Pd-GaSe and Pd3-GaSe Monolayers: Two Promising Candidates for Detecting Dissolved Gases in Transformer Oil
Abstract
:1. Introduction
2. Computation Methods
3. Results and Discussion
3.1. Structures of Characteristic Gases, Pd-GaSe, and Pd3-GaSe Monolayers
3.2. H2 Adsorption
3.3. CO Adsorption
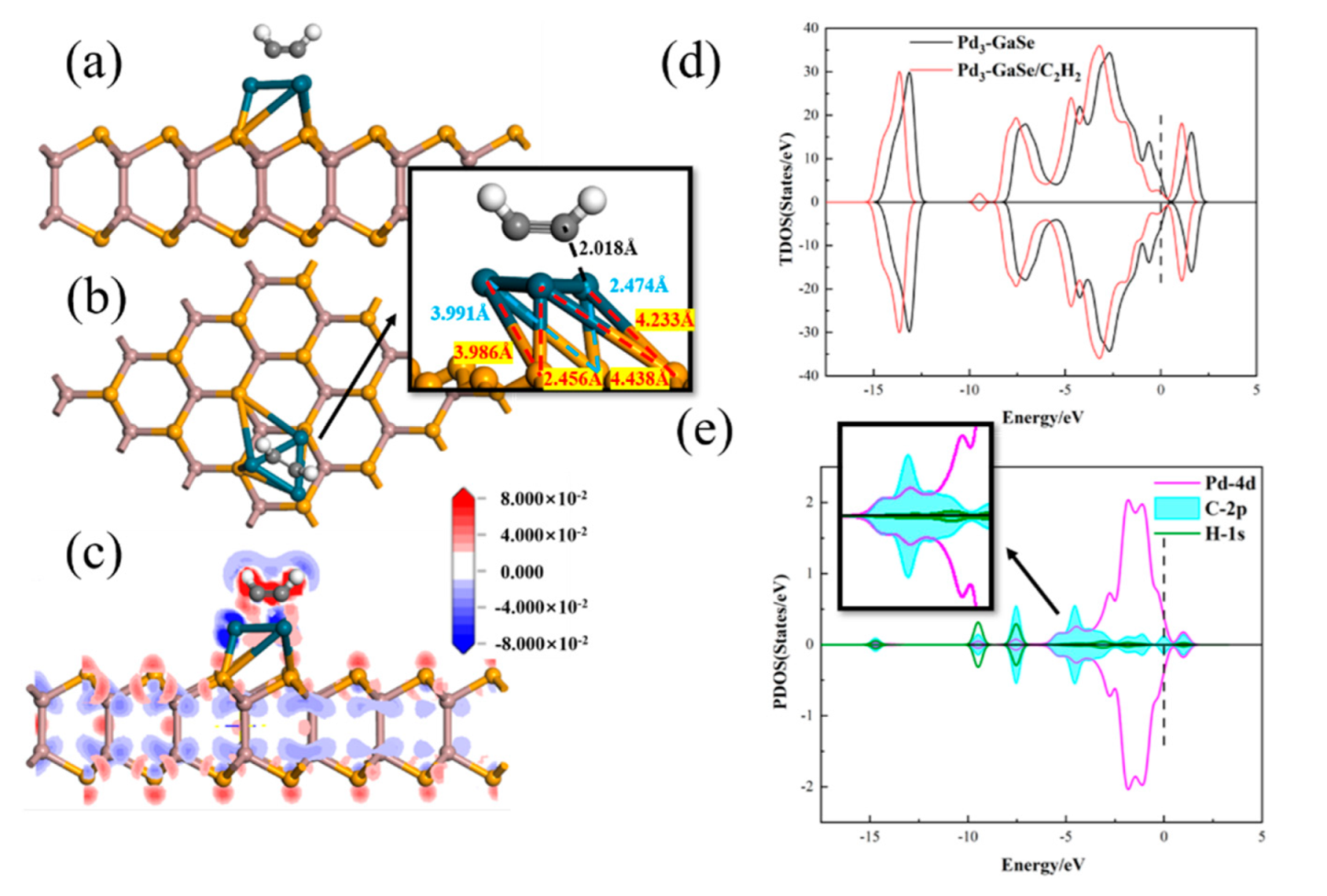
3.4. C2H2 Adsorption
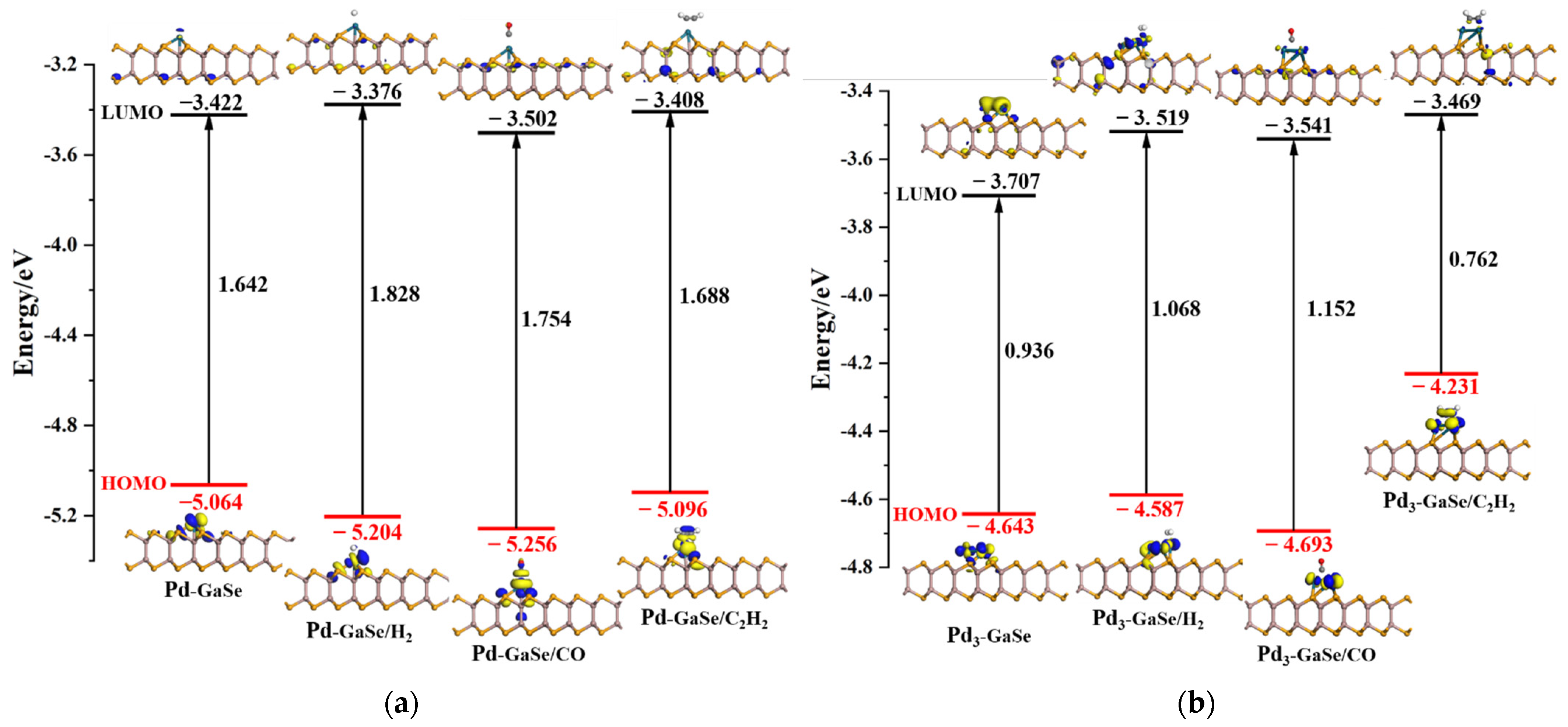
3.5. Frontier Orbital Theory Analysis
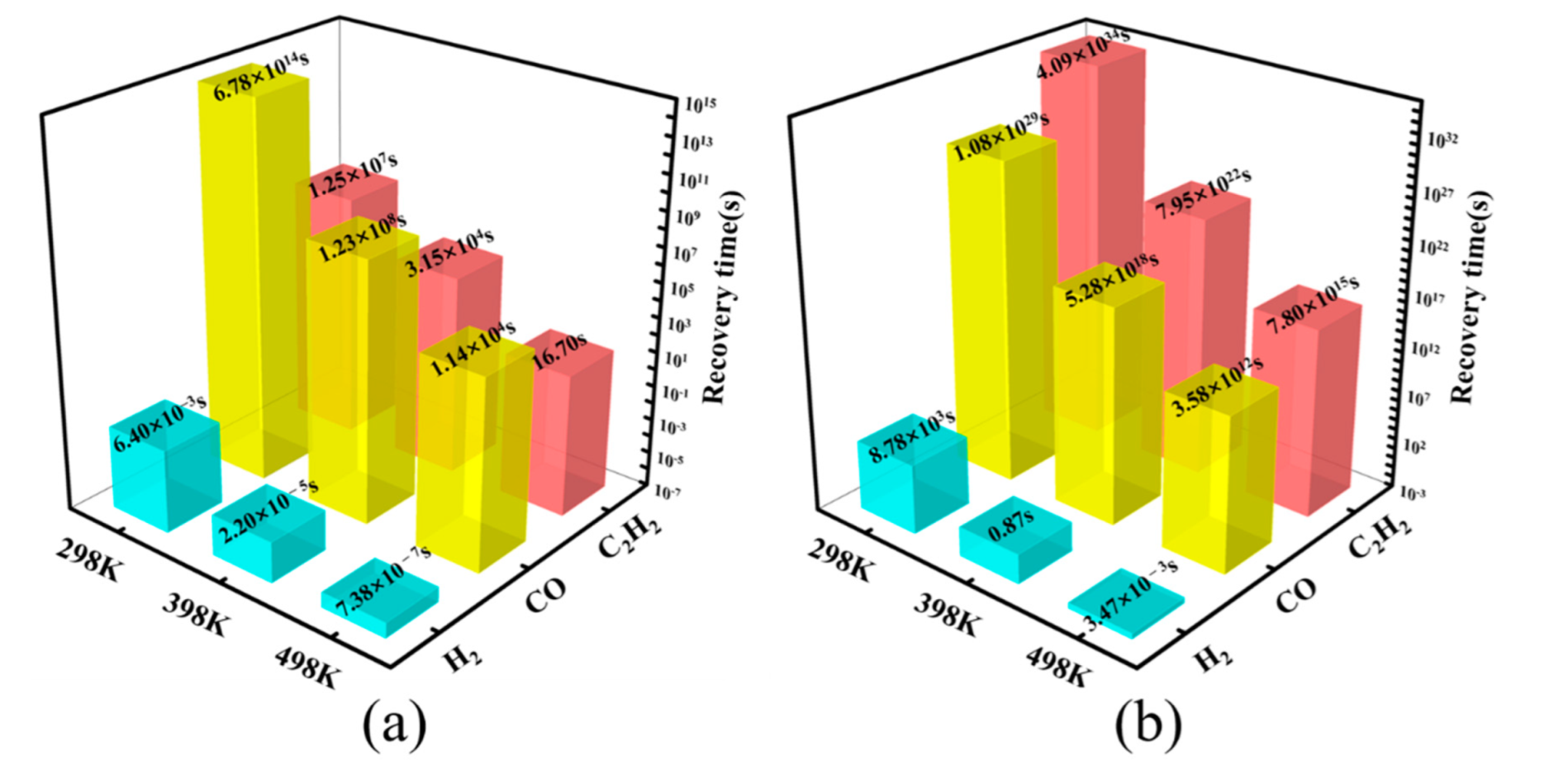
3.6. Analysis of Recovery Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tenbohlen, S.; Coenen, S.; Djamali, M.; Muller, A.; Samimi, M.H.; Siegel, M. Diagnostic Measurements for Power Transformers. Energies 2016, 9, 347. [Google Scholar] [CrossRef]
- Wang, J.C.; Zhang, X.X.; Liu, L.; Wang, Z.T. Dissolved gas analysis in transformer oil using Ni-Doped GaN monolayer: A DFT study. Superlattices Microstruct. 2021, 159, 107055. [Google Scholar] [CrossRef]
- Alsuhaibani, S.; Khan, Y.; Beroual, A.; Malik, N.H. A Review of Frequency Response Analysis Methods for Power Transformer Diagnostics. Energies 2016, 9, 879. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, S.; Manana, M.; Arroyo, A.; Castro, P.; Laso, A.; Martinez, R. Dissolved Gas Analysis Equipment for Online Monitoring of Transformer Oil: A Review. Sensors 2019, 19, 4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Gui, Y.G.; Xie, J.F.; Liu, X.; Wang, Q.; Tang, C. A DFT study of dissolved gas (C2H2, H2, CH4) detection in oil on CuO-modified BNNT. Appl. Surf. Sci. 2020, 500, 144030. [Google Scholar] [CrossRef]
- Singh, S.; Bandyopadhyay, M.N. Dissolved Gas Analysis Technique for Incipient Fault Diagnosis in Power Transformers: A Bibliographic Survey. IEEE Electr. Insul. Mag. 2010, 26, 41–46. [Google Scholar] [CrossRef]
- Yang, X.; Wu, X.; Li, J. Recent advances in screening two-dimensional materials for high-performance energy storage and conversion devices based on electronic structure theory. Chin. Sci. Bull. Chin. 2021, 66, 640–656. [Google Scholar] [CrossRef]
