Abstract
Healthcare is undergoing large transformations, and it is imperative to leverage new technologies to support the advent of personalized medicine and disease prevention. It is now well accepted that the levels of certain biological molecules found in blood and other bodily fluids, as well as in exhaled breath, are an indication of the onset of many human diseases and reflect the health status of the person. Blood, urine, sweat, or saliva biomarkers can therefore serve in early diagnosis of diseases such as cancer, but also in monitoring disease progression, detecting metabolic disfunctions, and predicting response to a given therapy. For most point-of-care sensors, the requirement that patients themselves can use and apply them is crucial not only regarding the diagnostic part, but also at the sample collection level. This has stimulated the development of such diagnostic approaches for the non-invasive analysis of disease-relevant analytes. Considering these timely efforts, this review article focuses on novel, sensitive, and selective sensing systems for the detection of different endogenous target biomarkers in bodily fluids as well as in exhaled breath, which are associated with human diseases.
1. Introduction
The recent COVID-19 pandemic has sparked interest in versatile types of bioanalytical tools, such as optical, electrochemical, and electronic biosensors, as they can be used to screen the patient’s infectious state, provide daily monitored information, and alert the respective bodies to react based on the acquired results. Biosensors must fulfill several vital performance characteristics, such as a nearly real-time response, long term stability of the sensor operated outside, specialized laboratories, and ability to sense molecules at low concentration. The increasing demand for highly efficient and fast treatments in the biomedical field has been stimulating researchers toward the development of innovative technologies, notably devices that can provide continuous information about the health state of individuals. Wearable biosensors, defined as flexible sensing devices that incorporate a biological recognition element, are part of this next generation of healthcare monitoring systems. While early efforts in this field were devoted to simpler sensors monitoring heart rate and burned calories, more recent efforts are targeted towards more complex systems for sensing small biomolecules, such as glucose, dopamine, serotonin, lactose, cortisol, and physiological ions. Sensing biomarkers in sweat rather than blood is at the forefront of diagnostic research due to its potential direct applicability for personalized point of care testing. Most water-soluble constituents present in the blood leach into sweat in about 30 min. However, they typically occur at concentrations that are an order of magnitude lower compared to blood, which imposes a challenge and requires improving the sensitivity of most affinity-based biosensors for such analyses. This, in addition to other clinical needs for ultrasensitive molecular analysis, have motivated the development of several endpoint-assay technologies primarily limited by the affinity and specificity of molecular-recognition agents for the analyte of interest and the transducer used to read out. Sensitivity and specificity vary widely between different probe–analyte pairs. For example, it is now almost routine to quantify genomic DNA with single-copy sensitivity, while the detection limits of microRNAs remains challenging [1,2]. Probe affinity and sensitivity also vary between samples. Differences in the composition of analyzed samples (varying pH and ion content, constituents that non-specifically block the sensor surface) further complicate the sensor construction and data analysis. In terms of signal transduction, various implementations of physico-chemical readout principles are available, mainly based on optical and electronic means.
The optical (bio)sensors for the analysis of chemical and biological species can be constructed based on fingerprinting approaches, enabled by infrared absorption and Raman spectroscopy or based on additional biomolecules that can specifically capture the target analyte in connection with optical probing of associated mass change. In these areas, the use of metallic nanostructures and sensor chips carrying thin metallic films offers a highly efficient means of optical signal amplification (surface enhanced infrared absorption spectroscopy-SEIRA or surface enhanced Raman spectroscopy–SERS). Sensitivity of biosensor assays can be further improved by taking advantage of fluorophore labels (surface plasmon-enhanced fluorescence-PEF) or use of versatile sensor platforms for label-free detection (based on surface plasmon-resonance–SPR or through simple colorimetric assays enabled by colloidal nanoparticle aggregation).
In the domain of electronic sensors, there is an increasing interest in electrolyte gated field effect transistors (FET) as they offer an alternative route for direct electronic, label-free transduction of bio-recognition events along with miniaturization, fast data handling, and processing. Even a small change of a chemical or biological quantity may significantly alter the output electronic signal and is thus ideal for highly sensitive sensing. Electronic biosensor-based detection and quantitative estimation of analytes in bodily fluids remains a challenge, however, both from the fundamental point of view as well as in the context of practical applications. The major problems persisting for diagnostic use are: (i) maintaining high charge mobility (on the biosensor) after surface modification with specific receptors to achieve high sensitivity and selectivity; (ii) reproducibility of device preparation, and (iii) limitations due to Debye screening length in biological fluids with high salt concentrations such as blood and serum. Several reports over the last few years have shown that Graphene Field-Effect Transistors (GFETs) stand out for their small size, excellent electrical characteristics and high sensitivity to near surface charges and electrical fields, making them ideal devices for sensitive sensing [3,4,5,6]. Specificity to the target analyte can be integrated into GFETs via surface attachment of target-specific receptors. To overcome issues related to the low Debye screening length, the choice of the right surface receptor becomes paramount, along with choosing the right biosample medium.
2. Chemosensor Types
2.1. State-of-the-Art of Plasmonic Sensors
Plasmonics represent a field of nano photonics that concerns tight confinement of light intensity on surfaces of metallic nanostructures and thin metallic films [7]. This phenomenon is associated with the excitation of surface plasmon (SP) modes that originate from coupled collective oscillations of the electron density and associated electromagnetic field at the metal surface. The optical excitation of the confined field of SPs is accompanied by strongly enhanced electromagnetic field intensity |E/E0|2 and local density of optical states. Plasmonics has been used for surface plasmon resonance–SPR–biosensors as well as for the amplification in numerous spectroscopic techniques (see Figure 1), including surface-enhanced Raman spectroscopy-SERS, surface-enhanced infrared absorption spectroscopy-SEIRA, and surface plasmon-enhanced fluorescence-PEF [8,9,10,11]. In PEF affinity biosensors, fluorophore labels are used as tracers of the specific analyte capture and their coupling with the intense field of SPs allows for increasing the sensitivity of assay readout to rapidly detect trace amounts of molecular analytes present in analyzed samples. The enhancement factor of emitted fluorescence light intensity (per attached fluorophore label) typically reaches values up to 102 and several works [9,12] show even higher amplification factors exceeding 103. PEF takes advantage of the interaction between intense SP field and fluorophore emitters at their absorption and emission wavelengths that (locally within the SP probing depth Lp) increases the excitation rate, enhances quantum yield, and improves extraction efficiency of fluorescence light from the sensor surface. Various lithographically prepared metallic nanostructures have been used for signal amplification including thin metallic films with arrays of nanoholes (NHA) [13,14], nanoparticle arrays (NP) [15], and combined materials [16,17] that support a rich spectrum of interacting propagating surface plasmons (PSPs) and localized surface plasmons (LSPs). It is worth noting that plasmonic structures that provide significantly stronger field enhancement |E/E0|2 through tighter confinement of light intensity (probing depth Lp < 10 nm on e.g.,: narrow gaps between nanoparticles [18]) are typically not suitable for fluorescence enhancement due to the competing effect of quenching. However, such plasmonic nanoparticle geometries are attractive for SERS measurements that are increasingly utilized for vibrational spectroscopy-based analysis of trace amounts of molecular analytes. Similarly to PEF, the plasmonic nanostructures need to be adjusted to support strongly locally enhanced field intensity at spectral regions that match the incident probing laser wavelength and overlap with bands, where (Stokes or anti-Stokes) Raman scattering bands occur [19,20].
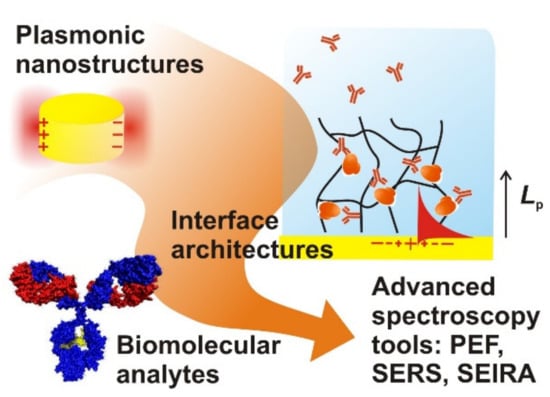
Figure 1.
Plasmonic nanostructures for the amplification of weak optical spectroscopy signals.
Plasmonic optical schemes have been implemented in range of affinity biosensors for rapid and sensitive detection of chemical and biological species with limits of detection (LOD) ranging from nM (regular label-free SPR [21]), fM (PEF [9]), to aM concentrations for aggregation-based assays [22] and SPR spectroscopy with enzymatically reacting labels [23]. It is worth noting that this performance was achieved for samples with liquid sample volumes of >μL and measurements were performed to detect a multitude of biomolecules. In addition, the strong confinement of the surface plasmon field was used to reduce of the optically probed volumes (down to aL) to monitor affinity binding events of individual biomolecules. These are typically operated at high concentrations of analyte (µM–mM) in order to reveal heterogeneity in biomolecular interactions beyond those that can be observed on a multitude of biomolecules [24]. In general, the optical schemes probing small volumes (<pL confocal volume) are not suitable for ultrasensitive detection in macroscopic samples due to slow biomolecule diffusion to the sensing spot, which translates to impracticable (long) detection times [25].
Rapid detection of very low amounts of biochemical markers with medical relevance has been made possible by the recent advancement of such dedicated sensors, as illustrated by the following examples that were selected to highlight several key important aspects. In the field of liquid biopsy pursued for early diagnosis and residual disease treatment of cancer, SPR biosensor technology was used for the analysis of cell-free DNA mutations specific to colon cancer in blood plasma [22]. This work demonstrated the limit of detection in RAS mutation at a low aM level by metallic nanoparticle–enhanced assay and it allowed for accurate discriminating of colorectal cancer patients from healthy donors (see Figure 2). In general, detection of low amounts of analyte in complex biological fluids (such as blood plasma) with plasmonic affinity biosensors is complicated by the presence of unspecific sorption of the molecules on the sensor surface. One of the possible routes to minimize this effect is the concept of the nanoparticle-release assay [26]. It was developed to enhance the specific SPR sensor response associated with the binding of target miRNA related to myelodysplastic syndromes (miR-125b, miR-16) in blood plasma and demonstrate that the assay enables detection with a limit of detection (LOD) of 349 aM. Another route to mitigate the impact of the unspecific sorption and thus allow the analysis of biological samples is the development of antifouling coatings that can carry biofunctional moieties. Direct SPR readout format on a sensor chip with poly[(N-(2-hydroxypropyl) methacrylamide)-co-(carboxybetaine methacrylamide)] brushes provided excellent resistance to fouling even after the functionalization. It enabled the detection of antibodies against hepatitis B surface antigen (antiHBs) in clinical serum samples and discriminated anti-HBs positive and negative clinical samples in 10 min [27]. The same biointerface was then used for the analysis of antiHBs in saliva samples, where, due to the lower concentration compared to blood plasma, the analysis had to be performed with the sandwich assay format and PEF readout [27]. Let us note that a thorough review of these developments has been conducted by more specialized publications [28,29,30].
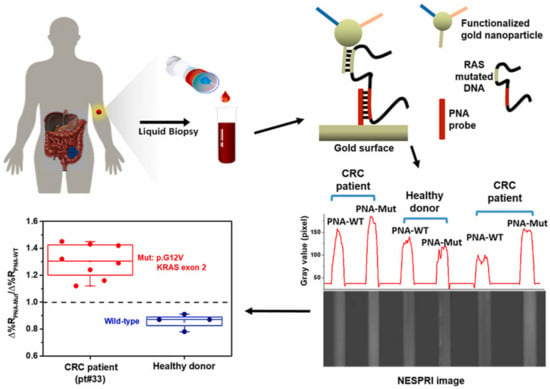
Figure 2.
Surface plasmon resonance imaging biosensor for liquid biopsy–based diagnosis of colorectal cancer. Reproduced from Biosensors and Bioelectronics, vol 170, D’Agata et al. Direct plasmonic detection of circulating RAS mutated DNA in colorectal cancer patients, 2020 [22] with permission from Elsevier.
2.2. Future Direction of Research in Plasmonic Sensors
Plasmonic biosensors have become an established technology in the field of affinity interaction analysis of a multitude of biomolecules (surface plasmon resonance–SPR–biosensors) and research has delivered a vast range of plasmonic materials that are tailored to amplify weak spectroscopy signals. Among future key research and development directions in plasmonic sensors and biosensors, we witness the efforts in exploiting the surface plasmon-based optics for new types of biomolecular interaction studies at the single molecule level, towards delivering a practical means for simplified and multiplexed ultrasensitive detection of target analytes, and to establish portable miniature optical devices enabling long-term monitoring of various analyte species in real-life complex samples (such as biomarkers in bodily fluids). The developed plasmonic nanostructures providing highly efficient means for amplification of Raman and fluorescence signals need to be translated to materials that can be prepared with scaled up production means and provide reproducible properties to address practical application and impact the analytical market. Moreover, the development of appropriate biocompatible polymer architectures that enable the contacting the optical sensor chips with complex liquid samples and avoid the sensor surface blocking and false response due to the fouling effects are of utmost importance.
2.3. State of the Art of Field Effect Transistor (FET) Type Sensors
FETs are semiconducting devices–also called unipolar transistors–since they only depend on the majority carrier in the semiconductor to finally become conductive. Their low cost, low volume, and low weight, as well as their excellent integration possibilities in portable electronic devices, make them ideal candidates for point of care medical applications. They can be used as biosensor materials since binding of analytes to the semiconductor surface influences the behavior with which they react to application of the gating voltage with direct correlation to the analyte concentration, charge, and molecular weight. FET type sensors can be used in gaseous or liquid media for the detection of biochemical markers or the detection of environmental pollutants [31].
Various types of FET sensors are available. An ion-sensitive field-effect transistor (ISFET) is a field-effect transistor used for measuring ion concentrations in solution; when the ion concentration (such as H+, see pH scale) changes, the current through the transistor will change accordingly [32,33]. ISFETs have been mainly used for pH detection [34] and are optimized for minimized interaction with interfering molecules. Hence, they depend only on specific ion species for transduction in a capacitive principle and are not suitable for the discrimination of a wide range of analytes. Successful specific DNA detection has been reported with differently modified FET type sensors (ISFET [35], EGFET [36]).
Special functionalized FETs with the aim to identify biomarkers are known as biosensor field-effect transistors (Bio-FETs or BioFETs). In such devices the voltage is changed by the concentration of the biomolecule, and the biomolecule itself, present in the gate channel. Hence, the charge transfer from the biomolecules to the transducing nanomaterial induces a change in the gate voltage. The overall amount of charge carriers thereby depends on the concentration of the biomolecules and so quantification is enabled by measuring the change in conductance or the change in source-drain current (or shift in the Dirac point (see Figure 3). Due to their outstanding sensitivity and selectivity, BioFETs are in the limelight for early diagnosis, drug screening, and disease screening.
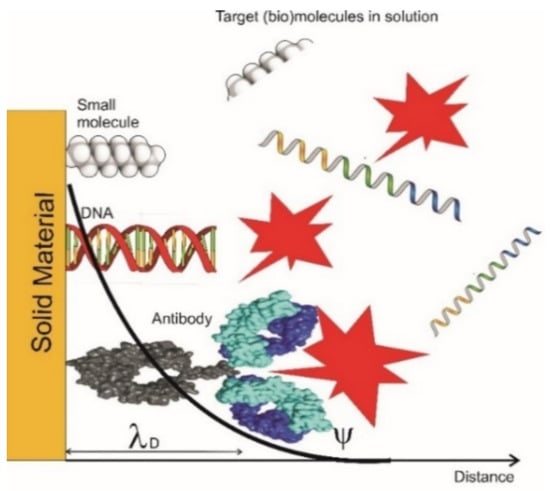
Figure 3.
Different bindings of biomolecules inside and outside the electrical double layer. Only the changes inside the electrical double layer are detected [lD, Debye length]. Modified from [37].
Transducing materials for BioFETs are inorganic semiconductors (e.g.,: SnO2, ZnO2, CuO, WO3) which might also be used for the detection of gases (CO, NOx, NH3, CH4, SO2, H2S) [38,39,40,41,42,43,44] and also, for the detection of simple organic molecules (hydrocarbons and alcohols). Here the need of the development of novel (mixed) oxides for the development of optimal platforms is necessary. Organic FET (OFET) type sensors use semiconducting polymers (CPs) for transduction. The main types of semiconducting polymers used for this purpose are Polyaniline, Polypyrrole, Phthalocyanine, and Thiophenes. Polymethylmethacrylate is commonly used as a gate insulator. Contrary to most of the metal oxide FET sensors the conductive polymer FET sensors can be operated at room temperature providing adequate sensitivity [45]. A disadvantage of polymer FET sensors is that they are usually not stable for extended times in their properties and tend to degrade in their performance. The advantage of using polymers in FET type sensors is their great variability enabled by using differently modified monomers, by adding additives (e.g.,: oxidic Nanoparticles [46]) or by using copolymerization schemes in the production of sensitive layers. Furthermore, pristine conductive polymers are unspecific towards a single analyte, but cover a wide range of sensitivity for a variety of volatile organic compounds. An OFET gas sensor with Pentacene for NH3 detection and phthalocyanine for NO2 has been successfully demonstrated [47,48]. Due to their nanostructured dimensions nanomaterials [silicon nanowires, metal oxides, nanoparticles, or carbon nanomaterials] used in FET sensors can be operated as well at room temperature thereby saving power and increase their usability for point of care applications. Further advantages are a decreased response time, good stability and a high selectivity paired with ultrasensitive properties down to low ppb range. FET sensors with nanostructured sensing principle are well suited to be embedded in silicon technology due to their small size and weight and low energy consumption. FET based on nanowires have been developed for CO and ethanol detection with CuO as sensing element [49]. In2O3 nanowires have been used for the detection of NO2 [50]. In order to reach a higher sensitivity a great number of nanowires can be arranged in a parallel fashion in a FET sensor. The sensitivity for NO2 could be reduced down to a single digit ppb value by such an arrangement [51].
The relentless miniaturization of silicon-based electronics coupled with the advent of carbon nanotubes most notably graphene, or MoS2 has led the electronic materials community towards atomically thin two-dimensional semiconductors [52,53,54].
