Scholar] [CrossRef]
- Kato, Y.; Yanagisawa, T.; Nakai, M.; Komatsu, K.; Inoue, M.N. Direct and Sensitive Detection of a Microsporidian Parasite of Bumblebees Using Loop-Mediated Isothermal Amplification (LAMP). Sci. Rep. 2020, 10, 1118. [Google Scholar] [CrossRef] [PubMed]
- Lannutti, L.; Mira, A.; Basualdo, M.; Rodriguez, G.; Erler, S.; Silva, V.; Gisder, S.; Genersch, E.; Florin-Christensen, M.; Schnittger, L. Development of a Loop-Mediated Isothermal Amplification (LAMP) and a Direct LAMP for the Specific Detection of Nosema Ceranae, a Parasite of Honey Bees. Parasitol. Res. 2020, 119, 3947–3956. [Google Scholar] [CrossRef]
- Vincent, M.; Xu, Y.; Kong, H. Helicase-Dependent Isothermal DNA Amplification. EMBO Rep. 2004, 5, 795–800. [Google Scholar] [CrossRef]
- Deng, H.; Gao, Z. Bioanalytical Applications of Isothermal Nucleic Acid Amplification Techniques. Anal. Chim. Acta 2015, 853, 30–45. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J.; Stetten, F. von Review: A Comprehensive Summary of a Decade Development of the Recombinase Polymerase Amplification. Analyst 2019, 144, 31–67. [Google Scholar] [CrossRef] [Green Version]
- Daher, R.K.; Stewart, G.; Boissinot, M.; Bergeron, M.G. Recombinase Polymerase Amplification for Diagnostic Applications. Clin. Chem. 2016, 62, 947–958. [Google Scholar] [CrossRef]
- Lutz, S.; Weber, P.; Focke, M.; Faltin, B.; Hoffmann, J.; Müller, C.; Mark, D.; Roth, G.; Munday, P.; Armes, N.; et al. Microfluidic Lab-on-a-Foil for Nucleic Acid Analysis Based on Isothermal Recombinase Polymerase Amplification (RPA). Lab. Chip 2010, 10, 887–893. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA Detection Using Recombination Proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef]