- Zhou, Z.X.; Yap, Y.K. Two-Dimensional Electronics and Optoelectronics: Present and Future. Electronics 2017, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Bag, A.; Lee, N.E. Gas sensing with heterostructures based on two-dimensional nanostructured materials: A review. J. Mater. Chem. C 2019, 7, 13367–13383. [Google Scholar] [CrossRef]
- Chaudhari, N.K.; Jin, H.; Kim, B.; Baek, D.S.; Joo, S.H.; Lee, K. MXene: An emerging two-dimensional material for future energy conversion and storage applications. J. Mater. Chem. A 2017, 5, 24564–24579. [Google Scholar] [CrossRef]
- Bao, J.; Jeppson, K.; Edwards, M.; Fu, Y.F.; Ye, L.L.; Lu, X.Z.; Liu, J. Synthesis and Applications of Two-Dimensional Hexagonal Boron Nitride in Electronics Manufacturing. Electron. Mater. Lett. 2016, 12, 1–16. [Google Scholar] [CrossRef]
- Yang, T.; Cui, Y.N.; Chen, H.Y.; Li, W.H. Controllable Preparation of Two Dimensional Metal- or Covalent Organic Frameworks for Chemical Sensing and Biosensing. Acta Chim. Sin. 2017, 75, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Abu Bakar, N.; Abu-Siada, A.; Islam, S. A Review of Dissolved Gas Analysis Measurement and Interpretation Techniques. IEEE Electr. Insul. Mag. 2014, 30, 39–49. [Google Scholar] [CrossRef]
- Cilliyuz, Y.; Bicen, Y.; Aras, F.; Aydugan, G. Measurements and performance evaluations of natural ester and mineral oil-immersed identical transformers. Int. J. Electr. Power Energy Syst. 2021, 125, 106517. [Google Scholar] [CrossRef]
- Faiz, J.; Soleimani, M. Dissolved Gas Analysis Evaluation in Electric Power Transformers using Conventional Methods a Review. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1239–1248. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Park, J.; Lee, K. MEMS hydrogen gas sensor for in-situ monitoring of hydrogen gas in transformer oil. Sens. Actuators B Chem. 2021, 326, 128989. [Google Scholar] [CrossRef]