A key advantage of carbon nanomaterial-based FETs like carbon nanotubes reduced graphene oxide (rGO), as well as graphene is the facile functionalization strategies based often on simple π-π stacking interactions [55,56] enabling easy attachment of biorecognition elements (e.g.,: antibodies, aptamers, DNA). Highly sensitive (down to single digit fM levels) single stranded DNA detection devices based on nanowires have been developed and demonstrated [55,57,58,59,60,61,62,63]. Protein detection (e.g., cancer markers) can be accomplished by specifically prepared antibody layers on the sensing surface [64]. Selective detection of (e.g., PSA [prostate specific antigen]) has been accomplished [65]. Pathogen species like viruses, bacteria [66], and molds can be detected too by specific surface proteins employing antibody-antigen interactions [67,68]. The detection of Dengue virus has been shown with a detection limit down to 10 fM [69]. Further the detection of specific enzymes [70,71] and even cell types has recently been demonstrated [72]. As a promising alternative to standard biorecognition units like antibodies for sensor surface functionalization, aptamers (ssDNA) have been demonstrated in a wide range of applications for the realization of healthcare devices [73].
Generally, independent of transduction material type, FET can be used as sensors in gas phase by application of a back-gate architecture, where the channel material is exposed to the environment gas phase. In this context, Hayasaka et al. have shown a single graphene FET system to discriminate between water, methanol, and ethanol in gas phase measurements with a 96% success rate utilizing machine learning [74]. Furthermore, inorganic semiconductor FETs are suitable candidates for this architecture but need to be operated at high temperatures. A modified building setup in SGFET sensors (suspended Gate FET) includes an air gap in order that the gas can reach the sensing gate surface directly. Such SGFET haven been shown to operate successfully at room temperature for the detection of H2, NH3, NOx, and CO [75].
Anyhow, FET transduction surfaces functionalized with biorecognition molecules suffer from sensor element degradation and instability due to the direct exposure to outer factors if these effects are not counteracted. On one hand, the application of hydrogel layers for shielding of surface functionalized biological components has been demonstrated before and enables application of the sensing principle also in the gaseous phase. The approach is highly compatible with capacitive sensing since the formation of electric double layers has been demonstrated for supercapacitor separator films [76]. Accordingly, displacement of such electric double layers due to analyte binding to the gate electrode would result in a significant change in gate capacitance, leading to high device sensitivity. For the detection in electrolyte solutions, the capabilities of the capacitive sensing approach were demonstrated by the group of Torsi et al. showing that such sensor architectures can potentially lead to single molecule detection (zM range) of genomic markers in whole blood serum [77]. On the other hand, the advantages of aptamers as functionalization elements are a much higher stability, allowing for application in harsher environments, as well as decreasing fabrication costs as tailor-made aptamers are becoming commercially more accessible due to improved synthesis routes. The applicability of aptamer-based FETs for gas sensing has been recently demonstrated [78] and enables to use back-gated device architecture for gas sensing without the need of an encapsulation layer to shield the bio-components from deterioration since aptamers withstand the gaseous phase environment.
2.4. Future Direction of Research in FET Type Sensors
Although biosensor FET applicability has been thoroughly demonstrated in literature, for the application of such biosensors in real-life scenarios still some challenges need to be addressed. Surface passivation strategies to allow for detection in complex media reach from encapsulation with high-k dielectric materials, zwitterionic brushes, PEG components, and not interchained components like ethylamine or bovine serum albumin. Investigations on sampling in saliva or breath condensates allows for pinpointing the optimized strategies depending on the transducing material and the sample matrix. Sensor data evaluation is generally performed by trained personnel and research towards automatized response evaluation principles and the implementation of machine learning can overcome this challenge to enable the FET biosensor technology for the application as point of care devices. Detailed understanding of the electrochemical and electrostatic interactions during FET transduction can be achieved via sensor fusion with optical methods and reveal the key parameters necessary for FET biosensor application with reduced baseline drifts, environmental influence, and independent data quality for sampling in complex media.
2.5. State of the Art of Chemiresistive Sensors
A chemiresistive configuration is essentially like a FET configuration, but instead of a three-electrode configuration a two-electrode configuration ensures simplified design while the analyte (e.g., VOCs) cause an analyte specific (or passive) gating effect (see Figure 4). The advantages of chemiresistors are low-manufacturing costs, simplicity (as no complex control electronics are required), and that they are more sustainable for integration in large sensors array to increase sensor performance and redundancy. The last two features are especially important in the context of VOC-sensors (e.g., gas sensors or electronic nose systems) as they offer portable, cost-efficient, and energy efficient sensors for trend predictions (e.g., fingerprint analysis). They are an enabling technology for breath biopsy as a direct medial application but also for environmental monitoring of potential hazard conditions, exogenous components, and air quality. Furthermore, due to the advantages of chemiresistive sensors they are ideal devices for Internet of Things (IoT) applications [79] (see Figure 5). Not all semi-conductive materials used for FETs are suitable candidates for the realization in a chemiresistive setup. As the working point is not adjusted by a gate electrode, only semi-conductors with suitable threshold voltages according to the n-type, p-type, or ambipolar behavior can be used. However, specific doping of the materials is a feasible strategy to expand the applicability of semiconducting materials for application in chemiresistors [80]. Such sensors include metal oxides (MOx), nanomaterials, and conductive polymers. Although chemiresistors can operate in a liquid environment, their key advantage resulting from the simplified structure is the operation in the gas phase.
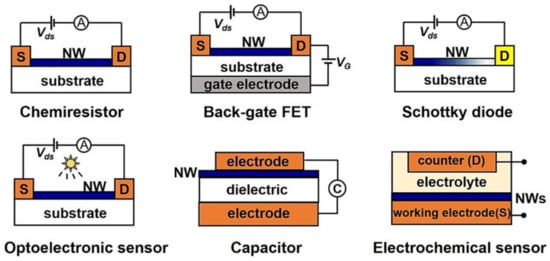
Figure 4.
Schematic illustration of electrically transduced gas sensors [31].
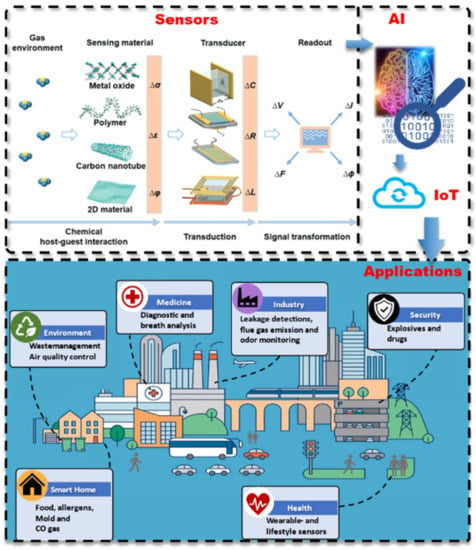
Figure 5.
Schematic overview of smart sensor systems from the transduction of sensor signals, via the artificial intelligence and IoT connection for applications in various sectors. Adapted from [78].
MOx sensors are generally realized in a chemiresistor configuration with an underlying heating element. Metal oxides used as sensing elements in MOx sensor types exhibit their sensing performance due to a significant change in electrical conductivity when they get in contact with the defined analyte gas component. When semiconducting metal oxides are heated to high temperatures (200–450 °C), oxygen species are absorbed to the surfaces, forming a depletion layer. Upon binding of the oxygen molecules to exposed reducing gases, electrons are released in the metal oxide, and current flow is observed. The generated electrical signal can be readily processed, thus making real time sensing devices and lab-on-a-chip platforms possible. Depending on the type of metal oxide and dopant used, a wide range of gases can be detected, but the downsides of the system are the high operating temperature and narrow range of detectable analytes. MOx based chemical sensors are used in the detection of obnoxious gases for the purpose of environmental monitoring SO2 [37,81,82,83], NOx [84,85,86], NH3 [87,88], H2S [89], CO [90,91] hydrocarbons [detection of oil spills], and VOCs [volatile organic compounds]. Such sensors have been light-powered and realized on flexible substrates for the detection of NO2 by ultrathin heterostructure configuration (see Figure 6). VOCs successfully detected are acetone [92,93], ethanol [94,95], formaldehyde [96,97], benzene [98], benzaldehyde [99,100], toluene [101,102], xylene [103,104,105], isoprene [99,106], propane [100], butanol [107,108], phenol [109,110], acetonitrile [111], dodecane [112,113]. Furthermore, palladium has been used successfully as the sensing material for hydrogen detection with a sensitivity of 10 ppm, to be operated at 150 °C [114].
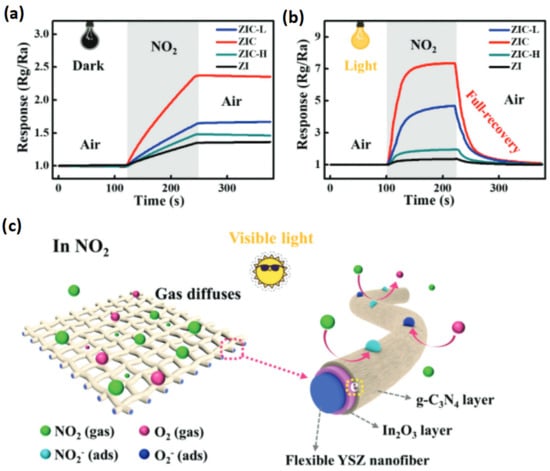
Figure 6.
(a) The dynamic response curves of visible light powered, flexible ZI, ZIC-L, ZIC, ZIC-H sensors to 1 ppm NO2 at room temperature in dark, (b) under visible-light illumination. (c) Schematic diagrams of the NO2 detection process with visible-light illumination, oxygen molecules in air freely diffuse through the porous structure of the ZIC networks. Adapted from [85].
The achieved detection limits are in the low ppm range and for some working principles even beyond in the ppb range. Normally the sensors have to be operated at high temperatures well above room temperature to achieve the required sensitivities. The sensitivity of MOx sensors is increasing with temperature up to a plateau of an optimal operating temperature. Above this individual temperature the sensitivity is decreasing again. For most of the MOx sensors this operating temperature is well beyond room temperature. MOx sensors have domain application for gaseous analytes, but due to operating needs at higher temperatures need more power and are not as easily to be integrated and have an interference problem by humidity and a low selectivity. Humidity is disturbing (decreasing) the sensitivity and operating the sensors at higher temperatures is overcoming this problem. When sensors work at lower temperatures the humidity problem is surfacing again, and solutions must be worked out to overcome this challenge for MOx sensors operating at room temperature. To be used for point of care applications the operating temperature must be decreased in the ideal case to room temperature. This can be achieved by using nanostructures for the sensing surfaces (e.g.,: [110], Cr2O3 decorated nanotubes for sensing of phenol) and should be further developed for other analytes. To improve the selectivity of MOx sensors, they can be arranged as a matrix [115] of sensors with similar but slightly adapted properties or in a micro sensor array of different sensing materials [116]. The response time is usually in the single digit seconds range, most of the recovery time is in the double-digit seconds range. MOx sensors have been used for applications in agriculture and food packing industry, breath and sweat analysis, for clinical markers diagnosis and pharmaceutical analysis, and in hazard analysis and forensics. In pharmaceutical analysis they can be used by forming an [electronic] e-tongue. The so-called e-tongue is used to detect small quantities of certain pharmaceutical drugs in bodily fluids. A CuO nano-sensor was used for the detection of Rifampicin [117]. The anticancer drug Doxorubicin and Dasatinib was detected with a ZnO sensor [118] Ranolazine, an antianginal drug was detected in very low concentration by a MOx sensor based on WO3 and graphene [119]. MOx sensors are proposed and have been used in food analysis for quality assessment. Also MOx sensors for ascorbic acid in fruits basing on ZnO/CNT were described [120]. An electronic nose based on the combination of MOx sensors comprising SnO2, MoO3, and SnO2 doped with Mo was shown to detect a combination of specific VOC’s generated by Enterobacteriaceae in vegetable preparations [121], Methyl-propyl sulfide and 2-Nonanon could be detected by MOx sensors based on Sn and W oxides for quality control of onions [122]. It can be expected that similar other developments for the quality control of prepacked food will emerge soon. MOx sensors have been proven also very valuable for Hazard analysis, Crime prevention, concealed explosives detection, and identification of fires. A MOx sensor based on ZnO doped with Indium [In] was successfully used to detect TNT, trinitrotoluene, DNT, dinitrotoluene, picric acid, and paranitrotoluene [123]. The sensor worked at room temperature and had a rapid response time of 6 s. Furthermore, fast and remote detection of fires with MOx sensors was shown [124,125]. Also, for agricultural applications MOx sensors have been developed and used. MOx sensors based on NiO could detect the pesticide parathion in low concentration in green vegetable samples [126]. Other pesticides could be analyzed as well with selected MOx sensors [127,128].
Similarly, to the application of (semi-) conducting polymers for OFETs, a wide range of organic polymers can be used for the realization of CP sensors in a chemiresistive configuration [129]. To improve the applicability of polymers used in OFET configuration, a differential sensing principle can be applied, measuring the resistance changes by comparison of a measurement and reference channel; one exposed to the sensing environment, while the reference sensor element is kept insulated. This approach allows for the use of a wide range of CP materials in chemiresistive configuration, while at the same time compensating the influence of environmental temperature [130]. The key technological advantages using polymers instead of metal oxides are room temperature applicability, low production costs, and a wide range of available materials with different specificities for target analytes [78]. Four technological leaps with high impact on the facilitation of a polymer based nose have been achieved since, namely: (i) Shirakawa’s Nobel prize work in 2000 [131] enabling chemical modifications for polymer conductivity allowing for pi-conjugated materials using different functional groups within the pi-system but also their corresponding side-chain modifications, which opened a plethora of new organic electronic materials to be used and optimized for tailormade applications like eNoses (e.g., increase in specificity); (ii) recent progresses in microfabrication of electronic circuit boards [132,133], providing smaller electrode distance patterns (reproducibility increase); (iii) and the enormous advances in machine learning and artificial intelligence [134], providing a perfectly suitable route for data evaluation of eNose response data (applicability increase); and (iv) the advances in printing technology allowing for high precision printing of micrometer scale polymer dots and the use of polymer solutions with low limitation to solution properties [78].
Hence, most pitfalls of previous attempts of utilizing polymer-based materials for eNose realization have been overcome in recent years and recent work has shown the state-of-the-art progress in this direction [135]. Still, commonly used polymer materials are not strictly specific against a single analyte, but rather show variable sensitivity against various volatile compounds. Various conductive polymers are known for their affinity to a variety of these compounds, e.g., polythiopenes (e.g., P3HT) for ammonia and amines [136], polyanilines for hydrogen sulfide [137] (see Figure 7), xylenes [138], or NO2 [139], polypyrrole for ammonia and indoles [140], poly(vinyl alcohol) for hazardous gases [141], poly(methyl methacrylate) for ethyl acetate and toluene [142]. Furthermore, a wide range of composites for conductive polymers (e.g., graphene composites) and nanowire application of CPs have been demonstrated as suitable approaches for gas sensing [143,144]. Such polymeric arrays have been demonstrated for the analysis of food freshness [145], diagnostics via breath analysis [146,147], analysis of human sweat [148], phytopathogenic microbes [149], meat spoilage detection [150], methanol detection in liquors [151], and Alzheimer markers [152], among other applications. Arrays of CPs are especially efficient and capable to discriminate between a wide range of analytes when p-type and n-type materials are used at the same time.
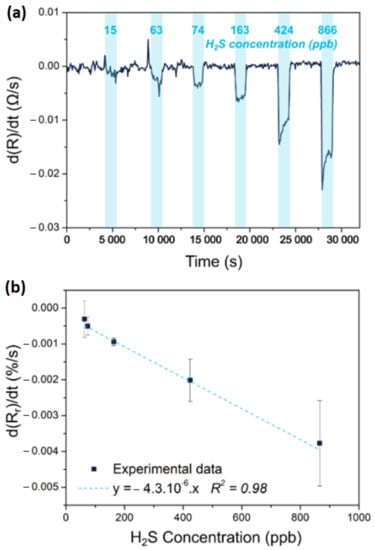
Figure 7.
Response to hydrogen sulfide of a chemiresistor based on a Polyaniline-SnCl2-PEDOT:PSS blend. (a) shows the derivative of the response evolution and (b) a corresponding calibration curve expressed as the variation of the relative response as a function of the concentration of hydrogen sulfide. Adapted from [137].
2.6. Future Direction of Research in Chemiresistive Sensors
On one hand, the narrow specificity of metal oxide sensors does not allow for monitoring of complex smell mixtures, odors, fragrances, or the bouquet of products. On the other hand, conductive polymers provide a wide range of detectable analytes and can support, supplement, or replace MOx sensors for a range of applications in the medical sector. A downside of CP sensor materials is the lack in long-term stability, since the materials are prone to degradation due to their interaction with environmental interferents, a downside that comes with the wide range of analyte sensitivity. To prolong long-term stability, deposition of graphene films on the CP [153], addition of nonvolatile dopants [154], and application of PU foams [155] and aerogels [156] were demonstrated. Furthermore, most CPs have dependence on the environmental humidity, which can pose an issue for sensitive biosensing in high humidity environments. Sensor drift adjustment [157] or addition of fatty acid filler materials have been developed to overcome this challenge. Furthermore, modern artificial intelligence algorithms allow for specified training of CP based e-Nose systems and provide solutions for medical applications from personalized and/or objectified patient care and sense complex mixtures for early diagnostics when the key challenges are addressed and can render CP based sensors as an enabling technology.
2.7. State of the Art of Sensor Arrays
The next step of sensor development is to deploy a group of sensors in a certain geometry pattern—a so-called sensor array—which allows increasing the signal-to-noise ratio (SNR), improving robustness by providing redundancy of the devices. An e-Nose is an electronic array of different semiconducting materials which change their resistivity based on the chemical composition of the exposed environment with an implemented analytical algorithm for training of the device in a biomimetic approach. On one hand, e-Nose systems based on metal oxides are already commercially available and resemble the current state-of-the-art [135,158]. They suffer from high fabrication costs, the need to be operated above 300 °C and a very low specificity against relevant volatile organic compounds. On the other hand, polymer-based e-Nose systems have been investigated widely from 1980 to the 2000s and did not yet perform sufficient for commercialization. With the recent progress in artificial intelligence and machine learning capabilities polymer-based e-Nose systems have again received attention and have been commercialized only in certain sectors. Recently also the field of multivariable sensors has received considerable attention, especially for environmental IoT applications, as they provide independent outputs to recognize gases and vapors, by using a sensing material designed to have diverse, and in the best case orthogonal, responses to different analytes (see Figure 8). These electrical, -optical-, and electrochemical outputs correspond to different sensing responses, thus yielding high dispersion which in turn allows for discrimination of up to four different gases in mixtures [78,159]. This concept has been superbly highlighted in two reviews by Potyrailo [160,161] (see Figure 9). Theoretically, they can quantify individual components in mixtures, prove to be immune to interferences, offer self-correction ability against environmental parameters (e.g.,: T, RH), and offer a more stable response over sensor arrays. Again, CP are interesting candidates for these sensors as their chemical tunability allows for the creation of novel sensing materials with multi-response mechanisms to different gases, [159] based on modulations of charge carrier density, polymer swelling, and conformational transitions of polymer chains. Due to the combination of the main four thrusts such as (1) tunability of sensing materials, (2) transducer design, (3) data analytics, and (4) manufacturability we foresee a bright future for chemiresistive (multivariable) gas sensors.