- Brett, C.M.A.; Brett, A.M.O. Electrochemistry: Principles, Methods, and Applications; Oxford Science Publications; Oxford University Press: New York, NY, USA, 1993; ISBN 978-0-19-855389-2. [Google Scholar]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry, 2nd ed.; Imperial College Press: London, UK, 2010; ISBN 978-1-84816-585-4. [Google Scholar]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Palchetti, I.; Laschi, S.; Marrazza, G.; Mascini, M. Electrochemical Imaging of Localized Sandwich DNA Hybridization Using Scanning Electrochemical Microscopy. Anal. Chem. 2007, 79, 7206–7213. [Google Scholar] [CrossRef]
- Voccia, D.; Sosnowska, M.; Bettazzi, F.; Roscigno, G.; Fratini, E.; De Franciscis, V.; Condorelli, G.; Chitta, R.; D’Souza, F.; Kutner, W.; et al. Direct Determination of Small RNAs Using a Biotinylated Polythiophene Impedimetric Genosensor. Biosens. Bioelectron. 2017, 87, 1012–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odenthal, K.J.; Gooding, J.J. An Introduction to Electrochemical DNAbiosensors. Analyst 2007, 132, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Meric, B.; Kerman, K.; Marrazza, G.; Palchetti, I.; Mascini, M.; Ozsoz, M. Disposable Genosensor, a New Tool for the Detection of NOS-Terminator, a Genetic Element Present in GMOs. Food Control 2004, 15, 621–626. [Google Scholar] [CrossRef]
- Lai, R.Y.; Lagally, E.T.; Lee, S.-H.; Soh, H.T.; Plaxco, K.W.; Heeger, A.J. Rapid, Sequence-Specific Detection of Unpurified PCR Amplicons via a Reusable, Electrochemical Sensor. Proc. Natl. Acad. Sci. USA 2006, 103, 4017–4021. [Google Scholar] [CrossRef] [Green Version]
- Laschi, S.; Miranda-Castro, R.; González-Fernández, E.; Palchetti, I.; Reymond, F.; Rossier, J.S.; Marrazza, G. A New Gravity-Driven Microfluidic-Based Electrochemical Assay Coupled to Magnetic Beads for Nucleic Acid Detection. Electrophoresis 2010, 31, 3727–3736. [Google Scholar] [CrossRef]
- Voccia, D.; Bettazzi, F.; Fratini, E.; Berti, D.; Palchetti, I. Improving Impedimetric Nucleic Acid Detection by Using Enzyme-Decorated Liposomes and Nanostructured Screen-Printed Electrodes. Anal. Bioanal. Chem. 2016, 408, 7271–7281. [Google Scholar] [CrossRef]