- Qian, G.C.; Peng, Q.J.; Zou, D.X.; Wang, S.; Yan, B.; Zhou, Q. First-Principles Insight into Au-Doped MoS2 for Sensing C2H6 and C2H4. Front. Mater. 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, S.; Bafghi, Z.G.; Manavizadeh, N.; Kordmahale, S.B. Highly Sensitive Detection of Dissolved Gases in Transformer Oil with Carbon-Doped ZnO Nanotube: A DFT Study. IEEE Sens. J. 2022, 22, 82–89. [Google Scholar] [CrossRef]
- Mi, H.W.; Zhou, Q.; Zeng, W. A density functional theory study of the adsorption of Cl2, NH3, and NO2 on Ag3-doped WSe2 monolayers. Appl. Surf. Sci. 2021, 563, 150329. [Google Scholar] [CrossRef]
- Yuan, T.; Fu, C.; Gong, Y.J.; Tong, Y.; Zhang, J.; Wang, Y.Q. First-principles Insights into Cu-Decorated GaN Monolayers for Sensing CO and HCHO in Dry-Type Transformers. ACS Omega 2021, 6, 19127–19133. [Google Scholar] [CrossRef]
- Hu, P.A.; Wen, Z.Z.; Wang, L.F.; Tan, P.H.; Xiao, K. Synthesis of Few-Layer GaSe Nanosheets for High Performance Photodetectors. ACS Nano 2012, 6, 5988–5994. [Google Scholar] [CrossRef]
- Late, D.J.; Liu, B.; Luo, J.J.; Yan, A.M.; Matte, H.; Grayson, M.; Rao, C.N.R.; Dravid, V.P. GaS and GaSe Ultrathin Layer Transistors. Adv. Mater. 2012, 24, 3549–3554. [Google Scholar] [CrossRef]
- Zhuang, H.L.L.; Hennig, R.G. Single-Layer Group-III Monochalcogenide Photocatalysts for Water Splitting. Chem. Mater. 2013, 25, 3232–3238. [Google Scholar] [CrossRef]
- Jie, W.J.; Chen, X.; Li, D.; Xie, L.; Hui, Y.Y.; Lau, S.P.; Cui, X.D.; Hao, J.H. Layer-Dependent Nonlinear Optical Properties and Stability of Non-Centrosymmetric Modification in Few-Layer GaSe Sheets. Angew. Chem. Int. Ed. 2015, 54, 1185–1189. [Google Scholar] [CrossRef]
- Zhou, C.J.; Zhu, H.L.; Wu, Y.P.; Lin, W.; Yang, W.H.; Dong, L.X. Effect of external strain on the charge transfer: Adsorption of gas molecules on monolayer GaSe. Mater. Chem. Phys. 2017, 198, 49–56. [Google Scholar] [CrossRef]
- Chen, D.C.; Zhang, X.X.; Tang, J.; Cui, H.; Li, Y. Noble metal (Pt or Au)-doped monolayer MoS2 as a promising adsorbent and gas-sensing material to SO2, SOF2 and SO2F2: A DFT study. Appl. Phys. A 2018, 124, 194. [Google Scholar] [CrossRef]
