Semiconductor-Type Gas Sensors Based on γ-Fe2O3 Nanoparticles and Its Derivatives in Conjunction with SnO2 and Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of γ-Fe2O3 Nanospheres
2.3. Preparation of γ-Fe2O3/SnO2 Nanoparticles
2.4. Preparation of γ-Fe2O3/SnO2/RGO Hybrid Nanoparticles
2.5. Characterization of Phases and Microstructures
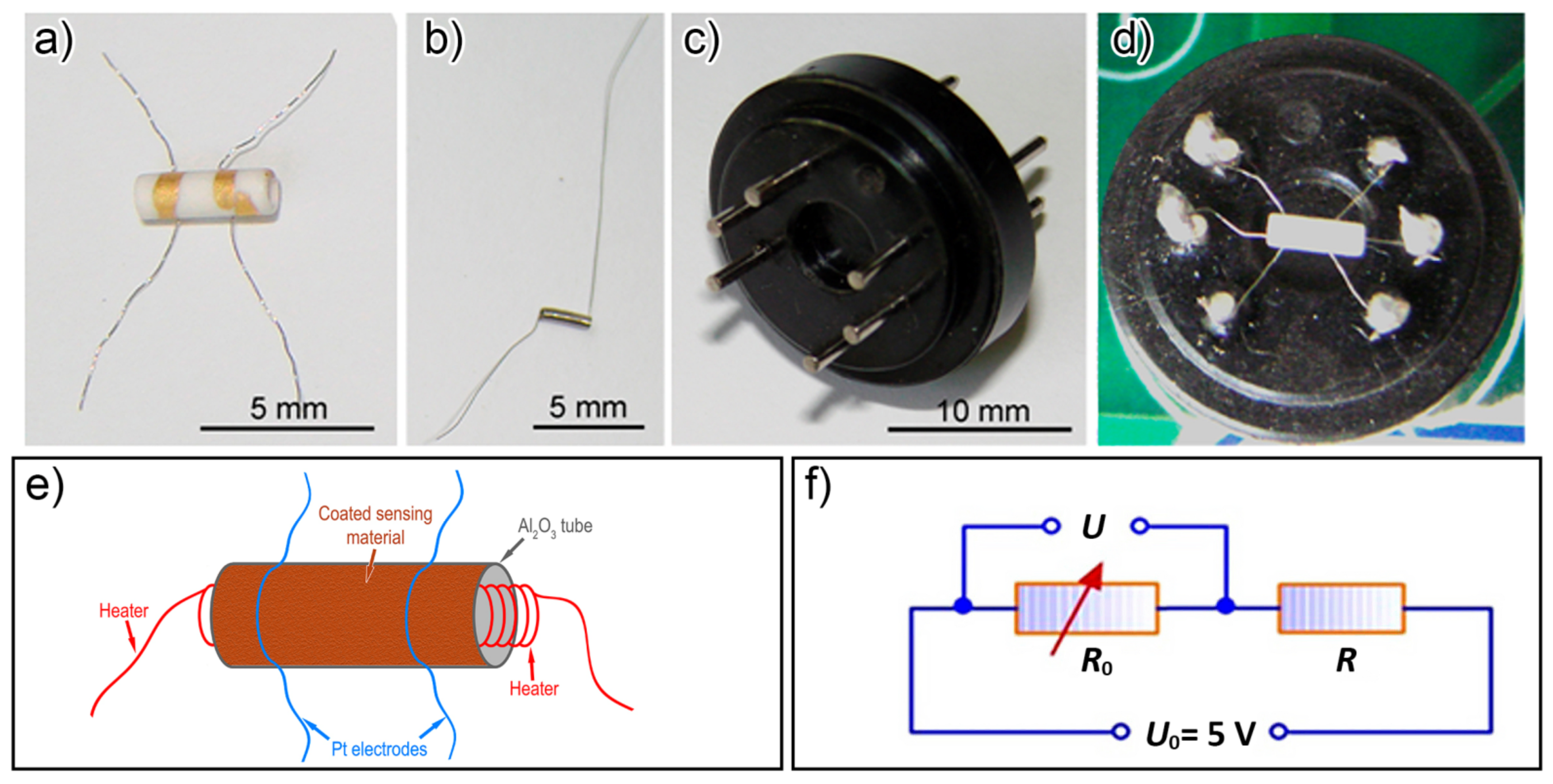
2.6. Fabrication of Gas-Sensing Devices
2.7. Measurement of Gas-Sensitivity Performance of the Sensors
3. Results and Discussion
3.1. Characterization
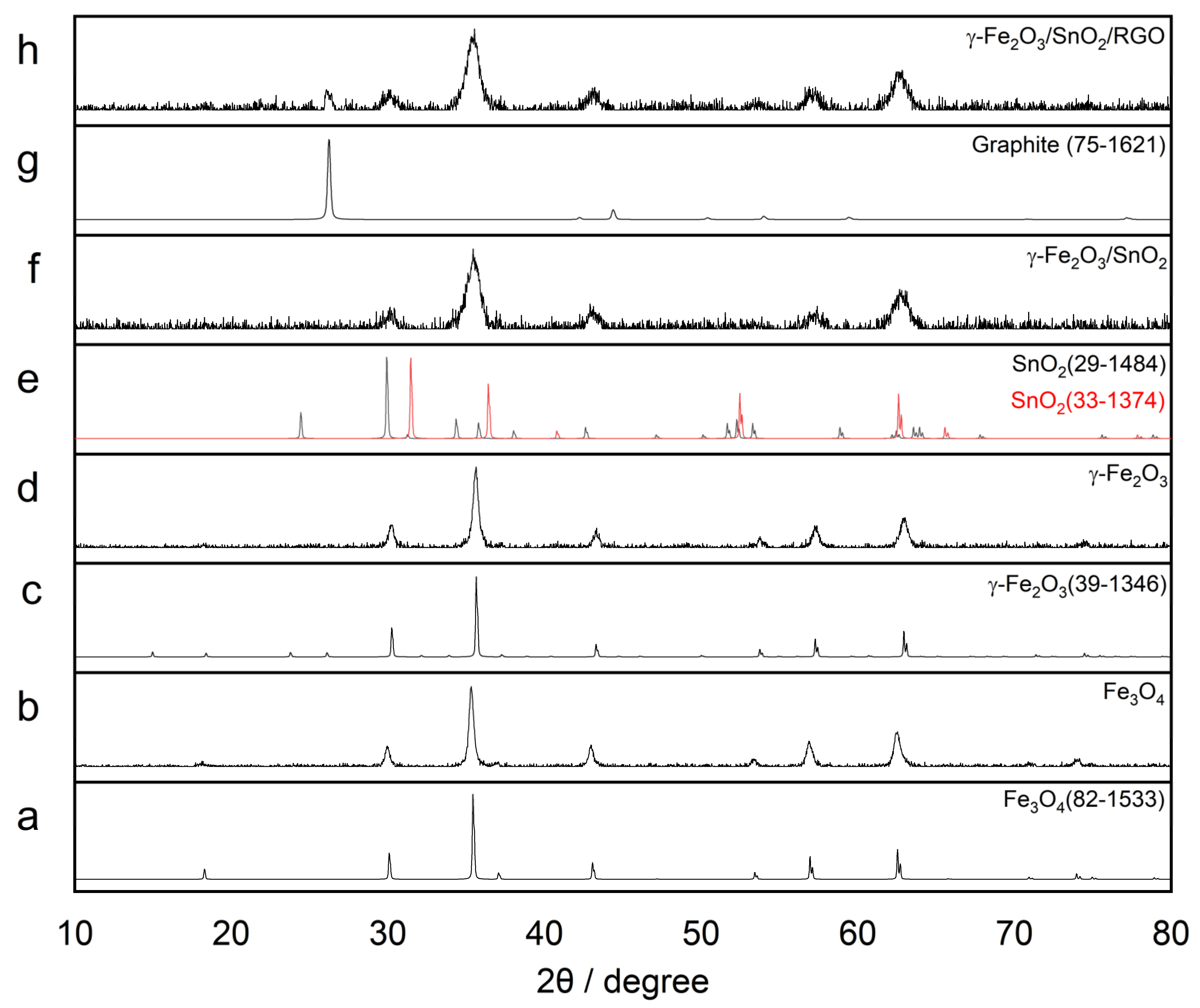
3.1.1. Characterization of γ-Fe2O3
3.1.2. Characterization of γ-Fe2O3/SnO2
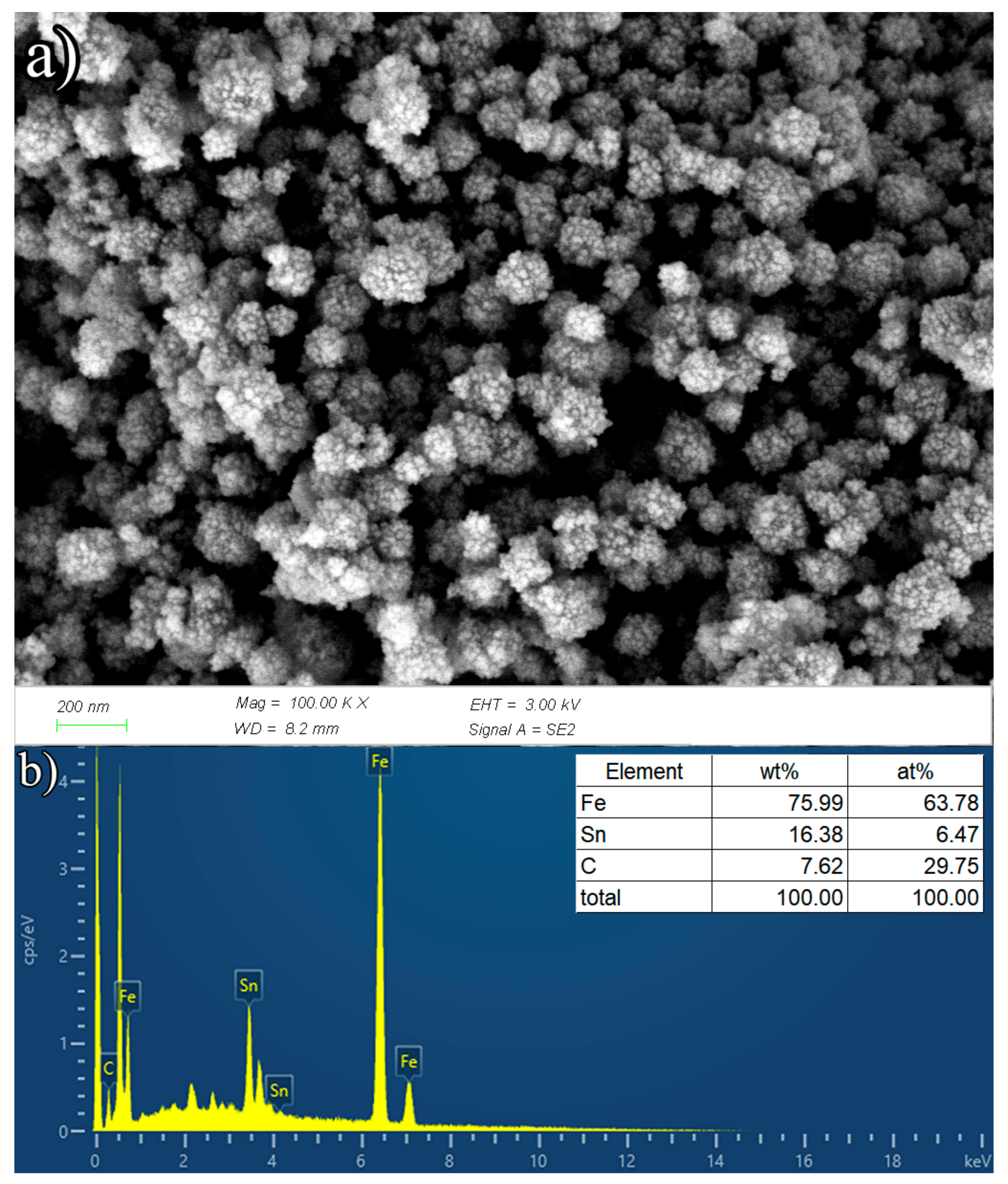
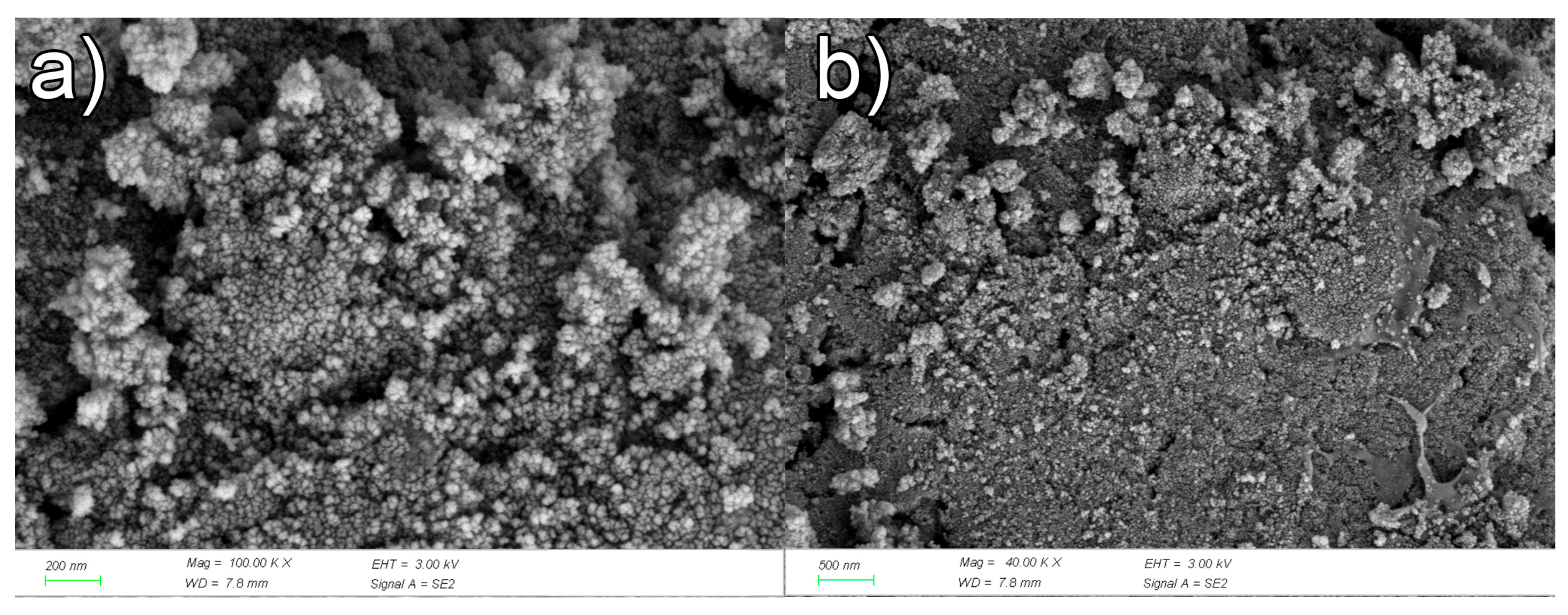
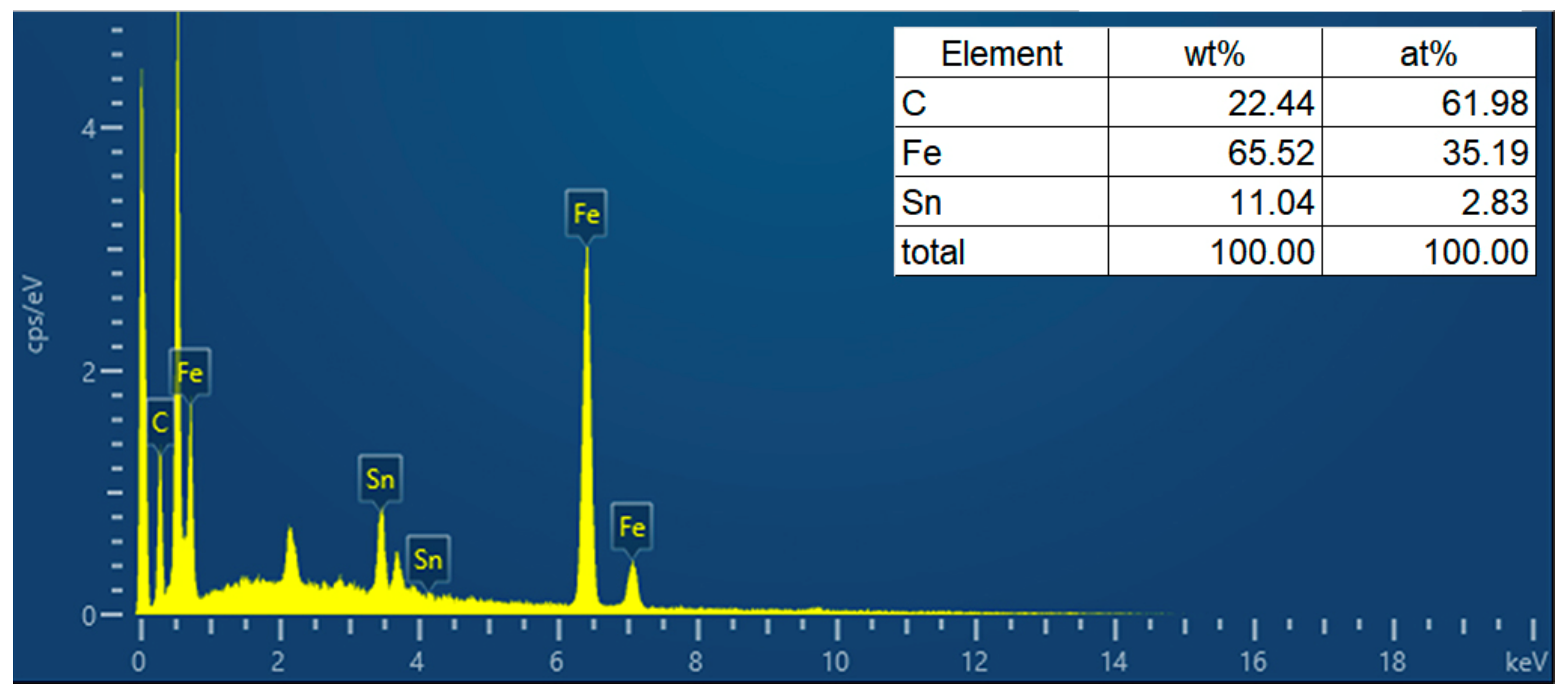
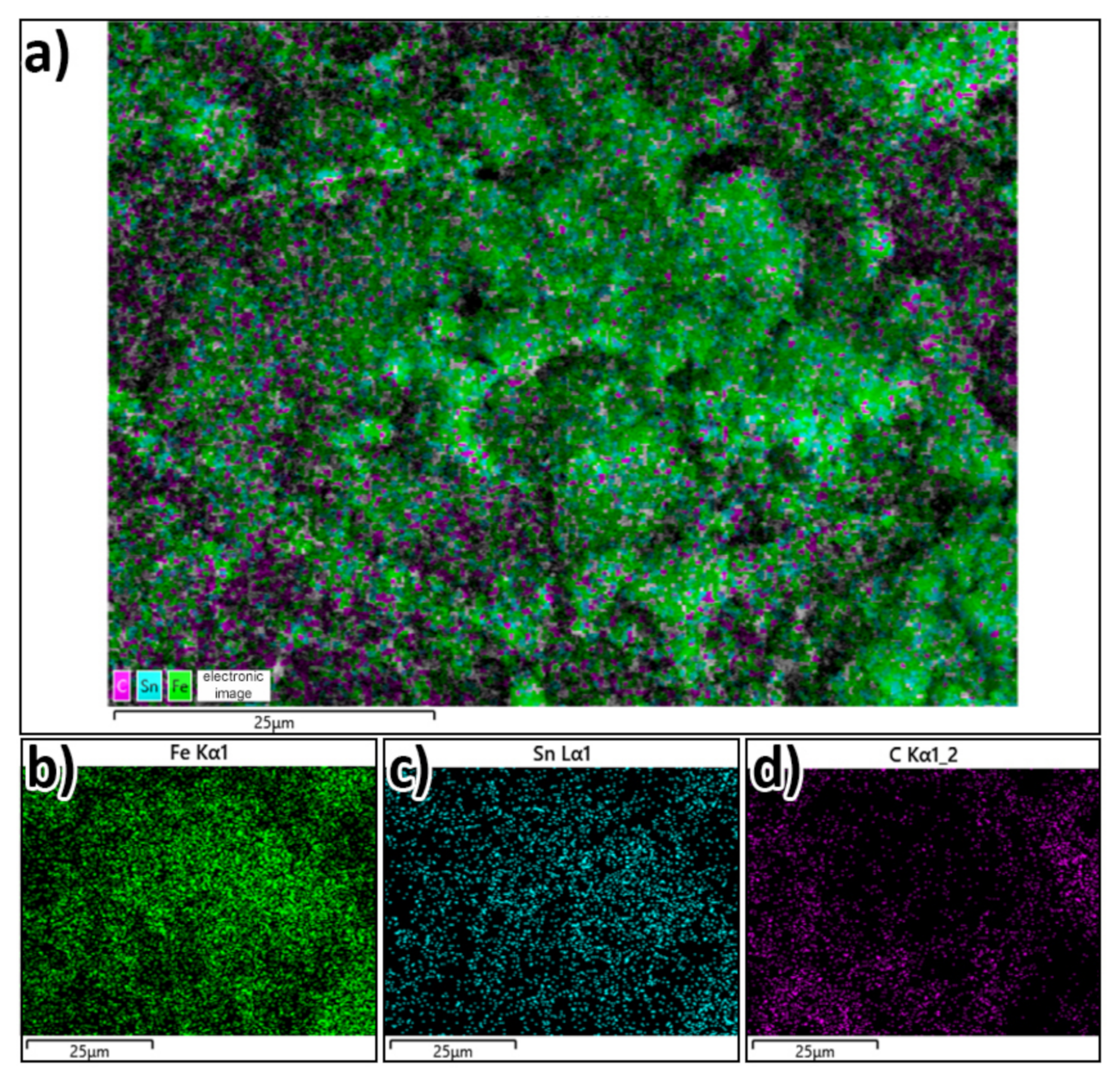
3.1.3. Characterization of γ-Fe2O3/SnO2/RGO
3.2. Gas-Sensing Mechanism
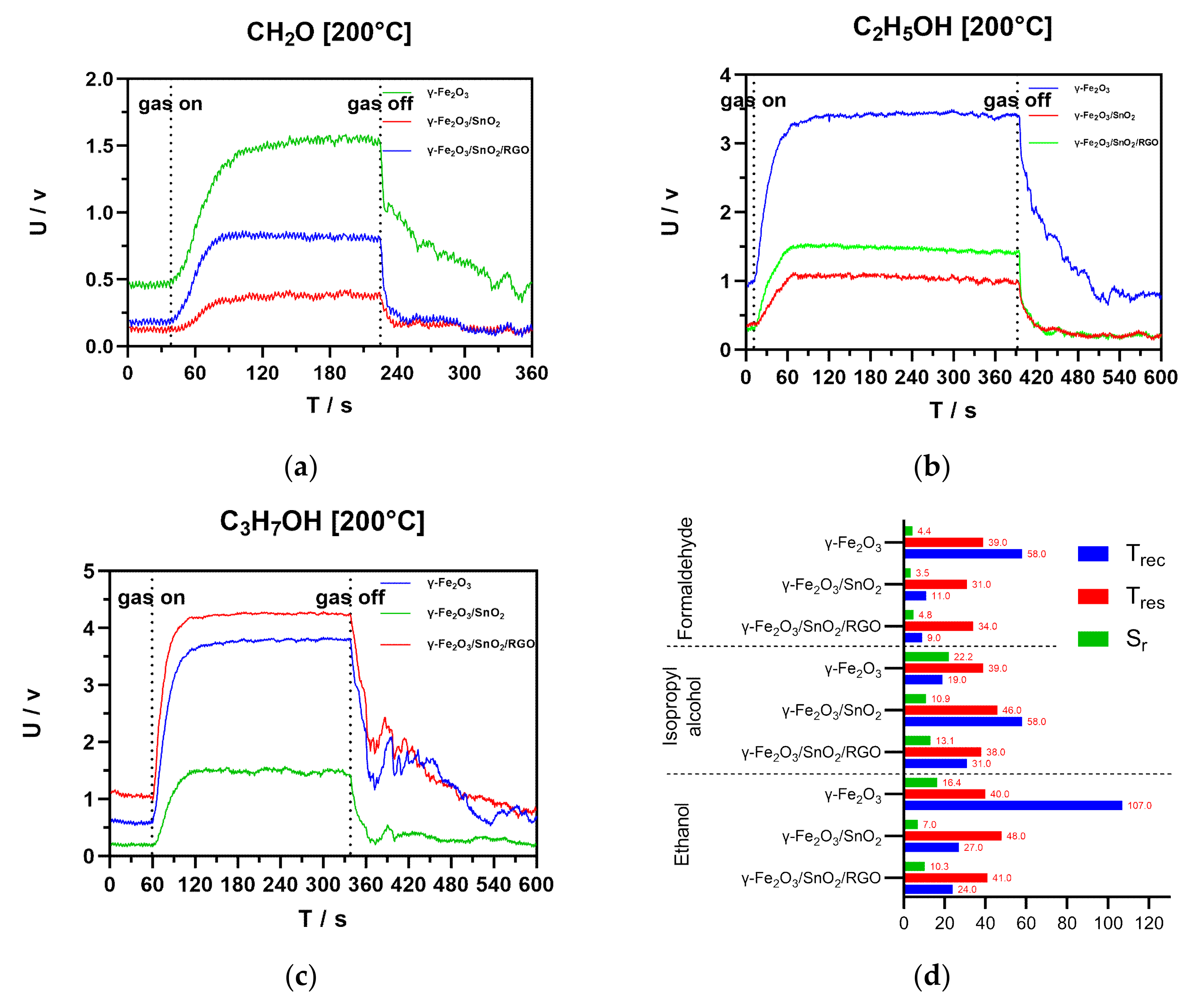
3.3. Results and Discussion on the Gas-Sensitivity Test for Organic Vapors
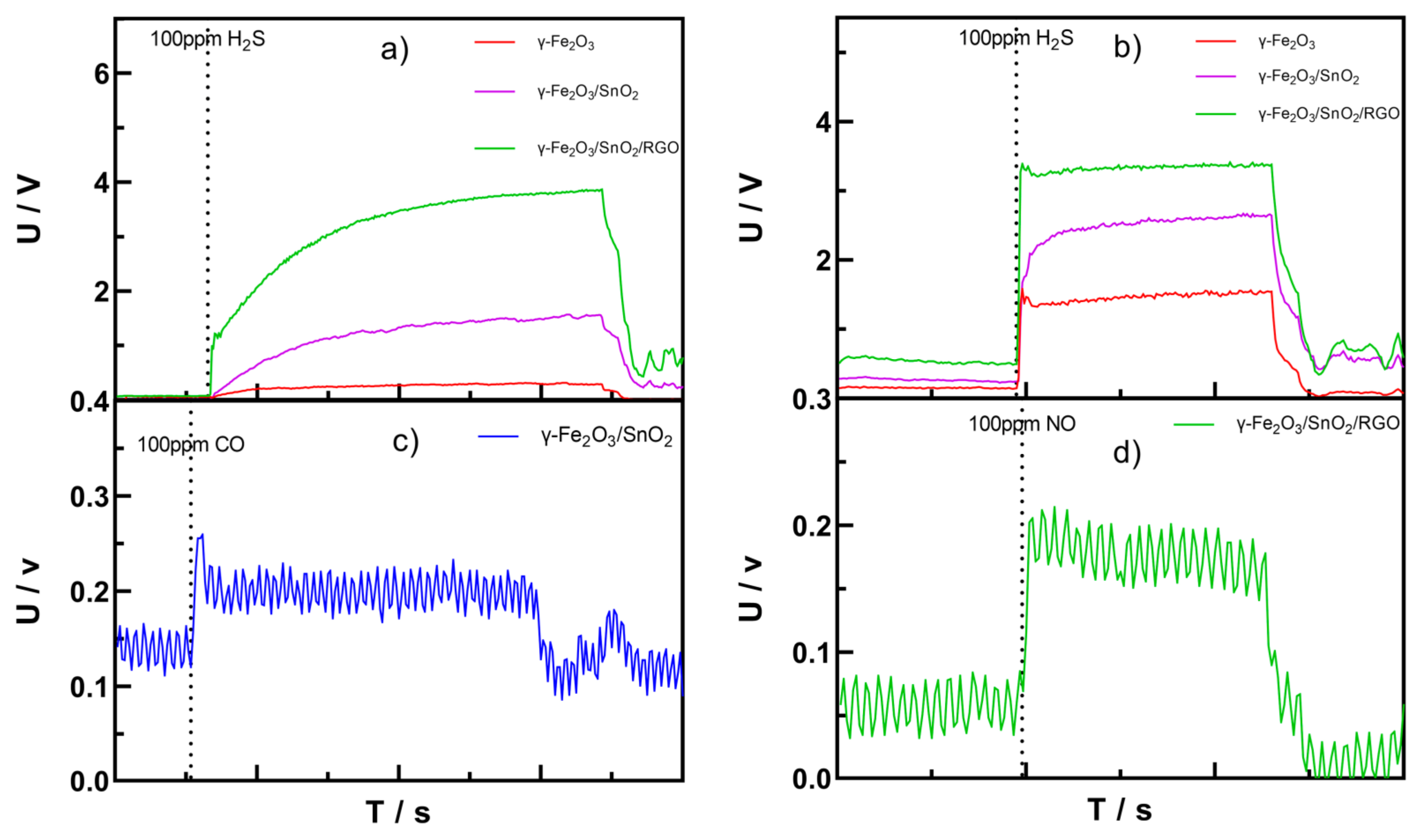
3.4. Results and Discussion on the Gas-Sensitivity Test for H2S, CO, and NO Gases
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, M.S.; Mandloi, R.K.; Singh, I. Environmental Impact Assessment of Human Error to Exhaust Toxic Gases and Oil Spilling for Prevention of Natural Disaster Through Fault Tree Analysis. IJERT 2020, 9, 82–91. [Google Scholar] [CrossRef]
- Dhall, S.; Mehta, B.R.; Tyagi, A.K.; Sood, K. A review on environmental gas sensors: Materials and technologies. Sens. Int. 2021, 2, 100116. [Google Scholar] [CrossRef]
- Winkless, L. Breathalyzer gets the nanotech treatment. Mater. Today 2015, 18, 421–422. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Rab, S.; Singh, R.P.; Suman, R. Sensors for daily life: A review. Sens. Int. 2021, 2, 100121. [Google Scholar] [CrossRef]