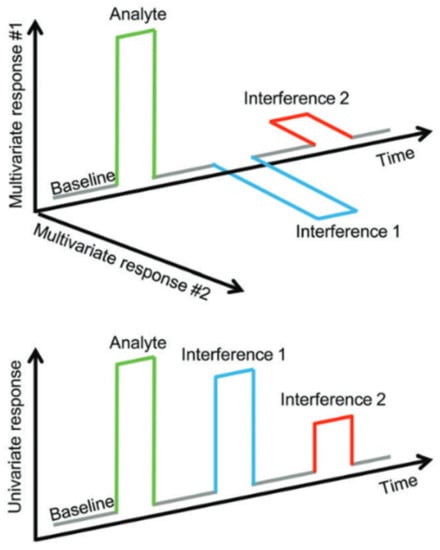
Figure 8.
Illustration of the advantages of multivariate sensor systems. Analyte and interference responses are well distinguishable. Picture from [160].
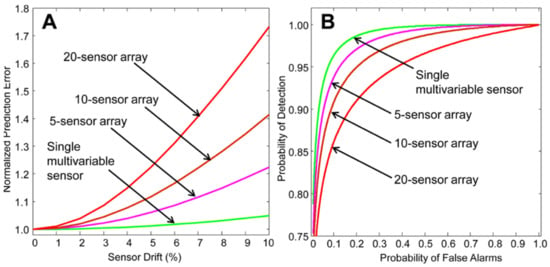
Figure 9.
(A) Demonstration of the sensor prediction rate error increase with increasing sensor drift for the comparison of arrays and multivariate sensors. (B) Probability of detection against false alarm predictions for multivariable sensors against sensor arrays. Figure from [161].
2.8. Future Direction of Research in Sensor Arrays
Recent advances in the fabrication of organic polymer materials with suitable conductivity have shown an increase in eNose research based on CPs. Among other applications, the suitability of the approach was shown for the detection of Penicillium digitatum in oranges [162], wood identification [163], air quality monitoring [164], oil pollution in sea ware [165], liquor discrimination [166], fruit aroma detection [167], bile species and fermentation states [168] or BTX components [169] with various implementation of artificial intelligence via principle component analysis [162,163], ensemble learning [165], Neural Networks [166], and Support Vector Machines [168,169]. Similar to the combinatory code used by the human brain it is not the response from one of the sensor materials that enables to differentiate between different smells, but the array formation of many different sensing components and the complex differences that occur from material to material when exposed to a certain smell [170]. Microfabrication techniques enable fabrication of interdigitated electrodes with distances below 50 µm, which enables a wide range of conductive polymer materials as sensing elements as an isotropic domain of interlinked polymer chains can be realized only on this scale for many polymers. Furthermore, novel doping methods, especially enabling the doping for n-type [171,172,173] materials, allow for adjustment of polymeric matrices for acidic and alkaline transduction processes enabling a wider range of analyte detection. Hence, research is driven to investigate combinations of polymer composites for the realization of a robust e-Nose system with a wide range of applicability.
3. Applications of Chemosensors in BioMedical Context
3.1. State-of-the-Art of Single Molecule Detection Technologies
Biosensors have been attracting increasing attention over the last decade with rapidly increasing market size and numbers of publications [174]. Individual biomolecules and their interactions are a key subject of research in the bioanalytical community and single molecule detection has become an enabling technology in the important fields of biology and biophysics. For the detection of pathogenic and exogenic compounds, various types of sensors have been developed to operate either in the liquid or in the gas phase. Arguably, optical technologies outperform electric sensors in liquid phase, but the most promising approaches for gas phase analytics are based on electrical signal transduction. Single molecule detection is typically implemented by using optical measurements in small sample volumes (<pL) with µM analyte concentration and they serve to reveal heterogeneities in biomolecular interactions that are otherwise hidden in measurements on a multitude of biomolecules [24]. In addition, single molecule detection in liquid samples of macroscopic volume represents the goal of ultimate sensitivity of analytical technologies [175]. Among these, detection of nucleic acids takes advantage of established enzyme-based amplifications including polymerase chain reaction (PCR). In order to enable quantitative detection of individual copies of oligonucleotide biomolecules in samples with macroscopic volume, the compartmenting of the analyzed sample is performed for digital type of readout. The majority of digital polymerase chain reaction (dPCR) assays rely on the compartmenting via microdroplets [176], which is performed in a microfluidic device that splits the analyzed sample to multiple small (nL) reactors that carry mostly 0 or 1 analyte biomolecules. The readout of the presence of the analyte in each compartment is performed after the PCR amplification by fluorescence. Most often used is the Förster energy transfer with quencher [177] that switches a fluorophore from its dark to its bright state (by increasing its quantum yield by up to 102-fold) in the analyte presence. For the sensitive analysis of protein analytes, the direct exponential amplification strategy of analyte itself (such as PCR) is not available and therefore enzymes are used as labels in conjunction with e.g., immunoassays. Fluorescence detection by the linear enzymatic amplification strategy has been adopted by using of beta-galactosidase label attached to a detection antibody and reacting with a substrate (e.g.,: resorufin-beta-D-galactopyranoside [175] with the quantum yield change of up to 102–103 triggered by the enzymatic reaction [178]).
3.2. Future Direction of Research Single Molecule Detection
In order to expand the applications of ultrasensitive detection platforms that are capable of detection of individual target molecules, research is pursued in the direction of enzyme–free amplification strategies, towards parallel detection of multiple analytes, and by overcoming the need of sample compartmenting. Through these efforts, new and simpler platforms for single molecule detection of chemical and biological species can be delivered to performing field analyses outside the specialized analytical laboratories.
Especially bioelectronic sensors based on large-area interfaces offer intriguing single-molecule label-free detection in combination with a compact design to detect the onset of a disease at the earliest possible stage [179,180,181,182]. Pioneered by the Torsi group, biolayer functionalized electrolyte-gated field effect transistors have been used for both protein and DNA sensing in the zepto-molar range. The remarkable sensitivity is attributed to an amplification mechanism, which is speculated to be triggered by the affinity binding event that induces a work-function change in the FET which is assumed to propagate in the gating-field through the electrostatic hydrogen-bonding network within the biolayer [182].
3.3. State of the Art in Biomarkers
The advent of technologies for discovery and validation of biomarkers and biomarker panels at the omic-wide level (DNA- and protein microarrays, next generation sequencing (NGS), mass-spectrometry based proteomics, and metabolomics) have paved the way for development of personalized treatment approaches. Some molecular biomarkers panels have already found their way into every-day life and clinical applications and are opening new possibilities for optimizing personal lifestyles and therapy of patients. It is undisputed that these biomarkers offer great potential, which has not yet been fully exploited. However, there is the big disadvantage of–still–complex analysis, requiring well-trained personnel and expensive lab equipment as well as complex protocols. That might be one reason why many biomarkers have failed to translate into clinical routine so far.
3.4. Future Direction of Research in Biomarkers
Upcoming concepts based on samples collected by minimal invasive methods, such as plasma, serum, saliva, and interstitial fluid are on the way and can accelerate the use of different biomarkers. Ideal targets for biomarker development, which can also be used for novel, improved sensing systems belong to different omic-layers of nucleic acids, like DNA methylation, miRNAs, mutations, as well as proteins and peptides. Many of the aforementioned analytes are ideal targets for disease detection and disease monitoring and for monitoring lifestyle adaptations and offer the advantage that they are stable in different body fluids like blood (including serum and plasma), saliva, and urine, being also highly specific for certain types of diseases. A current unmet clinical need is the straightforward, fast, and affordable analysis of these biomarkers and this is exactly where the great potential of chemosensors lies, particularly as they can be used at the point of care.
3.5. State of the Art in Personalized Medicine and Liquid Biopsy
“One size fits all” has been the standard in the medical world for a long time. The shift to personalized medicine started around 20 years ago and has accelerated over the past few years. The breakthrough of the Human Genome Project, which charted the complete human DNA in 2001 [183] was an important step in this process. Technological developments such as DNA microarrays and next generation sequencing (NGS) and ever improving data processing enabled genetics to take off. Charting somebody’s genetic profile has become increasingly faster and less costly and enables us to gain insight into the genetic profile and thereby to predict the risks of specific diseases for a specific person and which medication will work best. This kind of detailed genomic analysis is especially exploited in the cancer field with respect to clinically actionable mutation profiling (via whole-genome-or targeted mutation sequencing) and thereby tailoring targeted cancer therapies to the specific molecular profile of the tumor rather than to the tumor type.
3.6. Future Direction of Research in Personalized Medicine and Liquid Biopsy
In general biopsies are usually taken from the primary tumor, whereas samples from the metastases are often scarce and additionally overlooked for the purpose of treatment decisions. Novel approaches have arisen to detect tumor products from bodily fluids such as blood, urine, cerebrospinal fluid, or saliva including the analysis of circulating tumor cells (CTCs), circulating tumor nucleic acids (ctDNA and ctRNA), or tumor-derived extracellular vesicles (EVs), also termed exosomes [184,185,186]. This so-called “liquid biopsy” is a convenient, fast, non-invasive, and reproducible sampling method that can dynamically reflect the changes in tumor tissue and provide a robust basis for individualized therapy and early diagnosis of cancer. Though mutation profiling of circulating tumor DNA (ctDNA) in plasma and enumeration of circulating tumor cells (CTCs) from whole blood samples are the currently approved cancer liquid biopsy approaches (as not least evident from corresponding recently FDA approved assays such as the Roche Cobas EGFR Mutation Test v2, the FoundationOne® Liquid CDx test and the CELLSEARCH® Circulating Tumor Cell Kit) there are also examples of other biomarker types as well as bodily fluids with diagnostic potential as elaborated in more detail in the following.
3.7. State of the Art in DNA Methylation Biomarkers for Disease Diagnosis and Therapy Stratification
Being vital in embryogenesis and affecting such processes as imprinting or X-chromosome inactivation and/or silencing of repetitive DNA the methylation in eukaryotes is an important epigenetic modification regulating gene expression [187]. Hence, its deregulation is associated with a range of human diseases [188]. Every cell type has a unique DNA methylation fingerprint that changes during normal biological processes but also in many diseases, in particular cancer, whereby most work on disease-associated DNA methylation marks has been done starting in the early 80s. As DNA-methylation changes already occur early in tumorigenesis and can not only be detected in tissue but also in cell-free DNA present e.g., in plasma/serum, stool, urine, or other bodily fluids, DNA-methylation based biomarkers are particularly suited for early cancer detection. As illustrated in Figure 10 underneath numerous freely circulating DNA-methylation marks have been described e.g., for the big four tumor entities (breast-, lung-, prostate-, and colon cancer) which even overlap between the tumor types [189]. The application of cancer methylation markers is not limited to early diagnosis but also includes other stages of the cancer patient’s journey such as disease monitoring or detecting minimal residual disease [e.g., [190,191]. Though still very scarce, there are even first examples of chemobiosensors which determine the methylation status such as a carbon dot-modified liquid-exfoliated graphene Field Effect Transistor targeting the cancer marker Sept9 via a multiprobe approach [192].
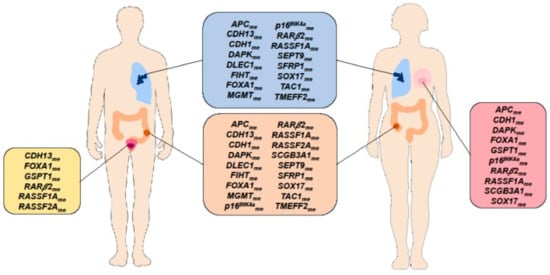
Figure 10.
Circulating cell-free DNA methylation-based biomarkers described in literature for cancer detection which are common to at least two cancer types [Breast Cancer (pink box), Lung Cancer (blue box), Prostate Cancer (yellow box), Colorectal Cancer (orange box)]. Reprinted with permission from [189]. 2020, Cells, MDPI.
In past years more and more DNA methylation biomarkers have also emerged for diseases other than cancer. Along these lines numerous DNA methylation biomarkers have been described in cardiovascular diseases such as for example atherosclerosis [193], hypertension [194], and myocardial infarction [195]. Further areas where circulating DNA methylation biomarkers have been identified in recent years include metabolic disorders such as type 2 diabetes [196] and obesity [197] as well as neurological diseases [198,199].
3.8. Future Direction of Research in DNA Methylation Biomarkers for Disease Diagnosis and Therapy Stratification
Despite the promise of epigenetic biomarkers, so far only a few DNA methylation-based candidate biomarkers have reached their potential for use in a clinical setting, and all these are mainly related to the field of cancer and typically comprise single diagnostic markers (see Table 1). Differences in DNA methylation between patients and controls may be large (e.g., more than 50%) in cancer but in other non-communicable diseases may be less than 5%. Methods used to measure methylation must therefore be accurate to well below this level of resolution. A recent landmark study by Liu et al. [200] revealed that plasma whole-genome bisulfite sequencing to analyze methylation patterns outperformed whole genome sequencing and targeted mutation methods for cancer detection. The authors further demonstrated that a targeted NGS-based methylation-based multi-cancer early detection test using a machine-learning classifier can simultaneously detect more than 50 cancer types with a single, fixed, low false positive rate of less than 1%, and can accurately localize the tissue of origin.

Table 1.
Characteristics of clinically approved DNA methylation assays for the detection of different cancers (derived from [201]).
Whereas the majority of DNA-methylation biomarkers have been described in the context of (early) cancer diagnosis there are also examples for DNA-methylation based therapy response markers [202,203]. Technologies typically applied for genome-wide and targeted DNA methylation analysis comprise DNA microarrays (e.g.,: Illumina EPIC bead array), NGS and quantitative PCR (qPCR).
3.9. State of the Art in Auto-Antibody (AAb) Biomarkers for Early Diagnosis
AAbs are produced as an immune-response against tumor-associated antigens (TAAs) comprising mutant, aberrantly post-processed, or locally over-expressed proteins in tumors [204]. AAbs can amplify a signal from antigens at very low concentrations, and at an early stage in tumorigenesis when the corresponding antigens may not themselves still be detectable. To search for such autoantibodies, several state-of-the art technologies and methodologies have been developed, including SEREX, phage display, protein microarrays, reverse-capture microarrays, and SERPA [205]. Using high density protein microarrays covering the whole human proteome, not only diagnostically relevant TAA panels for the big four cancer entities (colon, breast, lung, prostate) could be identified (e.g., [206,207]), but also the usefulness of serum/plasma-derived autoantibody biomarkers in non-cancer diseases such as e.g., ulcerative colitis has been demonstrated including pre-diagnostic samples [208].
3.10. Future Direction of Research in Autoantibody Biomarkers for Early Diagnosis
Although a high number of discovery studies have been published on various non-cancerous and cancerous diseases, AAb validation is still missing for many of these studies. The only exception is lung cancer diagnostics, where autoantibody-based early diagnostic assays are on their way to clinical practice and commercialization. Although the Oncimmune early CDT lung cancer assays are commercially available, these are lacking diagnostic performance [209]. Thus, a new direction followed in autoantibody biomarker based diagnostics is to use peptides derived from the diagnostically relevant autoantibody reactive antigens and to implement them instead of the whole proteins into multiplexed assay platforms. An additional new and promising avenue in antibody-based diagnostics has recently come up as it could be shown that immunoglobulin profiles are highly similar in blood and saliva, which opens not least new and promising applications for AAb biomarkers to be used along point-of-care chemosensors [210].
3.11. State of the Art in Extracellular Vesicles/Exosomes
EVs is an umbrella term for a heterogenous group of membranous vesicular structures, released to virtually all human bodily fluids. As EVs represent vehicles for the transfer of molecular cargo including proteins, DNA fragments, different RNA species, metabolites, and lipids, according to their role in intercellular communication, their cargo is also representative for the physiological (or pathological) state and origin of the releasing cell and therefore make them ideal biomarker candidates for minimally invasive systemic disease diagnostic purposes. A further plus is that EV cargo biomolecules are most efficiently protected from enzymatic degradation in bodily fluids and that, for example, tumor cells may release up to 10-fold more EVs than released by healthy cells. A commonly accepted classification divides EVs into three groups based on their distinctive biogenesis pathways: apoptotic bodies, microvesicles, and exosomes (see Figure 11) [211].
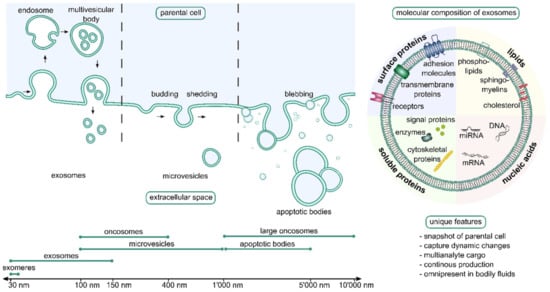
Figure 11.
Schematic depiction of the biogenesis pathway of apoptotic bodies, micro vesicles, and exosomes and the respective characteristic size ranges reported for these vesicle types (left side). Molecules which can be typically detected in exosomes are shown (right side). Reprinted with permission from [211]. 2021, Adv. Drug Deliv. Rev. is open access journal where copyright stays with authors. C Noehammer.
3.12. Future Direction of Research in EVs
There have been many studies on EVs and their various types of cargo in cancer as we recently summarized in a review [212] together with elaborating on the specific challenges associated with using saliva- and blood derived EVs and their cargo as diagnostic biomarkers. Beside cancer, EVs have also been proposed as biological indicators for a number of diseases affecting multiple organs including the central nervous system, liver, kidney, lung, and arteries, as recently reviewed by Barili & Vassali, highlighting the great, however not yet fully exploited potential of EVs as therapy delivery tool [213].
3.13. State of the Art of microRNA (miRNA) Biomarkers
Extracellular miRNAs are stable (i.e., protected from ribonucleases) and can be detected in many bodily fluids including blood, urine, or saliva [214]. miRNAs are secreted by cells in (i) a free form, i.e., not bound to any biomolecule; (ii) a complex with RNA-binding proteins including Argonaute 2 protein (AGO2), high-density lipoprotein (HDL), or nucleophosmin I (NPM1); (iii) cell delivered vesicles such as micro vesicles and exosomes. As miRNAs are tissue-specific, stable in bodily fluids and regulate a wide range of cellular processes in health, disease, and development (see Figure 12 underneath e.g., for their putative role in cardiac remodeling) they have raised enormous interest as potential and promising biomarkers and have been heavily studied in many diseases in recent years. A lot of research has been done here again in cancer, where many different miRNA biomarker panels for different tumor types have been described [215,216]. Cardiovascular diseases, neurological diseases as well as autoimmune diseases have been other areas of intensive research on circulating miRNA markers, which all have been nicely summarized in a recent review [217].
