A Sensitive Co-MOF/CNTs/SiO2 Composite Based Electrode for Determination of Gallic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments
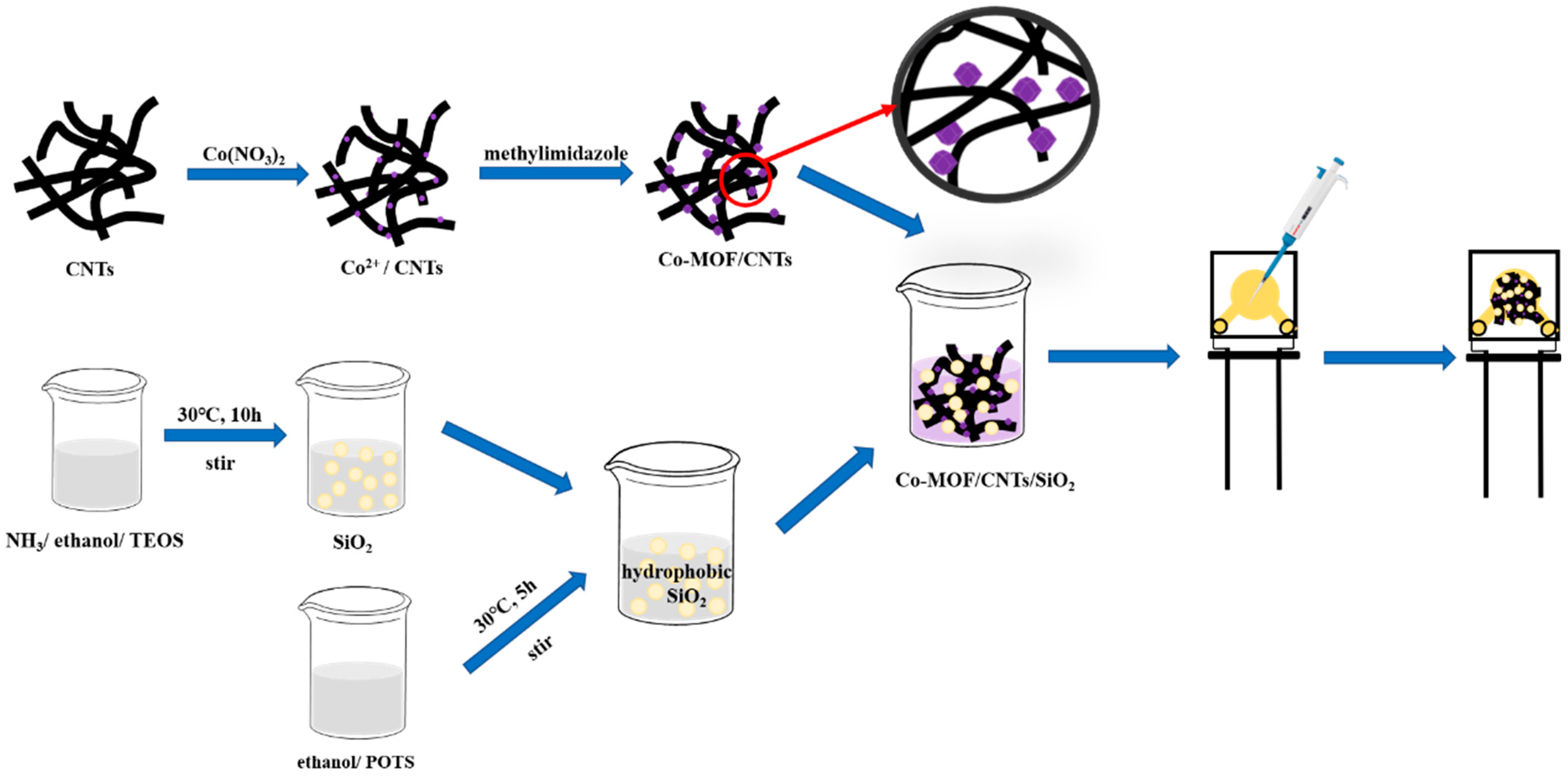
2.3. Synthesis of Co-MOF/CNTs and Co-MOF
2.4. Synthesis of Co-MOF/CNTs/SiO2
2.5. Preparation of Modified AuE
3. Results and Discussion
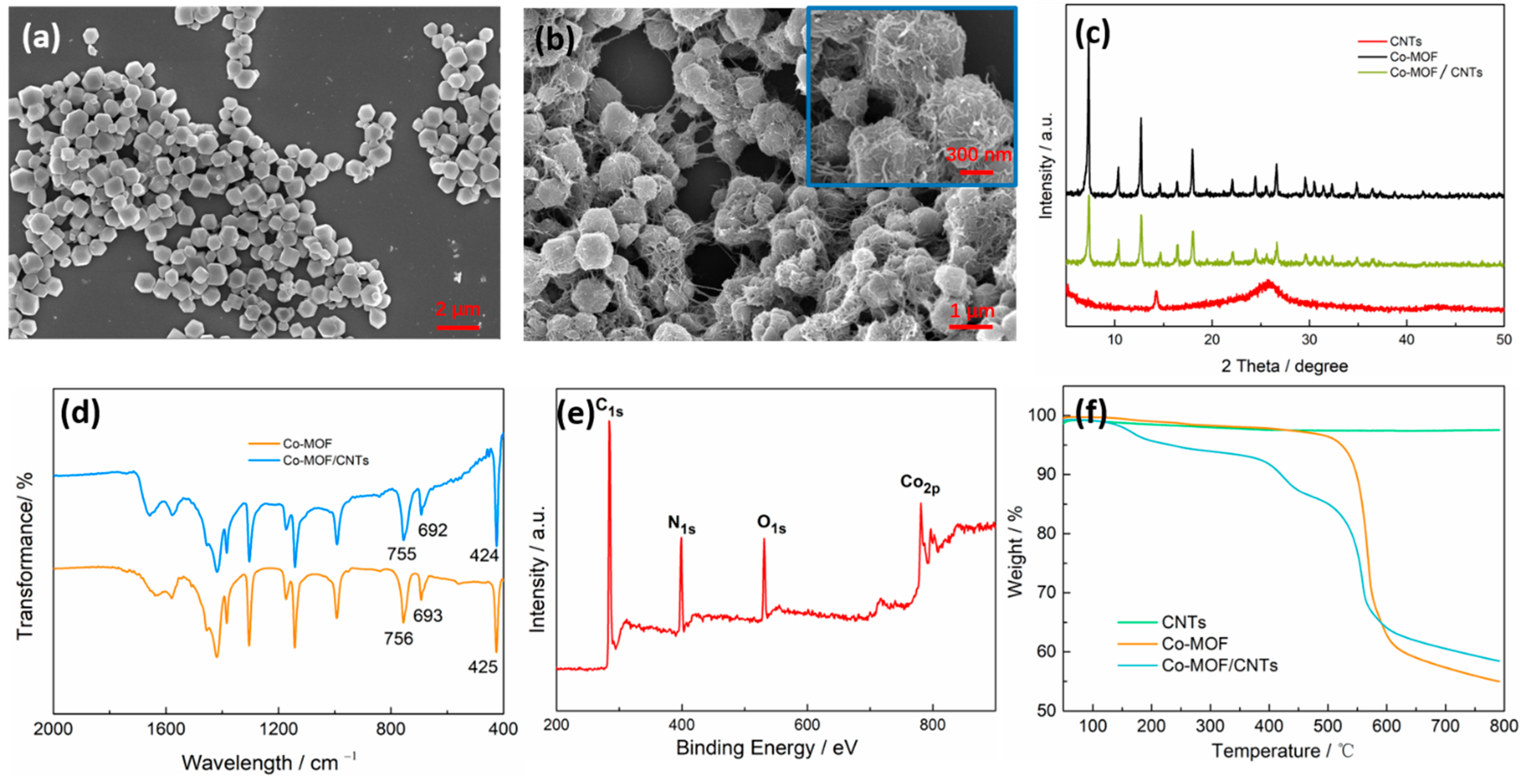
3.1. Characterization of Co-MOF/CNTs and Co-MOF
3.2. Characterization of Co-MOF/CNTs/SiO2
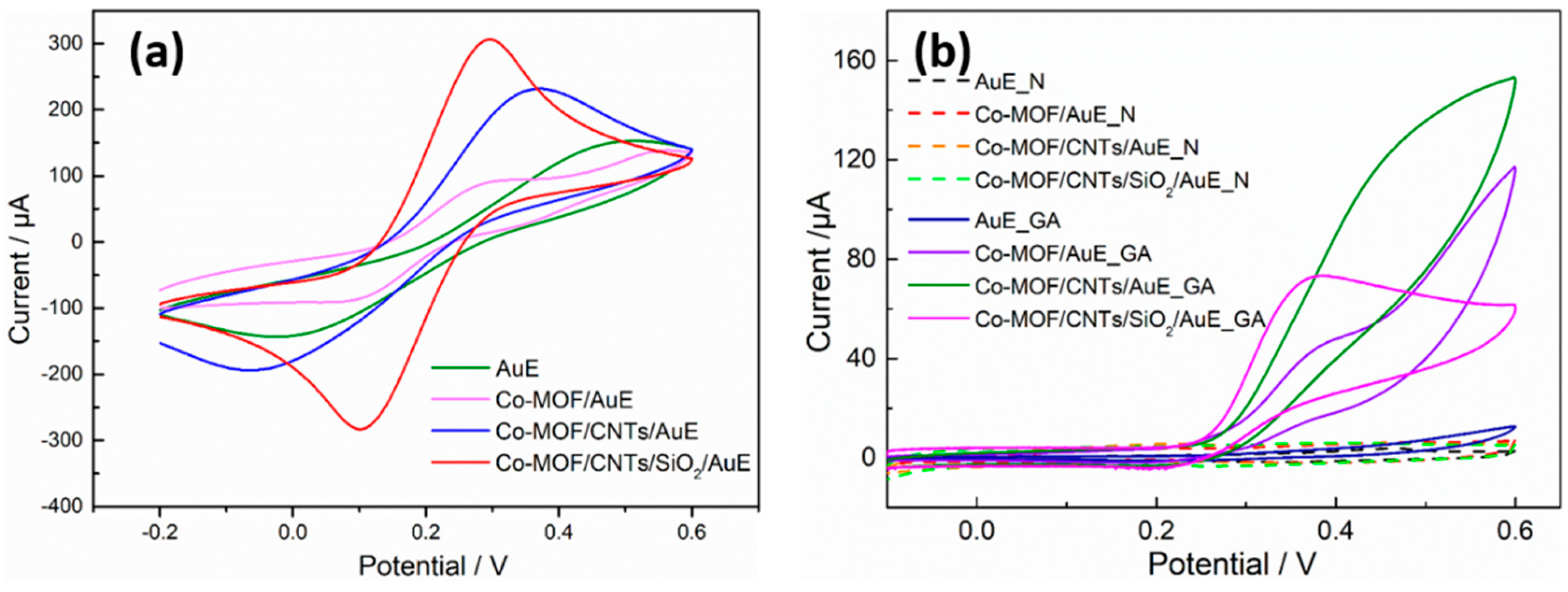
3.3. Electrochemical Characterization of Co-MOF/CNTs/SiO2-Modified Au Electrodes
3.4. Characterization of Surface Quality Change by EQCM
3.5. Electrochemical Behavior of Gallic Acid on Modified AuE
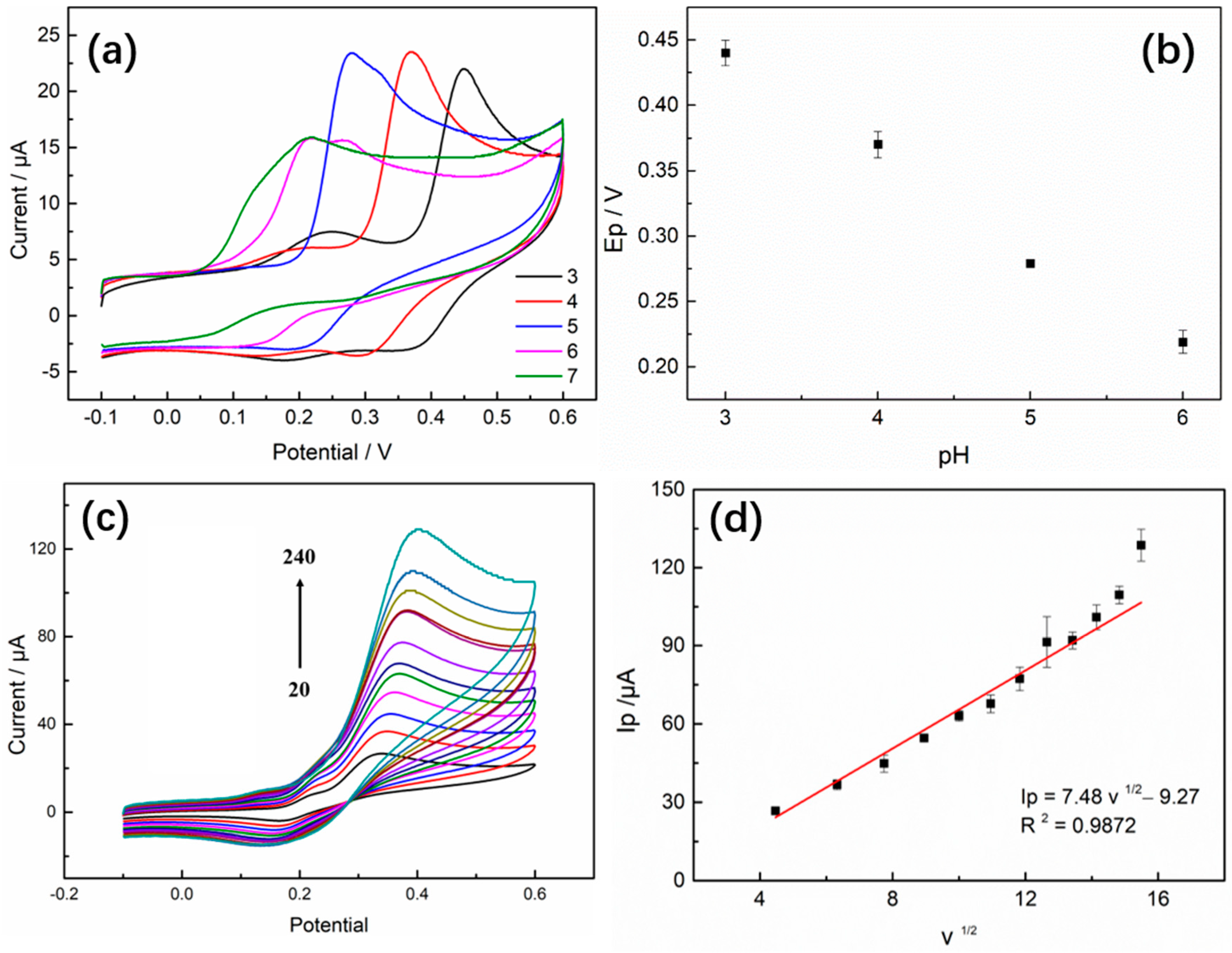
3.6. The Effect of pH and Scan Rate
3.7. Analysis of Electrooxidation Mechanism of GA
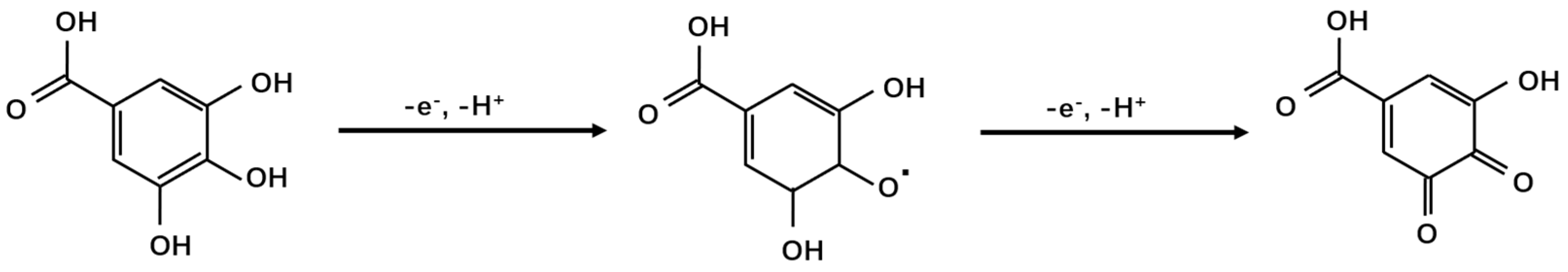
3.7.1. The Oxidation Process of GA
3.7.2. The Catalysis of Co-MOF to GA
- (1)
- 2Co-MOF—Co(II) → 2Co-MOF—Co(III) + 2e−
- (2)
- C7H6O6 − 2e− − 2H+ → C7H4O6
- (3)
- 2Co-MOF—Co(III) + 2e− → 2Co-MOF—Co(II)
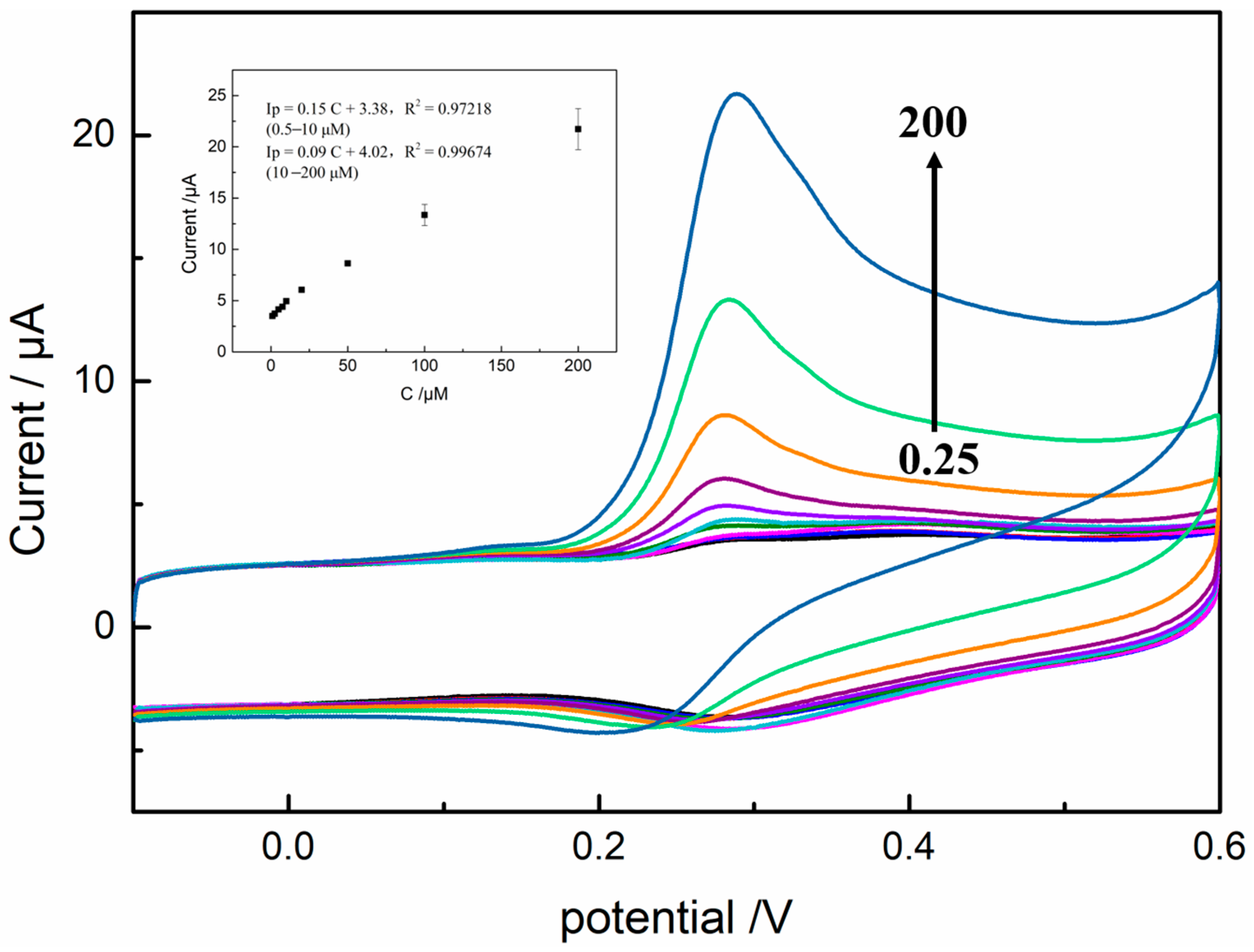
3.8. Electrochemical Determination of GA
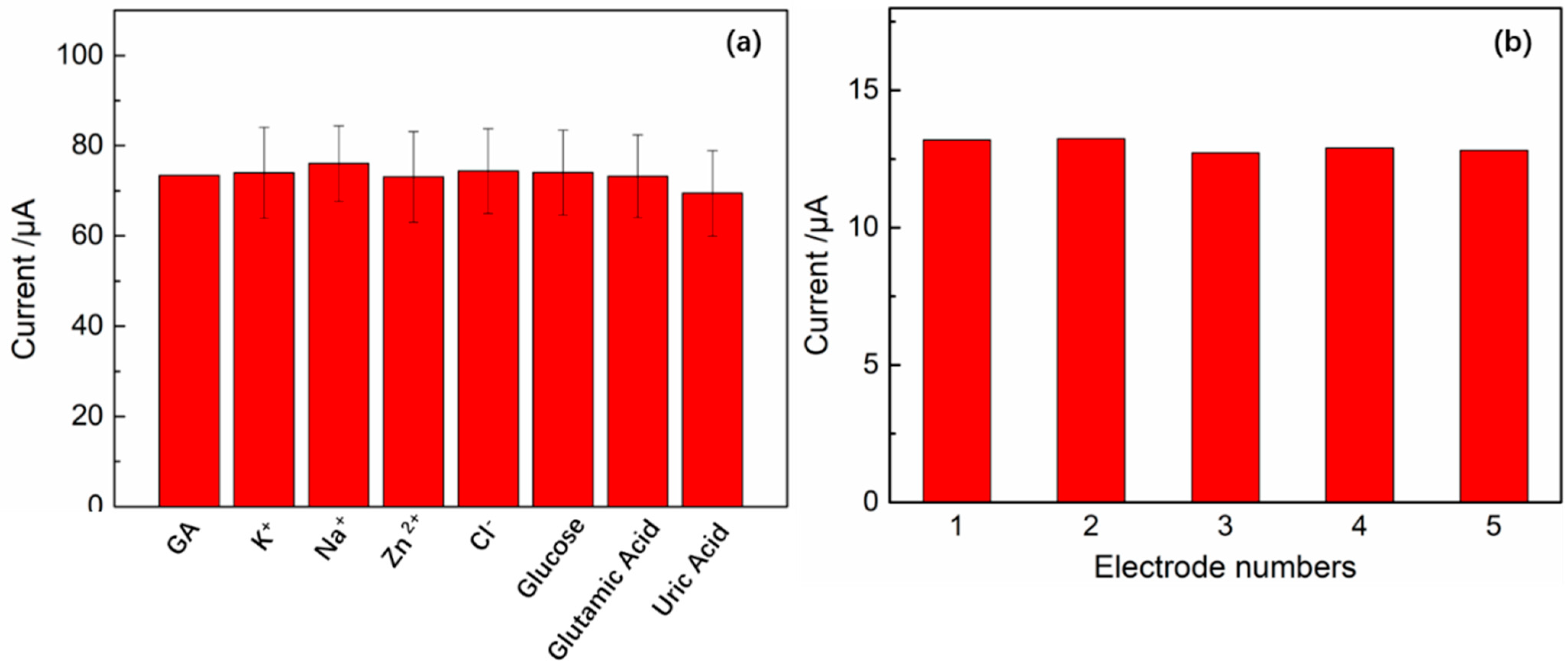
3.9. The Selectivity, Reproducibility and Stability of the GA Sensor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vilian, A.T.E.; Song, J.Y.; Lee, Y.S.; Hwang, S.K.; Kim, H.J.; Jun, Y.S.; Huh, Y.S.; Han, Y.K. Salt-templated three-dimensional porous carbon for electrochemical determination of gallic acid. Biosens. Bioelectron. 2018, 117, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Sainz-Urruela, C.; Vera-Lopez, S.; San Andres, M.P.; Diez-Pascual, A.M. Graphene-based sensors for the detection of bioactive compounds: A review. Int. J. Mol. Sci. 2021, 22, 3316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ran, Q.; Li, Y.; Li, B.; Liu, B.; Ma, H.; Zhang, M.; Komarneni, S. Highly sensitive detection of gallic acid based on 3D interconnected porous carbon nanotubes/carbon nanosheets modified glassy carbon electrode. J. Mater. Res. Technol. 2020, 9, 9422–9433. [Google Scholar] [CrossRef]
