Abstract
Novel, sensitive, selective, efficient and portable electrochemical biosensors are needed to detect residual contaminants of the pesticide 1-naphthyl methylcarbamate (carbaryl) in the environment, food, and essential biological fluids. In this work, a study of nanocomposite-based Ag reduced graphene oxide (rGO) and chitosan (CS) that optimise surface conditions for immobilisation of acetylcholinesterase (AChE) enzyme to improve the performance of catalytic biosensors is examined. The Ag/rGO/CS nanocomposite membrane was used to determine carbaryl pesticide using a potentiometer transducer. The AChE enzyme-based biosensor exhibits a good affinity for acetylthiocholine chloride (ATCl). It can catalyse the hydrolysis of ATCl with a potential value of 197.06 mV, which is then oxidised to produce a detectable and rapid response. Under optimal conditions, the biosensor detected carbaryl pesticide at concentrations in the linear range of 1.0 × 10−8 to 1.0 μg mL−1 with a limit of detection (LoD) of 1.0 × 10−9 μg mL−1. The developed biosensor exhibits a wide working concentration range, detection at low concentrations, high sensitivity, acceptable stability, reproducibility and simple fabrication, thus providing a promising tool for pesticide residue analysis.
1. Introduction
The biosensor is a point-of-care device with advantages, such as ease to use, fast detection, small device, accuracy, detection limit, portability, cost-effectiveness and direct detection in the field [1,2,3]. Biosensors are tools that have been continuously developed in recent decades to detect pesticides by converting the identification of analytes into signals that can be physically measured, such as optical, magnetic and electronic [1,4]. An electrochemical method-based biosensor with the enzyme acetylcholinesterase (AChE) selectively interacts with the target analyte and generates a signal from the measured analyte concentration [5].
Pesticides prevent losses caused by plant pest organisms (PPO) or pests and increase food production. Thus, it encourages using pesticides with high efficacy without considering the negative impact on the environment. In recent decades, carbamate and organophosphate pesticides are often used in agriculture due to their high stability in the environment compared to organochlorine compounds [6]. However, its improper use, improper handling and contaminated environment can pose risks to human health [7]. The carbamate pesticides are systemic insecticides with a broad spectrum as nematicides and acaricides [8]. Carbamate insecticides widely used in the field consist of carbofuran, aldicarb and carbaryl [9].
According to estimates from the World Health Organization (WHO), in 2017, cases of pesticide poisoning in farmers in developing countries were 18.2% of 100,000 farmers worldwide, and more than 168,000 people died every year. For health, it is necessary to control food safety from pesticide residues. Pesticide analysis is often carried out by gas chromatography (GC) or high-pressure liquid chromatography (HPLC) [10,11,12]. The weakness of the analytical method with GC and HPLC is the extraction and purification treatment in the laboratory, which requires more chemical solvents and longer analysis time [13]. Due to the weakness of conventional methods, detection methods with biosensors have been developed in recent decades. In this context, enzyme-based electrochemical biosensors are tools and applications in pesticide detection [14]. The carbaryl pesticide (1-naphthyl methylcarbamate) is a versatile pesticide in agriculture. The mechanism of action of carbaryl is the same as the mechanism of pesticides in general, namely the inhibition of the AChE enzyme. The AChE enzyme is essential in transmitting messages in the central nervous system in humans and animals [14,15]. The maximum tolerated level of carbaryl residue set by the European Union is 5.0 × 10−2 μg mL−1 [16]. Therefore, an appropriate detection tool for analysing carbaryl residues in the field is needed.
The development of electrochemical biosensors is practically applied. Advances in nanotechnology for the fabrication of AChE electrochemical biosensors have provided a variety of nanostructured materials with unique chemical and physical properties and exceptional properties, such as high specific surface area and high electrocatalytic activity [17,18]. For carbaryl detection, several studies have reported nanocomposite-based biosensors (polypyrrole, Au/graphene and silver/graphene) [7,14,15]. Rahmani et al. (2018) [7], has developed a carbaryl pesticide electrochemical sensor based on Au/graphene nanocomposite electrodes. The linear range for the determination of carbaryl is 8.0 × 10−4 to 6.0 × 10−2 μg mL−1 with a LoD of 2.4 × 10−4 μg mL−1 [7]. The LoD of this sensor for carbaryl detection is below the maximum residue level; stability and reproducibility are still limited. To improve the performance of the biosensor, which is much better than this carbaryl sensor. Developing a nanostructured membrane with AChE enzyme immobilized by the electrochemical method is necessary.