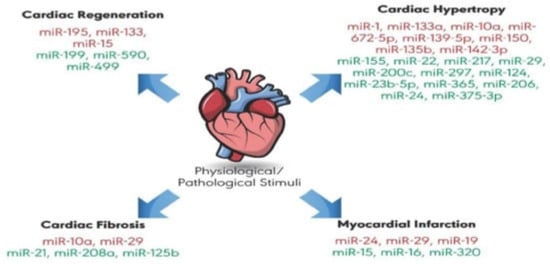
Figure 12.
MicroRNAs suggested to play a role in physiological and pathological cardiac remodeling. Some micro RNAs have a protective role (green color) against cardiovascular diseases, whereas others promote extensive cardiac remodeling, leading to disease (red color). Reprinted with permission from [218]. 2019, Int. J. Mol. Sci. is open access journal of MDPI.
3.14. Future Direction of Research in Small RNA Biomarkers
As mentioned above, changes in levels of circulating miRNAs have been associated with a wide range of diseases including type 2 diabetes (T2D), obesity, cardiovascular disease, cancer, neurodegenerative disorders, and others [219]. Not least many exosome-derived miRNA disease biomarkers have been described and exosome isolation has been generally shown to improve the sensitivity of miRNA amplification from human biologic fluids. Along these lines it is recommended to be the starting point for early biomarker studies to reduce the probability of false negative results involving low abundance miRNAs that may be missed by using unfractionated serum or saliva.
3.15. State of the Art of Saliva Biopsy
The bio-liquid saliva is easy and non-invasive to obtain, transport, and store, even by laypeople. Saliva is a unique, readily, and repeatedly available bodily fluid, which can be obtained via non-invasive, painless collection. In addition, saliva represents a basically unlimited sample matrix (healthy adults produce 500–1500 mL of saliva per day) and further offers the advantage of not requiring any special skills for collection, which makes it a most promising sample matrix for disease diagnostics and biomarker search. Systemic diseases influence the composition of saliva and thus the analysis of saliva is a promising medical branch of the future for point-of-care medicine. This enables patients to monitor their health status easily and continuously with portable tools such as smartphones that are already practically everywhere available. As a diagnostic indicator, saliva can be used to measure stress hormones, enzyme levels, and many biomarkers for acute and chronic diseases, micro-RNA, and microbiome. Liquid saliva biopsy is an innovative, rapid, and non-invasive means of examining the presence of cancer in a patient by detection of circulating tumor cells (CTCs) and fragments of tumor DNA. As soon as such biomarkers are validated and can be reliably measured with high-quality diagnostic sensors, saliva diagnostics opens a new promising area in future high-quality health care. This enables real-time health monitoring for effective and personalized preventive medicine for patients and doctors. Biosensors for saliva diagnostics have been used to detect chronic kidney disease [220] for Hyperuricemia [221] and prostate cancer [222]. Also, in dental medicine, saliva diagnostics employing immunosensors or enzyme-based sensors have been applied successfully for oral cancer [223,224], Periimplantitis [225], and Periodontitis [226,227,228,229,230]. The presence of bacteria and viruses can be detected, as well in the saliva with biosensors like Bacillus cereus [231,232], COVID-19 [181], HIV [233], Zika virus [234,235], Pseudomonas aeruginosa [236], and Staphylococcus aureus [237]. A recent review summarizes the advancements in this field of biochip development and innovative applications for disease recognition [238].
As a bodily fluid of the oral cavity, comprising a plethora of different biomarker types (DNA, mRNA, miRNA, proteins, antibodies, EVs, microbiome), saliva makes intuitively sense for detection of oral diseases, as underlined by the numerous biomarker studies performed in oral cancer and oral cavity-related diseases such as caries, periodontitis, and Sjögren’s syndrome. There is a wide spectrum of more than 100 suggested salivary biomarkers for oral cancer so far, comprising many proteins, but also DNAs, mRNAs, and miRNAs [239]. A review by Javaid et al. [240] summarizes commercially available test kits for oral diseases such as the OraRisk® HPV test for identification of individuals at risk for oral squamous cell carcinoma, the CRT bacteria® caries risk test, which determines streptococci mutants and lactobacilli counts in saliva and the MyPerioPath® salivary test (Oral DNA® Labs, Eden Prairie, MN, USA) which detects most of the periodontal pathogens. More and more studies have come up recently which demonstrated that saliva is also useful for detection of non-oral, systemic diseases including type 2 diabetes, melanoma, lung cancer, pancreatic cancer, breast cancer, and ovarian cancer [241]. In addition, a couple of salivary tests for non-oral diseases are commercially available, such as the FDA-approved point-of-care saliva test Oraquick advance rapid HIV-1/2 or the CE marked NarcoCheck® for the detection of five different drugs of abuse (cannabis, cocaine, heroin, amphetamines, and ecstasy). Finally, steroid hormones, such as estradiol, progesterone, testosterone, DHEA-S, and cortisol, are meanwhile often routinely measured in saliva.
3.16. Future Direction of Research Needs in Saliva Biopsy
More biomarkers must be discovered, detected, and validated. The development of biosensors with higher sensitivity integrated on a lab on chip using biocompatible materials will be performed for home testing, telemedicine, and remote patient monitoring. Easy to use home tests with non-invasive testing will result. Integrated artificial intelligence and machine learning will create an evolution of the monitoring process, resulting in improved sensitivity of detection and streamlined protocols for an established point of care diagnostic. Not at least due to the COVID-19 pandemic and the fact that COVID PCR testing was successfully performed from gurgle solutions and the feasibility of detection of COVID-specific antibodies in saliva could be demonstrated [242,243], saliva has most recently attracted wide interest as promising sample matrix for point of care and home testing. Though the understanding how diagnostically relevant biomolecules from distant tissues and organs reach saliva and thus can reflect disease states is still limited and respective blood-saliva barrier models have only started to be developed, e.g., [244,245] there has been recent evidence for saliva as liquid biopsy for successful detection of EGFR mutation analysis in lung cancer patients taking advantage of a novel core technology, electric field-induced release and measurement (EFIRM), which relies on a multiple flexible electrochemical sensor [246]. In addition, there has been support from a human lung cancer mouse xenograft model for the hypothesis that systemic nucleic acid biomarkers are carried and delivered into saliva via exosomes [247].
3.17. State of the Art in Breath Biopsy
Breath biopsy enables the non-invasive collection and analysis of biomarkers in the exhaled breath (Figure 13). Breath samples thus give a picture of the patient’s state of health and provide information about disease processes taking place in the body. A biomarker can show detectable changes in an existing disease, which is verified by statistically reliable tests on large patient populations. Metabolic changes occur in cancer cells in the earliest stages of the disease. The measurement of specific biomarkers and cancer-specific metabolites in the breath therefore has great diagnostic potential for the early detection of cancer. Diagnostics with breath biomarkers enable treatment decisions in a personalized medical approach by predicting drug resistance, toxicity, and therapeutic response. The health care system can be significantly improved by the application of non-invasive breath biopsy, since patients can be monitored without significant costs or radiation exposure. Increased patient acceptance of these new diagnostic and non-invasive options is to be expected.
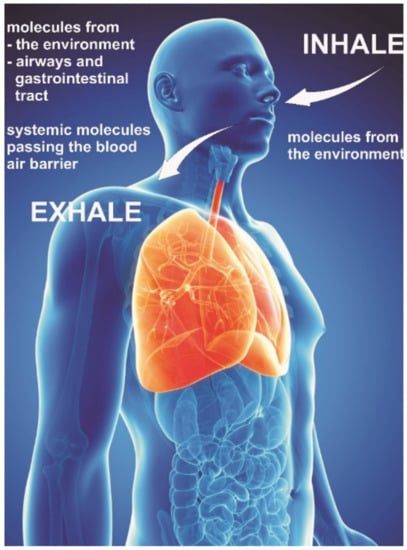
Figure 13.
Exhaled molecules in the body originate partly from the airways, gastrointestinal tract, or the whole body (i.e., systemic molecules passing the blood-air barrier in the lungs) and from environmental molecules inhaled (modified from [229]).
The diagnostic detection of specific volatile markers in the breath of persons or patients can give information about the health status of that person and can be used as a noninvasive early warning indicator of a starting health problem (see Figure 13). The detection of Acetone in the breath is an indicator of type1 and type2 diabetes. With a Carbon doped WO3 sensor a high sensitivity for Acetone was achieved. The lowest recorded sensitivity was down to 1.8 ppm [92]. Toluene in the breath is an indicator of lung cancer. A WO3 sensor doped with Pd has been shown to detect Toluene reliably within a response time of 10 s down to 1 ppm [248]. Other diseases which can be diagnosed by breath markers are Renal dysfunction [114], Myocardial infarction, Asthma [114], and unspecific Infections. A combination of different sensors in a single device forming an electronic nose is promising to detect reliably biomarkers indicating the mentioned health problems and additional ones. This principle has been demonstrated for the sensing of VOC’s resulting in a 3D printed artificial nose which appears 100 times more sensitive than the human nose [249]. The miniaturization of sensors in a portable device is promising and valuable to diagnostic tools for point of care diagnostics [250].
Breath biopsy has been used for the early detection of breast cancer in the exhaled air [251,252] using an array of sensors sensitive for VOC´s in an electronic nose setup. For improving the prediction accuracy machine learning techniques have been successfully applied [253] in a clinical study involving 899 subjects, both positive and negative predictive value was 97%.
A great potential is provided by breath biopsy also for the detection and monitoring of lung diseases like asthma and COPD [254]. Metabolic changes associated with the development of cancer open up new fields of non-invasive diagnostic by breath analysis of other tumor types [255]. Other diseases having been investigated to be suitable for detection by breath analysis such as cystic fibrosis [256,257] and diabetes [258]. A comprehensive review on breath biopsy describing state of the art of collecting breath samples and sensing means has recently been issued [259].
3.18. Future Direction of Research in Breath Biopsy
Additional respiratory biomarkers for early cancer detection and monitoring of treatment efficiency are identified and investigated. The costs of the sensors required for this are to be reduced and the operability of the monitoring devices is improved through the integration of AI and IOT. Examinations are carried out both in exhaled air (EB) and in exhaled condensate (EBC). The stability, sensitivity, and accuracy of the biosensors are improved. The measurement results obtained are compared with other standardized analysis methods. An improvement and standardization of the sampling procedure by checking the humidity and temperature in the event that the samples may need to be stored after the sampling is to be developed.
3.19. State of the Art in Wearable Sweat-Based Diagnostic Textile-Based Sensors
For monitoring chronic health conditions wearable sensors for analysis of sweat are of great medical, clinical, and commercial interest. Such wearable sensors must monitor with multiple measurements the composition of sweat during a 24 h period and they must keep their integrity and sensitivity at least during the addressed period. Electrochemical sensors provide high sensitivity and non-invasive detection of biomarkers. Specific antibodies must be immobilized on the surface of the electrochemical sensors and must retain their intact chemical structure for prolonged and stable sensing of the biomarkers. Physical based sensors can be integrated into textiles giving information about temperature, pressure, respiration monitoring, and are used as a sensor for obstacle monitoring. A recent review is analyzing comprehensively the obtained progress of integrated sensors in textiles for healthcare monitoring [260]. The analytes to be monitored are proteins and enzymes, e.g., Interleukin-6 and Cortisol. Other analytes detectable are pH, H2O2, Glucose, Lactate, Ammonia, Ions (Na, K, Cl, Pb, Cd), Adrenaline, and Uric acid.
3.20. Future Direction of Research in Textile-Based Sensors
Appropriate wearable sensing systems must be developed for other important biomarkers in human sweat. The stability of electrochemical sensors must be enhanced to prolong their functionality and to keep their biochemical integrity using room temperature ionic liquids. Electrochemical sensors on flexible and wearable substrates need to be developed and the reliability, stability, and sensitivity of such sensors well beyond the 24 h period must be reached by closely monitoring manufacturing parameters for the start of a successful series production. The energy needs of such sensors need to be diminished and easy readout provisions must be provided. An advanced integration of AI into the readout algorithms for pattern recognition needs to be established to obtain a well enhanced information gain.
4. Conclusions
Across all enabling technologies for biomedical technologies, recent advances have included de-convolution of data sets, in the manner of single molecule detection, complex microfluidic setups, multivariable sensing methods, functionalization schemes, and the implementation of artificial intelligence for data analytics. Biomedical applications already rely not only on the underlying technology but benefit strongly from more sophisticated algorithms, which can be readily tuned to overcome technological challenges digitally, allowing diagnosis and predictions for personalized medicine, handling of databases, cross validations, and classifications.
In the field of plasmonic-based sensors, SPR biosensors represent an established method that is routinely used for the biomolecular interaction analysis of (ensembles of) biomolecules enabled by a range of commercial instruments deployed in specialized laboratories. In addition, this optics concerning tailored metallic nanostructures paved the way for rapid portable analysis of low molecular weight compounds based on vibrational spectroscopy fingerprinting (SERS) and we witness research efforts to deliver new affinity-based tools for rapid ultrasensitive detection of biomarkers suitable for point of care applications as well as those aimed at molecular interaction studies at single molecule level serving in life sciences and, potentially, drug development areas.
In the domain of electronic sensors, there is an increasing interest in electrolyte gated field effect transistors, (FET) as they offer an alternative route for direct electronic, label-free transduction of bio-recognition events along with miniaturization and fast data handling and processing. Even a small change of a chemical or biological quantity may significantly alter the output electronic signal and are thus adapted for highly sensitive sensing. Electronic biosensor-based detection and quantitative estimation of analytes in bodily fluids remains a challenge both from the fundamental point of view as well as in the context of practical applications. The major problems persisting for diagnostic use are: (i) maintaining of high charge mobility (on the biosensor) after surface modification with specific receptors to achieve high sensitivity and selectivity; (ii) reproducibility of device preparation, and (iii) limitations due to Debye screening length in biological fluids with high salt concentrations such as blood and serum. Several reports over the last few years have shown that Graphene Field-Effect Transistors (GFETs) stand out for their small size, excellent electrical characteristics, and high sensitivity to near surface charges and electrical fields, making them ideal devices for sensitive sensing [3,4,5,6]. Specificity to the target analyte can be integrated into GFETs via surface attachment of target-specific receptors. To overcome issues related to the low Debye screening length, the choice of the right surface receptor becomes paramount, along with choosing the right bio sample medium.
Chemosensors can serve many application fields and without a doubt are most exciting tools for non-invasive personalized medicine and health monitoring. Nevertheless, specificity and sensitivity of the measured analytes for health or disease-state are key and the biggest challenge, beside limitations related to detection limits given by the abundance and stability of the studied diagnostic biomarkers in bodily fluids or breath. In addition, inter-individual as well as intra-individual differences and variabilities must be considered and understood as biomarker base line levels might differ from one individual to the other and can also depend on the time of the day or situation when a bio sample is taken as well as on the age of the individual. As manifold experiences and studies have shown, there is typically no one single marker to reliably indicate a certain disease. Therefore, successful sensor concepts need to detect multiple markers either from the same analyte type, or even markers from different biomolecule classes such as DNA, mRNA, microRNA, metabolites, or proteins, which, especially for the latter case, is technically not trivial. Finally, diagnostic results and their interpretation, even when in future strongly supported by machine learning and artificial intelligence approaches, need to lead to clear next steps and potential intervention measures, and by no means shall leave the patient in uncertainty.