- Toldrà, A.; Alcaraz, C.; Diogène, J.; O’Sullivan, C.K.; Campàs, M. Detection of Ostreopsis cf. ovata in Environmental Samples Using an Electrochemical DNA-Based Biosensor. Sci. Total Environ. 2019, 689, 655–661. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Kawde, A.-N.; Polsky, R. Metal Nanoparticle-Based Electrochemical Stripping Potentiometric Detection of DNA Hybridization. Anal. Chem. 2001, 73, 5576–5581. [Google Scholar] [CrossRef] [PubMed]
- Berti, F.; Lozzi, L.; Palchetti, I.; Santucci, S.; Marrazza, G. Aligned Carbon Nanotube Thin Films for DNA Electrochemical Sensing. Electrochim. Acta 2009, 54, 5035–5041. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Tailoring Sensitivity in Electrochemical Nucleic Acid Hybridization Biosensing: Role of Surface Chemistry and Labeling Strategies. ChemElectroChem 2019, 6, 60–72. [Google Scholar] [CrossRef]
- Ingrosso, C.; Corricelli, M.; Bettazzi, F.; Konstantinidou, E.; Bianco, G.V.; Depalo, N.; Striccoli, M.; Agostiano, A.; Curri, M.L.; Palchetti, I. Au Nanoparticle in Situ Decorated RGO Nanocomposites for Highly Sensitive Electrochemical Genosensors. J. Mater. Chem. B 2019, 7, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Bettazzi, F.; Palchetti, I. Photoelectrochemical Genosensors for the Determination of Nucleic Acid Cancer Biomarkers. Curr. Opin. Electrochem. 2018, 12, 51–59. [Google Scholar] [CrossRef]
- Holmberg, S.D.; Blake, P.A. Staphylococcal Food Poisoning in the United States: New Facts and Old Misconceptions. JAMA 1984, 251, 487–489. [Google Scholar] [CrossRef]
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the Detection and Identification of Pathogenic Bacteria: Past, Present, and Future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef]
- Toldrà, A.; O’Sullivan, C.K.; Campàs, M. Detecting Harmful Algal Blooms with Isothermal Molecular Strategies. Trends Biotechnol. 2019, 37, 1278–1281. [Google Scholar] [CrossRef]