- Chen, G.X.; Li, H.F.; Wang, D.D.; Li, S.Q.; Fan, X.B.; Zhang, J.M. Adsorption of toxic gas molecules on pristine and transition metal doped hexagonal GaN monolayer: A first-principles study. Vacuum 2019, 165, 35–45. [Google Scholar] [CrossRef]
- Ni, J.M.; Quintana, M.; Song, S.X. Adsorption of small gas molecules on transition metal (Fe, Ni and Co, Cu) doped graphene: A systematic DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 116, 113768. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Zhang, T.; He, Q.Q.; Bi, M.Q.; Chen, X.; Zhou, X. Adsorption performance and gas-sensing properties of V-GaSe to SF6 decomposition components in gas-insulated switchgear. Appl. Surf. Sci. 2022, 577, 151854. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Zhang, G.Z.; Tang, J. Pd-doped MoS2 monolayer: A promising candidate for DGA in transformer oil based on DFT method. Appl. Surf. Sci. 2019, 470, 1035–1042. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.M.; Xu, K.W.; Ji, V. A first-principles study on gas sensing properties of graphene and Pd-doped graphene. Appl. Surf. Sci. 2015, 343, 121–127. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.F.; Peng, X.Y. Adsorption of SO2 and NO2 molecule on intrinsic and Pd-doped HfSe2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 513, 145863. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Li, A.; Liu, X.S.; Ma, C.Z.; Li, X.; Yang, W.J.; Ding, X.L. Density functional study of the adsorption of NO on Ni-n (n=1, 2, 3 and 4) clusters doped functionalized graphene support. Appl. Surf. Sci. 2019, 481, 940–950. [Google Scholar] [CrossRef]
- Cui, H.; Jia, P.F. Doping effect of small Rhn (n = 1–4) clusters on the geometric and electronic behaviors of MoS2 monolayer: A first-principles study. Appl. Surf. Sci. 2020, 526, 146659. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Lee, S.G.; Choi, J.I.; Koh, W.; Jang, S.S. Adsorption of beta-D-glucose and cellobiose on kaolinite surfaces: Density functional theory (DFT) approach. Appl. Clay Sci. 2013, 71, 73–81. [Google Scholar] [CrossRef]