- Gerstner, E. Nobel Prize 2010: Andre Geim & Konstantin Novoselov. Nat. Phys. 2010, 6, 836. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Guinea, F.; Katsnelson, M.I.; Geim, A.K. Energy gaps and a zero-field quantum Hall effect in graphene by strain engineering. Nat. Phys. 2010, 6, 30–33. [Google Scholar] [CrossRef] [Green Version]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N.; Harack, B.; Hilke, M.; Horth, A.; Majlis, N.; Massicotte, M.; Vandsburger, L.; Whiteway, E.; et al. Experimental Review of Graphene. ISRN Condens. Matter Phys. 2012, 2012, 1–56. [Google Scholar] [CrossRef] [Green Version]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Pal, P.; Yadav, A.; Chauhan, P.S.; Parida, P.K.; Gupta, A. Reduced graphene oxide based hybrid functionalized films for hydrogen detection: Theoretical and experimental studies. Sens. Int. 2021, 2, 100072. [Google Scholar] [CrossRef]
- Haridas, V.; Sukhananazerin, A.; Sneha, J.M.; Pullithadathil, B.; Narayanan, B. α-Fe2O3 loaded less-defective graphene sheets as chemiresistive gas sensor for selective sensing of NH3. Appl. Surf. Sci. 2020, 517, 146158. [Google Scholar] [CrossRef]
- Bai, S.; Chen, C.; Luo, R.; Chen, A.; Li, D. Synthesis of MoO3/reduced graphene oxide hybrids and mechanism of enhancing H2S sensing performances. Sens. Actuators B Chem. 2015, 216, 113–120. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Cheng, X.; Xu, Y.; Gao, S.; Zhao, H.; Huo, L. Hierarchical NiO Cube/Nitrogen-Doped Reduced Graphene Oxide Composite with Enhanced H2S Sensing Properties at Low Temperature. ACS Appl. Mater. Interfaces 2017, 9, 26293–26303. [Google Scholar] [CrossRef]
- MalekAlaie, M.; Jahangiri, M.; Rashidi, A.M.; HaghighiAsl, A.; Izadi, N. Selective hydrogen sulfide (H2S) sensors based on molybdenum trioxide (MoO3) nanoparticle decorated reduced graphene oxide. Mater. Sci. Semicond. Processing 2015, 38, 93–100. [Google Scholar] [CrossRef]