- Toldrà, A.; O’Sullivan, C.K.; Diogène, J.; Campàs, M. Detecting Harmful Algal Blooms with Nucleic Acid Amplification-Based Biotechnological Tools. Sci. Total Environ. 2020, 749, 141605. [Google Scholar] [CrossRef]
- Reynoso, E.C.; Laschi, S.; Palchetti, I.; Torres, E. Advances in Antimicrobial Resistance Monitoring Using Sensors and Biosensors: A Review. Chemosensors 2021, 9, 232. [Google Scholar] [CrossRef]
- Jaroenram, W.; Kampeera, J.; Arunrut, N.; Karuwan, C.; Sappat, A.; Khumwan, P.; Jaitrong, S.; Boonnak, K.; Prammananan, T.; Chaiprasert, A.; et al. Graphene-Based Electrochemical Genosensor Incorporated Loop-Mediated Isothermal Amplification for Rapid on-Site Detection of Mycobacterium Tuberculosis. J. Pharm. Biomed. Anal. 2020, 186, 113333. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.Y.C.; Xiao, W.; West, N.; Wee, E.; Wang, Y.; Trau, M. Rapid, Single-Cell Electrochemical Detection of Mycobacterium Tuberculosis Using Colloidal Gold Nanoparticles. Anal. Chem. 2015, 87, 10613–10618. [Google Scholar] [CrossRef] [PubMed]
- Torres-Chavolla, E.; Alocilja, E.C. Nanoparticle Based DNA Biosensor for Tuberculosis Detection Using Thermophilic Helicase-Dependent Isothermal Amplification. Biosens. Bioelectron. 2011, 26, 4614–4618. [Google Scholar] [CrossRef]
- Yang, A.K.L.; Lu, H.; Wu, S.Y.; Kwok, H.C.; Ho, H.P.; Yu, S.; Cheung, A.K.L.; Kong, S.K. Detection of Panton-Valentine Leukocidin DNA from Methicillin-Resistant Staphylococcus Aureus by Resistive Pulse Sensing and Loop-Mediated Isothermal Amplification with Gold Nanoparticles. Anal. Chim. Acta 2013, 782, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Cai, R.; Zhang, Z.; Chen, H.; Tian, Y.; Zhou, N. A Versatile Signal-on Electrochemical Biosensor for Staphylococcus Aureus Based on Triple-Helix Molecular Switch. Sens. Actuators B Chem. 2021, 326, 128842. [Google Scholar] [CrossRef]