- Tkatchenko, A.; DiStasio, R.A.; Head-Gordon, M.; Scheffler, M. Dispersion-corrected Moller-Plesset second-order perturbation theory. J. Chem. Phys. 2009, 131, 094106. [Google Scholar] [CrossRef]
- Zeng, F.P.; Feng, X.X.; Chen, X.Y.; Yao, Q.; Miao, Y.L.; Dai, L.J.; Li, Y.; Tang, J. First-principles analysis of Ti3C2Tx MXene as a promising candidate for SF6 decomposition characteristic components sensor. Appl. Surf. Sci. 2022, 578, 152020. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, X.X.; Chen, D.C.; Tang, J. Adsorption mechanism of SF6 decomposed species on pyridine-like PtN3 embedded CNT: A DFT study. Appl. Surf. Sci. 2018, 447, 594–598. [Google Scholar] [CrossRef]
- Gao, X.; Zhou, Q.; Wang, J.X.; Xu, L.N.; Zeng, W. DFT Study on the Selective Adsorption Properties of Modified Graphene for SF6 Decompositions. IEEE Sens. J. 2021, 21, 3193–3200. [Google Scholar] [CrossRef]
- Pakornchote, T.; Ektarawong, A.; Alling, B.; Pinsook, U.; Tancharakorn, S.; Busayaporn, W.; Bovornratanaraks, T. Phase stabilities and vibrational analysis of hydrogenated diamondized bilayer graphenes: A first principles investigation. Carbon 2019, 146, 468–475. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Mom, R.V.; Cheng, J.; Koper, M.T.M.; Sprik, M. Modeling the Oxygen Evolution Reaction on Metal Oxides: The Infuence of Unrestricted DFT Calculations. J. Phys. Chem. C 2014, 118, 4095–4102. [Google Scholar] [CrossRef]
- Li, B.L.; Zhou, Q.; Peng, R.C.; Liao, Y.M.; Zeng, W. Adsorption of SF6 decomposition gases (H2S, SO2, SOF2 and SO2F2) on Sc-doped MoS2 surface: A DFT study. Appl. Surf. Sci. 2021, 549, 149271. [Google Scholar]
- Zhang, X.X.; Wang, J.C.; Chen, D.C.; Liu, L. The adsorption performance of harmful gas on Cu doped WS2: A first-principle study. Mater. Today Commun. 2021, 28, 102488. [Google Scholar] [CrossRef]