- Das, D.; Biswas, D.; Hazarika, A.K.; Sabhapondit, S.; Roy, R.B.; Tudu, B.; Bandyopadhyay, R. CuO nanoparticles decorated MIP-based electrode for sensitive determination of gallic acid in green tea. IEEE Sens. J. 2021, 21, 5687–5694. [Google Scholar] [CrossRef]
- Lin, X.; Deng, Y.; He, Y.; Chen, J.; Hu, S. Construction of hydrophilic N, O-rich carboxylated triazine-covalent organic frameworks for the application in selective simultaneous electrochemical detection. Appl. Surf. Sci. 2021, 545, 149047. [Google Scholar] [CrossRef]
- Nazari, F.; Ghoreishi, S.M.; Khoobi, A. Bio-based Fe3O4/chitosan nanocomposite sensor for response surface methodology and sensitive determination of gallic acid. Int. J. Biol. Macromol. 2020, 160, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.H.; Salgado, H.R. Gallic acid: Review of the methods of determination and quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Liu, Y.C.; Hu, X.Y.; Xu, M.; Wang, Y.M. Studies on interactions of pentagalloyl glucose, ellagic acid and gallic acid with bovine serum albumin: A spectroscopic analysis. Food Chem. 2020, 324, 6. [Google Scholar] [CrossRef]
- Zaripour, M.; Zare-Shahabadi, V.; Jahromi, H.J. Application of ultrasonic-assisted inclusion complex formation with alpha-cyclodextrin for simultaneous spectrophotometric determination of gallic acid and vanillic acids in fruit samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117197. [Google Scholar] [CrossRef]
- Sangeetha, N.S.; Narayanan, S.S. A novel bimediator amperometric sensor for electrocatalytic oxidation of gallic acid and reduction of hydrogen peroxide. Anal. Chim. Acta 2014, 828, 34–45. [Google Scholar] [CrossRef]
- Kaur, S.; Shiekh, B.A.; Kaur, D.; Kaur, I. Highly sensitive sensing of Fe(III) harnessing schiff based ionophore: An electrochemical approach supported with spectroscopic and dft studies. J. Mol. Liq. 2021, 333, 115954. [Google Scholar] [CrossRef]