The nanocomposite is based on graphene as an enhanced sensing platform for biosensors because this type of nanocomposite film can produce a synergistic effect to increase sensitivity [19]. The graphene as a carbon nanomaterial has a large surface area, excellent thermal/chemical stability, high electronic/thermal conductivity, and superior mechanical flexibility [18,20]. Graphene materials, including graphene oxide (GO), three-dimensional GO [21,22], and porous GO [23], have been widely used in sensor detection of organophosphorus pesticides (OPs), achieving relatively good detection results [24,25]. However, the mechanical strength of the graphene material is weak, and the internal bond of the material is not strong, so it can cause the stability of this sensor to decrease [26]. In this case, incorporating graphene and chitosan (CS) is required. The CS is a natural biopolymer with hydrophilicity, biodegradability, biocompatibility, non-toxicity properties, excellent film-forming ability and outstanding mechanical strength. CS provides a natural microenvironment for enzymes and also offers sufficient access for electrons to move between enzymes and electrodes [27,28].
Xie et al. designed an electrochemical biosensor with GO/CS/parathion to determine OPs with a linear detection range of 1.0 × 10−3 to 1.5 μg mL−1 with a LoD of 0.012 to 0.23 × 10−3 μg mL−1, good stability and high sensitivity [29]. The nanocomposite of rGO/silver nanocluster/CS modified with glassy carbon electrode (GCE) prepared by Zhang et al. for the detection of phoxim pesticide with LoD is 0.25 × 10−4 μg mL−1 [19], which shows good sensitivity, stability and reproducibility, thus being able to provide a promising tool for the analysis of enzyme inhibitors and the direct examination of samples becomes more practical. In this work, an electrochemical Ag/rGO/CS biosensor was developed to detect carbaryl pesticides with a potentiometer design strategy as a detector that combines the ability of CPs to inhibit AChE enzyme activity immobilized on nanocomposite membranes at the working electrode of the biosensor.
2. Materials and Methods
2.1. Materials
Acetylcholinesterase (AChE, EC 3.1.1.7, C3889-500UN), acetylthiochline chloride (ATCl, A5626), hydrazine hydrate (85%), glutaraldehyde (GA), sodium citrate (Na3C6H5O7) and chitosan (CS) from shrimp shells, ≥95% (deacetylated), carbaryl pesticides (32055-250MG) were purchased from Sigma-Aldrich, St. Louis, MI, USA. Silver nitrate (AgNO3), graphene oxide (GO), sodium hydroxide (NaOH), potassium chloride (KCl) 1 × 10−1 M, citric acid (C6H8O7), acetic acid (CH3CO2H), ethyl alcohol (C5H6O, 98%), phosphate buffer solutions (PBS) with values of pH 8.0 were purchased from Merck, Darmstadt, Germany.
2.2. Apparatus and Instruments
Measurement of the potential value of pesticide detection using a potentiometer (SANFIX DM-888C) as a transducer with Au wire electrode coated with Ag/rGO/CS nanocomposite membrane as working electrode, and Ag/AgCl as reference electrode. Electrolysis of Ag/AgCl uses Pt wire as cathode and Ag wire as anode connected to a battery as a source of electric current. Morphology and elemental analysis of membrane modification was obtained using Scanning Electron Microscope-Energy Dispersive X-ray (SEM-EDX) from Phenom Desktop ProXL. X-ray diffraction (XRD) analysis of the prepared samples was carried out using a Bruker D2 Phaser with Cu Kα radiation (λ = 1.541).
2.3. Synthesis of Reduced Graphene Oxide
Graphene oxide (GO) powder, 15–20 sheets, 4–10% edge-oxidized chemically reduced using hydrazine served as a reducing agent to produce reduced-GO (rGO) [19]. Briefly, 150 mg of GO was dispersed in 100 mL of deionized water and ultrasonicated for more than 2 h. Furthermore, 3.0 mL of hydrazine hydrate solution was added and dispersed until homogeneous. The resulting solution was then refluxed for 24 h at 95 °C. The filter cake was washed with water and methyl alcohol several times and then dried for 24 h at 60 °C.