- Chen, D.C.; Li, Y.; Xiao, S.; Yang, C.X.; Zhou, J.; Xiao, B.B. Single Ni atom doped WS2 monolayer as sensing substrate for dissolved gases in transformer oil: A first-principles study. Appl. Surf. Sci. 2022, 579, 152141. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cui, H.; Zhang, J.; Tang, J. Adsorption characteristic of Pd-4 cluster carbon nanotube towards transformer oil dissolved components: A simulation. Appl. Surf. Sci. 2017, 419, 802–810. [Google Scholar] [CrossRef]
- Xiao, Z.; Wu, W.; Wu, X.W.; Zhang, Y.F. Adsorption of NO2 on monolayer MoS2 doped with Fe, Co, and Ni, Cu: A computational investigation. Chem. Phys. Lett. 2020, 755, 137768. [Google Scholar] [CrossRef]
- Ye, X.; Jiang, X.; Chen, L.; Jiang, W.J.; Wang, H.L.; Cen, W.L.; Ma, S.G. Effect of manganese dioxide crystal structure on adsorption of SO2 by DFT and experimental study. Appl. Surf. Sci. 2020, 521, 146477. [Google Scholar] [CrossRef]
- Cui, H.; Guo, Y.X.; Zhao, Q.; Zhang, G.Z. Pd-doped PtSe2 monolayer with strain-modulated effect for sensing SF6 decomposed species: A first-principles study. J. Mater. Res. Technol. 2022, 18, 629–636. [Google Scholar] [CrossRef]
- Gui, X.X.; Zhou, Q.; Peng, S.D.; Xu, L.N.; Zeng, W. Adsorption behavior of Rh -doped MoS2 monolayer towards SO2, SOF2, SO 2F2 based on DFT study. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 122, 114224. [Google Scholar] [CrossRef]
- Zhang, X.X.; Cui, H.; Dong, X.C.; Chen, D.C.; Tang, J. Adsorption performance of Rh decorated SWCNT upon SF6 decomposed components based on DFT method. Appl. Surf. Sci. 2017, 420, 825–832. [Google Scholar] [CrossRef]
- Liu, D.K.; Gui, Y.G.; Ji, C.; Tang, C.; Zhou, Q.; Li, J.; Zhang, X.X. Adsorption of SF6 decomposition components over Pd (111): A density functional theory study. Appl. Surf. Sci. 2019, 465, 172–179. [Google Scholar] [CrossRef]
- Gui, X.X.; Zhou, Q.; Peng, S.D.; Xu, L.N.; Zeng, W. Dissolved gas analysis in transformer oil using Sb-doped graphene: A DFT study. Appl. Surf. Sci. 2020, 533, 147509. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zhou, Q.; Hou, W.J.; Li, J.; Zeng, W. Adsorption properties of Cr modified GaN monolayer for H2, CO, C2H2 and C2H4. Chem. Phys. 2021, 550, 111304. [Google Scholar] [CrossRef]