Author Contributions
All authors (E.K., C.R.-R., J.D., A.-W.H., C.N., F.P.-M., S.S., V.W., W.K. and C.K.) contributed equally to this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schoepp, N.G.; Schlappi, T.S.; Curtis, M.S.; Butkovich, S.S.; Miller, S.; Humphries, R.M.; Ismagilov, R.F. Rapid Pathogen-Specific Phenotypic Antibiotic Susceptibility Testing Using Digital LAMP Quantification in Clinical Samples. Sci. Transl. Med. 2017, 9, eaal3693. [Google Scholar] [CrossRef] [PubMed]
- Rissin, D.M.; López-Longarela, B.; Pernagallo, S.; Ilyine, H.; Vliegenthart, A.D.B.; Dear, J.W.; Díaz-Mochón, J.J.; Duffy, D.C. Polymerase-Free Measurement of MicroRNA-122 with Single Base Specificity Using Single Molecule Arrays: Detection of Drug-Induced Liver Injury. PLoS ONE 2017, 12, e0179669. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Mishyn, V.; Leroux, Y.R.; Butruille, L.; Woitrain, E.; Barras, A.; Aspermair, P.; Happy, H.; Kleber, C.; Boukherroub, R.; et al. Highly Performing Graphene-Based Field Effect Transistor for the Differentiation between Mild-Moderate-Severe Myocardial Injury. Nano Today 2022, 43, 101391. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Graphene-Based Field-Effect Transistor Biosensors for the Rapid Detection and Analysis of Viruses: A Perspective in View of COVID-19. Carbon Trends 2021, 2, 100011. [Google Scholar] [CrossRef]
- Kwong Hong Tsang, D.; Lieberthal, T.J.; Watts, C.; Dunlop, I.E.; Ramadan, S.; del Rio Hernandez, A.E.; Klein, N. Chemically Functionalised Graphene FET Biosensor for the Label-Free Sensing of Exosomes. Sci. Rep. 2019, 9, 13946. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Schuller, J.A.; Barnard, E.S.; Cai, W.; Jun, Y.C.; White, J.S.; Brongersma, M.L. Plasmonics for Extreme Light Concentration and Manipulation. Nat. Mater. 2010, 9, 193–204. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Plasmonics in Biology and Plasmon-Controlled Fluorescence. Plasmonics 2006, 1, 5–33. [Google Scholar] [CrossRef]
- Bauch, M.; Toma, K.; Toma, M.; Zhang, Q.; Dostalek, J. Plasmon-Enhanced Fluorescence Biosensors: A Review. Plasmonics 2014, 9, 781–799. [Google Scholar] [CrossRef]
- Lal, S.; Grady, N.K.; Kundu, J.; Levin, C.S.; Lassiter, J.B.; Halas, N.J. Tailoring Plasmonic Substrates for Surface Enhanced Spectroscopies. Chem. Soc. Rev. 2008, 37, 898. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, Applications, and the Future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Kinkhabwala, A.; Yu, Z.; Fan, S.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large Single-Molecule Fluorescence Enhancements Produced by a Bowtie Nanoantenna. Nat. Photon. 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Höppener, C.; Novotny, L. Exploiting the Light–Metal Interaction for Biomolecular Sensing and Imaging. Quart. Rev. Biophys. 2012, 45, 209–255. [Google Scholar] [CrossRef]
- Masson, J.-F.; Murray-Méthot, M.-P.; Live, L.S. Nanohole Arrays in Chemical Analysis: Manufacturing Methods and Applications. Analyst 2010, 135, 1483. [Google Scholar] [CrossRef]
- Xie, F.; Pang, J.S.; Centeno, A.; Ryan, M.P.; Riley, D.J.; Alford, N.M. Nanoscale Control of Ag Nanostructures for Plasmonic Fluorescence Enhancement of Near-Infrared Dyes. Nano Res. 2013, 6, 496–510. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, F.; Li, W.-D.; Wang, Y.; Hu, J.; Chou, S.Y. Giant and Uniform Fluorescence Enhancement over Large Areas Using Plasmonic Nanodots in 3D Resonant Cavity Nanoantenna by Nanoimprinting. Nanotechnology 2012, 23, 225301. [Google Scholar] [CrossRef]
- Bauch, M.; Dostalek, J. Collective Localized Surface Plasmons for High Performance Fluorescence Biosensing. Opt. Express 2013, 21, 20470. [Google Scholar] [CrossRef]
- Kern, A.M.; Martin, O.J.F. Excitation and Reemission of Molecules near Realistic Plasmonic Nanostructures. Nano Lett. 2011, 11, 482–487. [Google Scholar] [CrossRef]
- Guillot, N.; de la Chapelle, M.L. The Electromagnetic Effect in Surface Enhanced Raman Scattering: Enhancement Optimization Using Precisely Controlled Nanostructures. J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 2321–2333. [Google Scholar] [CrossRef]
- Colas, F.J.; Cottat, M.; Gillibert, R.; Guillot, N.; Djaker, N.; Lidgi-Guigui, N.; Toury, T.; Barchiesi, D.; Toma, A.; Di Fabrizio, E.; et al. Red-Shift Effects in Surface Enhanced Raman Spectroscopy: Spectral or Intensity Dependence of the Near-Field? J. Phys. Chem. C 2016, 120, 13675–13683. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- D’Agata, R.; Breveglieri, G.; Zanoli, L.M.; Borgatti, M.; Spoto, G.; Gambari, R. Direct Detection of Point Mutations in Nonamplified Human Genomic DNA. Anal. Chem. 2011, 83, 8711–8717. [Google Scholar] [CrossRef] [PubMed]
- De la Rica, R.; Stevens, M.M. Plasmonic ELISA for the Ultrasensitive Detection of Disease Biomarkers with the Naked Eye. Nat. Nanotechnol. 2012, 7, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.B.; Zijlstra, P. Single-Molecule Plasmon Sensing: Current Status and Future Prospects. ACS Sens. 2017, 2, 1103–1122. [Google Scholar] [CrossRef]
- Sheehan, P.E.; Whitman, L.J. Detection Limits for Nanoscale Biosensors. Nano Lett. 2005, 5, 803–807. [Google Scholar] [CrossRef]
- Špringer, T.; Krejčík, Z.; Homola, J. Detecting Attomolar Concentrations of MicroRNA Related to Myelodysplastic Syndromes in Blood Plasma Using a Novel Sandwich Assay with Nanoparticle Release. Biosens. Bioelectron. 2021, 194, 113613. [Google Scholar] [CrossRef]
- Riedel, T.; Hageneder, S.; Surman, F.; Pop-Georgievski, O.; Noehammer, C.; Hofner, M.; Brynda, E.; Rodriguez-Emmenegger, C.; Dostálek, J. Plasmonic Hepatitis B Biosensor for the Analysis of Clinical Saliva. Anal. Chem. 2017, 89, 2972–2977. [Google Scholar] [CrossRef]
- Finotti, A.; Allegretti, M.; Gasparello, J.; Giacomini, P.; Spandidos, D.; Spoto, G.; Gambari, R. Liquid Biopsy and PCR-Free Ultrasensitive Detection Systems in Oncology (Review). Int. J. Oncol. 2018, 53, 1395–1434. [Google Scholar] [CrossRef]
- Altug, H.; Oh, S.-H.; Maier, S.A.; Homola, J. Advances and Applications of Nanophotonic Biosensors. Nat. Nanotechnol. 2022, 17, 5–16. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Cvelbar, U.; Abdulhalim, I. A Comprehensive Review on Plasmonic-Based Biosensors Used in Viral Diagnostics. Commun. Biol. 2021, 4, 70. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, L.; Deng, Z.; Liao, J. Electrically Transduced Gas Sensors Based on Semiconducting Metal Oxide Nanowires. Sensors 2020, 20, 6781. [Google Scholar] [CrossRef]
- Van Hal, R.E.G.; Eijkel, J.C.T.; Bergveld, P. A Novel Description of ISFET Sensitivity with the Buffer Capacity and Double-Layer Capacitance as Key Parameters. Sens. Actuators B Chem. 1995, 24, 201–205. [Google Scholar] [CrossRef]
- Yates, D.E.; Levine, S.; Healy, T.W. Site-Binding Model of the Electrical Double Layer at the Oxide/Water Interface. J. Chem. Soc. Faraday Trans. 1974, 70, 1807. [Google Scholar] [CrossRef]
- Da Silva Rodrigues, B.; Morimoto, N.; Sagazan, O.; Crand, S.; Le Bihan, F.; Mohammed-Brahim, T.; Bonnaud, O. Sensitive Continuous Monitoring of PH Thanks to Matrix of Several Suspended Gate Field Effect Transistors. ECS Trans. 2009, 23, 203–209. [Google Scholar]
- Ingebrandt, S.; Han, Y.; Nakamura, F.; Poghossian, A.; Schöning, M.J.; Offenhäusser, A. Label-Free Detection of Single Nucleotide Polymorphisms Utilizing the Differential Transfer Function of Field-Effect Transistors. Biosens. Bioelectron. 2007, 22, 2834–2840. [Google Scholar] [CrossRef]
- Kamahori, M.; Ishige, Y.; Shimoda, M. DNA Detection by an Extended-Gate FET Sensor with a High-Frequency Voltage Superimposed onto a Reference Electrode. Anal. Sci. 2007, 23, 75–79. [Google Scholar] [CrossRef][Green Version]
- Zhou, Q.; Zeng, W.; Chen, W.; Xu, L.; Kumar, R.; Umar, A. High Sensitive and Low-Concentration Sulfur Dioxide (SO2) Gas Sensor Application of Heterostructure NiO-ZnO Nanodisks. Sens. Actuators B Chem. 2019, 298, 126870. [Google Scholar] [CrossRef]
- Capone, S.; Zuppa, M.; Presicce, D.S.; Francioso, L.; Casino, F.; Siciliano, P. Metal Oxide Gas Sensor Array for the Detection of Diesel Fuel in Engine Oil. Sens. Actuators B Chem. 2008, 131, 125–133. [Google Scholar] [CrossRef]
- Fleischer, M. Advances in Application Potential of Adsorptive-Type Solid State Gas Sensors: High-Temperature Semiconducting Oxides and Ambient Temperature GasFET Devices. Meas. Sci. Technol. 2008, 19, 042001. [Google Scholar] [CrossRef]
- Korotcenkov, G. Gas Response Control through Structural and Chemical Modification of Metal Oxide Films: State of the Art and Approaches. Sens. Actuators B Chem. 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal Oxides for Solid-State Gas Sensors: What Determines Our Choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Meixner, H.; Gerblinger, J.; Lampe, U.; Fleischer, M. Thin-Film Gas Sensors Based on Semiconducting Metal Oxides. Sens. Actuators B Chem. 1995, 23, 119–125. [Google Scholar] [CrossRef]
- Ng, K.; Boussaid, F.; Bermak, A. A CMOS Single-Chip Gas Recognition Circuit for Metal Oxide Gas Sensor Arrays. Circuits Syst. I Regul. Pap. IEEE Trans. 2011, 58, 1569–1580. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.N.; Ling-Chung, S.K. Conducting Polymer Gas Sensors Part III: Results for Four Different Polymers and Five Different Vapours. Sens. Actuators 1989, 20, 287–292. [Google Scholar] [CrossRef]
- Han, S.; Huang, W.; Shi, W.; Yu, J. Performance Improvement of Organic Field-Effect Transistor Ammonia Gas Sensor Using ZnO/PMMA Hybrid as Dielectric Layer. Sens. Actuators B Chem. 2014, 203, 9–16. [Google Scholar] [CrossRef]
- Shi, W.; Yu, X.; Zheng, Y.; Yu, J. DNA Based Chemical Sensor for the Detection of Nitrogen Dioxide Enabled by Organic Field-Effect Transistor. Sens. Actuators B Chem. 2016, 222, 1003–1011. [Google Scholar] [CrossRef]
- Huang, W.; Diallo, A.K.; Dailey, J.L.; Besar, K.; Katz, H.E. Electrochemical Processes and Mechanistic Aspects of Field-Effect Sensors for Biomolecules. J. Mater. Chem. C 2015, 3, 6445–6470. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, Z.; Yan, B.; Zheng, Z.; Bao, Q.L.; Wu, T.; Li, C.M.; Shen, Z.X.; Zhang, J.X.; Gong, H.; et al. Multifunctional CuO Nanowire Devices: P-Type Field Effect Transistors and CO Gas Sensors. Nanotechnology 2009, 20, 085203. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Li, C.; Tang, T.; Liu, X.; Han, S.; Lei, B.; Zhou, C. Detection of NO2 down to Ppb Levels Using Individual and Multiple In2O3 Nanowire Devices. Nano Lett. 2004, 4, 1919–1924. [Google Scholar] [CrossRef]
- McAlpine, M.C.; Ahmad, H.; Wang, D.; Heath, J.R. Highly Ordered Nanowire Arrays on Plastic Substrates for Ultrasensitive Flexible Chemical Sensors. Nat. Mater. 2007, 6, 379–384. [Google Scholar] [CrossRef]
- Mabeck, J.T.; Malliaras, G.G. Chemical and Biological Sensors Based on Organic Thin-Film Transistors. Anal. Bioanal. Chem. 2005, 384, 343–353. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Chemical and Biological Sensing Applications Based on Graphene Field-Effect Transistors. Biosens. Bioelectron. 2010, 26, 1727–1730. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 Field-Effect Transistor for Next-Generation Label-Free Biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Patolsky, F.; Lieber, C.M. Nanowire Nanosensors. Mater. Today 2005, 8, 20–28. [Google Scholar] [CrossRef]
- Mishyn, V.; Hugo, A.; Rodrigues, T.; Aspermair, P.; Happy, H.; Marques, L.; Hurot, C.; Othmen, R.; Bouchiat, V.; Boukherroub, R.; et al. The Holy Grail of Pyrene-Based Surface Ligands on the Sensitivity of Graphene-Based Field Effect Transistors. Sens. Diagn. 2022, 1, 235–244. [Google Scholar] [CrossRef]
- Chia, J.S.Y.; Tan, M.T.T.; Khiew, P.S.; Chin, J.K.; Siong, C.W. A Bio-Electrochemical Sensing Platform for Glucose Based on Irreversible, Non-Covalent Pi–Pi Functionalization of Graphene Produced via a Novel, Green Synthesis Method. Sens. Actuators B Chem. 2015, 210, 558–565. [Google Scholar] [CrossRef]
- Streifer, J.A.; Kim, H.; Nichols, B.M.; Hamers, R.J. Covalent Functionalization and Biomolecular Recognition Properties of DNA-Modified Silicon Nanowires. Nanotechnology 2005, 16, 1868–1873. [Google Scholar] [CrossRef]
- Wenga, G.; Jacques, E.; Salaün, A.-C.; Rogel, R.; Pichon, L.; Geneste, F. Step-Gate Polysilicon Nanowires Field Effect Transistor Compatible with CMOS Technology for Label-Free DNA Biosensor. Biosens. Bioelectron. 2013, 40, 141–146. [Google Scholar] [CrossRef]
- Maki, W.C.; Mishra, N.N.; Cameron, E.G.; Filanoski, B.; Rastogi, S.K.; Maki, G.K. Nanowire-Transistor Based Ultra-Sensitive DNA Methylation Detection. Biosens. Bioelectron. 2008, 23, 780–787. [Google Scholar] [CrossRef]
- Singh, S.; Zack, J.; Kumar, D.; Srivastava, S.; Gupta, G.; Saluja, D.; Khan, M.A.; Singh, P. DNA Hybridization on Silicon Nanowires. Thin Solid Films 2010, 519, 1151–1155. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Chua, J.H.; Chee, R.-E.; Agarwal, A.; Wong, S.M.; Buddharaju, K.D.; Balasubramanian, N. Highly Sensitive Measurements of PNA-DNA Hybridization Using Oxide-Etched Silicon Nanowire Biosensors. Biosens. Bioelectron. 2008, 23, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Khamis, S.; Jones, R.; Johnson, A.T.C.; Preti, G.; Kwak, J.; Gelperin, A. DNA-Decorated Carbon Nanotube-Based FETs as Ultrasensitive Chemical Sensors: Discrimination of Homologues, Structural Isomers, and Optical Isomers. AIP Adv. 2012, 2, 022110. [Google Scholar] [CrossRef]
- Kong, T.; Su, R.; Zhang, B.; Zhang, Q.; Cheng, G. CMOS-Compatible, Label-Free Silicon-Nanowire Biosensors to Detect Cardiac Troponin I for Acute Myocardial Infarction Diagnosis. Biosens. Bioelectron. 2012, 34, 267–272. [Google Scholar] [CrossRef]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed Electrical Detection of Cancer Markers with Nanowire Sensor Arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Villamizar, R.A.; Maroto, A.; Rius, F.X.; Inza, I.; Figueras, M.J. Fast Detection of Salmonella Infantis with Carbon Nanotube Field Effect Transistors. Biosens. Bioelectron. 2008, 24, 279–283. [Google Scholar] [CrossRef]
- Mishra, N.N.; Maki, W.C.; Cameron, E.; Nelson, R.; Winterrowd, P.; Rastogi, S.K.; Filanoski, B.; Maki, G.K. Ultra-Sensitive Detection of Bacterial Toxin with Silicon Nanowire Transistor. Lab Chip 2008, 8, 868. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical Detection of Single Viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Zhang, L.; Huang, M.; Luo, Z.; Tay, G.; Lim, E.-J.; Kang, T.; Chen, Y. Silicon Nanowire Biosensor for Highly Sensitive and Rapid Detection of Dengue Virus. Sens. Actuators B Chem. 2010, 146, 138–144. [Google Scholar] [CrossRef]
- Juang, D.S.; Lin, C.-H.; Huo, Y.-R.; Tang, C.-Y.; Cheng, C.-R.; Wu, H.-S.; Huang, S.-F.; Kalnitsky, A.; Lin, C.-C. Proton-ELISA: Electrochemical Immunoassay on a Dual-Gated ISFET Array. Biosens. Bioelectron. 2018, 117, 175–182. [Google Scholar] [CrossRef]
- Mulla, Y.; Tuccori, E.; Magliulo, M.; Lattanzi, G.; Palazzo, G.; Persaud, K.; Torsi, L. Capacitance-Modulated Transistor Detects Odorant Binding Protein Chiral Interactions. Nat. Commun. 2015, 6, 6010. [Google Scholar] [CrossRef]
- Schöning, M.; Poghossian, A. Recent Advances in Biologically Sensitive Field-Effect Transistors (BioFETs). Analyst 2002, 127, 1137–1151. [Google Scholar] [CrossRef]