- Yin, L.; Wang, H.; Li, L.; Li, H.; Chen, D.; Zhang, R. Microwave-assisted preparation of hierarchical CuO@rGO nanostructures and their enhanced low-temperature H2S-sensing performance. Appl. Surf. Sci. 2019, 476, 107–114. [Google Scholar] [CrossRef]
- Shewale, P.S.; Yun, K.-S. Synthesis and characterization of Cu-doped ZnO/RGO nanocomposites for room-temperature H2S gas sensor. J. Alloys Compd. 2020, 837, 155527. [Google Scholar] [CrossRef]
- Patekari, M.D.; Pawar, K.K.; Salunkhe, G.B.; Kodam, P.M.; Padvi, M.N.; Waifalkar, P.P.; Sharma, K.K.; Patil, P.S. Synthesis of Maghemite nanoparticles for highly sensitive and selective NO2 sensing. Mater. Sci. Eng. B 2021, 272, 115339. [Google Scholar] [CrossRef]
- Radhakrishnan, J.K.; Kumara, M. Effect of temperature modulation, on the gas sensing characteristics of ZnO nanostructures, for gases O2, CO and CO2. Sens. Int. 2021, 2, 100059. [Google Scholar] [CrossRef]
- Aishwarya, K.; Nirmala, R.; Navamathavan, R. Recent advancements in liquefied petroleum gas sensors: A topical review. Sens. Int. 2021, 2, 100091. [Google Scholar] [CrossRef]
- Sharafeldin, I.; Garcia-Rios, S.; Ahmed, N.; Alvarado, M.; Vilanova, X.; Allam, N.K. Metal-decorated carbon nanotubes-based sensor array for simultaneous detection of toxic gases. J. Environ. Chem. Eng. 2021, 9, 104534. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Rab, S.; Suman, R. Exploring the potential of nanosensors: A brief overview. Sens. Int. 2021, 2, 100130. [Google Scholar] [CrossRef]
- Priyadarshi, H.; Singh, K.; Shrivastava, A. Experimental study of maghemite nanomaterials towards sustainable energy storage device application. Mater. Sci. Semicond. Processing 2022, 147, 106698. [Google Scholar] [CrossRef]
- Shokrollahi, H. A review of the magnetic properties, synthesis methods and applications of maghemite. J. Magn. Magn. Mater. 2017, 426, 74–81. [Google Scholar] [CrossRef]
