Progress in Fast and Red Plastic Scintillators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
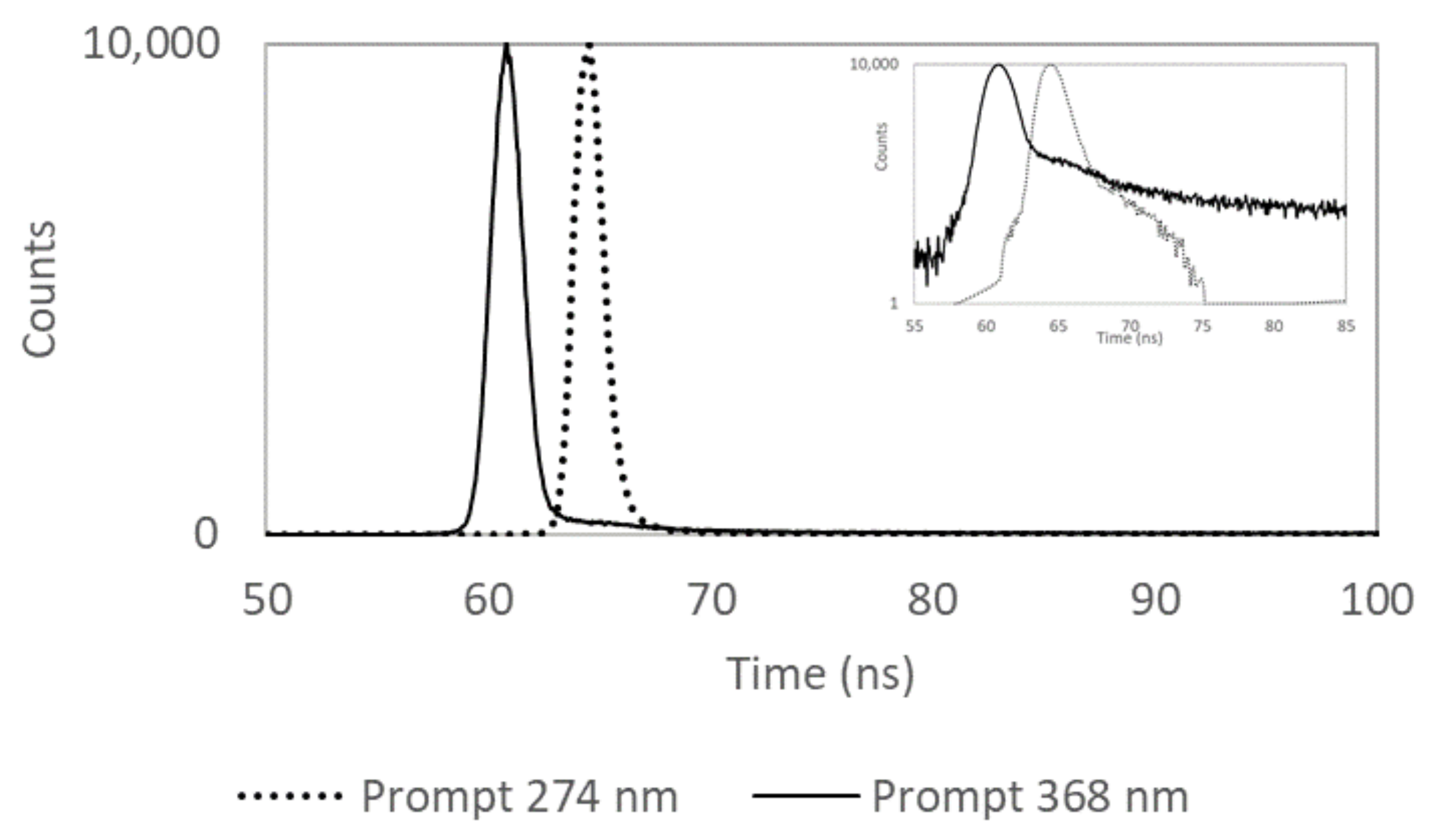
3.1. Intrinsic Limitations of the Spectrofluorometer Setup
3.2. Matrix + Primary Fluorophore Mixtures
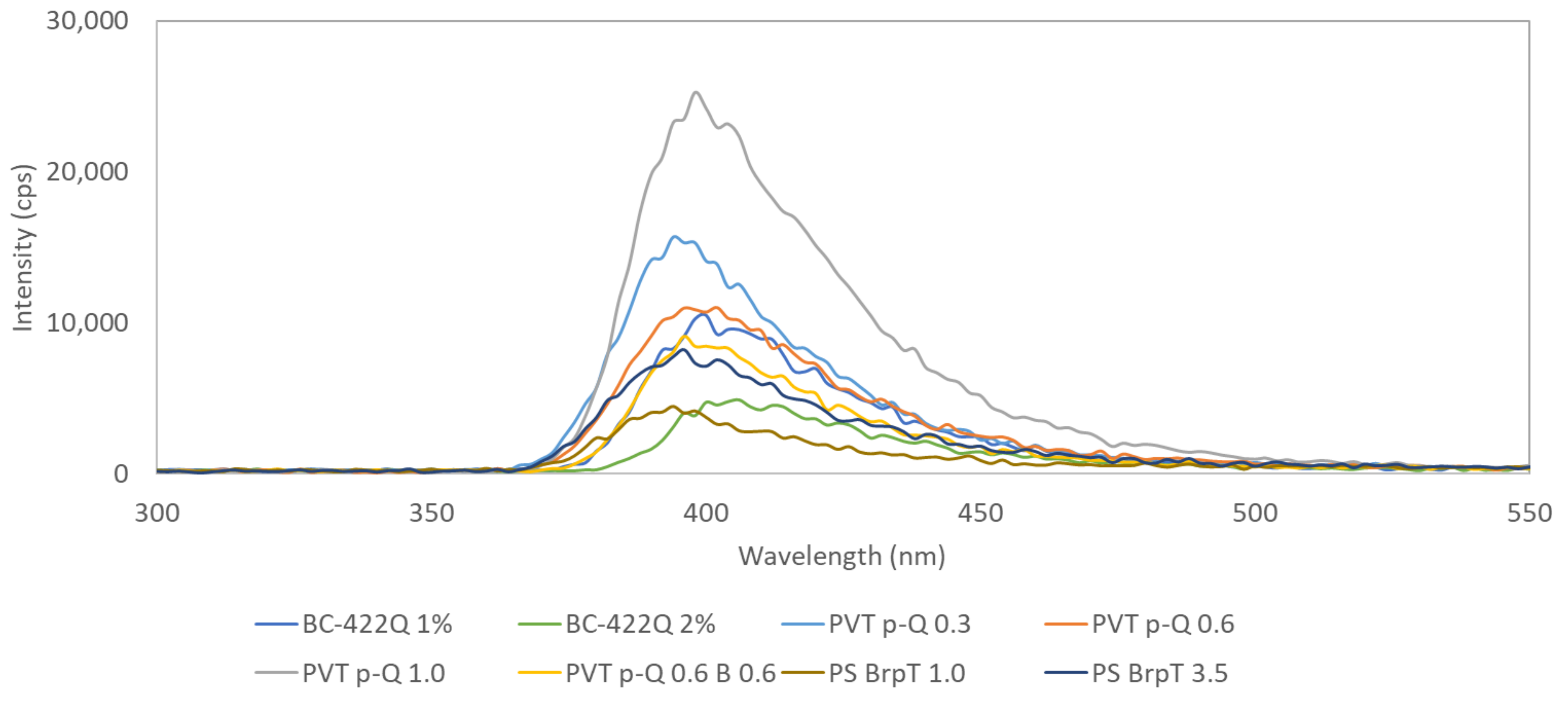
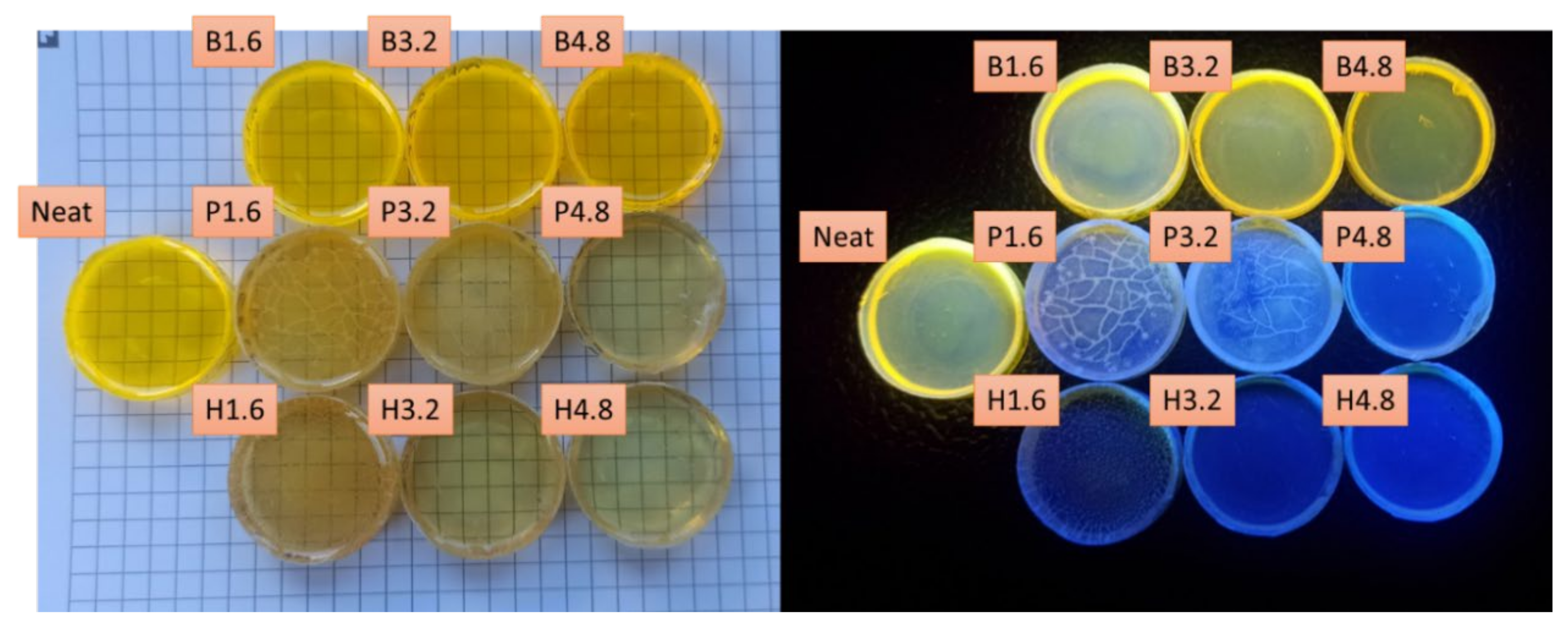
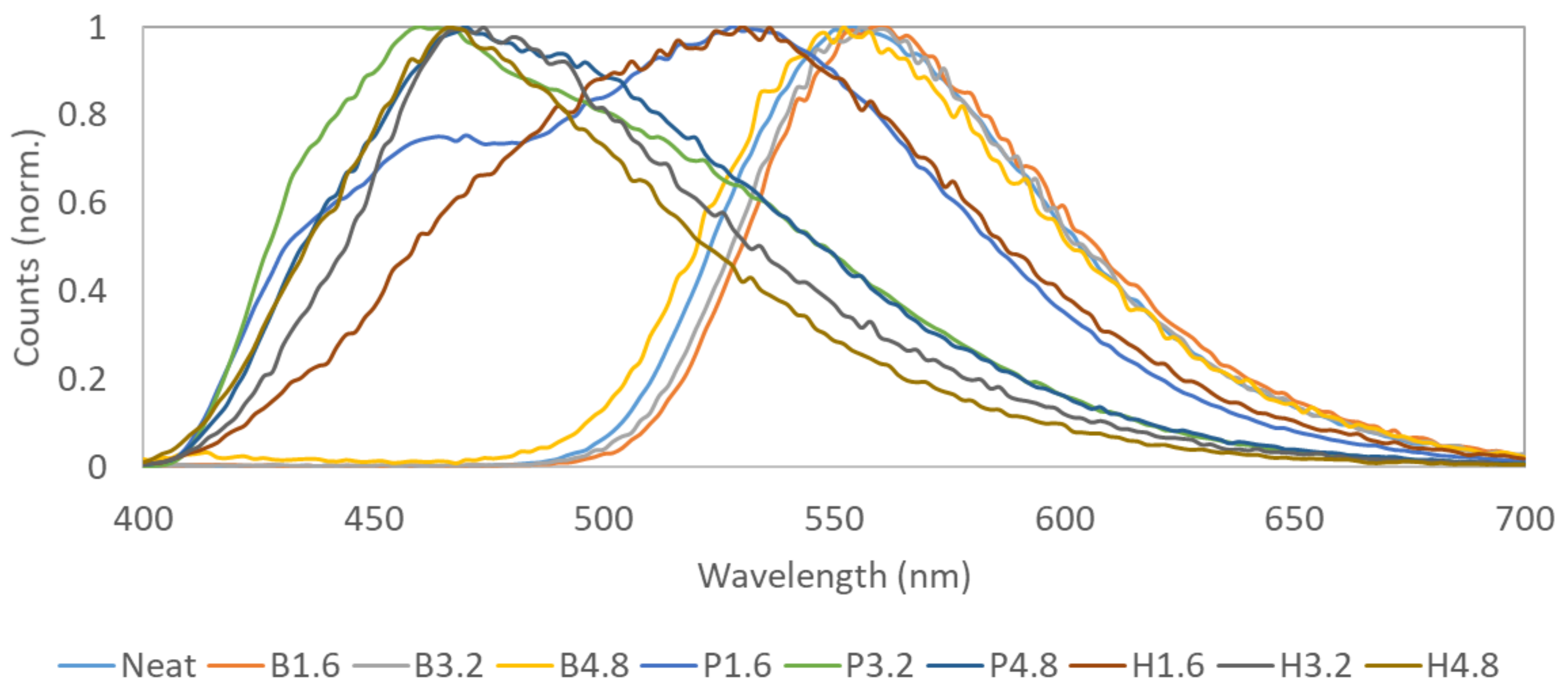
3.3. Matrix + Secondary Fluorophore Mixtures
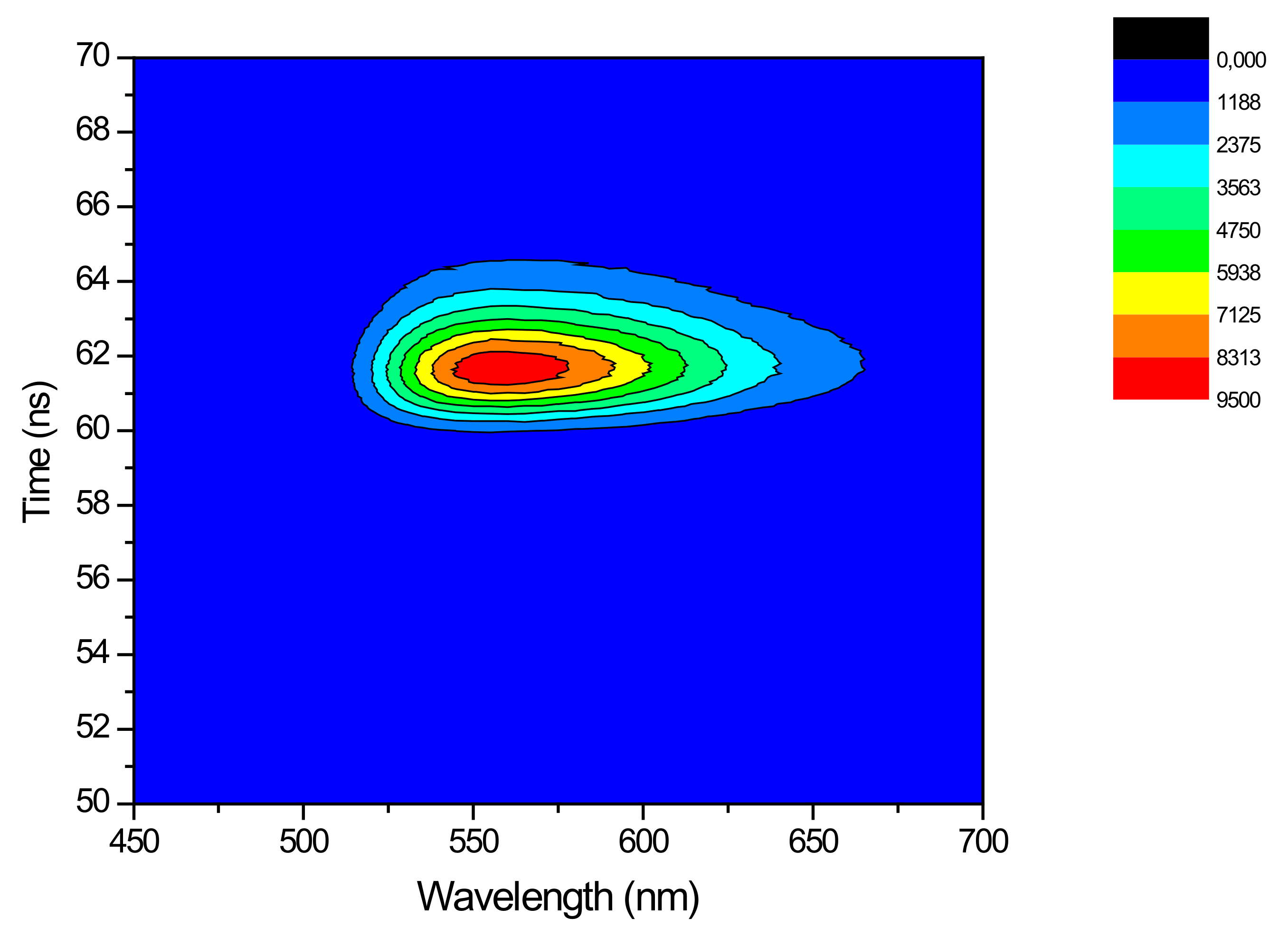
3.4. Full Red-and-Fast Plastic Systems
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dujardin, C.; Hamel, M. Introduction—Overview on plastics and inorganic scintillators. In Plastic Scintillators: Chemistry and Applications; Hamel, M., Ed.; Springer Nature: Cham, Switzerland, 2021; pp. 3–33. [Google Scholar] [CrossRef]
- Hamel, M.; Pjatkan, R.; Burešová, H. From the R&D to the commercialization of a new green-emitting plastic scintillator. Nucl. Instrum. Methods A 2020, 955, 163294. [Google Scholar] [CrossRef]
- Franks, L.A.; Lutz, S.; Lyons, P.B. Development of Long-Wavelength-Emitting Scintillators with Improved Decay Time Characteristics. IEEE Trans. Nucl. Sci. 1978, NS-25, 1024–1026. [Google Scholar] [CrossRef]
- Beddar, S.; Tendler, I.; Therriault-Proulx, F.; Archambault, L.; Beaulieu, L. Recent Advances and Clinical Applications of Plastic Scintillators in the Field of Radiation Therapy. In Plastic Scintillators: Chemistry and Applications; Hamel, M., Ed.; Springer Nature: Cham, Switzerland, 2021; pp. 425–460. [Google Scholar] [CrossRef]