2.4. Preparation of Ag/rGO/CS
The Ag/rGO/CS membrane preparation is a modification of the research by Zhang et al. (2015) [19]. The membrane was made with 2 mL of 0.5 M AgNO3 and 1 mL of 2.5 mg mL−1 rGO solution. Then, the AgNO3 and rGO mixture was vortexed and then sonicated for 30 min to form a light grey stable dispersion. Furthermore, 3.0 mL of 2% CS and 10 drops of 4 M NaOH were added. The formed Ag/rGO/CS membrane was then coated on the Au electrode to form an Au-Ag/rGO/CS membrane electrode. Au-Ag/rGO/CS membrane electrodes were immersed in citrate buffer solution, pH 5.5, for 1 h and rinsed with distilled water. It was next soaked in PBS pH 8 for 1 h.
2.5. Preparation of Acetylcholinesterase Enzyme Immobilization
The membrane electrodes of Au-Ag/rGO/CS nanocomposite were immersed in 25% GA solution for 6 h, then immobilized in AChE enzyme for 48 h at 4 °C. Finally, a modified electrode was formed, namely the Au-Ag/rGO/CS@AChE membrane electrode.
2.6. Measurement of Potential Value Biosensor
Measurement of electrode potential value based on the membrane of Au-Ag/rGO/chitosan@AChE nanocomposite using a potentiometer transducer to see the performance of biosensors in analyzing pesticides. Before measurement, the membrane electrode was immersed in PBS for 10 min, then the substrate potential of 1 × 10−3 M ATCl was measured to obtain a constant potential value (E0). Furthermore, the membrane electrode was taken and washed with distilled water and then immersed in a carbaryl pesticide solution for 30 min. Then, the membrane electrode was rinsed with PBS and then dipped again in the ATCl substrate solution. The potential measurement is repeated until a constant potential value is obtained (Ei). Variation of concentrations of carbaryl pesticide solution as inhibitor used was 1 × 10−8, 10−7, 10−6, 10−5, 10−4, 10−3, 10−2, 10−1 and 1.0 μg mL−1. The inhibition value of carbaryl pesticides was calculated using the following equation:
where, E0 is the potential value of ATCl in the biosensor without carbaryl pesticide and Ei is the potential value of ATCl in the biosensor with inhibition of the carbaryl pesticide. The performance of the biosensor was analyzed by observing the potential value (mV) of the AChE enzyme inhibition plotted against –log of pesticide concentration to obtain a linear calibration curve (y = ax + b), and the linear range is also observed.
2.7. Limit of Detection
The limit of detection (LoD) indicates the lowest analyte concentration detected by the electrode as the lowest measurement limit. The analysis results of the potential value of the biosensor electrode after interacting with pesticides at successive concentrations of 1.0 × 10−8 to 1.0 mg L−1 obtained a linear equation of the calibration curve, y = ax + b. The equation for the y value at the detection limit is based on Christian et al. (2014) [30]. Calculation for value of LoD = + 3 S(y/x), where S(y/x) is the standard deviation of the response and is the mean of the blank values.
2.8. Reproducibility
The precision is measured as standard deviation or coefficient of variation (% CV) and can be expressed as reproducibility. %RSD refers to the “coefficient of variation”, %RSD = (s/ā) × 100%, where s is the standard deviation, and ā is the mean of the measurement results [31]. Analysis of precision was carried out by making a carbaryl test solution with a concentration of 1.0 × 10−8 to 1.0 mg L−1. The measurement of the potential value was carried out 5 times at different times and different electrodes but with the same membrane composition as the designed biosensor electrode.
3. Results and Discussion
3.1. Characterizations of Ag/rGO/CS
The surface morphology of Ag/rGO/CS nanocomposite was observed by SEM. Figure 1A,B, show that on the surface of the Ag/rGO/CS nanocomposite, there are some fused pores and noticeable wrinkles, which can increase the surface area of the electrode. The wavy morphology was observed from Figure 1B, indicating large Ag on the colloidal surface. The EDX spectrum of the Ag/rGO/CS nanocomposite (Figure 1C), showed the presence of different elements where the most dominant was the Ag element. The percentages of C (11.55%) and O (53.37%) atoms in the table (Figure 1C inset) indicate that C and O atoms are present in the rGO/CS nanocomposite. Meanwhile, the chitosan coating was proven by the presence of N with a level of 17.65%. The graph in Figure 1C, strengthens the evidence that a certain amount of Ag has been successfully formed in the rGO/CS nanocomposite with an Ag atom percentage of 11.10%. The nanocomposites of Ag/rGO/CS (Figure 1D) and Ag are evenly distributed on the rGO/CS surface, attributed to “hot spots” that enhance the electrocatalytic performance of the biosensor [32,33]. The isolated bright dots of the first image in Figure 1D, can clearly show the individual Ag atoms based on the contrast difference between the masses of Ag atoms which are heavier than the other atoms. The SEM image identified the atomic dispersion of Ag added to rGO/CS. Ag is distributed uniformly, thereby minimizing electron transfer between the rGO/CS layers and improving electrochemical properties. This characterization proves the success of the Ag/rGO/CS nanocomposite.