- Barreda-García, S.; Miranda-Castro, R.; de-Los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Helicase-Dependent Isothermal Amplification: A Novel Tool in the Development of Molecular-Based Analytical Systems for Rapid Pathogen Detection. Anal. Bioanal. Chem. 2018, 410, 679–693. [Google Scholar] [CrossRef]
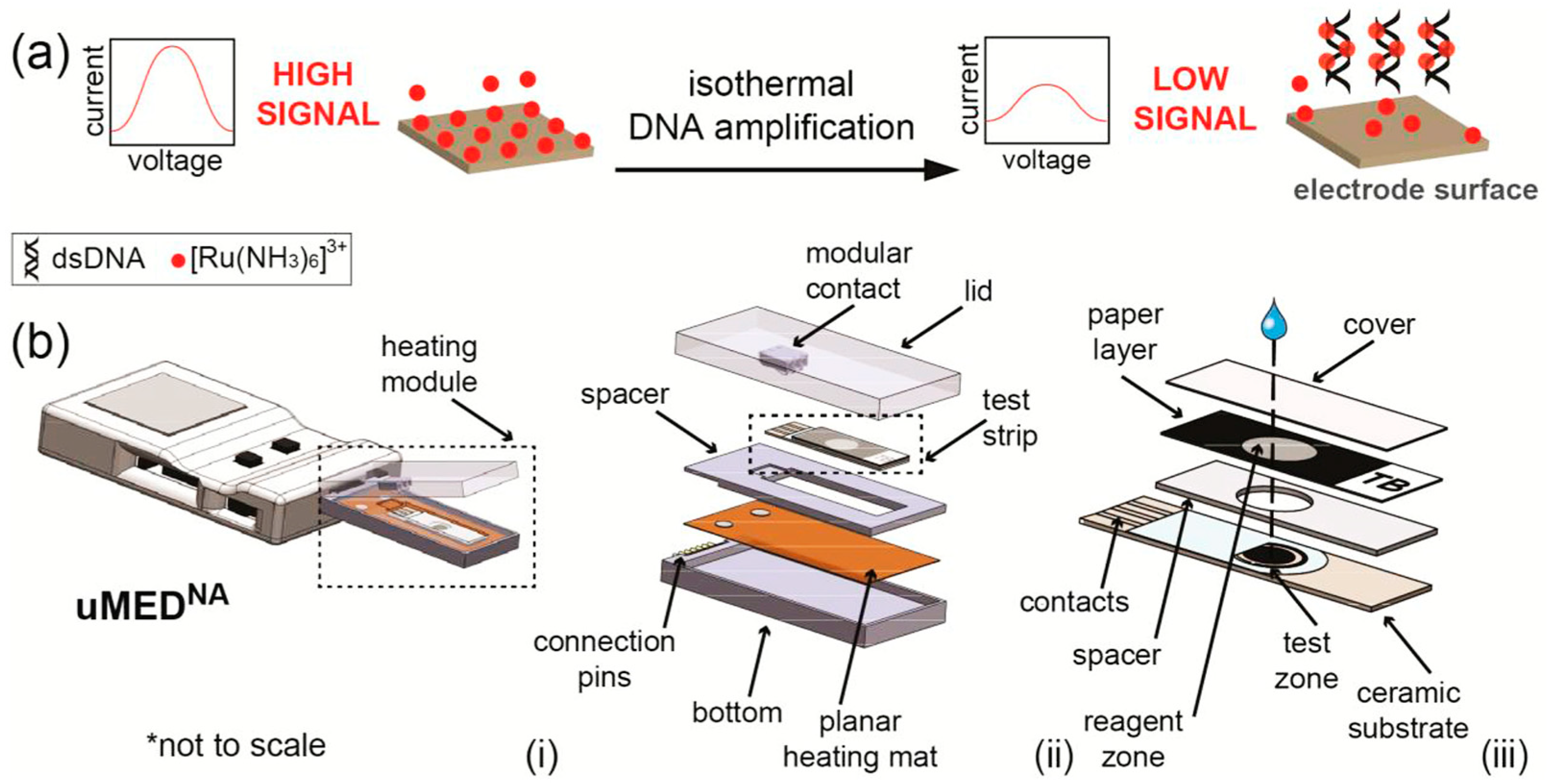
- Tsaloglou, M.-N.; Nemiroski, A.; Camci-Unal, G.; Christodouleas, D.C.; Murray, L.P.; Connelly, J.T.; Whitesides, G.M. Handheld Isothermal Amplification and Electrochemical Detection of DNA in Resource-Limited Settings. Anal. Biochem. 2018, 543, 116–121. [Google Scholar] [CrossRef]
- Huang, T.-T.; Liu, S.-C.; Huang, C.-H.; Lin, C.-J.; Huang, S.-T. An Integrated Real-Time Electrochemical LAMP Device for Pathogenic Bacteria Detection in Food. Electroanalysis 2018, 30, 2397–2404. [Google Scholar] [CrossRef]
- Kim, H.E.; Schuck, A.; Lee, S.H.; Lee, Y.; Kang, M.; Kim, Y.-S. Sensitive Electrochemical Biosensor Combined with Isothermal Amplification for Point-of-Care COVID-19 Tests. Biosens. Bioelectron. 2021, 182, 113168. [Google Scholar] [CrossRef]
- Xu, Z.; Yin, K.; Ding, X.; Li, Z.; Sun, X.; Li, B.; Lalla, R.V.; Gross, R.; Liu, C. An Integrated E-Tube Cap for Sample Preparation, Isothermal Amplification and Label-Free Electrochemical Detection of DNA. Biosens. Bioelectron. 2021, 186, 113306. [Google Scholar] [CrossRef]
- Gorbalenya, A.E.; Baker, S.C.; Baric, R.S.; de Groot, R.J.; Drosten, C.; Gulyaeva, A.A.; Haagmans, B.L.; Lauber, C.; Leontovich, A.M.; Neuman, B.W.; et al. The Species Severe Acute Respiratory Syndrome-Related Coronavirus: Classifying 2019-NCoV and Naming It SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miripour, Z.S.; Sarrami-Forooshani, R.; Sanati, H.; Makarem, J.; Taheri, M.S.; Shojaeian, F.; Eskafi, A.H.; Abbasvandi, F.; Namdar, N.; Ghafari, H.; et al. Real-Time Diagnosis of Reactive Oxygen Species (ROS) in Fresh Sputum by Electrochemical Tracing; Correlation between COVID-19 and Viral-Induced ROS in Lung/Respiratory Epithelium during This Pandemic. Biosens. Bioelectron. 2020, 165, 112435. [Google Scholar] [CrossRef] [PubMed]