- Gandini, M.; Villa, I.; Beretta, M.; Gotti, C.; Imran, M.; Carulli, F.; Fantuzzi, E.; Sassi, M.; Zaffalon, M.; Brofferio, C.; et al. Efficient, fast and reabsorption-free perovskite nanocrystal-based sensitized plastic scintillators. Nat. Nanotechnol. 2020, 15, 462–468. [Google Scholar] [CrossRef]
- Ma, W.; Su, Y.; Zhang, Q.; Deng, C.; Pasquali, L.; Zhu, W.; Tian, Y.; Ran, P.; Chen, Z.; Yang, G.; et al. Thermally activated delayed fluorescence (TADF) organic molecules for efficient X-ray scintillation and imaging. Nat. Mater. 2021, 21, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Hamel, M.; Turk, G.; Rousseau, A.; Darbon, S.; Reverdin, C.; Normand, S. Preparation and characterization of highly lead-loaded red plastic scintillators under low energy X-rays. Nucl. Instrum. Methods A 2011, 660, 57–63. [Google Scholar] [CrossRef]
- Cahill, P.A. Toward red-emitting, radiation tolerant chromophores. Radiat. Phys. Chem. 1993, 41, 351–363. [Google Scholar] [CrossRef]
- Berlman, I.B.; Ogdan, Y.A. Fast red emitting plastic scintillator. Nucl. Instrum. Methods 1980, 178, 411–413. [Google Scholar] [CrossRef]
- Montbarbon, E.; Amiot, M.-N.; Tromson, D.; Gaillard, S.; Frangville, C.; Woo, R.; Bertrand, G.H.V.; Pansu, R.B.; Renaud, J.-L.; Hamel, M. Large irradiation doses can improve the fast neutron/gamma discriminating capability of plastic scintillators. Phys. Chem. Chem. Phys. 2017, 19, 28105–28115. [Google Scholar] [CrossRef]
- Hamel, M.; Trocmé, M.; Rousseau, A.; Darbon, S. Red-emitting liquid and plastic scintillators with nanosecond time response. J. Lumin. 2017, 190, 511–517. [Google Scholar] [CrossRef]