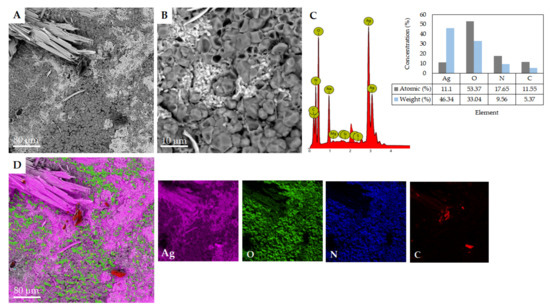
Figure 1.
(A,B) SEM images, (C) EDX analysis; (D) elemental mapping of the Ag/rGO/CS nanocomposite.
Figure 2 shows the X-ray diffraction (XRD) curve of the Ag/rGO/CS nanocomposite. The characteristic peaks of GO and rGO were observed at 2θ = 9.23° with high intensity and 2θ = 24.21° showing narrow reflection, respectively. For the Ag/rGO/CS nanocomposite, it showed characteristic peaks at 2θ = 38.02° and 44.13°, corresponding to the respective (111) and (200) planes of face-centered cubic (fcc) Ag crystals. The Ag lattice in Ag/rGO/CS nanocomposite shows that Ag is uniformly dispersed in the rGO sheet and CS molecular network. In addition, Ag did not change the crystal structure.
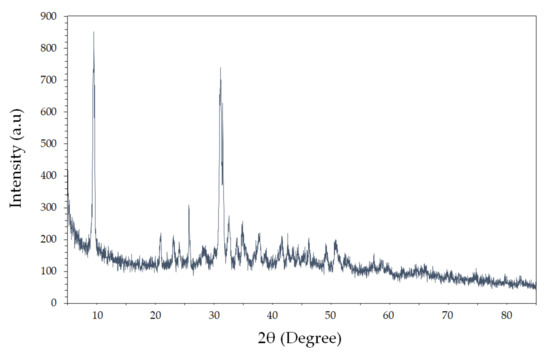
Figure 2.
XRD patterns of the Ag/rGO/CS nanocomposite.
3.2. The Modified Electrodes of Ag/rGO/CS Nanocomposite
To describe the modified electrode characteristics of Ag/rGO/CS nanocomposite by adding GA as a crosslinker to increase the immobilisation ability of AChE enzyme and incorporated into PBS (pH = 8). Under optimal conditions (see Supplementary), a potentiometer was used to measure the electrochemical reaction between AChE and ATCl. Table S1, shows the potential values for the determination of ATCl as a substrate. The concentration of ATCl 1.0 × 10−3 M resulted in a potentiometric response from the biosensor of 197.06 mV. The electrode of Ag/rGO/CS shows no current for carbaryl oxidation [7]. Meanwhile, the potential value for the blank (without the presence of carbaryl pesticide) is that the pesticide solvent used is 98% ethanol and PBS (pH = 8.0) in a ratio (3:7), resulting in a potential value of 186.72 mV. The potential value decreased from the blank compared to the initial potential value due to the inhibitory effect of ethanol on the performance of the AChE enzyme, although the difference was relatively low. These results are attributed to the increased active surface area and fast electron transfer from the electrodes due to the unique properties of rGO. The membrane electrodes of Ag/rGO/CS as bio-sensors have high affinity and catalytic properties for ATCl substrates.
3.3. Carbaryl Detection
In principle, the AChE enzyme catalyses the reaction of the substrate acetylthiocholine chloride (ATCl) to produce the electroactive product thiocholine (TCh), which is oxidised electrochemically [14,34]. This hydrolysis reaction is inhibited in the presence of pesticides because AChE binds to the pesticide, resulting in lower TCh concentrations and lower oxidation currents. Carbaryl (carbamate group) pesticides reversibly inhibit enzymes by forming covalent bonds with serine residues (Ser200) present at the AChE active site through nucleophilic attack and produce carbamoylation enzymes, which cannot catalyze acetylcholine. The inhibitory effect of different pesticides was measured by a potentiometry in measuring the biosensor response to 1.0 × 10−3 M ATCl after incubation with varying concentrations of carbaryl pesticide. As shown in Figure 3a, with biosensor responses before and after 6 min incubation in pesticide concentrations of 1.0 × 10−8 to 1.0 μg mL−1. The potential value (Figure 3a) gradually decreased with increasing carbaryl concentration. The potential value indicated by the potentiometer is the result of the hydrolysis reaction of the ATCl substrate with the enzyme AChE catalyst so that the presence of carbaryl pesticide compounds will inhibit the activity of the AChE enzyme catalyst. The higher the pesticide concentration, the higher the inhibitory ability, causing the potential value to decrease.