- Hanif, A.; Farooq, R.; Rehman, M.U.; Khan, R.; Majid, S.; Ganaie, M.A. Aptamer Based Nanobiosensors: Promising Healthcare Devices. Saudi Pharm. J. 2019, 27, 312–319. [Google Scholar] [CrossRef]
- Hayasaka, T.; Lin, A.; Copa, V.C.; Lopez, L.P.; Loberternos, R.A.; Ballesteros, L.I.M.; Kubota, Y.; Liu, Y.; Salvador, A.A.; Lin, L. An Electronic Nose Using a Single Graphene FET and Machine Learning for Water, Methanol, and Ethanol. Microsyst. Nanoeng. 2020, 6, 50. [Google Scholar] [CrossRef]
- Leu, M.; Doll, T.; Flietner, B.; Lechner, J.; Eisele, I. Evaluation of Gas Mixtures with Different Sensitive Layers Incorporated in Hybrid FET Structures. Sens. Actuators B Chem. 1994, 19, 678–681. [Google Scholar] [CrossRef]
- Li, L.; Lu, F.; Wang, C.; Zhang, F.; Liang, W.; Kuga, S.; Dong, Z.; Zhao, Y.; Huang, Y.; Wu, M. Flexible Double-Cross-Linked Cellulose-Based Hydrogel and Aerogel Membrane for Supercapacitor Separator. J. Mater. Chem. A 2018, 6, 24468–24478. [Google Scholar] [CrossRef]
- Macchia, E.; Sarcina, L.; Driescher, C.; Gounani, Z.; Tewari, A.; Osterbacka, R.; Palazzo, G.; Tricase, A.; Kovacs Vajna, Z.M.; Viola, F.; et al. Single-Molecule Bioelectronic Label-Free Assay of Both Protein and Genomic Markers of Pancreatic Mucinous Cysts’ in Whole Blood Serum. Adv. Electron. Mater. 2021, 7, 2100304. [Google Scholar] [CrossRef]
- Lee, K.; Yoo, Y.K.; Chae, M.-S.; Hwang, K.S.; Lee, J.; Kim, H.; Hur, D.; Lee, J.H. Highly Selective Reduced Graphene Oxide (RGO) Sensor Based on a Peptide Aptamer Receptor for Detecting Explosives. Sci. Rep. 2019, 9, 10297. [Google Scholar] [CrossRef]
- Dai, J.; Ogbeide, O.; Macadam, N.; Sun, Q.; Yu, W.; Li, Y.; Su, B.-L.; Hasan, T.; Huang, X.; Huang, W. Printed Gas Sensors. Chem. Soc. Rev. 2020, 49, 1756–1789. [Google Scholar] [CrossRef]
- Scaccabarozzi, A.D.; Basu, A.; Aniés, F.; Liu, J.; Zapata-Arteaga, O.; Warren, R.; Firdaus, Y.; Nugraha, M.I.; Lin, Y.; Campoy-Quiles, M.; et al. Doping Approaches for Organic Semiconductors. Chem. Rev. 2021, 122, 4420–4492. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. A Comparative Study of RGO-SnO2 and MWCNT-SnO2 Nanocomposites Based SO2 Gas Sensors. Sens. Actuators B Chem. 2017, 248, 980–986. [Google Scholar] [CrossRef]
- Palimar, S.; Kaushik, S.D.; Siruguri, V.; Swain, D.; Viegas, A.E.; Narayana, C.; Sundaram, N.G. Investigation of Ca Substitution on the Gas Sensing Potential of LaFeO3 Nanoparticles towards Low Concentration SO2 Gas. Dalton Trans. 2016, 45, 13547–13555. [Google Scholar] [CrossRef] [PubMed]
- Septiani, N.L.W.; Saputro, A.G.; Kaneti, Y.V.; Maulana, A.L.; Fathurrahman, F.; Lim, H.; Yuliarto, B.; Nugraha, M.I.; Dipojono, H.K.; Golberg, D.; et al. Hollow Zinc Oxide Microsphere–Multiwalled Carbon Nanotube Composites for Selective Detection of Sulfur Dioxide. ACS Appl. Nano Mater. 2020, 3, 8982–8996. [Google Scholar] [CrossRef]
- Joshi, N.; Hayasaka, T.; Liu, Y.; Liu, H.; Oliveira, O.N.; Lin, L. A Review on Chemiresistive Room Temperature Gas Sensors Based on Metal Oxide Nanostructures, Graphene and 2D Transition Metal Dichalcogenides. Microchim. Acta 2018, 185, 213. [Google Scholar] [CrossRef]
- Han, C.; Li, X.; Liu, Y.; Tang, Y.; Liu, M.; Li, X.; Shao, C.; Ma, J.; Liu, Y. Flexible All-Inorganic Room-Temperature Chemiresistors Based on Fibrous Ceramic Substrate and Visible-Light-Powered Semiconductor Sensing Layer. Adv. Sci. 2021, 8, 2102471. [Google Scholar] [CrossRef]
- Gholami, P.; Rashidi, A.; Khaleghi Abbasabadi, M.; Pourkhalil, M.; Jahangiri, M.; Izadi, N. Synthesis and Characterization of ZnO-Functionalized Multiwall Carbon Nanotubes Nanocomposite as NOx Gas Sensor. Res. Chem. Intermed. 2020, 46, 3911–3927. [Google Scholar] [CrossRef]
- Shetty, S.S.; Jayarama, A.; Bhat, S.; Karunasagar, I.; Pinto, R. A Review on Metal-Oxide Based Trace Ammonia Sensor for Detection of Renal Disease by Exhaled Breath Analysis. Mater. Today Proc. 2022, 55, 113–117. [Google Scholar] [CrossRef]
- Jung, S.; Baik, K.H.; Ren, F.; Pearton, S.J.; Jang, S. AlGaN/GaN Heterostructure Based Schottky Diode Sensors with ZnO Nanorods for Environmental Ammonia Monitoring Applications. ECS J. Solid State Sci. Technol. 2018, 7, Q3020–Q3024. [Google Scholar] [CrossRef]
- Kneer, J.; Knobelspies, S.; Bierer, B.; Wöllenstein, J.; Palzer, S. New Method to Selectively Determine Hydrogen Sulfide Concentrations Using CuO Layers. Sens. Actuators B Chem. 2016, 222, 625–631. [Google Scholar] [CrossRef]
- Hjiri, M.; Lassaad, E.M.; Leonardi, S.G.; Pistone, A.; Mavilia, L.; Neri, G. Al-Doped ZnO for Highly Sensitive CO Gas Sensors. Sens. Actuators B Chem. 2014, 196, 413–420. [Google Scholar] [CrossRef]
- Koralli, P.; Petropoulou, G.; Mouzakis, D.; Mousdis, G.; Kompitsas, M. Efficient CO Sensing by a CuO: Au Nanocomposite Thin Film Deposited by PLD on a Pyrex Tube. Sens. Actuators A Phys. 2021, 332, 113120. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, X.-Y.; Zhao, Z.-H.; Li, L.; Zhang, L.; Yao, H.-C.; Wang, J.-S.; Li, Z.-J. Highly Sensitive and Selective Acetone Sensor Based on C-Doped WO3 for Potential Diagnosis of Diabetes Mellitus. Sens. Actuators B Chem. 2014, 199, 210–219. [Google Scholar] [CrossRef]
- Lee, J.E.; Lim, C.K.; Park, H.J.; Song, H.; Choi, S.-Y.; Lee, D.-S. ZnO–CuO Core-Hollow Cube Nanostructures for Highly Sensitive Acetone Gas Sensors at the Ppb Level. ACS Appl. Mater. Interfaces 2020, 12, 35688–35697. [Google Scholar] [CrossRef] [PubMed]
- Nakate, U.T.; Patil, P.; Nakate, Y.T.; Na, S.-I.; Yu, Y.; Hahn, Y.-B. Ultrathin Ternary Metal Oxide Bi2MoO6 Nanosheets for High Performance Asymmetric Supercapacitor and Gas Sensor Applications. Appl. Surf. Sci. 2021, 551, 149422. [Google Scholar] [CrossRef]
- Rafiee, Z.; Roshan, H.; Sheikhi, M.H. Low Concentration Ethanol Sensor Based on Graphene/ZnO Nanowires. Ceram. Int. 2021, 47, 5311–5317. [Google Scholar] [CrossRef]
- Lou, C.; Lei, G.; Liu, X.; Xie, J.; Li, Z.; Zheng, W.; Goel, N.; Kumar, M.; Zhang, J. Design and Optimization Strategies of Metal Oxide Semiconductor Nanostructures for Advanced Formaldehyde Sensors. Coord. Chem. Rev. 2022, 452, 214280. [Google Scholar] [CrossRef]
- Jo, Y.K.; Jeong, S.-Y.; Moon, Y.K.; Jo, Y.-M.; Yoon, J.-W.; Lee, J.-H. Exclusive and Ultrasensitive Detection of Formaldehyde at Room Temperature Using a Flexible and Monolithic Chemiresistive Sensor. Nat. Commun. 2021, 12, 4955. [Google Scholar] [CrossRef]
- Moon, Y.K.; Jeong, S.-Y.; Jo, Y.-M.; Jo, Y.K.; Kang, Y.C.; Lee, J.-H. Highly Selective Detection of Benzene and Discrimination of Volatile Aromatic Compounds Using Oxide Chemiresistors with Tunable Rh-TiO2 Catalytic Overlayers. Adv. Sci. 2021, 8, 2004078. [Google Scholar] [CrossRef]
- Park, Y.; Yoo, R.; Ryull Park, S.; Lee, J.H.; Jung, H.; Lee, H.-S.; Lee, W. Highly Sensitive and Selective Isoprene Sensing Performance of ZnO Quantum Dots for a Breath Analyzer. Sens. Actuators B Chem. 2019, 290, 258–266. [Google Scholar] [CrossRef]
- Herrera-Rivera, R.; Olvera, M.; Maldonado, A. Propane Sensor Pellets Based on Nanopowders Cu-Doped ZnO. In Proceedings of the 2017 14th International Conference on Electrical Engineering, Computing Science and Automatic Control (CCE), Mexico City, Mexico, 20–22 October 2017; p. 5. [Google Scholar]
- Kim, J.-H.; Lee, J.-H.; Park, Y.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Toluene-and Benzene-Selective Gas Sensors Based on Pt-and Pd-Functionalized ZnO Nanowires in Self-Heating Mode. Sens. Actuators B Chem. 2019, 294, 78–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Qin, L.; Liu, D.; Liu, Y.; Liu, F.; Song, H.; Wang, Y.; Lu, G. Preparation of Au-Loaded TiO2 Pecan-Kernel-like and Its Enhanced Toluene Sensing Performance. Sens. Actuators B Chem. 2018, 255, 2240–2247. [Google Scholar] [CrossRef]
- Gao, H.; Wei, D.; Lin, P.; Liu, C.; Sun, P.; Shimanoe, K.; Yamazoe, N.; Lu, G. The Design of Excellent Xylene Gas Sensor Using Sn-Doped NiO Hierarchical Nanostructure. Sens. Actuators B Chem. 2017, 253, 1152–1162. [Google Scholar] [CrossRef]
- Moon, Y.K.; Jeong, S.-Y.; Kang, Y.C.; Lee, J.-H. Metal Oxide Gas Sensors with Au Nanocluster Catalytic Overlayer: Toward Tuning Gas Selectivity and Response Using a Novel Bilayer Sensor Design. ACS Appl. Mater. Interfaces 2019, 11, 32169–32177. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, Y.; Guo, J.; Zhao, L.; Wang, T.; Yan, X.; Wang, C.; Liu, F.; Sun, P.; Lu, G. Highly Sensitive and Selective Xylene Sensor Based on Pp Heterojunctions Composites Derived from Off-Stoichiometric Cobalt Tungstate. Sens. Actuators B Chem. 2022, 351, 130973. [Google Scholar] [CrossRef]
- Saito, N.; Haneda, H.; Watanabe, K.; Shimanoe, K.; Sakaguchi, I. Highly Sensitive Isoprene Gas Sensor Using Au-Loaded Pyramid-Shaped ZnO Particles. Sens. Actuators B Chem. 2021, 326, 128999. [Google Scholar] [CrossRef]
- Poloju, M.; Jayababu, N.; Rao, E.V.; Rao, R.G.; Reddy, M.R. Development of CdO/ZnO Nanocomposites for the Rapid Detection and Discrimination of n-Butanol. Surf. Interfaces 2020, 20, 100586. [Google Scholar] [CrossRef]
- Srinivasan, P.; Mehtre, S. Zinc Oxide Nanoparticles from Coriandrum Sativum as Sensor for Detection of N-Butanol and Nitric Oxide Gas. Mater. Today Proc. 2022, 51, 1760–1764. [Google Scholar] [CrossRef]
- Wahab, R.; Khan, F.; Ahmad, N.; Alam, M. Molybdenum Rods Assembled with Nanosheets: A High Catalytic Material for Phenol Sensing. Mater. Today Chem. 2020, 18, 100347. [Google Scholar] [CrossRef]
- Rahman, M.M.; Balkhoyor, H.B.; Asiri, A.M. Phenolic Sensor Development Based on Chromium Oxide-Decorated Carbon Nanotubes for Environmental Safety. J. Environ. Manag. 2017, 188, 228–237. [Google Scholar] [CrossRef]
- Maccato, C.; Bigiani, L.; Carraro, G.; Gasparotto, A.; Sada, C.; Comini, E.; Barreca, D. Toward the Detection of Poisonous Chemicals and Warfare Agents by Functional Mn3O4 Nanosystems. ACS Appl. Mater. Interfaces 2018, 10, 12305–12310. [Google Scholar] [CrossRef]
- Xu, X.; Arab Pour Yazdi, M.; Sanchez, J.-B.; Billard, A.; Berger, F.; Martin, N. Exploiting the Dodecane and Ozone Sensing Capabilities of Nanostructured Tungsten Oxide Films. Sens. Actuators B Chem. 2018, 266, 773–783. [Google Scholar] [CrossRef]
- Xu, X.; Yazdi, M.A.P.; Sanchez, J.-B.; Billard, A.; Berger, F.; Martin, N. Reactive Co-Sputtering of Tungsten Oxide Thin Films by Glancing Angle Deposition for Gas Sensors. Mater. Today Proc. 2019, 6, 314–318. [Google Scholar] [CrossRef]
- Moon, H.G.; Jung, Y.; Han, S.D.; Shim, Y.-S.; Jung, W.-S.; Lee, T.; Lee, S.; Park, J.H.; Baek, S.-H.; Kim, J.-S.; et al. All Villi-like Metal Oxide Nanostructures-Based Chemiresistive Electronic Nose for an Exhaled Breath Analyzer. Sens. Actuators B Chem. 2018, 257, 295–302. [Google Scholar] [CrossRef]
- Han, J.-K.; Kang, M.; Jeong, J.; Cho, I.; Yu, J.-M.; Yoon, K.-J.; Park, I.; Choi, Y.-K. Artificial Olfactory Neuron for an In-Sensor Neuromorphic Nose. Adv. Sci. 2022, 15, 2106017. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Wang, Y.; Zhang, C.; Pittman, Z.A.; Oliveira, A.M.; Fu, K.; Zhao, J.; Srivastava, R.; Willis, B.G. Classification of Tea Aromas Using Multi-Nanoparticle Based Chemiresistor Arrays. Sensors 2019, 19, 2547. [Google Scholar] [CrossRef] [PubMed]
- Bano, K.; Bajwa, S.Z.; Bassous, N.J.; Webster, T.J.; Shaheen, A.; Taj, A.; Hameed, S.; Tehseen, B.; Dai, Z.; Iqbal, M.Z.; et al. Development of Biocompatible 1D CuO Nanoneedles and Their Potential for Sensitive, Mass-Based Detection of Anti-Tuberculosis Drugs. Appl. Nanosci. 2019, 9, 1341–1351. [Google Scholar] [CrossRef]
- Alavi-Tabari, S.A.R.; Khalilzadeh, M.A.; Karimi-Maleh, H. Simultaneous Determination of Doxorubicin and Dasatinib as Two Breast Anticancer Drugs Uses an Amplified Sensor with Ionic Liquid and ZnO Nanoparticle. J. Electroanal. Chem. 2018, 811, 84–88. [Google Scholar] [CrossRef]
- Ansari, S.; Ansari, M.S.; Satsangee, S.P.; Jain, R. WO3 Decorated Graphene Nanocomposite Based Electrochemical Sensor: A Prospect for the Detection of Anti-Anginal Drug. Anal. Chim. Acta 2019, 1046, 99–109. [Google Scholar] [CrossRef]
- Gupta, V.; Karimi-Maleh, H.; Agarwal, S.; Karimi, F.; Bijad, M.; Farsi, M.; Shahidi, S.-A. Fabrication of a Food Nano-Platform Sensor for Determination of Vanillin in Food Samples. Sensors 2018, 18, 2817. [Google Scholar] [CrossRef]
- Gobbi, E.; Falasconi, M.; Zambotti, G.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Rapid Diagnosis of Enterobacteriaceae in Vegetable Soups by a Metal Oxide Sensor Based Electronic Nose. Sens. Actuators B Chem. 2015, 207, 1104–1113. [Google Scholar] [CrossRef]
- Konduru, T.; Rains, G.; Li, C. A Customized Metal Oxide Semiconductor-Based Gas Sensor Array for Onion Quality Evaluation: System Development and Characterization. Sensors 2015, 15, 1252–1273. [Google Scholar] [CrossRef]
- Ge, Y.; Wei, Z.; Li, Y.; Qu, J.; Zu, B.; Dou, X. Highly Sensitive and Rapid Chemiresistive Sensor towards Trace Nitro-Explosive Vapors Based on Oxygen Vacancy-Rich and Defective Crystallized In-Doped ZnO. Sens. Actuators B Chem. 2017, 244, 983–991. [Google Scholar] [CrossRef]
- Sowmya, B.; John, A.; Panda, P. A Review on Metal-Oxide Based Pn and Nn Heterostructured Nano-Materials for Gas Sensing Applications. Sens. Int. 2021, 2, 100085. [Google Scholar]
- Knoblauch, J.; Illyaskutty, N.; Kohler, H. Early Detection of Fires in Electrical Installations by Thermally Modulated SnO2/Additive-Multi Sensor Arrays. Sens. Actuators B Chem. 2015, 217, 36–40. [Google Scholar] [CrossRef]
- Khairy, M.; Ayoub, H.A.; Banks, C.E. Non-Enzymatic Electrochemical Platform for Parathion Pesticide Sensing Based on Nanometer-Sized Nickel Oxide Modified Screen-Printed Electrodes. Food Chem. 2018, 255, 104–111. [Google Scholar] [CrossRef]
- Khan, N.; Athar, T.; Fouad, H.; Umar, A.; Ansari, Z.A.; Ansari, S.G. Application of Pristine and Doped SnO2 Nanoparticles as a Matrix for Agro-Hazardous Material (Organophosphate) Detection. Sci. Rep. 2017, 7, 42510. [Google Scholar] [CrossRef]
- Ulloa, A.M.; Glassmaker, N.; Oduncu, M.R.; Xu, P.; Wei, A.; Cakmak, M.; Stanciu, L. Roll-to-Roll Manufactured Sensors for Nitroaromatic Organophosphorus Pesticides Detection. ACS Appl. Mater. Interfaces 2021, 13, 35961–35971. [Google Scholar] [CrossRef]
- Lange, U.; Mirsky, V.M. Chemiresistors Based on Conducting Polymers: A Review on Measurement Techniques. Anal. Chim. Acta 2011, 687, 105–113. [Google Scholar] [CrossRef]
- Yang, S.; Bintinger, J.; Park, S.; Jain, S.; Alexandrou, K.; Fruhmann, P.; Besar, K.; Katz, H.; Kymissis, I. Inexpensive, Versatile, and Robust USB-Driven Sensor Platform. IEEE Sens. Lett. 2017, 1, 5000304. [Google Scholar] [CrossRef]
- Shirakawa, H. Nobel Lecture: The Discovery of Polyacetylene Film—The Dawning of an Era of Conducting Polymers. Rev. Mod. Phys. 2001, 73, 713–718. [Google Scholar] [CrossRef]