- Trushkina, Y.; Tai, C.-W.; Salazar-Alvarez, G. Fabrication of Maghemite Nanoparticles with High Surface Area. Nanomaterials 2019, 9, 1004. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Wu, G.; Wang, L. Peculiar porous α-Fe2O3, γ-Fe2O3 and Fe3O4 nanospheres: Facile synthesis and electromagnetic properties. Powder Technol. 2015, 269, 443–451. [Google Scholar] [CrossRef]
- Kim, W.; Lee, J.S.; Jang, J. Facile synthesis of size-controlled Fe2O3 nanoparticle-decorated carbon nanotubes for highly sensitive H2S detection. RSC Adv. 2018, 8, 31874–31880. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Zhang, S.; Yang, Y.; Yu, H.; Dong, X. Highly sensitive H2S sensors based on metal-organic framework driven γ-Fe2O3 on reduced graphene oxide composites at room temperature. Sens. Actuators B Chem. 2020, 325, 128804. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Wang, Y.; Chen, Z. A Highly Sensitive and Room Temperature CNTs/SnO2/CuO Sensor for H2S Gas Sensing Applications. Nanoscale Res. Lett. 2020, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, S.; Dong, R.; Zhang, L.; Zhu, Z.; Gao, X. One-pot synthesis of SnO2 hollow microspheres and their formaldehyde sensor application. Mater. Lett. 2016, 184, 9–12. [Google Scholar] [CrossRef]
- Wang, T.T.; Ma, S.Y.; Cheng, L.; Jiang, X.H.; Zhang, M.; Li, W.Q.; Jin, W.X. Facile fabrication of multishelled SnO2 hollow microspheres for gas sensing application. Mater. Lett. 2016, 164, 56–59. [Google Scholar] [CrossRef]
- Vaishanv, V.S.; Patel, P.D.; Patel, N.G. Indium Tin Oxide Thin-Film Sensor for Detection of Volatile Organic Compounds (VOCs). Mater. Manuf. Processes 2006, 21, 257–261. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, Z.; Zong, X. Flexible and highly sensitive H2S gas sensor based on in-situ polymerized SnO2/rGO/PANI ternary nanocomposite with application in halitosis diagnosis. Sens. Actuators B Chem. 2019, 289, 32–41. [Google Scholar] [CrossRef]