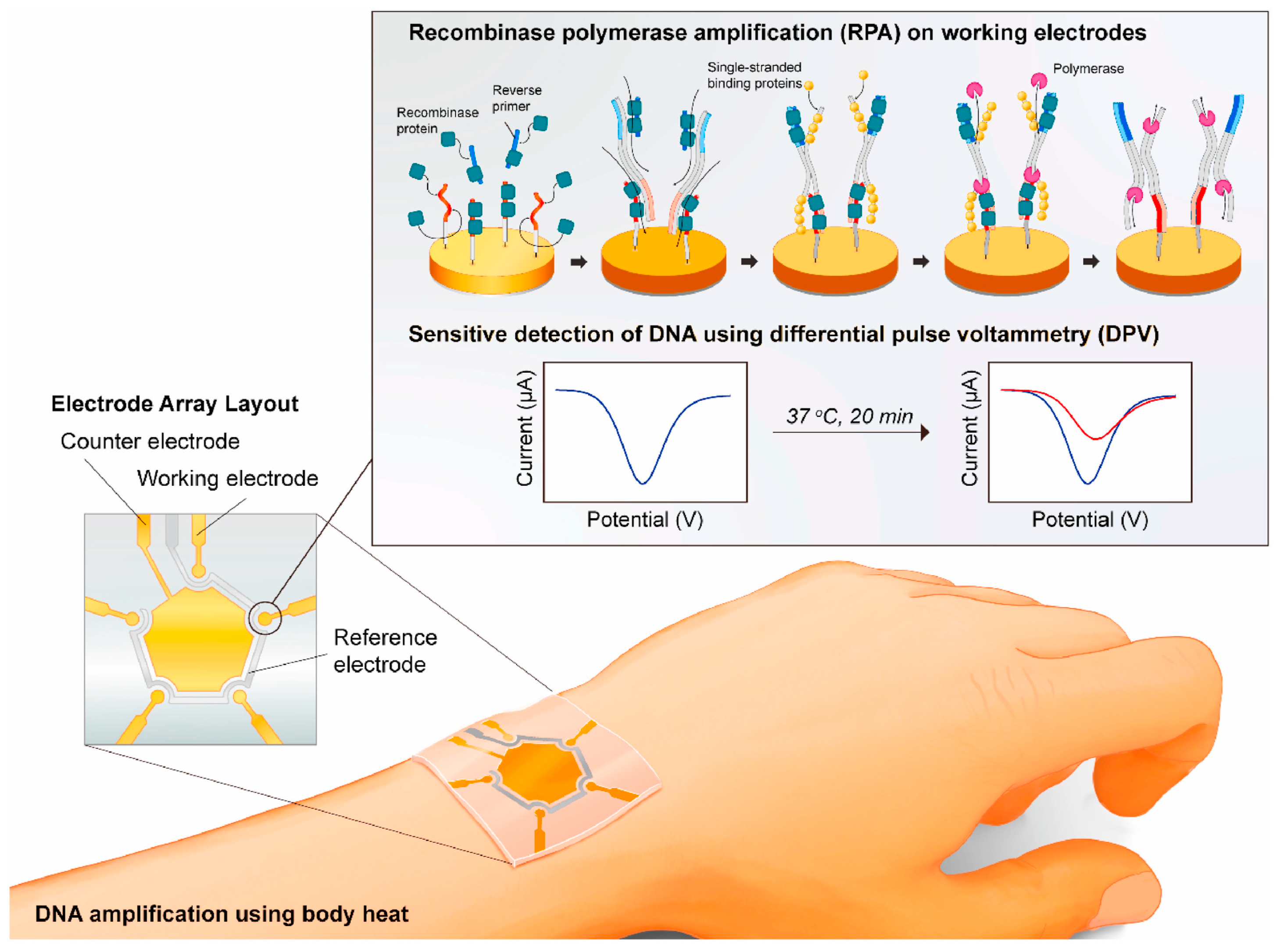
- Trinh, K.T.L.; Lee, N.Y. Fabrication of Wearable PDMS Device for Rapid Detection of Nucleic Acids via Recombinase Polymerase Amplification Operated by Human Body Heat. Biosensors 2022, 12, 72. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, C.; Arzul, I.; Carrasco, N.; Furones Nozal, D. A Literature Review as an Aid to Identify Strategies for Mitigating Ostreid Herpesvirus 1 in Crassostrea Gigas Hatchery and Nursery Systems. Rev. Aquac. 2019, 11, 565–585. [Google Scholar] [CrossRef] [Green Version]
- Toldrà, A.; Furones, M.D.; O’Sullivan, C.K.; Campàs, M. Detection of Isothermally Amplified Ostreid Herpesvirus 1 DNA in Pacific Oyster (Crassostrea gigas) Using a Miniaturised Electrochemical Biosensor. Talanta 2020, 207, 120308. [Google Scholar] [CrossRef]
- Martisova, A.; Holcakova, J.; Izadi, N.; Sebuyoya, R.; Hrstka, R.; Bartosik, M. DNA Methylation in Solid Tumors: Functions and Methods of Detection. Int. J. Mol. Sci. 2021, 22, 4247. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, X.; He, M.; Shang, Y.; Tetlow, A.L.; Godwin, A.K.; Zeng, Y. Ultrasensitive Detection of Circulating Exosomes with a 3D-Nanopatterned Microfluidic Chip. Nat. Biomed. Eng. 2019, 3, 438–451. [Google Scholar] [CrossRef]
- Oliveira, B.; Veigas, B.; Fernandes, A.R.; Águas, H.; Martins, R.; Fortunato, E.; Baptista, P.V. Fast Prototyping Microfluidics: Integrating Droplet Digital Lamp for Absolute Quantification of Cancer Biomarkers. Sensors 2020, 20, 1624. [Google Scholar] [CrossRef] [Green Version]
- Moranova, L.; Stanik, M.; Hrstka, R.; Campuzano, S.; Bartosik, M. Electrochemical LAMP-Based Assay for Detection of RNA Biomarkers in Prostate Cancer. Talanta 2022, 238, 123064. [Google Scholar] [CrossRef]