- Seenivasan, R.; Chang, W.J.; Gunasekaran, S. Highly sensitive detection and removal of lead ions in water using cysteine-functionalized graphene oxide/polypyrrole nanocomposite film electrode. ACS Appl. Mater. Interfaces 2015, 7, 15935–15943. [Google Scholar] [CrossRef] [PubMed]
- Angizi, S.; Hatamie, A.; Ghanbari, H.; Simchi, A. Mechanochemical green synthesis of exfoliated edge-functionalized boron nitride quantum dots: Application to vitamin c sensing through hybridization with gold electrodes. ACS Appl. Mater. Interfaces 2018, 10, 28819–28827. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wei, Z.; Wang, J.; Zhong, J. An electrochemical biosensor based on NiO nanoflowers/polymethylene blue composite for non-enzymatic glucose detection. J. Electrochem. Soc. 2020, 167, 146512. [Google Scholar] [CrossRef]
- Pwavodi, P.C.; Ozyurt, V.H.; Asir, S.; Ozsoz, M. Electrochemical sensor for determination of various phenolic compounds in wine samples using Fe3O4 nanoparticles modified carbon paste electrode. Micromachines 2021, 12, 312. [Google Scholar] [CrossRef]
- Goda, M.A.; El-Moghny, M.G.A.; El-Deab, M.S. Enhanced electrocatalytic oxidation of urea at CuOx-NiOx nanoparticle-based binary catalyst modified polyaniline/GC electrodes. J. Electrochem. Soc. 2020, 167, 064522. [Google Scholar] [CrossRef]
- Aryal, K.P.; Jeong, H.K. Electrochemical detection of ascorbic acid with chemically functionalized carbon nanofiber/beta-cyclodextrin composite. Chem. Phys. Lett. 2020, 757, 137881. [Google Scholar] [CrossRef]
- Zeng, X.; Li, N.; Wang, J. Electrochemical synthesis of (poly) dimethoxyaniline on glassy carbon electrodes and their applications in the detection of L- and D-glutamic acids. J. Electrochem. Soc. 2019, 166, B3066–B3071. [Google Scholar] [CrossRef]
- Phonklam, K.; Wannapob, R.; Sriwimol, W.; Thavarungkul, P.; Phairatana, T. A novel molecularly imprinted polymer PMB/MWCNTs sensor for highly-sensitive cardiac troponin T detection. Sens. Actuators B Chem. 2020, 308, 127630. [Google Scholar] [CrossRef]