- Tanaka, K.; Yanagida, T.; Hirose, A.; Yamane, H.; Yoshii, R.; Chujo, Y. Synthesis and color tuning of boron diiminate conjugated polymers with aggregation-induced scintillation properties. RSC Adv. 2015, 5, 966563. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Surin, N.M.; Borshchev, O.V.; Luponosov, Y.N.; Akimov, D.Y.; Alexandrov, I.S.; Burenkov, A.A.; Kovalenko, A.G.; Stekhanov, V.N.; Kleymyuk, E.A.; et al. Nanostructured organosilicon luminophores and their application in highly efficient plastic scintillators. Sci. Rep. 2014, 4, 6549. [Google Scholar] [CrossRef] [Green Version]
- Huijun, Y.; Xiangtuo, W.; Yuying, J.; Yuanli, Z. Energy transfer in multi component plastic scintillators. J. Lumin. 1984, 31-32, 833–835. [Google Scholar] [CrossRef]
- Dalla Palma, M.; Quaranta, A.; Marchi, T.; Collazuol, G.; Carturan, S.; Cinausero, M.; Degerlier, M.; Gramegna, F. Red Emitting Phenyl-Polysiloxane Based Scintillators for Neutron Detection. IEEE Trans. Nucl. Sci. 2014, 61, 2052–2058. [Google Scholar] [CrossRef]
- Huijun, Y. Fast red-emitting plastic scintillators. He Dianzixue Yu Tance Jishu. Yuanzineng Chubanshe: Beijing, China. 1990, 10, 353–355. [Google Scholar]
- Adadurov, A.F.; Zhmurin, P.N.; Lebedev, V.N.; Kovalenko, V.V. Plastic scintillator with phosphorescent dopants for α-particles registration. Nucl. Instrum. Methods A 2010, 621, 354–357. [Google Scholar] [CrossRef]
- Sytnik, A.; Kasha, M. Spectroscopic criteria for wavelength shifting, fast, and red-infrared scintillators. Radiat. Phys. Chem. 1993, 41, 331–349. [Google Scholar] [CrossRef]