- Camacho, C.; Matías, J.C.; Chico, B.; Cao, R.; Gómez, L.; Simpson, B.K.; Villalonga, R. Amperometric Biosensor for Hydrogen Peroxide, Using Supramolecularly Immobilized Horseradish Peroxidase on the β-Cyclodextrin-Coated Gold Electrode. Electroanalysis 2007, 19, 2538–2542. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, F.; Ma, Y.; Wang, J.; Li, H.; Zhang, J. Isolation and Detection Technologies of Extracellular Vesicles and Application on Cancer Diagnostic. Dose-Response Publ. Int. Hormesis Soc. 2019, 17, 1559325819891004. [Google Scholar] [CrossRef] [Green Version]
- Dilsiz, N. Role of Exosomes and Exosomal MicroRNAs in Cancer. Future Sci. OA 2020, 6, FSO465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
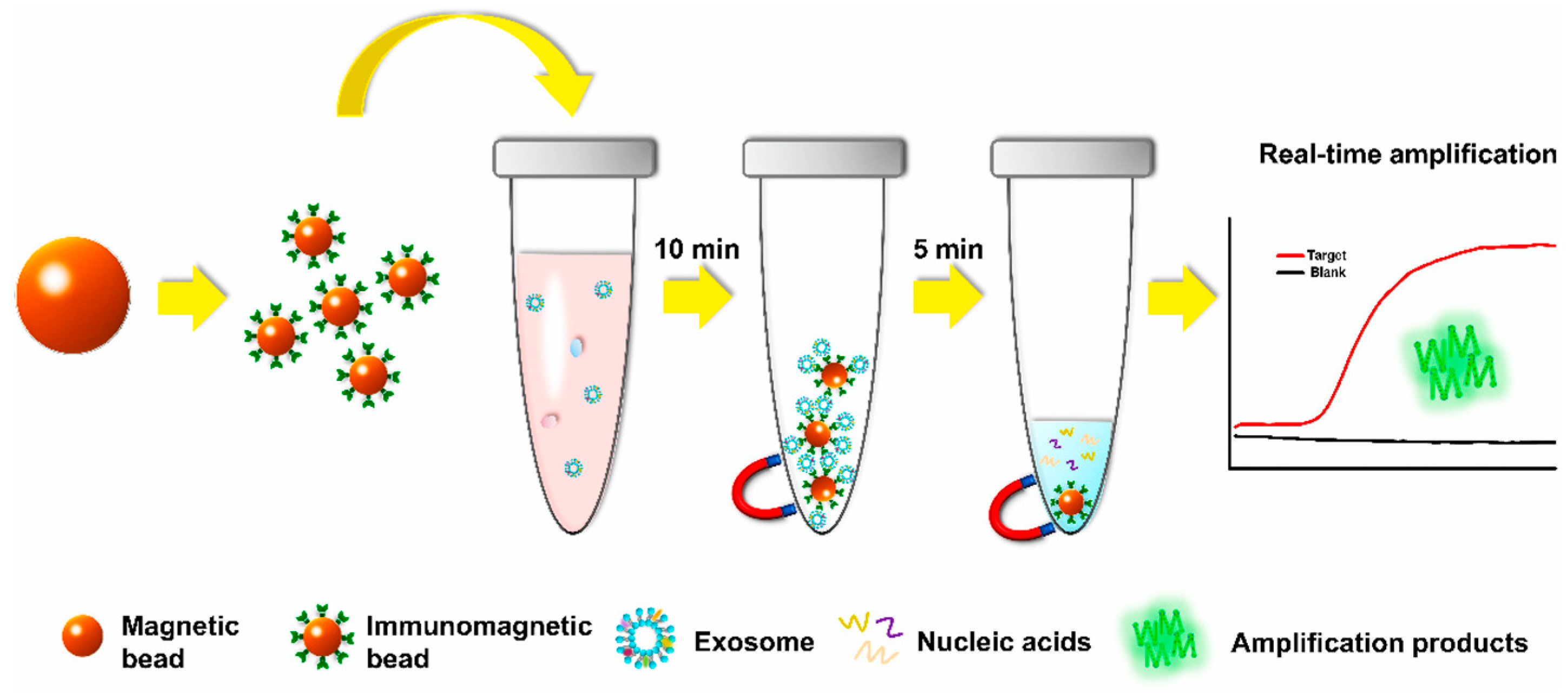
- Lin, Q.; Huang, Z.; Ye, X.; Yang, B.; Fang, X.; Liu, B.; Chen, H.; Kong, J. Lab in a Tube: Isolation, Extraction, and Isothermal Amplification Detection of Exosomal Long Noncoding RNA of Gastric Cancer. Talanta 2021, 225, 122090. [Google Scholar] [CrossRef]
- Hashimoto, K.; Inada, M.; Ito, K. Multiplex Real-Time Loop-Mediated Isothermal Amplification Using an Electrochemical DNA Chip Consisting of a Single Liquid-Flow Channel. Anal. Chem. 2019, 91, 3227–3232. [Google Scholar] [CrossRef]
- Izadi, N.; Sebuyoya, R.; Moranova, L.; Hrstka, R.; Anton, M.; Bartosik, M. Electrochemical Bioassay Coupled to LAMP Reaction for Determination of High-Risk HPV Infection in Crude Lysates. Anal. Chim. Acta 2021, 1187, 339145. [Google Scholar] [CrossRef]
- Li, Z.; Ding, X.; Yin, K.; Xu, Z.; Cooper, K.; Liu, C. Electric Field-Enhanced Electrochemical CRISPR Biosensor for DNA Detection. Biosens. Bioelectron. 2021, 192, 113498. [Google Scholar] [CrossRef]
- Liu, H.; He, T.; Gao, Y.; Yu, J. Novel Integrated Type Nucleic Acid Detecting Device. Available online: https://worldwide.espacenet.com/patent/search/family/067580275/publication/CN110129188A?q=CN110129188A (accessed on 13 July 2022).