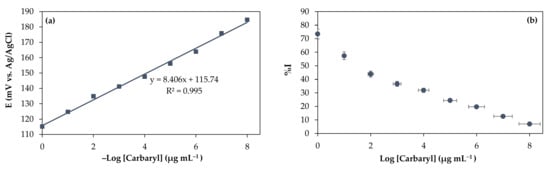
Figure 3.
(a) Graph of the relationship of –Log [Carbaryl] with the E (mV vs. Ag/AgCl) and (b) the %I of carbaryl pesticide.
The linear regression equation is expressed as y= 115.74 + 8.406 Log [carbaryl], R2 = 0.995 with LoD being 1.0 × 10−9 μg mL−1 at a potential value of 194.21 mV. This LoD is far below the maximum pesticide residue limit, which is 5.0 × 10−2 μg mL−1 [16]. The linear range of carbaryl indicates that the biosensor is more sensitive to detect pesticides at low concentrations. The performance of the proposed biosensor in the determination of carbaryl has been compared with other reported modified electrodes. The linear range and LoD of the proposed Ag/rGO/CS electrode are comparable to and even better than in Table 1. When carbaryl molecules adsorb to the surface of the Ag/rGO/CS electrode, the concentration of ions in the solution will decrease, so will be reduced the gate voltage differential and carrier mobility [16]. In this work, Ag/rGO/CS nanocomposite membrane electrodes can analyze carbaryl pesticide residues with a wide range of working concentrations and detection at low concentrations using potentiometer transducers. The potentiometer has the nature of a “small device” that is easy to carry out in the field and inexpensive [13].

Table 1.
Comparison of the linear range and LOD of modified membranes for carbaryl detection with current biosensors.
The calibration curve of the inhibition percentage versus the log of pesticide concentration is shown in Figure 3b. The percentage of inhibition (%I) is the inhibitory power of the pesticide (inhibitor) on the performance of the enzyme. Generally, pesticide biosensor designs rely on quantitative enzyme activity measurements before and after contact with the substrate. The %I produced after interacting with the inhibitor will correlate with the inhibitor concentration and the interaction time (incubation time). As a result, the enzyme residue activity is inversely related to the inhibitor concentration. Giving inhibitors can affect enzyme activity and the concentration of the resulting product so that the potential value obtained is small. Some pesticides are irreversible and reversible inhibitors. Irreversible inhibitors are inhibitors whose chemical reactions run in one direction, which can cause damage to enzymes; this can be caused by hydrolysis or oxidation reactions. If the inhibitors are reversible, the chemical reactions run in both directions to reduce enzyme damage. The interaction between the acetylcholinesterase enzyme and the inhibitor occurs between the enzyme’s active site and the inhibitor [36]. The highest inhibition percentage was 72.02% with a carbaryl concentration of 1.0 μg mL−1, and the lowest was 7.27% at a carbaryl concentration of 1.0 × 10−8 μg mL−1 (see Supplementary Table S3). Ag/rGO/CS nanocomposite membrane is suitable for pesticide biosensor design. Both CS and rGO can serve as excellent immobilization platforms for enzymes, antibodies or DNA [37].
3.4. Precision of Measurements and Stability of Biosensor
The evaluation of biosensor precision was by testing one electrode for five replicate determinations in 1.0 × 10−3 M ATCl after immersion in carbaryl pesticide for 30 min. The reproducibility and stability of Ag/rGO/CS electrodes were checked. A series of five iterations of the modified electrode was provided in the same manner and tested and obtained an RSD of 2.23%, indicating acceptable reproducibility. When the enzyme electrode was not used, the electrodes were stored at 4 °C. No clear potential difference in ATCl response was observed after 5 repetitions. This method shows good reproducibility according to the RSD value and long-term stability of the sensor [7].