- Tanaka, K.; Yanagida, T.; Yamane, H.; Hirose, A.; Yoshii, R.; Chujo, Y. Liquid scintillators with near infrared emission based on organoboron conjugated polymers. Bioorg. Med. Chem. Lett. 2015, 25, 5331–5334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakirova, J.R.; Grachova, E.V.; Melekhova, A.A.; Krupenya, D.V.; Gurzhiy, V.V.; Karttunen, A.J.; Koshevoy, I.O.; Melnikov, A.S.; Tunik, S.P. Luminescent AuI—CuI Triphosphane Clusters That Contain Extended Linear Arylacetylenes. Eur. J. Inorg. Chem. 2012, 4048–4056. [Google Scholar] [CrossRef]
- Montbarbon, E.; Sguerra, F.; Bertrand, G.H.V.; Magnier, É.; Coulon, R.; Pansu, R.B.; Hamel, M. N-(2-ethylhexyl)carbazole: A new fluorophore highly suitable for liquid scintillation and n/γ discrimination. Chem.-Eur. J. 2016, 22, 12074–12080. [Google Scholar] [CrossRef]
- Ebran, A.; Taieb, J.; Belier, G.; Chatillon, A.; Laurent, B.; Martin, J.-F.; Pellereau, E. Picosecond resolution on relativistic heavy ions’ time-of-flight measurement. Nucl. Instrum. Methods A 2013, 728, 40–46. [Google Scholar] [CrossRef]
- Moszyński, M. Study of light collection process from cylindrical scintillators. Nucl. Instrum. Methods 1976, 134, 77–85. [Google Scholar] [CrossRef]
- Moszyński, M.; Bengtson, B. Status of timing with plastic scintillation detectors. Nucl. Instrum. Methods 1979, 158, 1–31. [Google Scholar] [CrossRef]
- Nanoled, pulsed laser and LED light sources. Available online: https://www.horiba.com/us/en/scientific/products/fluorescence-spectroscopy/lifetime/tcspc-components/nanoled/nanoled-618/ (accessed on 17 January 2022).