- Liao, Y.M.; Zhou, Q.; Hou, W.J.; Li, J.; Zeng, W. Theoretical study of dissolved gas molecules in transformer oil adsorbed on intrinsic and Cr-doped InP3 monolayer. Appl. Surf. Sci. 2021, 561, 149816. [Google Scholar] [CrossRef]
- Li, Z.H.; Jia, L.F.; Chen, J.X.; Cui, X.S.; Zeng, W.; Zhou, Q. Ag-modified hexagonal GaN monolayer as an innovative gas detector toward SF6 decomposed species: Insights from the first-principles computations. Appl. Surf. Sci. 2022, 589, 153000. [Google Scholar] [CrossRef]
- Boerner, E.D.; Bertram, H.N. Non-Arrhenius behavior in single domain particles. IEEE Trans. Magn. 1998, 34, 1678–1680. [Google Scholar] [CrossRef]
- Lee, J.M.; Lim, S.H. Thermally activated magnetization switching in a nanostructured synthetic ferrimagnet. J. Appl. Phys. 2013, 113, 063914. [Google Scholar]
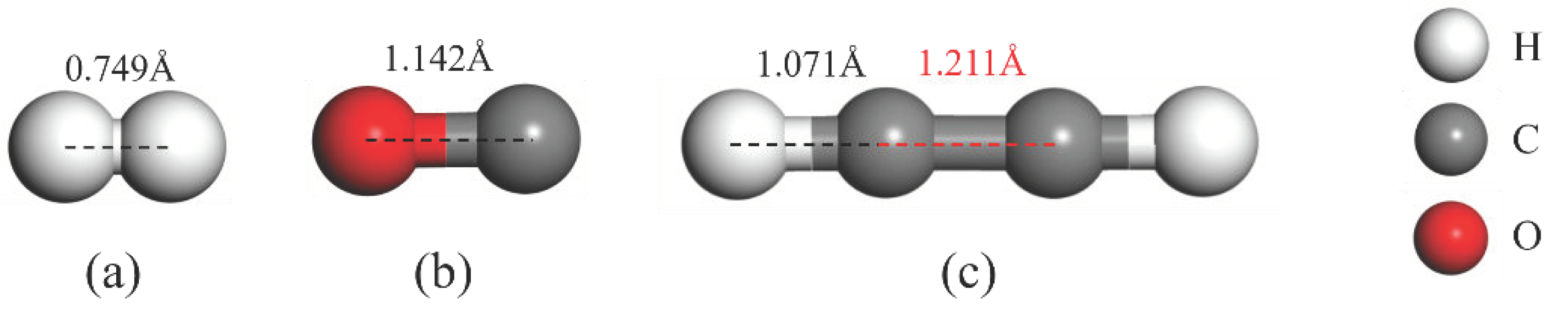

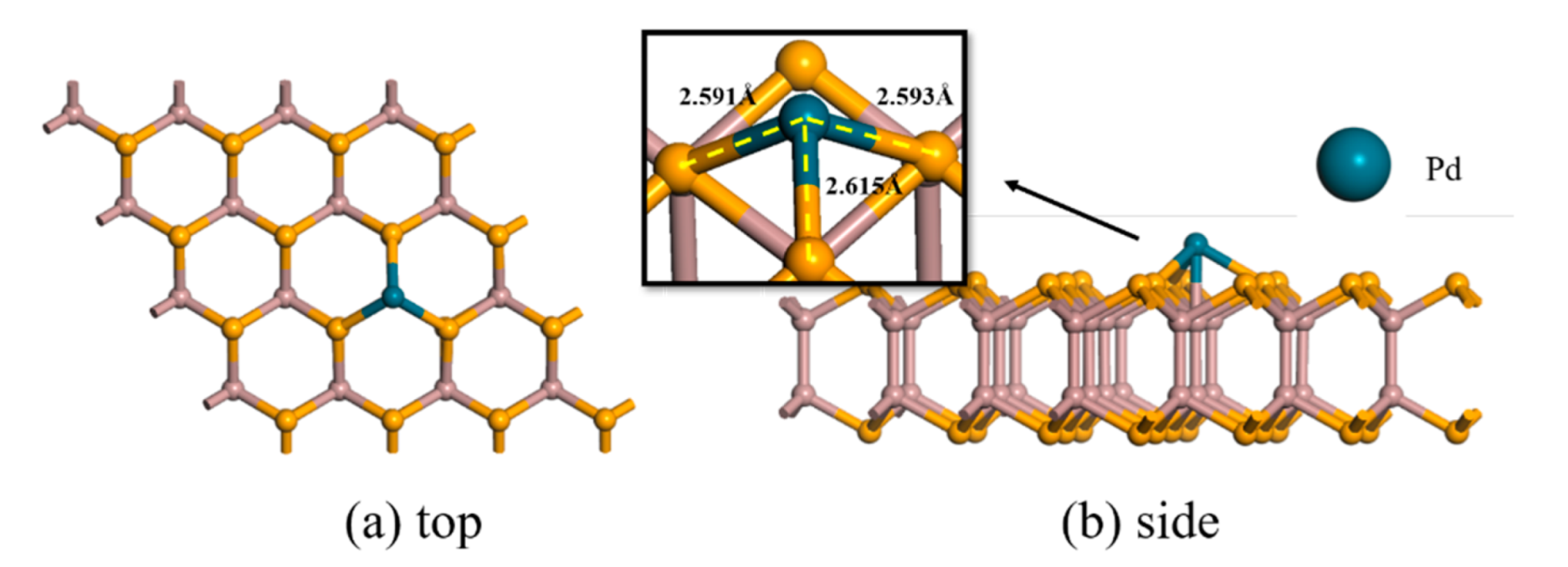


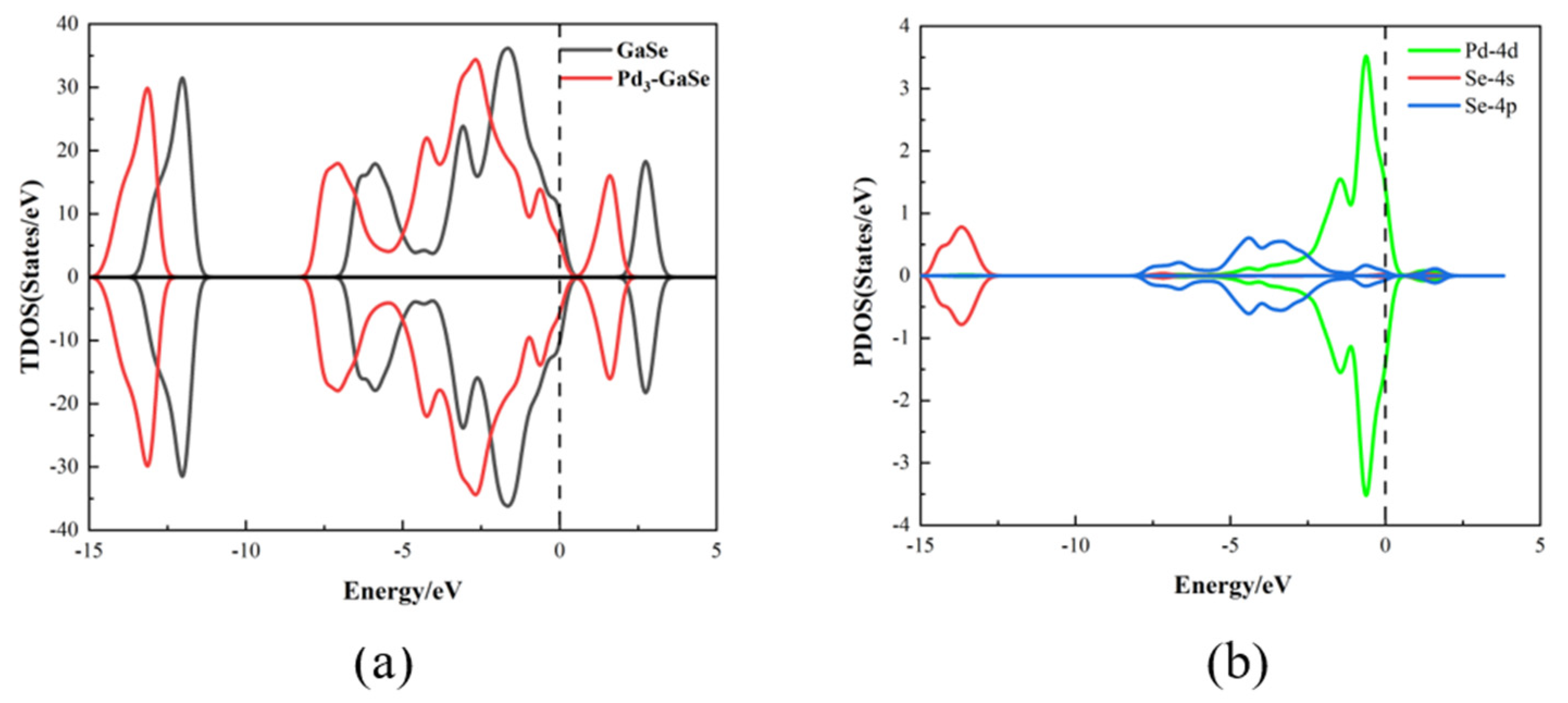
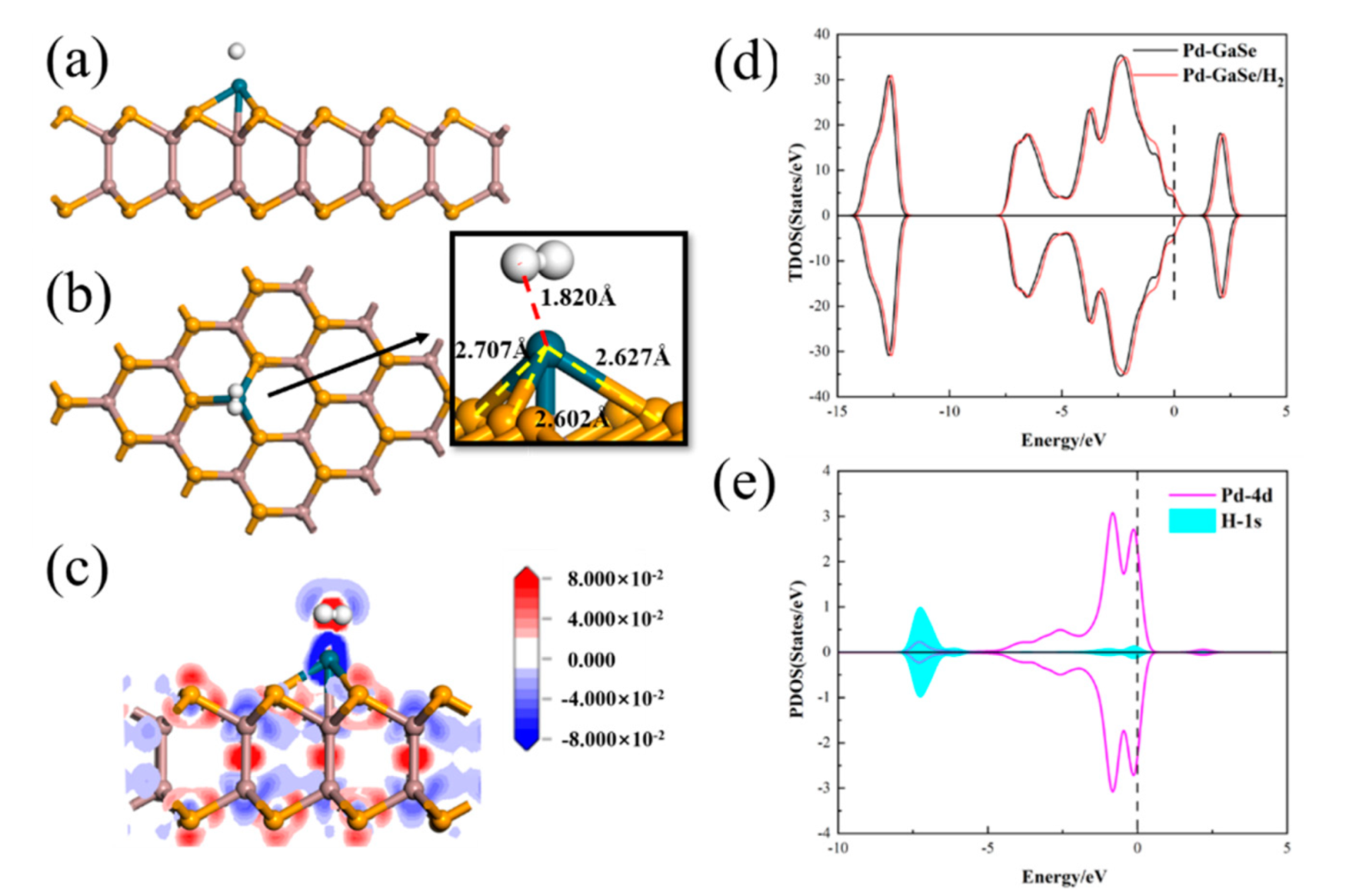
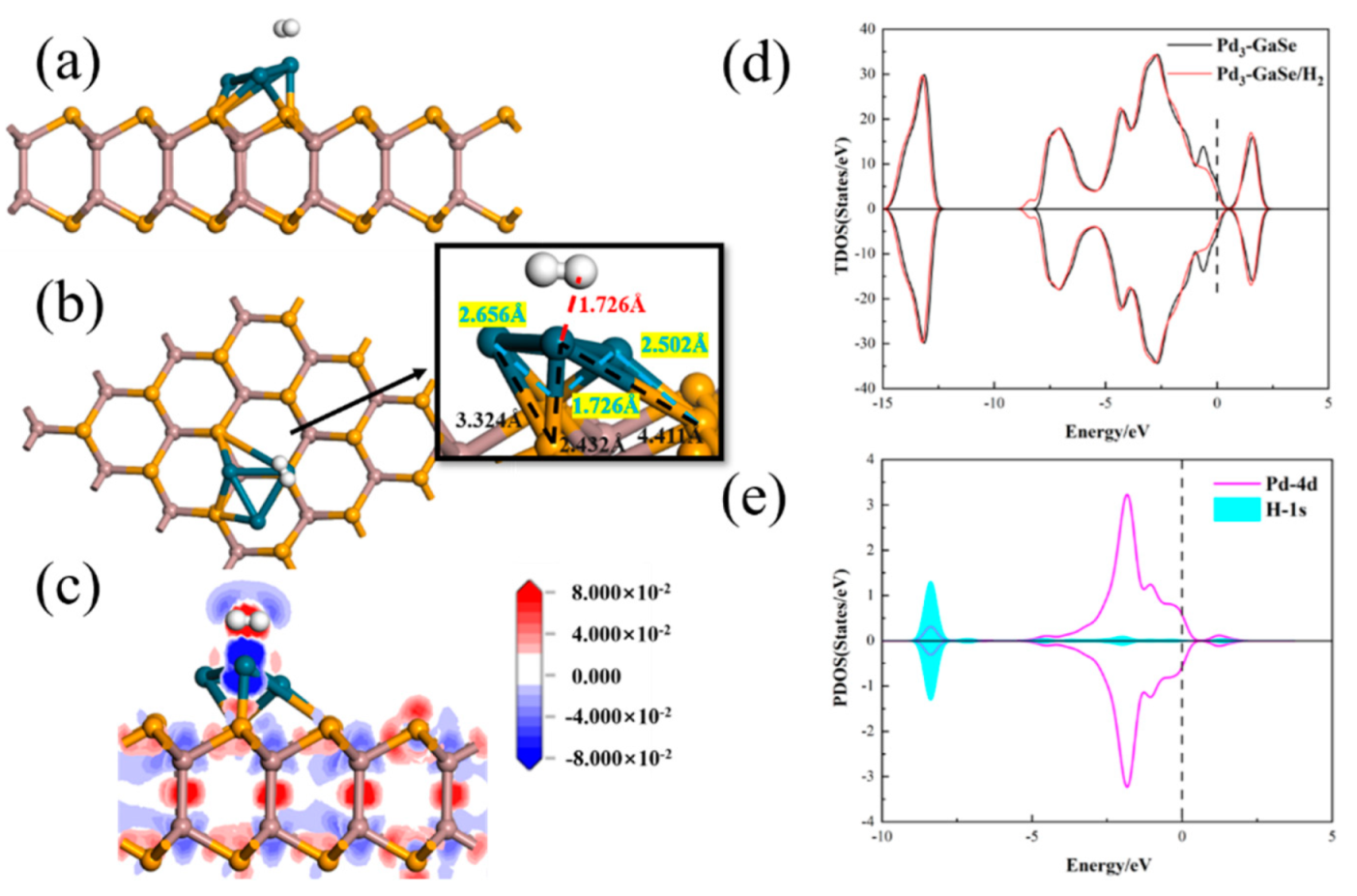
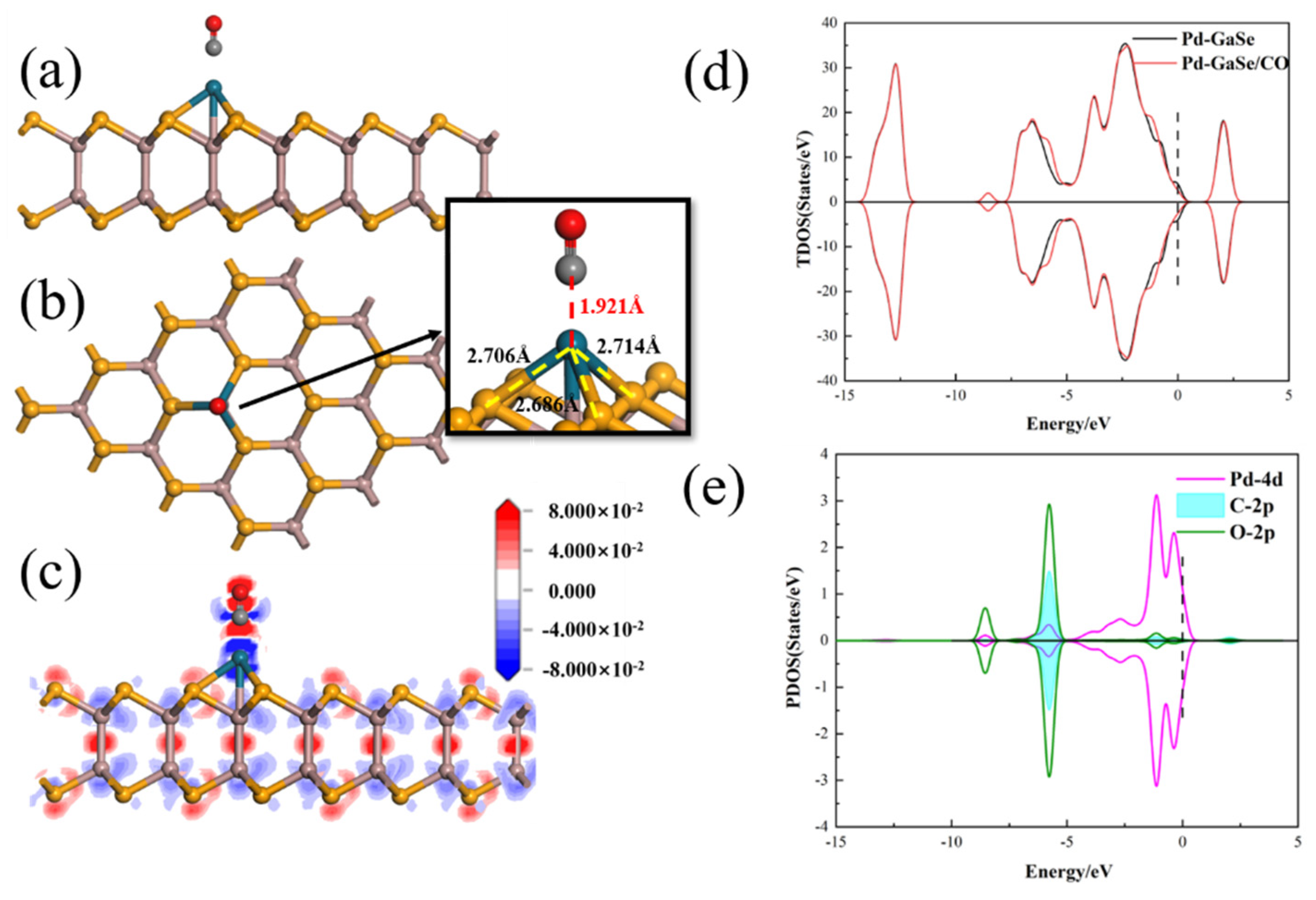
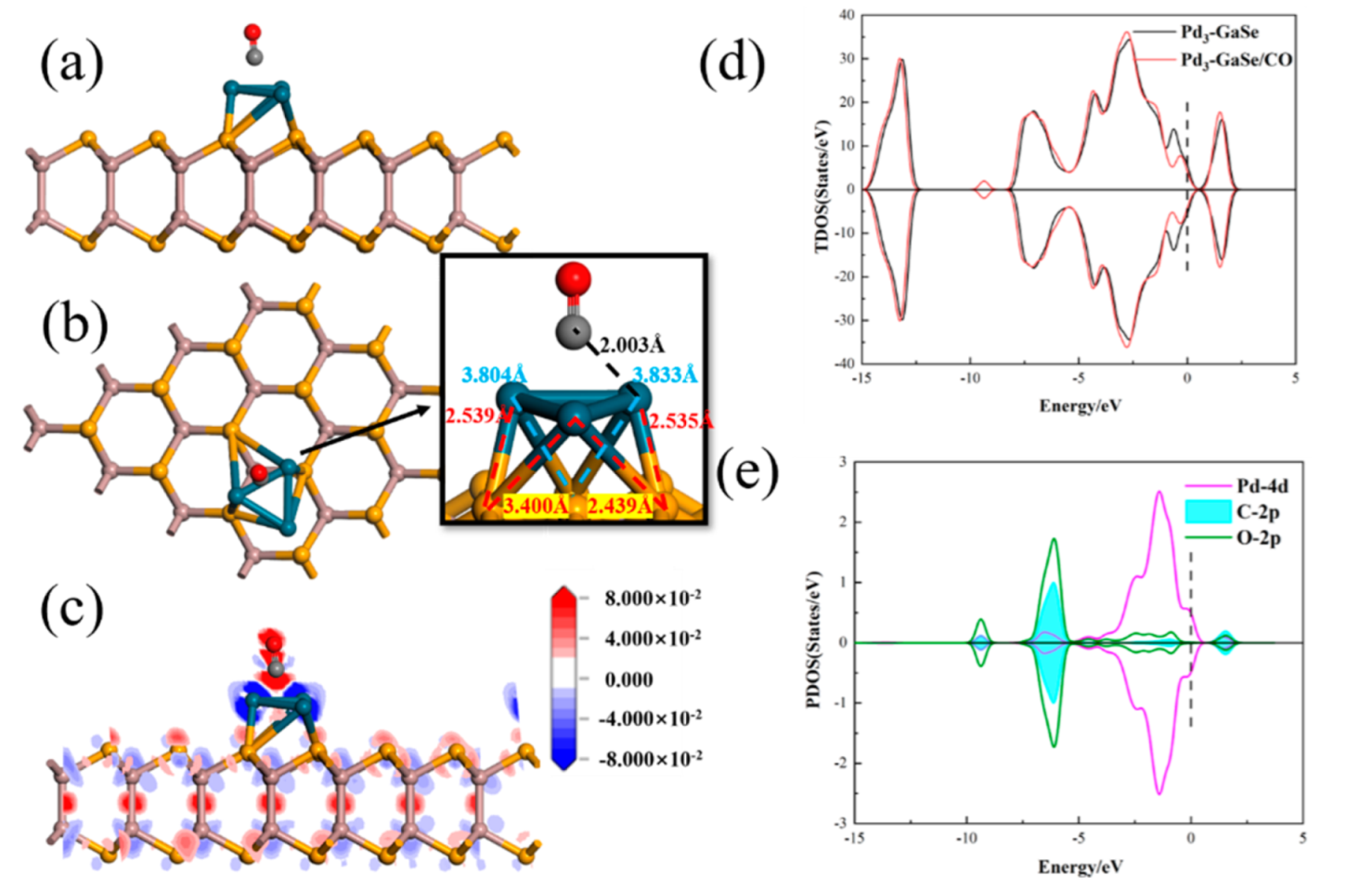
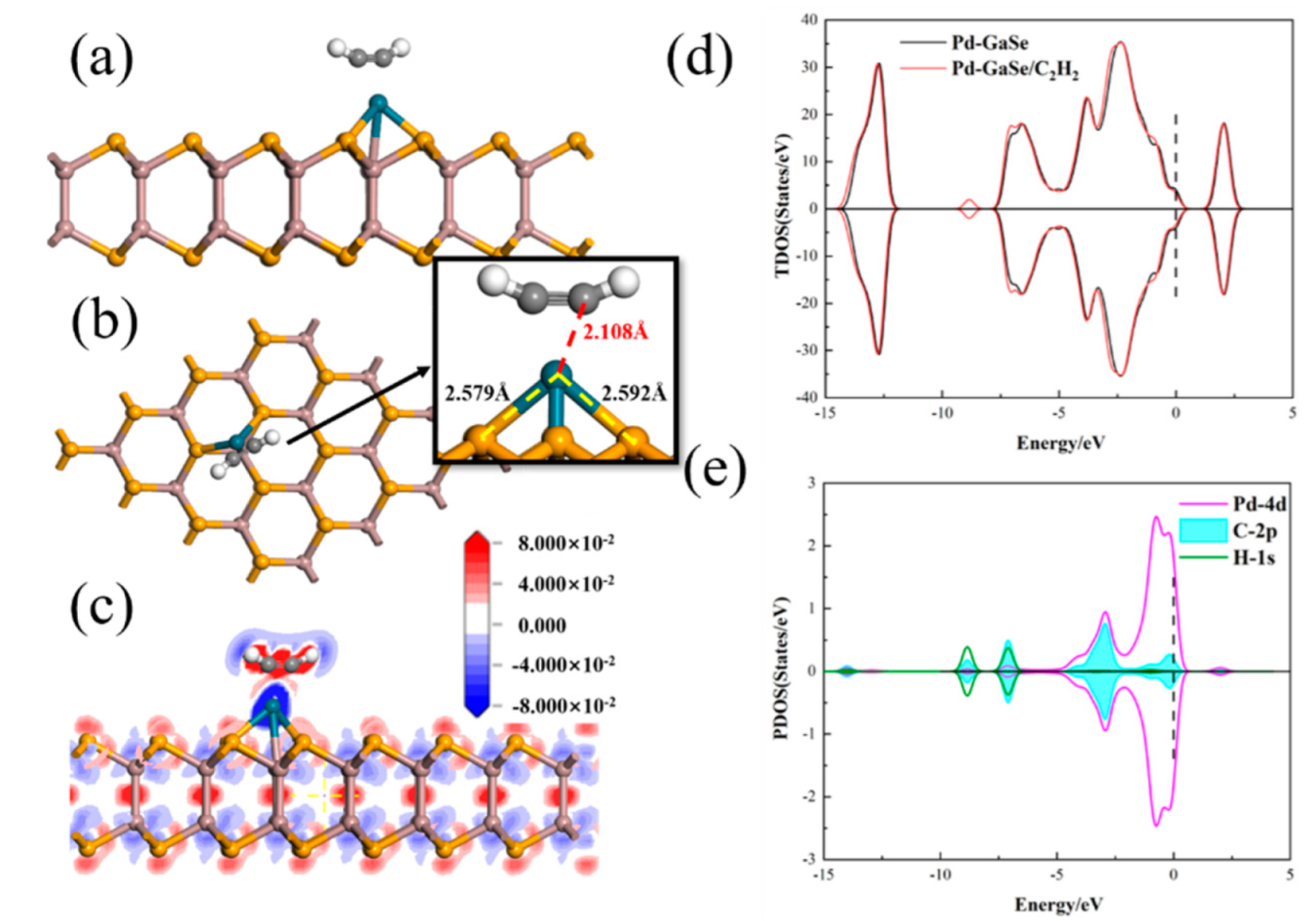
| Structure | The Length of Band (Å) | Adsorption Distance (Å) | Atom | Charge (e) | QT (e) | Ead (eV) | |
|---|---|---|---|---|---|---|---|
| GaSe/H2 | H1-H2 | 0.751 | 3.504 | H1 | 0.001 | 0.002 | −0.544 |
| H2 | 0.001 | ||||||
| Pd-GaSe/H2 | H1-H2 | 0.822 | 1.820 | H1 | 0.058 | 0.108 | −0.58 |
| H2 | 0.050 | ||||||
| Pd | −0.249 | ||||||
| Pd3-GaSe/H2 | H1-H2 | 0.847 | 1.726 | H1 | 0.100 | 0.199 | −0.943 |
| H2 | 0.099 | ||||||
| Pd1 | −0.153 | ||||||
| Pd2 | −0.336 | ||||||
| Pd3 | −0.161 | ||||||
| Structure | The Length of Bond (Å) | Adsorption Distance (Å) | Atom | Charge (e) | QT (e) | Ead (eV) | |
|---|---|---|---|---|---|---|---|
| GaSe/CO | C-O | 1.141 | 3.804 | C | 0.111 | 0.006 | −0.545 |
| O | −0.105 | ||||||
| Pd-GaSe/CO | C-O | 1.158 | 1.921 | C | 0.307 | 0.163 | −1.587 |
| O | −0.144 | ||||||