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z.; Xu, S.; Zhang, W.; Yu, H.; Li, M.; Huang, Z.; Zang, J.; et al. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- He, H. Metal oxide semiconductors and conductors. In Solution Processed Metal Oxide Thin Films for Electronic Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–30. [Google Scholar] [CrossRef]
- Firtat, B.; Moldovan, C.; Brasoveanu, C.; Muscalu, G.; Gartner, M.; Zaharescu, M.; Chesler, P.; Hornoiu, C.; Mihaiu, S.; Vladut, C.; et al. Miniaturised MOX based sensors for pollutant and explosive gases detection. Sens. Actuators B Chem. 2017, 249, 647–655. [Google Scholar] [CrossRef]
- Song, Z.; Liu, J.; Liu, Q.; Yu, H.; Zhang, W.; Wang, Y.; Huang, Z.; Zang, J.; Liu, H. Enhanced H2S gas sensing properties based on SnO2 quantum wire/reduced graphene oxide nanocomposites: Equilibrium and kinetics modeling. Sens. Actuators B Chem. 2017, 249, 632–638. [Google Scholar] [CrossRef]
- Rashad, M.M.; Ibrahim, I.A.; Osama, I.; Shalan, A.E. Distinction between SnO2 nanoparticles synthesized using co-precipitation and solvothermal methods for the photovoltaic efficiency of dye-sensitized solar cells. Bull. Mater. Sci. 2014, 37, 903–909. [Google Scholar] [CrossRef]
- Wang, W.W.; Yao, J.L. Hydrothermal Synthesis of SnO2/Fe3O4 Nanocomposites and Their Magnetic Property. J. Phys. Chem. C 2009, 113, 3070–3075. [Google Scholar] [CrossRef]
- Zou, C.; Hu, J.; Su, Y.; Zhou, Z.; Cai, B.; Tao, Z.; Huo, T.; Hu, N.; Zhang, Y. Highly repeatable and sensitive three-dimensional γ-Fe2O3@reduced graphene oxide gas sensors by magnetic-field assisted assembly process. Sens. Actuators B Chem. 2020, 306, 127546. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Chen, D.; Yin, L.; Ge, L.; Fan, B.; Zhang, R.; Sun, J.; Shao, G. Low-temperature and highly selective NO-sensing performance of WO3 nanoplates decorated with silver nanoparticles. Sens. Actuators B Chem. 2013, 185, 445–455. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Yamazoe, N.; Shimanoe, K. Fundamentals of semiconductor gas sensors. In Semiconductor Gas Sensors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 3–34. [Google Scholar] [CrossRef]
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.C.; Miller, D.; Oralkan, O.; Hesketh, P.J.; et al. Editors’ Choice—Critical Review—A Critical Review of Solid State Gas Sensors. J. Electrochem. Soc. 2020, 167, 037570. [Google Scholar] [CrossRef]
- Madrid, S.I.U.; Pal, U.; Sanchez-De Jesus, F. Controlling size and magnetic properties of Fe3O4 clusters in solvothermal process. Adv. Nano Res. 2014, 2, 187–198. [Google Scholar] [CrossRef] [Green Version]
- De Villiers, J.P.; Lu, L. Quantitative XRD analysis and evaluation of iron ore, sinter, and pellets. In Iron Ore; Elsevier: Amsterdam, The Netherlands, 2022; pp. 109–126. [Google Scholar] [CrossRef]
- Schroder, D.K. Semiconductor Material and Device Characterization, 3rd ed.; IEEE Press: Hoboken, NJ, USA; Wiley: Piscataway, NJ, USA, 2006. [Google Scholar]
- Zuo, J.; Tavakoli, S.; Mathavakrishnan, D.; Ma, T.; Lim, M.; Rotondo, B.; Pauzauskie, P.; Pavinatto, F.; MacKenzie, D. Additive Manufacturing of a Flexible Carbon Monoxide Sensor Based on a SnO2-Graphene Nanoink. Chemosensors 2020, 8, 36. [Google Scholar] [CrossRef]
- Choi, K.S.; Park, S.; Chang, S.-P. Enhanced ethanol sensing properties based on SnO2 nanowires coated with Fe2O3 nanoparticles. Sens. Actuators B Chem. 2017, 238, 871–879. [Google Scholar] [CrossRef]
- Ye, Z.; Yang, W.; Yuan, Z.; Zhang, K.; Tai, H. Facile depositing strategy to fabricate a hetero-affinity hybrid film for improving gas-sensing performance. Nanotechnology 2021, 32, 205502. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, V.; Sureshkumar, S.; Rao, T.S.; Sridhar, T.M. Impedance Spectroscopy-Based Reduced Graphene Oxide-Incorporated ZnO Composite Sensor for H2S Investigations. ACS Omega 2019, 4, 9976–9982. [Google Scholar] [CrossRef] [Green Version]