- Berlman, I.B.; Lutz, S.S.; Flournoy, J.M.; Ashford, C.B.; Franks, L.A.; Lyons, P.B. New fast organic scintillators using intramolecular bromine quenching. Nucl. Instrum. Methods 1984, 225, 78–84. [Google Scholar] [CrossRef]
- Andreeshchev, E.A.; Avedisyan, V.S.; Veronyan, S.M.; Zyablin, V.L.; Kovyrzina, K.A.; Kushakevich, Y.P.; Rozman, I.M.; Shoniya, V.M. SPS-B18 high-speed plastic scintillator. Instrum. Exp. Techn. 1988, 31, 593–595, Translated from Prib. Tekh. Eksp. 1988, 67–68. [Google Scholar]
- Lutz, S.S.; Franks, L.A.; Flournoy, J.M. High speed liquid scintillators for optical fiber applications. Nucl. Instrum. Methods 1982, 193, 623–629. [Google Scholar] [CrossRef]
- Zheng, H.; Baumbaugh, B.; Gerig, A.; Hurlbut, C.; Kauffman, J.; Marchant, J.; Pla-Dalmau, A.; Reynolds, K.; Ruchti, R.; Warchol, J.; et al. New scintillator and waveshifter materials. AIP Conf. Proc. 1998, 450, 371–380. [Google Scholar] [CrossRef]
- Bondarev, S.L.; Knyukshto, V.N.; Stepuro, V.I.; Stupak, A.P.; Turban, A.A. Fluorescence and electronic structure of the laser dye dcm in solutions and in polymethylmethacrylate. J. Appl. Spectrosc. 2004, 71, 194–201, Translated from Zh. Prikl. Spektrosk. 2004, 71, 179–186. [Google Scholar] [CrossRef]
- Muniz-Miranda, F.; Pedone, A.; Muniz-Miranda, M. Spectroscopic and DFT investigation on the photo-chemical properties of a push-pull chromophore: 4-Dimethylamino-4′-nitrostilbene. Spectrochim. Acta Part A 2018, 190, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Papper, V.; Pines, D.; Likhtenshtein, G.; Pines, E. Photophysical characterization of trans-4,4′-disubstituted stilbenes. J. Photochem. Photobiol. A 1997, 111, 87–96. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for use in PhotochemCAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef] [Green Version]
- BC-430 plastic scintillator. Available online: https://www.crystals.saint-gobain.com/sites/imdf.crystals.com/files/documents/bc430-data-sheet.pdf (accessed on 17 January 2022).