| Pd | −0.334 | ||||||
| Pd3-GaSe/CO | C-O | 1.180 | 2.003 | C | 0.373 | 0.187 | −2.427 |
| O | −0.186 | ||||||
| Pd1 | −0.203 | ||||||
| Pd2 | −0.206 | ||||||
| Pd3 | −0.165 | ||||||
| Structure | The Length of Bond (Å) | Bond Angle (°) | Adsorption Distance (Å) | Atom | Charge (e) | QT (e) | Ead (eV) | ||
|---|---|---|---|---|---|---|---|---|---|
| GaSe/C2H2 | C1-C2 | 1.212 | H1-C1-C2 | 179.704 | 3.593 | C1 | −0.123 | 0.004 | −0.700 |
| C2 | −0.127 | ||||||||
| C1-H1 | 1.072 | C1-C2-H2 | 179.283 | H1 | 0.126 | ||||
| C2-H2 | 1.072 | H2 | 0.128 | ||||||
| Pd-GaSe/C2H2 | C1-C2 | 1.261 | H1-C1-C2 | 155.279 | 2.108 | C1 | −0.062 | 0.100 | −1.307 |
| C2 | −0.060 | ||||||||
| C1-H1 | 1.079 | C1-C2-H2 | 155.552 | H1 | 0.111 | ||||
| H2 | 0.111 | ||||||||
| C2-H2 | 1.079 | Pd | −0.296 | ||||||
| Pd3-GaSe/C2H2 | C1-C2 | 1.337 | H1-C1-C2 | 131.730 | 2.018 | C1 | −0.026 | 0.178 | −2.757 |
| C2 | −0.035 | ||||||||
| H1 | 0.119 | ||||||||
| C1-H1 | 1.097 | C1-C2-H2 | 131.258 | H2 | 0.120 | ||||
| Pd1 | −0.212 | ||||||||
| C2-H2 | 1.096 | Pd2 | −0.204 | ||||||
| Pd3 | −0.208 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, T.; Zeng, W.; Zhou, Q. Pd-GaSe and Pd3-GaSe Monolayers: Two Promising Candidates for Detecting Dissolved Gases in Transformer Oil. Chemosensors 2022, 10, 236. https://doi.org/10.3390/chemosensors10070236
Hou T, Zeng W, Zhou Q. Pd-GaSe and Pd3-GaSe Monolayers: Two Promising Candidates for Detecting Dissolved Gases in Transformer Oil. Chemosensors. 2022; 10(7):236. https://doi.org/10.3390/chemosensors10070236
Chicago/Turabian StyleHou, Tianyu, Wen Zeng, and Qu Zhou. 2022. "Pd-GaSe and Pd3-GaSe Monolayers: Two Promising Candidates for Detecting Dissolved Gases in Transformer Oil" Chemosensors 10, no. 7: 236. https://doi.org/10.3390/chemosensors10070236
APA StyleHou, T., Zeng, W., & Zhou, Q. (2022). Pd-GaSe and Pd3-GaSe Monolayers: Two Promising Candidates for Detecting Dissolved Gases in Transformer Oil. Chemosensors, 10(7), 236. https://doi.org/10.3390/chemosensors10070236