- Wei, Q.; Song, P.; Yang, Z.; Wang, Q. Hierarchical assembly of Fe2O3 nanorods on SnO2 nanospheres with enhanced ethanol sensing properties. Phys. E Low-Dimens. Syst. Nanostructures 2018, 103, 156–163. [Google Scholar] [CrossRef]
- Sowmya, B.; John, A.; Panda, P.K. A review on metal-oxide based p-n and n-n heterostructured nano-materials for gas sensing applications. Sens. Int. 2021, 2, 100085. [Google Scholar] [CrossRef]
- Bo, Z.; Wei, X.; Guo, X.; Yang, H.; Mao, S.; Yan, J.; Cen, K. SnO2 nanoparticles incorporated CuO nanopetals on graphene for high-performance room-temperature NO2 sensor. Chem. Phys. Lett. 2020, 750, 137485. [Google Scholar] [CrossRef]


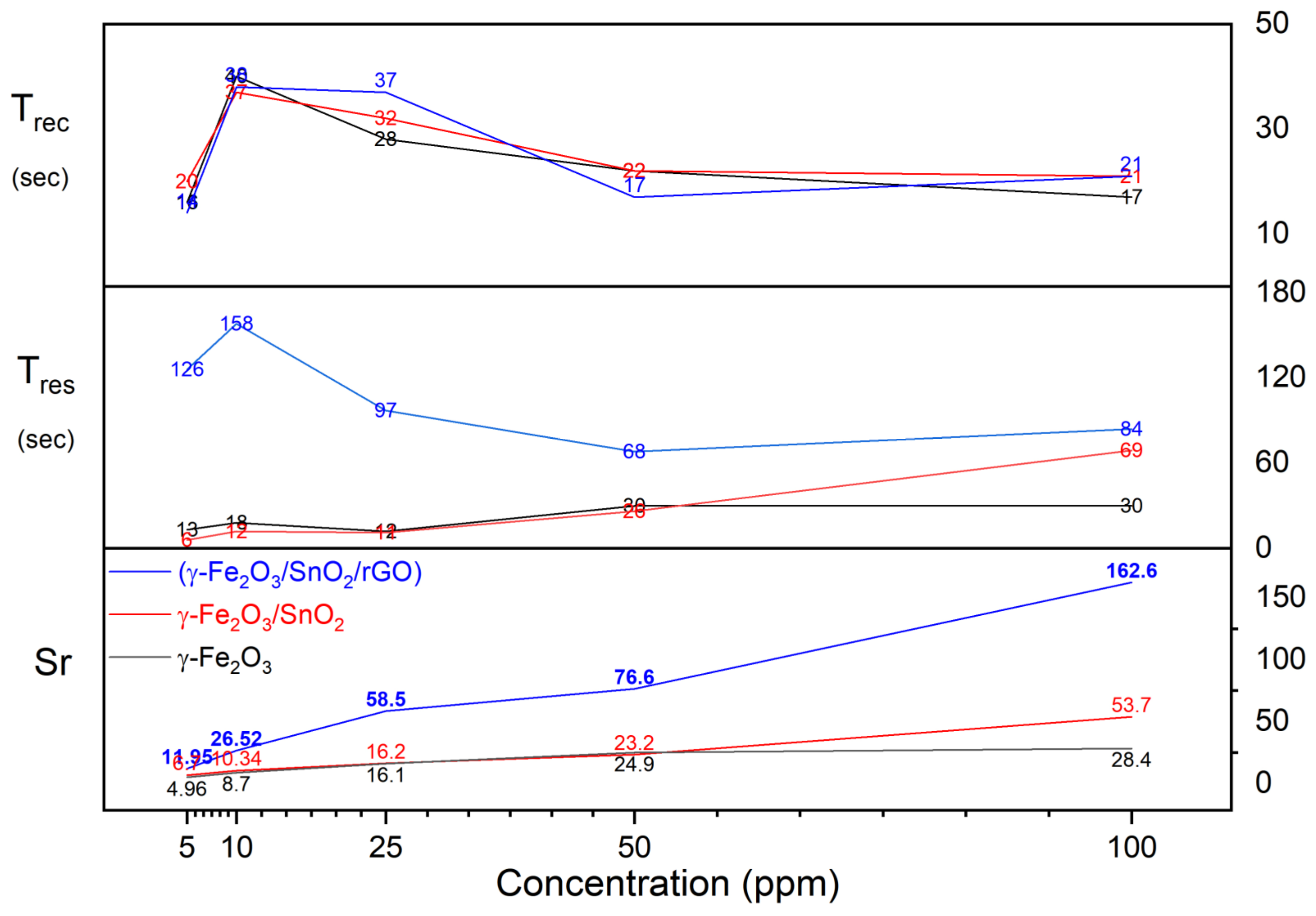
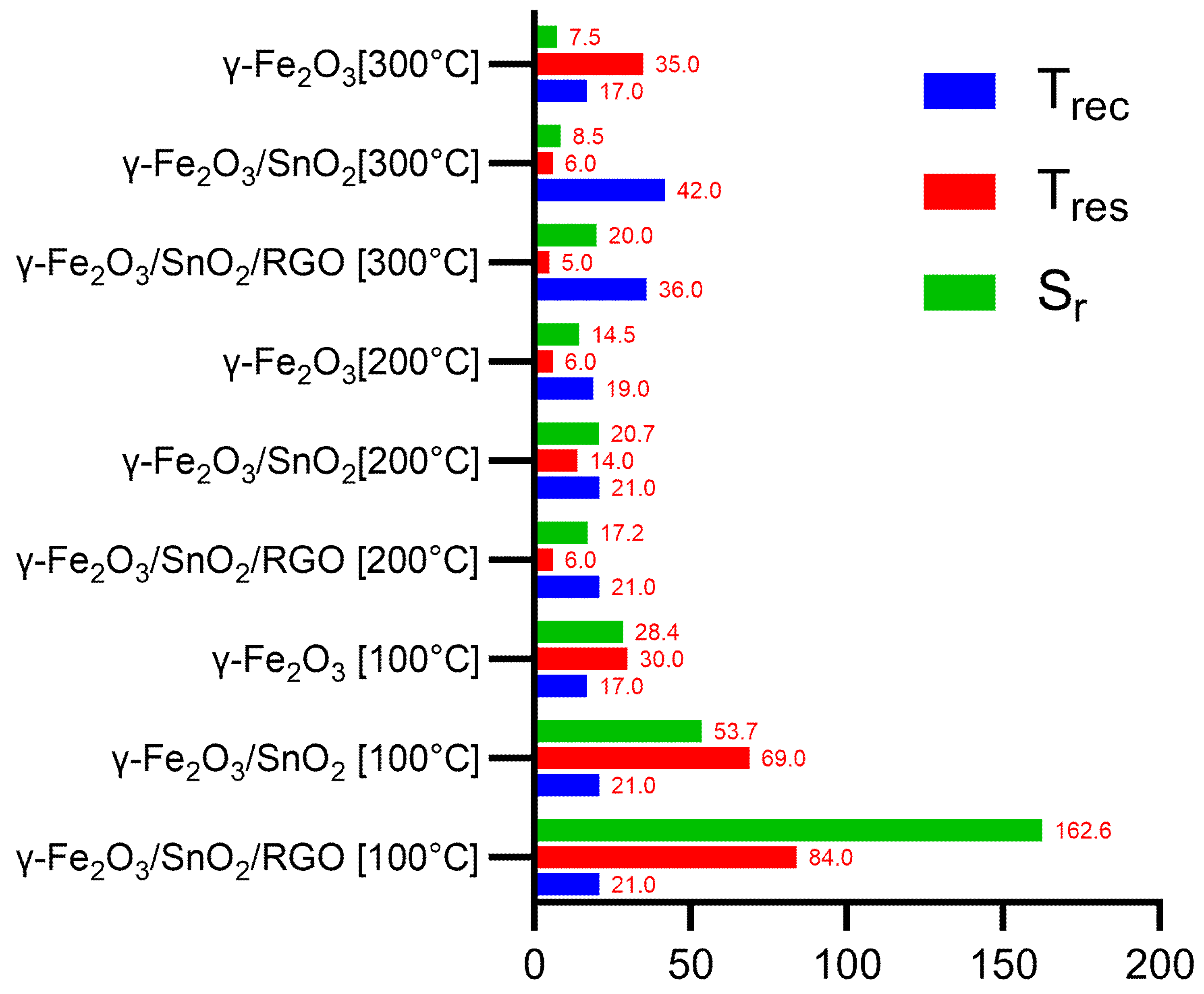
| Organic Liquids | Concentration (ppm) | Volume (μL) | |
|---|---|---|---|
| 1 | Methanol | 100 | 3.21 |
| 2 | Ethanol | 100 | 4.63 |
| 3 | Isopropyl alcohol | 100 | 6.08 |
| 4 | Formaldehyde | 100 | 2.2 |
| Gases | Concentration (ppm) | Volume (mL) | |
| 1 | CO | 100 | 1.8 |
| 2 | NO | 100 | 1.8 |
| 3 | H2S | 5 | 0.09 |
| 10 | 0.18 | ||
| 25 | 0.45 | ||
| 50 | 0.9 | ||
| 100 | 1.8 |
| Gas | Compound | Temperature | Sr | Tres (s) | Trec (s) |
|---|---|---|---|---|---|
| Methanol | γ-Fe2O3 | 100 °C | - | - | - |
| γ-Fe2O3/SnO2 | - | - | - | ||
| γ-Fe2O3/SnO2/RGO | 6.4 | 15 | 14 | ||
| γ-Fe2O3 | 160 °C | 1.9 | 22 | 32 | |
| γ-Fe2O3/SnO2 | - | - | - | ||
| γ-Fe2O3/SnO2/RGO | 2.8 | 23 | 24 | ||
| γ-Fe2O3 | 200 °C | 5.3 | 18 | 34 | |
| γ-Fe2O3/SnO2 | - | - | - | ||
| γ-Fe2O3/SnO2/RGO | 7.9 | 12 | 18 | ||
| Ethanol | γ-Fe2O3 | 100 °C | - | - | - |
| γ-Fe2O3/SnO2 | - | - | - | ||
| γ-Fe2O3/SnO2/RGO | 5.3 | 19 | 16 | ||
| γ-Fe2O3 | 160 °C | 3.6 | 21 | 16 | |