- Li, Y.; Chen, L.; Gao, R.; Liu, B.; Zheng, W.; Zhu, Y.; Ruan, J.; Ouyang, X.; Xu, Q. Nanosecond and highly Sensitive Scintillator Based on All-Inorganic Perovskite Single Crystals. ACS Appl. Mater. Interfaces 2021, 14, 1489–1495. [Google Scholar] [CrossRef] [PubMed]

| State | Last Fluorophore | Light Output (ph/MeV) | Decay Time (ns)c | Ref. | |
|---|---|---|---|---|---|
| YAG:Ce | Y3Al5O12(Ce) | 550 | 8000 | 70 | - |
| PVT scintillator | BC-430 | 580 | 6900 | 16.8 | - |
| Polymer thin film |  | 584 | n.d. a | 0.46 (60) + 1.0 (40) | [12] |
| Polystyrene scintillator | 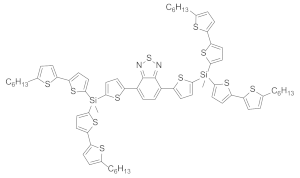 | 588 | 9780 | n.d. | [13] |
| Polystyrene scintillator |  | 591 | 80 | 9.22–13.26 | [7] |
| Polystyrene scintillator | 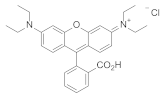 | 595 | n.d. | 5.94 | [14] |
| Polystyrene scintillator | 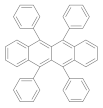 | >600 | n.d. | 5 | [9] |
| Polysiloxane scintillator | Lumogen Red | ≈600 | 8300 | n.d. | [15] |
| Polystyrene scintillator | 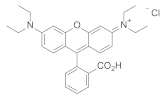 | 600 | ≈16,000 | ≈6 | [3] |
| PMMA scintillator | CsPbBr3 Perovskite +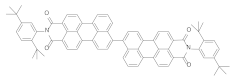 | ≈600 | ≈9000 | 3.4 (87) + 14.1 (13) | [5] |
| Poly(styrene-co-acrylonitrile) scintillator | 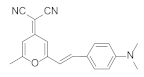 | ≈610 | n.d. | n.d. | [16] |
| Polystyrene scintillator | 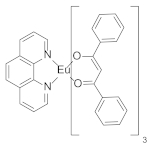 | 614 | 5650 b | 469,000 | [17] |
| Sucrose octaacetate | 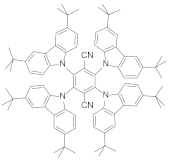 | 618 | 37,500 | 10.9 + 1960 | [6] |
| Polystyrene scintillator | 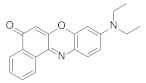 | 610–620 | 70–300 | 8.7 | [11] |
| PMMA scintillator | 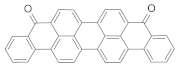 | ≈620 | n.d. | 3.94 | [18] |
| Polymer in liquid | 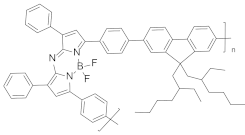 | 750 | n.d. | 1.92 | [19] |
| Molecule | Structure | Mono- or Biexponential τ (ns) a | <τ> (ns) b |
|---|---|---|---|
| PPO | 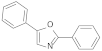 | 2.27 | 2.27 |
| 1,1,4,4-tetraphenylbutadiene | 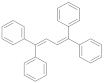 | 1.45 (99) + 34.7 (1) | 1.78 |
| Biphenyl | 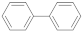 | 4.81 | 4.81 |
| p-terphenyl | 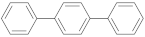 | 1.45 | 1.45 |
| p-quaterphenyl | 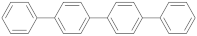 | 0.85 (73) + 1.82 (27) | 1.11 |
| p-sexiphenyl | 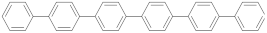 | 1.67 (66) + 2.83 (34) | 2.06 |
| m-terphenyl | 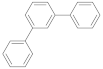 | 1.15 (59) + 8.96 (41) | 4.35 |
| 4-bromo-p-terphenyl | 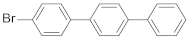 | 0.72 (58) + 3.81 (42) | 2.01 |
| 4,4′-dibromo-p-terphenyl | 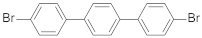 | 1.90 (64) + 12.54 (36) | 5.73 |
| PMP1-phenyl-3-(mesityl)-2-pyrazoline | 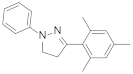 | 4.11 | 4.11 |
| Naphthalene | 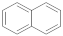 | 8.67 | 8.67 |
| Anthracene | 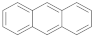 | 2.42 (90) + 8.51 (10) | 3.03 |
| 4-isopropylbiphenyl | 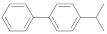 | 0.80 (5) + 4.94 (95) | 4.73 |
| Pyrene | 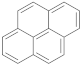 | 23.0 | 23.0 |
| N-ethylcarbazole | 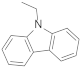 | 7.91 | 7.91 |