4. Conclusions
This work obtained favourable characteristics of Ag, rGO and CS incorporation. An AChE enzyme-based biosensor with Ag/rGO/CS nanocomposite membrane electrode has been developed to detect carbaryl pesticides belonging to the carbamate group. This electrode exhibits a good electrocatalytic effect for the determination of carbaryl pesticides. This biosensor electrode’s calibration curve shows a range linear carbaryl pesticide concentration from 1.0 × 10−8 to 1.0 μg mL−1 with an LoD of 1.0 × 10−9 μg mL−1 and good reproducibility and stability. The biosensors with Ag/rGO/CS nanocomposite membrane electrodes have advantages: high application potential, wide working concentration range, detection at low concentrations, high sensitivity, acceptable stability, reproducibility and simple fabrication. It is concluded that the biosensor has been successfully made to have better advantages than the previously reported electrochemical sensor because the detection tool used is a small-device potentiometer with good potential as an alternative to enzymatic biosensors. Therefore, this biosensor has potential applications in the biomonitoring of carbaryl pesticides and allows for the analysis of other pesticides. This method can inhibit the performance of other enzymes from building a variety of biosensors that require a suitable membrane to maintain enzyme stability.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/chemosensors10040138/s1, Table S1. Measurement of potential value, Table S2. Calculations for determination of Limit of Detection (LOD), Table S3. Calculations for determination of inhibition percentage, Table S4. Calculations for determination of measurements precision, Figure S1. Calibration curve of the relationship of –Log [Carbaryl] with the potential value (i) first, (ii) second, (iii) third, (iv) fourth, (v) fifth and (vi) mean of measurement.
Author Contributions
Data curation, M.M., H.R. and B.R.; writing—original draft preparation, M.M. and F.H.H.; writing—review and editing, M.M., H.R. and F.H.H.; supervision, M.M., H.R. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia (Kemdikbudristek) on an applied research scheme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request due to restrictions e.g., privacy or ethical.
Acknowledgments
The authors thank all the team for their contribution to this research, University of Halu Oleo (UHO) and Ministry of Education, Culture, Research, and Technology (Kemdikbudristek) of the Republic of Indonesia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kalyani, N.; Goel, S.; Jaiswal, S. On-site sensing of pesticides using point-of-care biosensors: A review. Environ. Chem. Lett. 2021, 19, 345–354. [Google Scholar] [CrossRef]
- Patel, H.; Rawtani, D.; Agrawal, Y.K. A newly emerging trend of chitosan-based sensing platform for the organophosphate pesticide detection using Acetylcholinesterase—A review. Trends Food Sci. Technol. 2019, 85, 78–91. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Su, X. Review of optical sensors for pesticides. TrAC-Trends Anal. Chem. 2018, 103, 1–20. [Google Scholar] [CrossRef]
- Verma, M.L. Nanobiotechnology advances in enzymatic biosensors for the agri-food industry. Environ. Chem. Lett. 2017, 15, 555–560. [Google Scholar] [CrossRef]
- Bacciu, A.; Arrigo, P.; Delogu, G.; Marceddu, S.; Monti, P.; Rocchitta, G.; Serra, P.A. A new perspective on using glycols in glutamate biosensor design: From stabilizing agents to a new containment net. Chemosensors 2020, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Mashuni; Ramadhan, L.O.A.N.; Jahiding, M. Syarfiah Design of Pesticide Biosensor Using Glutaraldehyde Crosslinked-Cellulose Acetate Membrane in Gold Electrode. In Proceedings of the International Journal of Chemical, Environmental & Biological Sciences; Hakim, L., Ed.; Brawijaya University: Malang, India, 2016; Volume 4, pp. 147–151. [Google Scholar]