- Chen, S.; Xu, Y.; Yuan, X. Preparation and Application of Electrochemical Micro-Fluidic Device for Nucleic Acid Isothermal Amplification. 2015. Available online: https://worldwide.espacenet.com/patent/search/family/052644683/publication/CN104407036A?q=pn%3DCN104407036A (accessed on 13 July 2022).
- Chen, S.; Lai, J. Micro-Fluidic Electrochemical Sensor Capable of Rapidly Detecting Viruses. 2021. Available online: https://worldwide.espacenet.com/patent/search/family/075442249/publication/CN213012858U?q=pn%3DCN213012858U (accessed on 13 July 2022).

| Isothermal Amplification Method | Limit of Detection | Lab-on-a-Chip/Wearable Platform | Real Samples | Electrochemical Detection | Type of Electrode | Analysis Time | References | |
|---|---|---|---|---|---|---|---|---|
| Mycobacterium tuberculosis | ||||||||
| LAMP | 40 CFU/equivalence | - | Sputum | (CV) | SPGE | <65 min | [55] | |
| RPA | 1 CFU/mL | - | None | (DPV) | SPCE | - | [56] | |
| HDA | 0.5 aM | - | None | (DPV) | SPCE | From 2 to 8 h | [57] | |
| RPA | 11 CFU/mL | Yes | None | (SWV) | SPCE | - | [61] | |
| Staphylucoccus aureus | ||||||||
| LAMP | 530 copies of target | - | None | Resistive pulse sensing | SPCE | - | [58] | |
| SDA | 8 CFU/mL | - | Lake water and honey | DPV | SPCE | - | [59] | |
| Salmonella spp./E. coli | ||||||||
| LAMP | 10 and 1 Bacteria DNA copies | Yes | None | DPV | Gold electrodes | Less than 30 min | [62] | |
| SARS-CoV-2 | ||||||||
| RPA | 3.925 fg/μL | Yes | None | DPV | Gold Electrode | Less than 20 min | [63] | |
| Lambda DNA | ||||||||
| LAMP | 100 DNA copies/mL | Yes | Saliva | (EIS) | pH-sensitive electrode | 30 min | [64] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patiti, C.; Sfragano, P.S.; Laschi, S.; Pillozzi, S.; Boddi, A.; Crociani, O.; Bernini, A.; Palchetti, I. Chip-Based and Wearable Tools for Isothermal Amplification and Electrochemical Analysis of Nucleic Acids. Chemosensors 2022, 10, 278. https://doi.org/10.3390/chemosensors10070278
Patiti C, Sfragano PS, Laschi S, Pillozzi S, Boddi A, Crociani O, Bernini A, Palchetti I. Chip-Based and Wearable Tools for Isothermal Amplification and Electrochemical Analysis of Nucleic Acids. Chemosensors. 2022; 10(7):278. https://doi.org/10.3390/chemosensors10070278
Chicago/Turabian StylePatiti, Claudia, Patrick Severin Sfragano, Serena Laschi, Serena Pillozzi, Anna Boddi, Olivia Crociani, Andrea Bernini, and Ilaria Palchetti. 2022. "Chip-Based and Wearable Tools for Isothermal Amplification and Electrochemical Analysis of Nucleic Acids" Chemosensors 10, no. 7: 278. https://doi.org/10.3390/chemosensors10070278
APA StylePatiti, C., Sfragano, P. S., Laschi, S., Pillozzi, S., Boddi, A., Crociani, O., Bernini, A., & Palchetti, I. (2022). Chip-Based and Wearable Tools for Isothermal Amplification and Electrochemical Analysis of Nucleic Acids. Chemosensors, 10(7), 278. https://doi.org/10.3390/chemosensors10070278