| N-(2-ethylhexyl)carbazole | 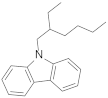 | 12.30 | 12.30 |
| BBD2,5-di(4′-biphenylyl)-1,3,4-oxadiazole | 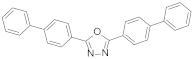 | 1.35 | 1.35 |
| PBD2-phenyl-5-(4′-biphenylyl)-1,3,4-oxadiazole |  | 1.51 | 1.51 |
| Butyl-PBD2-(p-tert-butylphenyl-5-(4′-biphenylyl)-1,3,4-oxadiazole | 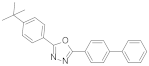 | 0.92 (54) + 1.64 (46) | 1.25 |
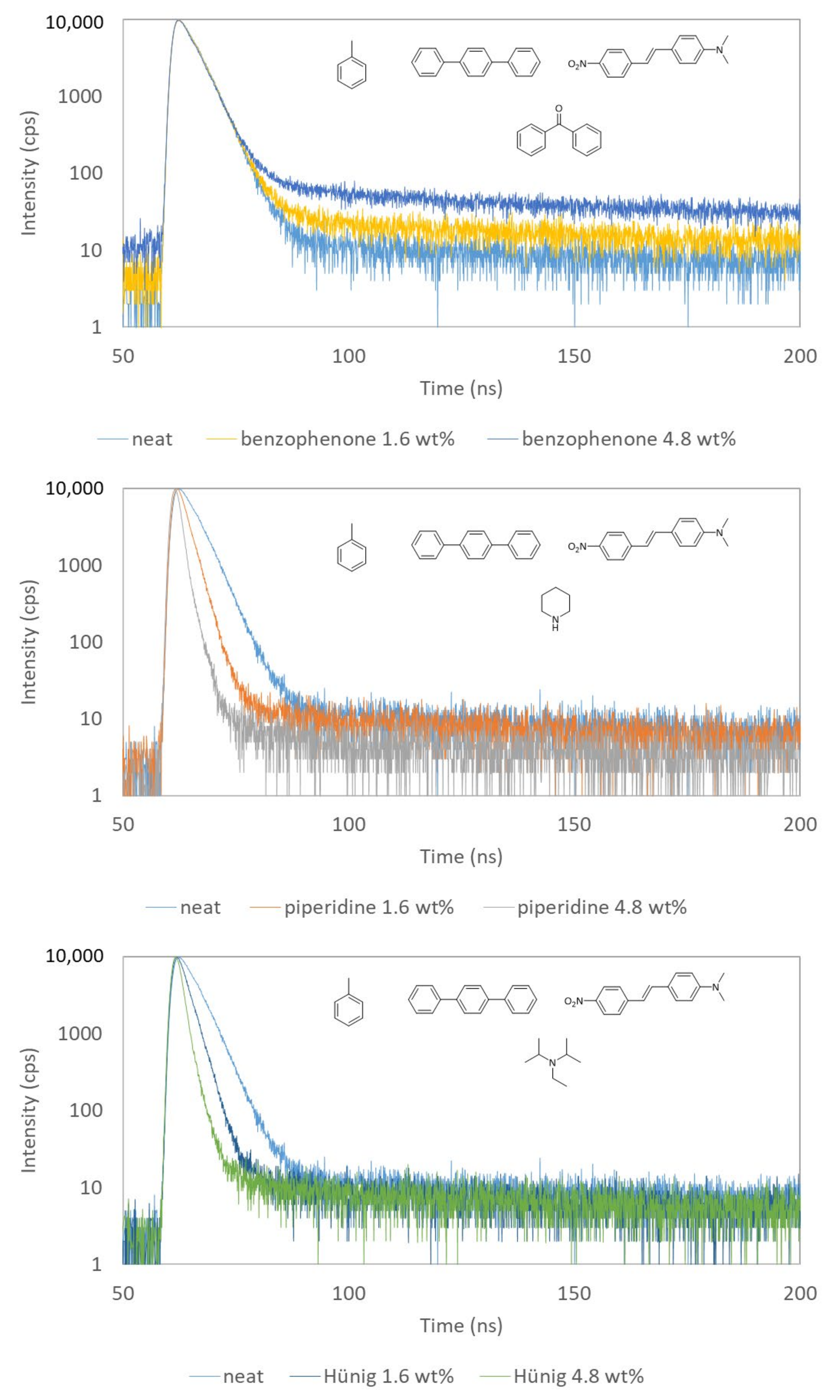
| Molecule | Quencher (wt %) | τ (ns) | <τ> (ns) |
|---|---|---|---|
| p-terphenyl | - | 1.45 | 1.45 |
| p-terphenyl | Benzophenone (0.4) | 0.59 (63.5) + 1.85 (36.5) | 1.05 |
| p-terphenyl | Piperidine (1.6) | 0.60 (71) + 1.92 (29) | 0.98 |
| p-terphenyl | Hünig’s base (1.6) | 0.66 (78.6) + 2.11 (21.4) | 0.97 |
| p-quaterphenyl | - | 0.85 (73) + 1.82 (27) | 1.11 |
| p-quaterphenyl | Benzophenone (0.4) | 0.77 (42.5) + 2.08 (57.5) | 1.52 |
| p-quaterphenyl | Piperidine (1.6) | 1.73 | 1.73 |
| p-quaterphenyl | Hünig’s base (1.6) | 0.71 (48.5) + 4.44 (51.5) | 2.6 |
| Composition * | Mono- or Biexponential τ (ns) * | <τ> (ns) | FWHM (ns) | |
|---|---|---|---|---|
| BC-422Q 1% | 0.78 (67) + 3.05 (32) | 1.52 | 360 | 2.05 |
| BC-422Q 2% | 0.81 (73) + 3.08 (27) | 1.42 | 402 | 1.96 |
| PVT p-Q 0.3 | 1.71 (52) + 11.6 (48) | 6.46 | 370 | 2.58 |
| PVT p-Q 0.6 | 1.50 (59) + 9.93 (41) | 4.69 | 370 | 2.52 |
| PVT p-Q 1.0 | 1.52 (66) + 8.41 (34) | 3.86 | 370 | 2.52 |
| PVT p-Q 0.6 B 0.6 | 1.23 (63) + 8.05 (37) | 3.86 | 370 | 2.25 |
| PS p-T 2 B 2 | 0.81 (48.5) + 4.09 (51.4) | 2.50 | 410 | 2.08 |
| PS 4-Br-p-T 0.5 | 0.56 (50) + 2.90 (34.9) + 10.9 (15.1) | 2.95 | 400 | 1.71 |
| PS 4-Br-p-T 1.0 | 0.99 (49.75) + 5.38 (50.25) | 3.19 | 400 | 2.14 |
| PS 4-Br-p-T 1.5 | 0.53 (46.5) + 2.80 (36.1) + 11.8 (17.4) | 3.31 | 400 | 1.71 |
| PS 4-Br-p-T 3.5 | 0.882 (73.9) + 4.00 (26.1) | 1.69 | 400 | 2.00 |
| Composition * | Radioluminescence vs. BC-422Q 1% (%) | Light Yield ϕ vs. BC-422Q 1% (ph/MeV) | ϕ/<τ> (ph/MeV/ns) | |
|---|---|---|---|---|
| BC-422Q 1% | 400 | 100 | 1700 | 1118 |
| BC-422Q 2% | 406 | 56 | 290 | 204 |
| PVT p-Q 0.3 | 394 | 140 | 1940 | 300 |
| PVT p-Q 0.6 | 402 | 118 | 1310 | 279 |
| PVT p-Q 1.0 | 398 | 229 | 2840 | 735 |
| PVT p-Q 0.6 B 0.6 | 396 | 87 | 610 | 158 |
| PS 4-Br-p-T 1.0 | 394 | 52 | 700 | 219 |
| PS 4-Br-p-T 3.5 | 396 | 90 | 1870 | 1106 |
| # | Molecule | Structure | τ (ns) | |
|---|---|---|---|---|
| 1 | Nile red | 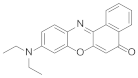 | 610 | 5.18 |
| 2 | DCM4-(Dicyanomethylene)-2-methyl-6-(4-dimethylaminostyryl)-4H-pyran | 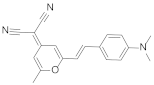 | 550 | 0.95 |
| 3 | Rubrene |  | 584 | 27.3 |
| 4 | N,N′-Bis(2,5-di-tert-butylphenyl)-3,4,9,10-perylenedicarboximide | 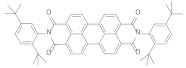 | 580 | 6.6 |
| 5 | N,N′-Bis-n-pentyl-1,6,7,12-tetrachloro-3,4,9,10-perylenedicarboximide | 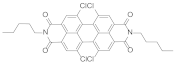 | 596 | 11.4 |
| 6 | Pyrromethene 605Difluoro [2-(4-ethyl-3,5-dimethyl-1H-pyrrol-2-yl-κN)-2-(4-ethyl-3,5-dimethyl-2H-pyrrol-2-ylidene-κN)ethyl acetato])boron | 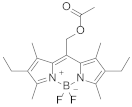 | 586 | 12.4 |
| 7 | Pyrromethene 597Difluoro(4-(1,1-dimethylethyl)-2-{1-[4-(1,1-dimethylethyl)-3,5-dimethyl-2H-pyrrol-2-ylidene-N]ethyl}-3,5-dimethyl-1H-pyrrol-2-ylidene-N]ethyl}-3,5-dimethyl-1H-pyrrolato-N)boron | 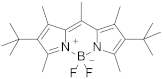 | 608 | 13.8 |