- Rahmani, T.; Bagheri, H.; Behbahani, M.; Hajian, A.; Afkhami, A. Modified 3D Graphene-Au as a Novel Sensing Layer for Direct and Sensitive Electrochemical Determination of Carbaryl Pesticide in Fruit, Vegetable, and Water Samples. Food Anal. Methods 2018, 11, 3005–3014. [Google Scholar] [CrossRef]
- Anshori, A.; Prasetiyono, C. Pestisida Pada Budidaya Kedelai Di Kabupaten Bantul D. I. Yogyakarta. Caraka Tani J. Sustain. Agric. 2016, 31, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Sudarma, N.; Luh, N.; Dilisca, N.; Putri, D.; Prihatiningsih, D. Identifikasi Residu Pestisida Organofosfat dan Karbamat Pada Buah dan Sayur yang Dijual di Pasar Badung Desa Dauh Puri Kangin Denpasar Bali Tahun 2019. J. Kesehat. Terpadu 2020, 4, 13–17. [Google Scholar]
- Song, N.E.; Lee, J.Y.; Mansur, A.R.; Jang, H.W.; Lim, M.C.; Lee, Y.; Yoo, M.; Nam, T.G. Determination of 60 pesticides in hen eggs using the QuEChERS procedure followed by LC-MS/MS and GC-MS/MS. Food Chem. 2019, 298, 1–10. [Google Scholar] [CrossRef]
- Anagnostopoulos, C.; Miliadis, G.E. Development and validation of an easy multiresidue method for the determination of multiclass pesticide residues using GC-MS/MS and LC-MS/MS in olive oil and olives. Talanta 2013, 112, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, T.; Luo, X.; Xiong, H.; Min, F.; Chen, Y.; Nie, S.; Xie, M. Determination of multi-pesticide residues in green tea with a modified QuEChERS protocol coupled to HPLC-MS/MS. Food Chem. 2018, 275, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Mashuni, M.; Ritonga, H.; Jahiding, M.; La Ode Ahmad Nur, R.; Kurniawati, D.; Hamid, F.H. The Performance of Organophosphate Pesticides Determination Using Biosensor Based on Small Device Potentiometer as a Transducer. Chem. Proc. 2021, 5, 69. [Google Scholar] [CrossRef]
- Loguercio, L.F.; Thesing, A.; Demingos, P.; de Albuquerque, C.D.L.; Rodrigues, R.S.B.; Brolo, A.G.; Santos, J.F.L. Efficient acetylcholinesterase immobilization for improved electrochemical performance in polypyrrole nanocomposite-based biosensors for carbaryl pesticide. Sens. Actuators B Chem. 2021, 339, 129875. [Google Scholar] [CrossRef]
- Pop, A.; Lung, S.; Orha, C.; Manea, F. Silver/graphene-modified boron doped diamond electrode for selective detection of carbaryl and paraquat from water. Int. J. Electrochem. Sci. 2018, 13, 2651–2660. [Google Scholar] [CrossRef]
- Thanh, C.T.; Binh, N.H.; Van Tu, N.; Thu, V.T.; Bayle, M.; Paillet, M.; Sauvajol, J.L.; Thang, P.B.; Lam, T.D.; Minh, P.N.; et al. An interdigitated ISFET-type sensor based on LPCVD grown graphene for ultrasensitive detection of carbaryl. Sens. Actuators B Chem. 2018, 260, 78–85. [Google Scholar] [CrossRef]
- Sundarmurugasan, R.; Gumpu, M.B.; Ramachandra, B.L.; Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Simultaneous detection of monocrotophos and dichlorvos in orange samples using acetylcholinesterase-zinc oxide modified platinum electrode with linear regression calibration. Sens. Actuators B Chem. 2016, 230, 306–313. [Google Scholar] [CrossRef]
- Cui, H.F.; Wu, W.W.; Li, M.M.; Song, X.; Lv, Y.; Zhang, T.T. A highly stable acetylcholinesterase biosensor based on chitosan-TiO2-graphene nanocomposites for detection of organophosphate pesticides. Biosens. Bioelectron. 2018, 99, 223–229. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Yang, Z.; Ji, S.; Wang, J.; Pang, P.; Feng, L.; Wang, H.; Wu, Z.; Yang, W. An acetylcholinesterase inhibition biosensor based on a reduced graphene oxide/silver nanocluster/chitosan nanocomposite for detection of organophosphorus pesticides. Anal. Methods 2015, 7, 6213–6219. [Google Scholar] [CrossRef]
- Ge, S.; Lan, F.; Liang, L.; Ren, N.; Li, L.; Liu, H.; Yan, M.; Yu, J. Ultrasensitive Photoelectrochemical Biosensing of Cell Surface N-Glycan Expression Based on the Enhancement of Nanogold-Assembled Mesoporous Silica Amplified by Graphene Quantum Dots and Hybridization Chain Reaction. ACS Appl. Mater. Interfaces 2017, 9, 6670–6678. [Google Scholar] [CrossRef]
- Bao, J.; Huang, T.; Wang, Z.; Yang, H.; Geng, X.; Xu, G.; Samalo, M.; Sakinati, M.; Huo, D.; Hou, C. 3D graphene/copper oxide nano-flowers based acetylcholinesterase biosensor for sensitive detection of organophosphate pesticides. Sens. Actuators B Chem. 2019, 279, 95–101. [Google Scholar] [CrossRef]