- Malinauskas, M.; Žukauskas, A.; Hasegawa, S.; Hayasaki, Y.; Mizeikis, V.; Buividas, R.; Juodkazis, S. Ultrafast Laser Processing of Materials: From Science to Industry. Light Sci. Appl. 2016, 5, e16133. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.-Q.; Liu, Y.; Zhong, D.; Nikzad, S.; Liu, S.; Yu, Z.; Liu, D.; Wu, H.-C.; Zhu, C.; Li, J.; et al. Monolithic Optical Microlithography of High-Density Elastic Circuits. Science 2021, 373, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Shahid, N.; Rappon, T.; Berta, W. Applications of Artificial Neural Networks in Health Care Organizational Decision-Making: A Scoping Review. PLoS ONE 2019, 14, e0212356. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.; Kim, T.; Eom, T.H.; Kim, S.Y.; Jang, H.W. Chemoresistive Materials for Electronic Nose: Progress, Perspectives, and Challenges. InfoMat 2019, 1, 289–316. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia Gas Sensors: A Comprehensive Review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Duc, C.; Boukhenane, M.L.; Fagniez, T.; Khouchaf, L.; Redon, N.; Wojkiewicz, J.-L. Conductive Polymer Composites for Hydrogen Sulphide Sensors Working at Sub-PPM Level and Room Temperature. Sensors 2021, 21, 6529. [Google Scholar] [CrossRef]
- Phillips, B.; Banerjee, S.; Tu, X.; Fang, L. Electrical Vapour Sensing with Macrocyclic Molecular Receptors. Supramol. Chem. 2020, 32, 165–177. [Google Scholar] [CrossRef]
- Park, H.; Kim, D.-H.; Ma, B.S.; Shin, E.; Kim, Y.; Kim, T.-S.; Kim, F.S.; Kim, I.-D.; Kim, B.J. High-Performance, Flexible NO2 Chemiresistors Achieved by Design of Imine-Incorporated n-Type Conjugated Polymers. Adv. Sci. 2022, 9, 2200270. [Google Scholar] [CrossRef]
- Amini, A.; Jafari, A.; Vafaei, M.; Mahmoodian, M. Stability Enhancement of Polypyrrole Thin Film Ammonia Sensor by Camphor Sulfonic Acid Dopant. J. Mater. Sci. Mater. Electron. 2022, 33, 1293–1306. [Google Scholar] [CrossRef]
- Tarek, M.; Khozemy, E.; Ghobashy, M. Radiation Synthesis of Gas Sensor Based on Polyaniline Nanoflake-Poly Vinyl Alcohol Film for Four Hazardous Gases (NH3, CO2, H2S and Phenol). Arab. J. Nucl. Sci. Appl. 2020, 53, 210–221. [Google Scholar]
- Danesh, E.; Ghaffarian, S.R.; Molla-Abbasi, P. Non-Solvent Induced Phase Separation as a Method for Making High-Performance Chemiresistors Based on Conductive Polymer Nanocomposites. Sens. Actuators B Chem. 2011, 155, 562–567. [Google Scholar] [CrossRef]
- Zamiri, G.; Haseeb, A.S.M.A. Recent Trends and Developments in Graphene/Conducting Polymer Nanocomposites Chemiresistive Sensors. Materials 2020, 13, 3311. [Google Scholar] [CrossRef]
- Yan, Y.; Yang, G.; Xu, J.-L.; Zhang, M.; Kuo, C.-C.; Wang, S.-D. Conducting Polymer-Inorganic Nanocomposite-Based Gas Sensors: A Review. Sci. Technol. Adv. Mater. 2020, 21, 768–786. [Google Scholar] [CrossRef]
- Chang, L.-Y.; Chuang, M.-Y.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J.; Yeh, P.-H.; Chen, J.-N. One-Minute Fish Freshness Evaluation by Testing the Volatile Amine Gas with an Ultrasensitive Porous-Electrode-Capped Organic Gas Sensor System. ACS Sens. 2017, 2, 531–539. [Google Scholar] [CrossRef]
- Ly, T.N.; Park, S. Highly Sensitive Ammonia Sensor for Diagnostic Purpose Using Reduced Graphene Oxide and Conductive Polymer. Sci. Rep. 2018, 8, 18030. [Google Scholar] [CrossRef]
- Jisha, P.; Suma, M. Synthesis and Electrical Characterization of Protonic Acid Doped Polyaniline for Detection of Monoterpene Vapours to Diagnose Malaria. In Proceedings of the 2019 International Conference on Data Science and Communication (IconDSC), Bangalore, India, 1–2 March 2019; pp. 1–5. [Google Scholar]
- Pirovano, P.; Dorrian, M.; Shinde, A.; Donohoe, A.; Brady, A.J.; Moyna, N.M.; Wallace, G.; Diamond, D.; McCaul, M. A Wearable Sensor for the Detection of Sodium and Potassium in Human Sweat during Exercise. Talanta 2020, 219, 121145. [Google Scholar] [CrossRef]
- Wilson, A.D.; Lester, D.G.; Oberle, C.S. Development of Conductive Polymer Analysis for the Rapid Detection and Identification of Phytopathogenic Microbes. Phytopathology 2004, 94, 419–431. [Google Scholar] [CrossRef]
- Matindoust, S.; Farzi, G.; Nejad, M.B.; Shahrokhabadi, M.H. Polymer-Based Gas Sensors to Detect Meat Spoilage: A Review. React. Funct. Polym. 2021, 165, 104962. [Google Scholar] [CrossRef]
- Samanta, S.; Roy, P.; Kar, P. Sensing of Ethanol and Other Alcohol Contaminated Ethanol by Conducting Functional Poly (o-Phenylenediamine). Mater. Sci. Eng. B 2020, 256, 114541. [Google Scholar] [CrossRef]
- Supraja, P.; Tripathy, S.; Singh, R.; Singh, V.; Chaudhury, G.; Singh, S.G. Towards Point-of-Care Diagnosis of Alzheimer’s Disease: Multi-Analyte Based Portable Chemiresistive Platform for Simultaneous Detection of β-Amyloid (1–40) and (1–42) in Plasma. Biosens. Bioelectron. 2021, 186, 113294. [Google Scholar] [CrossRef]
- Wang, M.-H.; Ji, B.-W.; Gu, X.-W.; Tian, H.-C.; Kang, X.-Y.; Yang, B.; Wang, X.-L.; Chen, X.; Li, C.-Y.; Liu, J.-Q. Direct Electrodeposition of Graphene Enhanced Conductive Polymer on Microelectrode for Biosensing Application. Biosens. Bioelectron. 2018, 99, 99–107. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, W.; Yan, K.; Pan, L.; Yu, G. Doping Engineering of Conductive Polymer Hydrogels and Their Application in Advanced Sensor Technologies. Chem. Sci. 2019, 10, 6232–6244. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, X.; Sui, G.; Du, R.; Zhang, Q.; Fu, Q. Plasma Modification of PU Foam for Piezoresistive Sensor with High Sensitivity, Mechanical Properties and Long-Term Stability. Chem. Eng. J. 2020, 381, 122666. [Google Scholar] [CrossRef]
- Khan, Z.U.; Edberg, J.; Hamedi, M.M.; Gabrielsson, R.; Granberg, H.; Wågberg, L.; Engquist, I.; Berggren, M.; Crispin, X. Thermoelectric Polymers and Their Elastic Aerogels. Adv. Mater. 2016, 28, 4556–4562. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-Y.; Zhang, Z. A Novel Gas Sensor Signal Drift Adjustment Method Based on Controlled Measurement. In Proceedings of the 2018 China Semiconductor Technology International Conference (CSTIC), Shanghai, China, 11–12 March 2018; pp. 1–5. [Google Scholar]
- John, A.T.; Murugappan, K.; Nisbet, D.R.; Tricoli, A. An Outlook of Recent Advances in Chemiresistive Sensor-Based Electronic Nose Systems for Food Quality and Environmental Monitoring. Sensors 2021, 21, 2271. [Google Scholar] [CrossRef] [PubMed]
- Amrani, M.E.H.; Dowdeswell, R.M.; Payne, P.A.; Persaud, K.C. An Intelligent Gas Sensing System. Sens. Actuators B Chem. 1997, 44, 512–516. [Google Scholar] [CrossRef]
- Potyrailo, R.A. Toward High Value Sensing: Monolayer-Protected Metal Nanoparticles in Multivariable Gas and Vapor Sensors. Chem. Soc. Rev. 2017, 46, 5311–5346. [Google Scholar] [CrossRef] [PubMed]
- Potyrailo, R.A. Multivariable Sensors for Ubiquitous Monitoring of Gases in the Era of Internet of Things and Industrial Internet. Chem. Rev. 2016, 116, 11877–11923. [Google Scholar] [CrossRef]
- Gruber, J.; Nascimento, H.M.; Yamauchi, E.Y.; Li, R.W.C.; Esteves, C.H.A.; Rehder, G.P.; Gaylarde, C.C.; Shirakawa, M.A. A Conductive Polymer Based Electronic Nose for Early Detection of Penicillium Digitatum in Post-Harvest Oranges. Mater. Sci. Eng. C 2013, 33, 2766–2769. [Google Scholar] [CrossRef]
- Cordeiro, J.R.; Li, R.W.C.; Takahashi, É.S.; Rehder, G.P.; Ceccantini, G.; Gruber, J. Wood Identification by a Portable Low-Cost Polymer-Based Electronic Nose. RSC Adv. 2016, 6, 109945–109949. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Yao, D.-J. Intelligent Gas-Sensing Systems and Their Applications. J. Micromech. Microeng. 2018, 28, 093001. [Google Scholar] [CrossRef]
- Chandler, R.; Das, A.; Gibson, T.; Dutta, R. Detection of Oil Pollution in Seawater: Biosecurity Prevention Using Electronic Nose Technology. In Proceedings of the 2015 31st IEEE International Conference on Data Engineering Workshops, Seoul, Korea, 13–17 April 2015; pp. 98–100. [Google Scholar]
- Yang, Y.; Liu, H.; Gu, Y. A Model Transfer Learning Framework with Back-Propagation Neural Network for Wine and Chinese Liquor Detection by Electronic Nose. IEEE Access 2020, 8, 105278–105285. [Google Scholar] [CrossRef]
- Tiggemann, L.; Ballen, S.; Bocalon, C.; Graboski, A.M.; Manzoli, A.; de Paula Herrmann, P.S.; Steffens, J.; Valduga, E.; Steffens, C. Low-Cost Gas Sensors with Polyaniline Film for Aroma Detection. J. Food Eng. 2016, 180, 16–21. [Google Scholar] [CrossRef]
- Tan, C.; Xie, D.; Liu, Y.; Peng, W.; Li, X.; Ai, L.; Wu, C.; Wen, C.; Huang, X.; Guo, J. Identification of Different Bile Species and Fermentation Times of Bile Arisaema Based on an Intelligent Electronic Nose and Least Squares Support Vector Machine. Anal. Chem. 2018, 90, 3460–3466. [Google Scholar] [CrossRef]
- Julian, T.; Hidayat, S.N.; Rianjanu, A.; Dharmawan, A.B.; Wasisto, H.S.; Triyana, K. Intelligent Mobile Electronic Nose System Comprising a Hybrid Polymer-Functionalized Quartz Crystal Microbalance Sensor Array. ACS Omega 2020, 5, 29492–29503. [Google Scholar] [CrossRef]
- Buck, L.; Axel, R. A Novel Multigene Family May Encode Odorant Receptors: A Molecular Basis for Odor Recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Schwarze, M.; Tietze, M.L.; Ortmann, F.; Kleemann, H.; Leo, K. Universal Limit for Air-Stable Molecular n-Doping in Organic Semiconductors. ACS Appl. Mater. Interfaces 2020, 12, 40566–40571. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, H.; Liu, A.; Zhu, H.-H.; Li, W.; Lin, Y.-F.; Noh, Y.-Y. Doping: A Key Enabler for Organic Transistors. Adv. Mater. 2018, 30, 1801830. [Google Scholar] [CrossRef]
- Salzmann, I.; Heimel, G.; Oehzelt, M.; Winkler, S.; Koch, N. Molecular Electrical Doping of Organic Semiconductors: Fundamental Mechanisms and Emerging Dopant Design Rules. Acc. Chem. Res. 2016, 49, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Olson, N.; Bae, J. Biosensors—Publication Trends and Knowledge Domain Visualization. Sensors 2019, 19, 2615. [Google Scholar] [CrossRef]
- Rissin, D.M.; Kan, C.W.; Campbell, T.G.; Howes, S.C.; Fournier, D.R.; Song, L.; Piech, T.; Patel, P.P.; Chang, L.; Rivnak, A.J.; et al. Single-Molecule Enzyme-Linked Immunosorbent Assay Detects Serum Proteins at Subfemtomolar Concentrations. Nat. Biotechnol. 2010, 28, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Hindson, B.J.; Ness, K.D.; Masquelier, D.A.; Belgrader, P.; Heredia, N.J.; Makarewicz, A.J.; Bright, I.J.; Lucero, M.Y.; Hiddessen, A.L.; Legler, T.C.; et al. High-Throughput Droplet Digital PCR System for Absolute Quantitation of DNA Copy Number. Anal. Chem. 2011, 83, 8604–8610. [Google Scholar] [CrossRef] [PubMed]
- Marras, S.A.E. Efficiencies of Fluorescence Resonance Energy Transfer and Contact-Mediated Quenching in Oligonucleotide Probes. Nucleic Acids Res. 2002, 30, 122e. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, D.; Sakabe, M.; Kamiya, M.; Yamamoto, K.; Hiratake, J.; Ogawa, M.; Kosaka, N.; Choyke, P.L.; Nagano, T.; Kobayashi, H.; et al. Sensitive β-Galactosidase-Targeting Fluorescence Probe for Visualizing Small Peritoneal Metastatic Tumours In Vivo. Nat. Commun. 2015, 6, 6463. [Google Scholar] [CrossRef]
- Tricase, A.; Imbriano, A.; Macchia, E.; Sarcina, L.; Scandurra, C.; Torricelli, F.; Cioffi, N.; Torsi, L.; Bollella, P. Enzyme Based Amperometric Wide Field Biosensors: Is Single-Molecule Detection Possible? Electrochem. Sci. Adv. 2022, e2100215. [Google Scholar] [CrossRef]
- Macchia, E.; Torricelli, F.; Bollella, P.; Sarcina, L.; Tricase, A.; Di Franco, C.; Österbacka, R.; Kovács-Vajna, Z.M.; Scamarcio, G.; Torsi, L. Large-Area Interfaces for Single-Molecule Label-Free Bioelectronic Detection. Chem. Rev. 2022, 122, 4636–4699. [Google Scholar] [CrossRef]
- Macchia, E.; Tiwari, A.; Manoli, K.; Holzer, B.; Ditaranto, N.; Picca, R.A.; Cioffi, N.; Di Franco, C.; Scamarcio, G.; Palazzo, G.; et al. Label-Free and Selective Single-Molecule Bioelectronic Sensing with a Millimeter-Wide Self-Assembled Monolayer of Anti-Immunoglobulins. Chem. Mater. 2019, 31, 6476–6483. [Google Scholar] [CrossRef]
- Macchia, E.; Manoli, K.; Holzer, B.; Franco, C.D.; Ghittorelli, M.; Torricelli, F.; Alberga, D.; Mangiatordi, G.F.; Palazzo, G.; Scamarcio, G.; et al. Single-Molecule Detection with a Millimetre-Sized Transistor. Nat. Commun. 2018, 9, 3223. [Google Scholar] [CrossRef]
- International Human Genome Sequencing Consortium; Whitehead Institute for Biomedical Research Center for Genome Research; Lander, E.S.; Linton, L.M.; Birren, B.; Nusbaum, C.; Zody, M.C.; Baldwin, J.; Devon, K.; Dewar, K.; et al. Initial Sequencing and Analysis of the Human Genome. Nature 2001, 409, 860–921. [Google Scholar] [CrossRef]
- De Wit, S.; van Dalum, G.; Lenferink, A.T.M.; Tibbe, A.G.J.; Hiltermann, T.J.N.; Groen, H.J.M.; van Rijn, C.J.M.; Terstappen, L.W.M.M. The Detection of EpCAM+ and EpCAM–Circulating Tumor Cells. Sci. Rep. 2015, 5, 12270. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.; Kinde, I.; Agrawal, N.; Bartlett, B.; Wang, H.; Luber, B.; Kinzler, K.; Vogelstein, B.; et al. Abstract 5606: Detection of Circulating Tumor DNA in Early and Late Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Nedaeinia, R.; Manian, M.; Jazayeri, M.H.; Ranjbar, M.; Salehi, R.; Sharifi, M.; Mohaghegh, F.; Goli, M.; Jahednia, S.H.; Avan, A.; et al. Circulating Exosomes and Exosomal MicroRNAs as Biomarkers in Gastrointestinal Cancer. Cancer Gene Ther. 2017, 24, 48–56. [Google Scholar] [CrossRef]
- Schübeler, D. Function and Information Content of DNA Methylation. Nature 2015, 517, 321–326. [Google Scholar] [CrossRef]
- Jin, Z.; Liu, Y. DNA Methylation in Human Diseases. Genes Dis. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Constâncio, V.; Nunes, S.P.; Henrique, R.; Jerónimo, C. DNA Methylation-Based Testing in Liquid Biopsies as Detection and Prognostic Biomarkers for the Four Major Cancer Types. Cells 2020, 9, 624. [Google Scholar] [CrossRef]
- Moss, J.; Zick, A.; Grinshpun, A.; Carmon, E.; Maoz, M.; Ochana, B.L.; Abraham, O.; Arieli, O.; Germansky, L.; Meir, K.; et al. Circulating Breast-Derived DNA Allows Universal Detection and Monitoring of Localized Breast Cancer. Ann. Oncol. 2020, 31, 395–403. [Google Scholar] [CrossRef]
- Tham, C.; Chew, M.; Soong, R.; Lim, J.; Ang, M.; Tang, C.; Zhao, Y.; Ong, S.Y.K.; Liu, Y. Postoperative Serum Methylation Levels of TAC1 and SEPT9 Are Independent Predictors of Recurrence and Survival of Patients with Colorectal Cancer. Cancer 2014, 120, 3131–3141. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, J.; Zhang, R.; Li, F.; Li, Y.; Jia, Y. Multiprobe Assay for Clinical SEPT9 Methylation Based on the Carbon Dot-Modified Liquid-Exfoliated Graphene Field Effect Transistor with a Potential to Present a Methylation Panorama. ACS Omega 2020, 5, 16228–16237. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, S.; Wang, G.; Zhang, S.; Luo, W.; Qin, Z.; Bi, X.; Tan, Y.; Meng, M.; Qin, J.; et al. SMAD7 Methylation as a Novel Marker in Atherosclerosis. Biochem. Biophys. Res. Commun. 2018, 496, 700–705. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Chen, X.; Han, L.; Li, L.; Liu, Y. MTHFD1 Promoter Hypermethylation Increases the Risk of Hypertension. Clin. Exp. Hypertens. 2019, 41, 422–427. [Google Scholar] [CrossRef]
- Nakatochi, M.; Ichihara, S.; Yamamoto, K.; Naruse, K.; Yokota, S.; Asano, H.; Matsubara, T.; Yokota, M. Epigenome-Wide Association of Myocardial Infarction with DNA Methylation Sites at Loci Related to Cardiovascular Disease. Clin. Epigenet. 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Xia, Y.; Bell, C.G.; Yet, I.; Ferreira, T.; Ward, K.J.; Gao, F.; Loomis, A.K.; Hyde, C.L.; Wu, H.; et al. An Integrated Epigenomic Analysis for Type 2 Diabetes Susceptibility Loci in Monozygotic Twins. Nat. Commun. 2014, 5, 5719. [Google Scholar] [CrossRef] [PubMed]