| γ-Fe2O3/SnO2 | 5.0 | 25 | 11 | ||
| γ-Fe2O3/SnO2/RGO | 10.1 | 27 | 15 | ||
| γ-Fe2O3 | 200 °C | 16.4 | 40 | 107 | |
| γ-Fe2O3/SnO2 | 7.0 | 48 | 27 | ||
| γ-Fe2O3/SnO2/RGO | 10.3 | 41 | 24 | ||
| Isopropyl alcohol | γ-Fe2O3 | 100 °C | - | - | - |
| γ-Fe2O3/SnO2 | - | - | - | ||
| γ-Fe2O3/SnO2/RGO | 16.2 | 33 | 21 | ||
| γ-Fe2O3 | 160 °C | 2.56 | 38 | 15 | |
| γ-Fe2O3/SnO2 | 10.6 | 36 | 34 | ||
| γ-Fe2O3/SnO2/RGO | 5.0 | 41 | 17 | ||
| γ-Fe2O3 | 200 °C | 22.16 | 39 | 31 | |
| γ-Fe2O3/SnO2 | 10.9 | 46 | 58 | ||
| γ-Fe2O3/SnO2/RGO | 13.1 | 38 | 19 | ||
| Formaldehyde | γ-Fe2O3 | 100 °C | - | - | - |
| γ-Fe2O3/SnO2 | - | - | - | ||
| γ-Fe2O3/SnO2/RGO | 4.5 | 22 | 20 | ||
| γ-Fe2O3 | 160 °C | - | - | - | |
| γ-Fe2O3/SnO2 | - | - | - | ||
| γ-Fe2O3/SnO2/RGO | 3.9 | 50 | 15 | ||
| γ-Fe2O3 | 200 °C | 4.4 | 39 | 58 | |
| γ-Fe2O3/SnO2 | 3.5 | 31 | 11 | ||
| γ-Fe2O3/SnO2/RGO | 4.8 | 34 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, Q.; Olimov, D.; Yin, L. Semiconductor-Type Gas Sensors Based on γ-Fe2O3 Nanoparticles and Its Derivatives in Conjunction with SnO2 and Graphene. Chemosensors 2022, 10, 267. https://doi.org/10.3390/chemosensors10070267
Qin Q, Olimov D, Yin L. Semiconductor-Type Gas Sensors Based on γ-Fe2O3 Nanoparticles and Its Derivatives in Conjunction with SnO2 and Graphene. Chemosensors. 2022; 10(7):267. https://doi.org/10.3390/chemosensors10070267
Chicago/Turabian StyleQin, Qi, Diyor Olimov, and Li Yin. 2022. "Semiconductor-Type Gas Sensors Based on γ-Fe2O3 Nanoparticles and Its Derivatives in Conjunction with SnO2 and Graphene" Chemosensors 10, no. 7: 267. https://doi.org/10.3390/chemosensors10070267
APA StyleQin, Q., Olimov, D., & Yin, L. (2022). Semiconductor-Type Gas Sensors Based on γ-Fe2O3 Nanoparticles and Its Derivatives in Conjunction with SnO2 and Graphene. Chemosensors, 10(7), 267. https://doi.org/10.3390/chemosensors10070267