- Dong, P.; Jiang, B.; Zheng, J. A novel acetylcholinesterase biosensor based on gold nanoparticles obtained by electroless plating on three-dimensional graphene for detecting organophosphorus pesticides in water and vegetable samples. Anal. Methods 2019, 11, 2428–2434. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, Q.; Li, X.; Wu, L.; Yu, A.; Lai, G.; Fu, L.; Dai, D.; Jiang, N.; Li, H.; et al. A double-deck structure of reduced graphene oxide modified porous ti3c2tx electrode towards ultrasensitive and simultaneous detection of dopamine and uric acid. Biosensors 2021, 11, 462. [Google Scholar] [CrossRef]
- Li, Y.; Shi, L.; Han, G.; Xiao, Y.; Zhou, W. Electrochemical biosensing of carbaryl based on acetylcholinesterase immobilized onto electrochemically inducing porous graphene oxide network. Sens. Actuators B Chem. 2017, 238, 945–953. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, R.; Shi, L.; Han, G.; Xiao, Y. Acetylcholinesterase biosensor based on electrochemically inducing 3D graphene oxide network/multi-walled carbon nanotube composites for detection of pesticides. RSC Adv. 2017, 7, 53570–53577. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Hu, H.; Wang, P.; Zhang, C.; Wu, M.; Yang, L. A stable biosensor for organophosphorus pesticide detection based on chitosan modified graphene. Biotechnol. Appl. Biochem. 2021, 1–9. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wu, A.; Wei, G. Designed graphene-peptide nanocomposites for biosensor applications: A review. Anal. Chim. Acta 2017, 985, 24–40. [Google Scholar] [CrossRef]
- Kaur, R.; Rana, S.; Lalit, K.; Singh, P.; Kaur, K. Electrochemical detection of methyl parathion via a novel biosensor tailored on highly biocompatible electrochemically reduced graphene oxide-chitosan-hemoglobin coatings. Biosens. Bioelectron. 2020, 167, 112486. [Google Scholar] [CrossRef]
- Xie, X.; Zhou, B.; Zhang, Y.; Zhao, G.; Zhao, B. A multi-residue electrochemical biosensor based on graphene/chitosan/parathion for sensitive organophosphorus pesticides detection. Chem. Phys. Lett. 2021, 767, 138355. [Google Scholar] [CrossRef]
- Christian, G.D.; Dasgupta, P.K.; Kevin, A. Schug Analytical Chemistry, 7th ed.; Wiley: Hoboken, NJ, USA, 2014; ISBN 9780470887578. [Google Scholar]
- Mark, H.; Workman, J. Limitations in Analytical Accuracy: Part 1—Horwitz’s Trumpet, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Khawaja, H.; Zahir, E.; Asghar, M.A.; Asghar, M.A. Graphene oxide, chitosan and silver nanocomposite as a highly effective antibacterial agent against pathogenic strains. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 555, 246–255. [Google Scholar] [CrossRef]
- An, J.; Guo, G.; Yin, R.; Luo, Q.; Li, X.; Liu, F.; Wang, D. Facile preparation of silver/reduced graphene oxide/chitosan colloid and application of the nanocomposite in antibacterial and catalytic activity. Polym. Int. 2018, 67, 515–527. [Google Scholar] [CrossRef]
- Akyüz, D.; Koca, A. An electrochemical sensor for the detection of pesticides based on the hybrid of manganese phthalocyanine and polyaniline. Sens. Actuators B Chem. 2019, 283, 848–856. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, G.; Li, C.; Zhou, Q.; Wang, M.; Yang, L. A novel acetylcholinesterase biosensor based on carboxylic graphene coated with silver nanoparticles for pesticide detection. Mater. Sci. Eng. C 2014, 35, 253–258. [Google Scholar] [CrossRef]
- Pundir, C.S.; Malik, A. Preety Bio-sensing of organophosphorus pesticides: A review. Biosens. Bioelectron. 2019, 140, 1–13. [Google Scholar] [CrossRef]
- Barkauskas, J.; Mikoliunaite, L.; Paklonskaite, I.; Genys, P.; Petroniene, J.J.; Morkvenaite-Vilkonciene, I.; Ramanaviciene, A.; Samukaite-Bubniene, U.; Ramanavicius, A. Single-walled carbon nanotube based coating modified with reduced graphene oxide for the design of amperometric biosensors. Mater. Sci. Eng. C 2019, 98, 515–523. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).