- Sayols-Baixeras, S.; Subirana, I.; Fernández-Sanlés, A.; Sentí, M.; Lluís-Ganella, C.; Marrugat, J.; Elosua, R. DNA Methylation and Obesity Traits: An Epigenome-Wide Association Study. The REGICOR Study. Epigenetics 2017, 12, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Arosio, B.; Falconi, A.; Micioni Di Bonaventura, M.V.; Karimi, M.; Mari, D.; Casati, M.; Maccarrone, M.; D’Addario, C. Global Changes in DNA Methylation in Alzheimer’s Disease Peripheral Blood Mononuclear Cells. Brain Behav. Immun. 2015, 45, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Dumaop, W.; Galasko, D.; Desplats, P. Distinctive Patterns of DNA Methylation Associated with Parkinson Disease: Identification of Concordant Epigenetic Changes in Brain and Peripheral Blood Leukocytes. Epigenetics 2013, 8, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Smith, D.; Richards, D.; et al. Sensitive and Specific Multi-Cancer Detection and Localization Using Methylation Signatures in Cell-Free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- Galardi, F.; De Luca, F.; Romagnoli, D.; Biagioni, C.; Moretti, E.; Biganzoli, L.; Di Leo, A.; Migliaccio, I.; Malorni, L.; Benelli, M. Cell-Free DNA-Methylation-Based Methods and Applications in Oncology. Biomolecules 2020, 10, 1677. [Google Scholar] [CrossRef]
- Ilijazi, D.; Pulverer, W.; Ertl, I.E.; Lemberger, U.; Kimura, S.; Abufaraj, M.; D’Andrea, D.; Pradere, B.; Bruchbacher, A.; Graf, A.; et al. Discovery of Molecular DNA Methylation-Based Biomarkers through Genome-Wide Analysis of Response Patterns to BCG for Bladder Cancer. Cells 2020, 9, 1839. [Google Scholar] [CrossRef]
- Gampenrieder, S.P.; Rinnerthaler, G.; Hackl, H.; Pulverer, W.; Weinhaeusel, A.; Ilic, S.; Hufnagl, C.; Hauser-Kronberger, C.; Egle, A.; Risch, A.; et al. DNA Methylation Signatures Predicting Bevacizumab Efficacy in Metastatic Breast Cancer. Theranostics 2018, 8, 2278–2288. [Google Scholar] [CrossRef]
- Zaenker, P.; Ziman, M.R. Serologic Autoantibodies as Diagnostic Cancer Biomarkers—A Review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2161–2181. [Google Scholar] [CrossRef]
- Luna Coronell, J.A.; Syed, P.; Sergelen, K.; Gyurján, I.; Weinhäusel, A. The Current Status of Cancer Biomarker Research Using Tumour-Associated Antigens for Minimal Invasive and Early Cancer Diagnostics. J. Proteom. 2012, 76, 102–115. [Google Scholar] [CrossRef]
- Luna-Coronell, J.A.; Vierlinger, K.; Gamperl, M.; Hofbauer, J.; Berger, I.; Weinhäusel, A. The Prostate Cancer Immunome: In Silico Functional Analysis of Antigenic Proteins from Microarray Profiling with IgG. Proteomics 2016, 16, 1204–1214. [Google Scholar] [CrossRef]
- Luna Coronell, J.A.; Sergelen, K.; Hofer, P.; Gyurján, I.; Brezina, S.; Hettegger, P.; Leeb, G.; Mach, K.; Gsur, A.; Weinhäusel, A. The Immunome of Colon Cancer: Functional In Silico Analysis of Antigenic Proteins Deduced from IgG Microarray Profiling. Genom. Proteom. Bioinform. 2018, 16, 73–84. [Google Scholar] [CrossRef]
- Jodeleit, H.; Milchram, L.; Soldo, R.; Beikircher, G.; Schönthaler, S.; Al-Amodi, O.; Wolf, E.; Beigel, F.; Weinhäusel, A.; Siebeck, M.; et al. Autoantibodies as Diagnostic Markers and Potential Drivers of Inflammation in Ulcerative Colitis. PLoS ONE 2020, 15, e0228615. [Google Scholar] [CrossRef]
- Sullivan, F.M.; Mair, F.S.; Anderson, W.; Armory, P.; Briggs, A.; Chew, C.; Dorward, A.; Haughney, J.; Hogarth, F.; Kendrick, D.; et al. Earlier Diagnosis of Lung Cancer in a Randomised Trial of an Autoantibody Blood Test Followed by Imaging. Eur. Respir. J. 2020, 57, 2000670. [Google Scholar] [CrossRef]
- Hettegger, P.; Huber, J.; Paßecker, K.; Soldo, R.; Kegler, U.; Nöhammer, C.; Weinhäusel, A. High Similarity of IgG Antibody Profiles in Blood and Saliva Opens Opportunities for Saliva Based Serology. PLoS ONE 2019, 14, e0218456. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Špilak, A.; Brachner, A.; Kegler, U.; Neuhaus, W.; Noehammer, C. Implications and Pitfalls for Cancer Diagnostics Exploiting Extracellular Vesicles. Adv. Drug Deliv. Rev. 2021, 175, 113819. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy Delivery Tools and Biomarkers of Diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Zhou, X.; Wen, W.; Shan, X.; Zhu, W.; Xu, J.; Guo, R.H.; Cheng, W.F.; Wang, F.; Qi, L.W.; Chen, Y.; et al. A six-microRNA panel in plasma was identified as a potential biomarker for lung adenocarcinoma diagnosis. Oncotarget 2016, 8, 6513–6525. [Google Scholar] [CrossRef] [PubMed]
- Jacob, H.; Stanisavljevic, L.; Storli, K.; Hestetun, K.; Dahl, O.; Myklebust, M. A four-microRNA classifier as a novel prognostic marker for tumor recurrence in stage II colon cancer. Sci. Rep. 2018, 8, 6157. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.D.; Li, Q.; Zhang, R.S.; Dai, X.L.; Chen, W.J.; Xing, D.M. Circulating microRNAs: Biomarkers of disease. Clin. Chem. 2021, 516, 46–54. [Google Scholar] [CrossRef]
- Wehbe, N.; Nasser, S.A.; Pintus, G.; Badran, A.; Eid, A.H.; Baydoun, E. MicroRNAs in Cardiac Hypertrophy. Int. J. Mol. Sci. 2019, 20, 4714. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular MiRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Wang, K.-H.; Hsieh, J.-C.; Chen, C.-C.; Zan, H.-W.; Meng, H.-F.; Kuo, S.-Y.; Nguyễn, M.T.N. A Low-Cost, Portable and Easy-Operated Salivary Urea Sensor for Point-of-Care Application. Biosens. Bioelectron. 2019, 132, 352–359. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; de Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable Salivary Uric Acid Mouthguard Biosensor with Integrated Wireless Electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef]
- Khan, M.S.; Dighe, K.; Wang, Z.; Srivastava, I.; Daza, E.; Schwartz-Dual, A.S.; Ghannam, J.; Misra, S.K.; Pan, D. Detection of Prostate Specific Antigen (PSA) in Human Saliva Using an Ultra-Sensitive Nanocomposite of Graphene Nanoplatelets with Diblock-Co-Polymers and Au Electrodes. Analyst 2018, 143, 1094–1103. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wu, C.-C.; Peng, Y.-S.; Wu, C.-W.; Chang, Y.-T.; Chang, K.-P. Detection of Anti-P53 Autoantibodies in Saliva Using Microfluidic Chips for the Rapid Screening of Oral Cancer. RSC Adv. 2018, 8, 15513–15521. [Google Scholar] [CrossRef]
- Dong, T.; Pires, N.M.M. Immunodetection of Salivary Biomarkers by an Optical Microfluidic Biosensor with Polyethylenimine-Modified Polythiophene-C70 Organic Photodetectors. Biosens. Bioelectron. 2017, 94, 321–327. [Google Scholar] [CrossRef]
- Rathnayake, N.; Gieselmann, D.-R.; Heikkinen, A.M.; Tervahartiala, T.; Sorsa, T. Salivary Diagnostics-Point-of-Care Diagnostics of MMP-8 in Dentistry and Medicine. Diagnostics 2017, 7, 7. [Google Scholar] [CrossRef]
- Wignarajah, S.; Suaifan, G.A.R.Y.; Bizzarro, S.; Bikker, F.J.; Kaman, W.E.; Zourob, M. Colorimetric Assay for the Detection of Typical Biomarkers for Periodontitis Using a Magnetic Nanoparticle Biosensor. Anal. Chem. 2015, 87, 12161–12168. [Google Scholar] [CrossRef]
- Bhakta, S.A.; Borba, R.; Taba, M.; Garcia, C.D.; Carrilho, E. Determination of Nitrite in Saliva Using Microfluidic Paper-Based Analytical Devices. Anal. Chim. Acta 2014, 809, 117–122. [Google Scholar] [CrossRef]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernandez, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of Matrix Metalloproteinases, Especially MMP-8, in Gingival Crevicular Fluid, Mouthrinse and Saliva for Monitoring Periodontal Diseases. Periodontol 2000 2016, 70, 142–163. [Google Scholar] [CrossRef]
- De Castro, L.F.; de Freitas, S.V.; Duarte, L.C.; de Souza, J.A.C.; Paixão, T.R.L.C.; Coltro, W.K.T. Salivary Diagnostics on Paper Microfluidic Devices and Their Use as Wearable Sensors for Glucose Monitoring. Anal. Bioanal. Chem. 2019, 411, 4919–4928. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, S.H.; Ko, Y.; Park, J.C.; Ji, S.; Gu, M.B. The Sensitive Detection of ODAM by Using Sandwich-Type Biosensors with a Cognate Pair of Aptamers for the Early Diagnosis of Periodontal Disease. Biosens. Bioelectron. 2019, 126, 122–128. [Google Scholar] [CrossRef]
- Chen, Z.; Mauk, M.G.; Wang, J.; Abrams, W.R.; Corstjens, P.L.A.M.; Niedbala, R.S.; Malamud, D.; Bau, H.H. A Microfluidic System for Saliva-Based Detection of Infectious Diseases. Ann. N. Y. Acad. Sci. 2007, 1098, 429–436. [Google Scholar] [CrossRef]
- Chen, D.; Mauk, M.; Qiu, X.; Liu, C.; Kim, J.; Ramprasad, S.; Ongagna, S.; Abrams, W.R.; Malamud, D.; Corstjens, P.L.A.M.; et al. An Integrated, Self-Contained Microfluidic Cassette for Isolation, Amplification, and Detection of Nucleic Acids. Biomed. Microdevices 2010, 12, 705–719. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, H.; Malamud, D.; Barber, C.; Ongagna Yhombi, S.; Yasmin, R.; Modak, S.; Janal, M.; Abrams, W.; Montagna, R. A Rapid, Self-Confirming Assay for HIV: Simultaneous Detection of Anti-HIV Antibodies and Viral RNA. J. AIDS Clin. Res. 2016, 7, 540. [Google Scholar] [CrossRef]
- Sabalza, M.; Yasmin, R.; Barber, C.A.; Castro, T.; Malamud, D.; Kim, B.J.; Zhu, H.; Montagna, R.A.; Abrams, W.R. Detection of Zika Virus Using Reverse-Transcription LAMP Coupled with Reverse Dot Blot Analysis in Saliva. PLoS ONE 2018, 13, e0192398. [Google Scholar] [CrossRef]
- Cabral-Miranda, G.; Cardoso, A.R.; Ferreira, L.C.S.; Sales, M.G.F.; Bachmann, M.F. Biosensor-Based Selective Detection of Zika Virus Specific Antibodies in Infected Individuals. Biosens. Bioelectron. 2018, 113, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Žukovskaja, O.; Jahn, I.; Weber, K.; Cialla-May, D.; Popp, J. Detection of Pseudomonas Aeruginosa Metabolite Pyocyanin in Water and Saliva by Employing the SERS Technique. Sensors 2017, 17, 1704. [Google Scholar] [CrossRef] [PubMed]
- Oblath, E.A.; Henley, W.H.; Alarie, J.P.; Ramsey, J.M. A Microfluidic Chip Integrating DNA Extraction and Real-Time PCR for the Detection of Bacteria in Saliva. Lab Chip 2013, 13, 1325. [Google Scholar] [CrossRef] [PubMed]
- Haji Mohammadi, M.; Mulder, S.; Khashayar, P.; Kalbasi, A.; Azimzadeh, M.; Aref, A.R. Saliva Lab-on-a-Chip Biosensors: Recent Novel Ideas and Applications in Disease Detection. Microchem. J. 2021, 168, 106506. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Rees, T.; Wright, J. A Review of Research on Salivary Biomarkers for Oral Cancer Detection. Clin. Transl. Med. 2014, 3, 3. [Google Scholar] [CrossRef]
- Javaid, M.A.; Ahmed, A.S.; Durand, R.; Tran, S.D. Saliva as a Diagnostic Tool for Oral and Systemic Diseases. J. Oral Biol. Craniofac. Res. 2016, 6, 67–76. [Google Scholar] [CrossRef]
- Aro, K.; Wei, F.; Wong, D.T.; Tu, M. Saliva Liquid Biopsy for Point-of-Care Applications. Front. Public Health 2017, 5, 77. [Google Scholar] [CrossRef]
- Fernandes, L.L.; Pacheco, V.B.; Borges, L.; Athwal, H.K.; de Paula Eduardo, F.; Bezinelli, L.; Correa, L.; Jimenez, M.; Dame-Teixeira, N.; Lombaert, I.M.A.; et al. Saliva in the Diagnosis of COVID-19: A Review and New Research Directions. J. Dent. Res. 2020, 99, 1435–1443. [Google Scholar] [CrossRef]
- Isho, B.; Abe, K.T.; Zuo, M.; Jamal, A.J.; Rathod, B.; Wang, J.H.; Li, Z.; Chao, G.; Rojas, O.L.; Bang, Y.M.; et al. Persistence of Serum and Saliva Antibody Responses to SARS-CoV-2 Spike Antigens in COVID-19 Patients. Sci. Immunol. 2020, 5, eabe5511. [Google Scholar] [CrossRef]
- Lin, G.C.; Smajlhodzic, M.; Bandian, A.-M.; Friedl, H.-P.; Leitgeb, T.; Oerter, S.; Stadler, K.; Giese, U.; Peham, J.R.; Bingle, L.; et al. An In Vitro Barrier Model of the Human Submandibular Salivary Gland Epithelium Based on a Single Cell Clone of Cell Line HTB-41: Establishment and Application for Biomarker Transport Studies. Biomedicines 2020, 8, 302. [Google Scholar] [CrossRef]
- Lin, G.C.; Leitgeb, T.; Vladetic, A.; Friedl, H.-P.; Rhodes, N.; Rossi, A.; Roblegg, E.; Neuhaus, W. Optimization of an Oral Mucosa In Vitro Model Based on Cell Line TR146. Tissue Barriers 2020, 8, 1748459. [Google Scholar] [CrossRef]
- Wei, F.; Lin, C.-C.; Joon, A.; Feng, Z.; Troche, G.; Lira, M.E.; Chia, D.; Mao, M.; Ho, C.-L.; Su, W.-C.; et al. Noninvasive Saliva-Based EGFR Gene Mutation Detection in Patients with Lung Cancer. Am. J. Respir. Crit. Care Med. 2014, 190, 1117–1126. [Google Scholar] [CrossRef]
- Yang, J.; Wei, F.; Schafer, C.; Wong, D.T.W. Detection of Tumor Cell-Specific MRNA and Protein in Exosome-Like Microvesicles from Blood and Saliva. PLoS ONE 2014, 9, e110641. [Google Scholar] [CrossRef]
- Kim, N.-H.; Choi, S.-J.; Yang, D.-J.; Bae, J.; Park, J.; Kim, I.-D. Highly Sensitive and Selective Hydrogen Sulfide and Toluene Sensors Using Pd Functionalized WO3 Nanofibers for Potential Diagnosis of Halitosis and Lung Cancer. Sens. Actuators B Chem. 2014, 193, 574–581. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of Electronic-Nose Technologies and VOC-Biomarkers for the Noninvasive Early Diagnosis of Gastrointestinal Diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Lee, J.-H. Toward Breath Analysis on a Chip for Disease Diagnosis Using Semiconductor-Based Chemiresistors: Recent Progress and Future Perspectives. Lab Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef]
- Queralto, N.; Berliner, A.N.; Goldsmith, B.; Martino, R.; Rhodes, P.; Lim, S.H. Detecting Cancer by Breath Volatile Organic Compound Analysis: A Review of Array-Based Sensors. J. Breath Res. 2014, 8, 027112. [Google Scholar] [CrossRef]
- Shirasu, M.; Touhara, K. The Scent of Disease: Volatile Organic Compounds of the Human Body Related to Disease and Disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Wang, Y.-C.; Peng, H.-Y.; Huang, C.-H. Breath Biopsy of Breast Cancer Using Sensor Array Signals and Machine Learning Analysis. Sci. Rep. 2021, 11, 103. [Google Scholar] [CrossRef]
- Bos, L.D.; Sterk, P.J.; Fowler, S.J. Breathomics in the Setting of Asthma and Chronic Obstructive Pulmonary Disease. J. Allergy Clin. Immunol. 2016, 138, 970–976. [Google Scholar] [CrossRef]
- Van der Schee, M.; Pinheiro, H.; Gaude, E. Breath Biopsy for Early Detection and Precision Medicine in Cancer. Ecancermedicalscience 2018, 12, ed84. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Monge, M.E.; McCarty, N.A.; Stecenko, A.A.; Fernández, F.M. Feasibility of Early Detection of Cystic Fibrosis Acute Pulmonary Exacerbations by Exhaled Breath Condensate Metabolomics: A Pilot Study. J. Proteome Res. 2017, 16, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Paris, D.; Melck, D.; Lucidi, V.; Ciabattoni, G.; Raia, V.; Calabrese, C.; Bush, A.; Barnes, P.J.; Motta, A. NMR Spectroscopy Metabolomic Profiling of Exhaled Breath Condensate in Patients with Stable and Unstable Cystic Fibrosis. Thorax 2012, 67, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Trefz, P.; Obermeier, J.; Lehbrink, R.; Schubert, J.K.; Miekisch, W.; Fischer, D.-C. Exhaled Volatile Substances in Children Suffering from Type 1 Diabetes Mellitus: Results from a Cross-Sectional Study. Sci. Rep. 2019, 9, 15707. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Hrinczenko, B.; Swain, G.; Peteu, S. Exhaled Breath Biomarker Sensing. Biosens. Bioelectron. 2021, 182, 113193. [Google Scholar] [CrossRef]
- Hatamie, A.; Angizi, S.; Kumar, S.; Pandey, C.M.; Simchi, A.; Willander, M.; Malhotra, B.D. Review—Textile Based Chemical and Physical Sensors for Healthcare Monitoring. J. Electrochem. Soc. 2020, 167, 037546. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).