- Marsh, C.; Shearer, G.C.; Knight, B.T.; Paul-Taylor, J.; Burrows, A.D. Supramolecular aspects of biomolecule interactions in metal–organic frameworks. Coord. Chem. Rev. 2021, 439, 213928. [Google Scholar] [CrossRef]
- Wang, B.; Xie, L.-H.; Wang, X.; Liu, X.-M.; Li, J.; Li, J.-R. Applications of metal–organic frameworks for green energy and environment: New advances in adsorptive gas separation, storage and removal. Green Energy Env. 2018, 3, 191–228. [Google Scholar] [CrossRef]
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the art and prospects in metal-organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Liao, X.; Fu, H.; Yan, T.; Lei, J. Electroactive metal-organic framework composites: Design and biosensing application. Biosens. Bioelectron. 2019, 146, 111743. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Koinuma, M.; Hayami, S. Proton conductors produced by chemical modifications of carbon allotropes, perovskites and metal organic frameworks. J. Mater. Chem. A 2017, 5, 7243–7256. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Y.; Zhang, Y.; Zeng, T.; Wan, Q.; Lai, G.; Yang, N. A UiO-66-NH2/carbon nanotube nanocomposite for simultaneous sensing of dopamine and acetaminophen. Anal. Chim. Acta 2021, 1158, 338419. [Google Scholar] [CrossRef]
- Lei, J.; Ju, H. Nanotubes in biosensing. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Keser Demir, N.; Chen, J.P.; Li, K. Applications of water stable metal-organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar] [CrossRef] [PubMed]
- Raza, W.; Kukkar, D.; Saulat, H.; Raza, N.; Azam, M.; Mehmood, A.; Kim, K.-H. Metal-organic frameworks as an emerging tool for sensing various targets in aqueous and biological media. TrAC Trend. Anal. Chem. 2019, 120, 115654. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Z.; Gu, S.; Wang, J. Detection of hexanal in humid circumstances using hydrophobic molecularly imprinted polymers composite. Sens. Actuators B Chem. 2019, 291, 141–147. [Google Scholar] [CrossRef]