| 8 | DANSTrans-4-dimethylamino-4′-nitrostilbene | 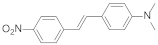 | 560 | 3.28 |
| Matrix | τ (ns) | Quantum Yield | ||
|---|---|---|---|---|
| PMMA | Bibliography | 550 | 2.0 | 0.76 |
| PMMA | Measurement | 550 | 2.63 | n.d. |
| Toluene | Bibliography | 567 | 0.02 | 0.08 |
| Styrene | Measurement | 550 | 0.95 | n.d. |
| Polystyrene | Measurement | 550 | 3.80 | n.d. |
| Quencher | Concentration (wt %) | τ (ns) | <τ> (ns) | FWHM ± 0.05 (ns) |
|---|---|---|---|---|
| - | 0 | 3.28 | 5.00 | |
| Benzophenone | 1.6 | 3.37 | 4.78 | |
| Benzophenone | 4.8 | 3.44 (89.1) + 61.1 (10.9) | 9.72 | 4.72 |
| Piperidine | 1.6 | 2.02 | 3.13 | |
| Piperidine | 4.8 | 0.91 (72.8) + 2.39 (27.2) | 1.31 | 2.25 |
| Hünig’s base | 1.6 | 2.16 | 3.24 | |
| Hünig’s base | 4.8 | 1.17 (91.8) + 5.14 (8.2) | 1.50 | 2.41 |
| Plastic Scintillator a | τ (ns) λex 274 nm | FWHM ± 0.11 (ns) | ϕ (ph/MeV) c | ϕ (ph/MeV) d | ϕ/<τ> (ph/MeV/ns) e | |
|---|---|---|---|---|---|---|
| BC-430 | 580 | 12.5 | 23.00 | 6900 | 6900 | 552 |
| BC-422Q 1% | 380 | 1.52 | 2.05 | n.d. f | 1700 | 1118 |
| PS + p-T 1.5 + DCM (2) 0.01 | 564 | 3.08 (85.5) + 22.1 (14.5) | 5.15 | 1300 | 1120 | 192 |
| PS + p-T 1.5 + DCM (2) 0.03 | 574 | 3.27 (89.7) + 22.4 (10.3) | 5.27 | 1500 | 1430 | 273 |
| PS + p-T 1.5 + DCM (2) 0.05 | 578 | 3.42 (92.5) + 23.0 (7.5) | 5.27 | 1300 | 1220 | 250 |
| PS + 4-Br-p-T 1.5 + DCM (2) 0.03 + B 2 | 560 | 3.30 | 2.08 | n.d. | 360 | 109 |
| PS + p-T 1.5 + 3 0.01 | 582 | 23.78 | 25.35 | 2330 | 2850 | 120 |
| PS + p-T 1.5 + 3 0.03 | 590 | 27.35 | 40.71 | 2500 | 2560 | 94 |
| PS + p-T 1.5 + 3 0.05 | 590 | 29.19 | 37.64 | 2460 | 2450 | 84 |
| PS + p-T 1.5 + 4 0.01 | 578 | 7.27 (95.3) + 41.8 (4.7) | 8.34 | 3670 | 4370 | 491 |
| PS + p-T 1.5 + 4 0.03 | 582 | 9.52 (73.15) + 34.9 (26.85) | 11.85 | 4400 | 4860 | 297 |
| PS + p-T 1.5 + 4 0.05 | 592 | 9.44 (52.65) + 35.0 (47.35) | 12.07 | 3700 | 4290 | 199 |
| PS + p-T 1.5 + 5 0.01 | 582 | 10.82 | 12.94 | 3210 | 2510 | 232 |
| PS + p-T 1.5 + 5 0.03 | 592 | 11.44 | 15.25 | 3240 | 3070 | 268 |
| PS + p-T 1.5 + 5 0.05 | 596 | 11.59 | 13.50 | 3240 | 3220 | 278 |
| PS + p-T 1.5 + 7 0.01 | 596 | 12.4 | 19.20 | 4250 | 3800 | 306 |
| PS + p-T 1.5 + 7 0.03 | 602 | 13.8 | 20.74 | 4500 | 4610 | 334 |
| PS + p-T 1.5 + 7 0.05 | 604 | 13.9 | 22.99 | 5070 | 5160 | 371 |
| PS + p-T 1.5 + DANS 0.01 | 554 | 4.23 | 7.13 | 6280 | 6100 | 1442 |
| PS + p-T 1.5 + DANS 0.03 | 570 | 4.40 | 7.68 | 5470 | 5390 | 1225 |
| PS + p-T 1.5 + DANS 0.05 | 572 | 4.68 | 7.90 | 5480 | 5600 | 1196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamel, M. Progress in Fast and Red Plastic Scintillators. Chemosensors 2022, 10, 86. https://doi.org/10.3390/chemosensors10020086
Hamel M. Progress in Fast and Red Plastic Scintillators. Chemosensors. 2022; 10(2):86. https://doi.org/10.3390/chemosensors10020086
Chicago/Turabian StyleHamel, Matthieu. 2022. "Progress in Fast and Red Plastic Scintillators" Chemosensors 10, no. 2: 86. https://doi.org/10.3390/chemosensors10020086
APA StyleHamel, M. (2022). Progress in Fast and Red Plastic Scintillators. Chemosensors, 10(2), 86. https://doi.org/10.3390/chemosensors10020086