- Li, L.; Li, B.; Dong, J.; Zhang, J. Roles of silanes and silicones in forming superhydrophobic and superoleophobic materials. J. Mater. Chem. A 2016, 4, 13677–13725. [Google Scholar] [CrossRef]
- Ren, T.; He, J. Substrate-versatile approach to robust antireflective and superhydrophobic coatings with excellent self-cleaning property in varied environments. ACS Appl. Mater. Interfaces 2017, 9, 34367–34376. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Yang, M.; Wang, K.; Zhang, Y.; He, J. CuO nanoparticles-containing highly transparent and superhydrophobic coatings with extremely low bacterial adhesion and excellent bactericidal property. ACS Appl. Mater. Interfaces 2018, 10, 25717–25725. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, G.; Dai, H.; Yang, M.; Fu, Y.; Ying, Y.; Li, Y. Biomineralization-mimetic preparation of hybrid membranes with ultra-high loading of pristine metal–organic frameworks grown on silk nanofibers for hazard collection in water. J. Mater. Chem. A 2018, 6, 3402–3413. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Synthesis of graphene oxide nanosheets decorated by nanoporous zeolite-imidazole (ZIF-67) based metal-organic framework with controlled-release corrosion inhibitor performance: Experimental and detailed DFT-D theoretical explorations. J. Hazard. Mater. 2021, 404, 124068. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von schwingquarzen zur wagung dunner schichten und zur mikrowagung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Yan, Y.; Bo, X.; Guo, L. MOF-818 Metal-organic framework-reduced graphene oxide/multiwalled carbon nanotubes composite for electrochemical sensitive detection of phenolic acids. Talanta 2020, 218, 121123. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baseggio, O.; Bonfa, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; de Gironcoli, S.; Delugas, P.; Ferrari Ruffino, F.; et al. Quantum espresso toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A Gen. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry.3. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Petersson, G.A.; Allaham, M.A. A complete basis set model chemistry.2. open-shell systems and the total energies of the 1st-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Allaham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry.1. the total energies of closed-shell atoms and hydrides of the 1st-row elements. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Chikere, C.O.; Hobben, E.; Faisal, N.H.; Kong-Thoo-Lin, P.; Fernandez, C. Electroanalytical determination of gallic acid in red and white wine samples using cobalt oxide nanoparticles-modified carbon-paste electrodes. Microchem. J. 2021, 160, 105668. [Google Scholar] [CrossRef]
- Chikere, C.O.; Faisal, N.H.; Kong Thoo Lin, P.; Fernandez, C. The synergistic effect between graphene oxide nanocolloids and silicon dioxide nanoparticles for gallic acid sensing. J. Solid State Electr. 2019, 23, 1795–1809. [Google Scholar] [CrossRef]
- Tashkhourian, J.; Nami-Ana, S.F. A sensitive electrochemical sensor for determination of gallic acid based on SiO2 nanoparticle modified carbon paste electrode. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 103–110. [Google Scholar] [CrossRef]
- Liang, Z.; Zhai, H.; Chen, Z.; Wang, H.; Wang, S.; Zhou, Q.; Huang, X. A simple, ultrasensitive sensor for gallic acid and uric acid based on gold microclusters/sulfonate functionalized graphene modified glassy carbon electrode. Sen. Actuators B Chem. 2016, 224, 915–925. [Google Scholar] [CrossRef]
- Wang, H.; Feng, X.; Bo, X.; Zhou, M.; Guo, L. Nickel-based metal-organic framework/crosslinked tubular poly(3,4-ethylenedioxythiophene) composite as an electrocatalyst for the detection of gallic acid and tinidazole. ChemElectroChem 2020, 7, 4031–4037. [Google Scholar] [CrossRef]
- Datta, S.; Kanjilal, B.; Sarkar, P. Electrochemical sensor for detection of polyphenols in tea and wine with differential pulse voltammetry and electrochemical impedance spectroscopy utilizing tyrosinase and gold nanoparticles decorated biomembrane. J. Electrochem. Soc. 2017, 164, B118–B126. [Google Scholar] [CrossRef]
- Luo, J.H.; Li, B.L.; Li, N.B.; Luo, H.Q. Sensitive detection of gallic acid based on polyethyleneimine-functionalized graphene modified glassy carbon electrode. Sens. Actuators B Chem. 2013, 186, 84–89. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Morozova, E.; Budnikov, H.; Davletshin, R.; Vorobev, V.; Osin, Y. Simultaneous voltammetric determination of gallic and ellagic acids in cognac and brandy using electrode modified with functionalized SWNT and poly(pyrocatechol violet). Food Anal. Method. 2019, 12, 2250–2261. [Google Scholar] [CrossRef]
- Madhusudhana; Manasa, G.; Bhakta, A.K.; Mekhalif, Z.; Mascarenhas, R.J. Bismuth-nanoparticles decorated multi-wall-carbon-nanotubes cast-coated on carbon paste electrode; an electrochemical sensor for sensitive determination of gallic acid at neutral pH. Mater. Sci. Energy Tech. 2020, 3, 174–182. [Google Scholar] [CrossRef]
- Refat, A.H.; Ahmed, B.; Emad, F.N.; François, G. Simultaneous voltammetric determination of gallic and protocatechuic acids in mango juice using a reduced graphene oxide-based electrochemical sensor. Beverages 2019, 5, 17. [Google Scholar] [CrossRef]


| Bond | Bond Order |
|---|---|
| O1-H13 | 0.9261 |
| O7-H15 | 0.9256 |
| O11-H17 | 0.9196 |
| O12-H18 | 0.8975 |
| Atom | q(N) | q(N + 1) | q(N − 1) | f− | f+ | f0 |
|---|---|---|---|---|---|---|
| 1(O) | −0.1753 | −0.2358 | −0.1493 | 0.0260 | 0.0605 | 0.0433 |
| 2(C) | 0.2068 | 0.0975 | 0.2284 | 0.0216 | 0.1093 | 0.0655 |
| 3(O) | −0.2833 | −0.4062 | −0.2175 | 0.0658 | 0.1228 | 0.0943 |
| 4(C) | −0.0337 | −0.1039 | 0.0563 | 0.0900 | 0.0702 | 0.0801 |
| 5(C) | −0.0793 | −0.1476 | −0.0175 | 0.0618 | 0.0683 | 0.0651 |
| 6(C) | 0.0508 | 0.0076 | 0.1014 | 0.0506 | 0.0433 | 0.0469 |
| 7(O) | −0.1867 | −0.2223 | −0.1344 | 0.0523 | 0.0356 | 0.0440 |
| 8(C) | 0.0443 | −0.0402 | 0.1498 | 0.1056 | 0.0845 | 0.0950 |
| 9(C) | 0.0666 | 0.0208 | 0.1403 | 0.0737 | 0.0458 | 0.0597 |
| 10(C) | −0.0564 | −0.1276 | −0.0188 | 0.0376 | 0.0712 | 0.0544 |
| 11(O) | −0.1839 | −0.2248 | −0.0804 | 0.1035 | 0.0409 | 0.0722 |
| 12(O) | −0.1832 | −0.2414 | −0.0692 | 0.1141 | 0.0582 | 0.0861 |
| 13(H) | 0.1844 | 0.1432 | 0.2099 | 0.0256 | 0.0412 | 0.0334 |
| 14(H) | 0.0396 | 0.0018 | 0.0767 | 0.0371 | 0.0378 | 0.0374 |
| 15(H) | 0.1886 | 0.1655 | 0.2193 | 0.0307 | 0.0231 | 0.0269 |
| 16(H) | 0.0528 | 0.0145 | 0.0855 | 0.0328 | 0.0383 | 0.0355 |
| 17(H) | 0.1713 | 0.1488 | 0.2031 | 0.0318 | 0.0224 | 0.0271 |
| 18(H) | 0.1766 | 0.1501 | 0.2163 | 0.0396 | 0.0266 | 0.0331 |
| Electrodes | Electrochemical Method | Linear Range (μM) | LOD (μM) | Sensitivities (μA μM−1 cm−2) | Ref. |
|---|---|---|---|---|---|
| CoONPs/CPE | DPV | 100–10,000 | 1.52 | — | [47] |
| Nano-GO/SiO2/GCE | DPV | 6.25–1000 | 2.09 | — | [48] |
| Nano-SiO2/CPE | DPV | 0.8–100 | 0.25 | 47.12 | [49] |
| AuMCs/SF-GR/GCE | DPV | 0.05–8 | 0.01 | — | [50] |
| MOF818@RGO/MWCNTs/GCE | DPV | 4–150 150–500 | 0.18 | 7.50 2.83 | [37] |
| Ni-MOF/PEDOT-2/GCE | DPV | 0.8–25.5 25.5–150 | 0.25 | 12.38 | [51] |
| ESM/AuNPs/Tyr/GCE | DPV | 5–65 | 1.71 | 0.08 | [52] |
| PEI-rGO/GCE | LSV | 0.59–58.78 | 0.41 | — | [53] |
| polyPCV/f-SWNT/GCE | DPV | 0.75–10 10–100 | 0.12 | — | [54] |
| Bi-MWCNT/MCPE | DPV | 1–100 | 0.16 | — | [55] |
| rGO/GCE | SWV | 20–144 | 30.80 | — | [56] |
| Co-MOF/CNTs/SiO2/AuE | CV | 0.05–10 10–200 | 0.2 | 2.48 2.29 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Zhou, Q.; Shao, W.; Wei, Z.; Wang, J. A Sensitive Co-MOF/CNTs/SiO2 Composite Based Electrode for Determination of Gallic Acid. Chemosensors 2022, 10, 443. https://doi.org/10.3390/chemosensors10110443
Zhu L, Zhou Q, Shao W, Wei Z, Wang J. A Sensitive Co-MOF/CNTs/SiO2 Composite Based Electrode for Determination of Gallic Acid. Chemosensors. 2022; 10(11):443. https://doi.org/10.3390/chemosensors10110443
Chicago/Turabian StyleZhu, Luyi, Qinan Zhou, Wenqing Shao, Zhenbo Wei, and Jun Wang. 2022. "A Sensitive Co-MOF/CNTs/SiO2 Composite Based Electrode for Determination of Gallic Acid" Chemosensors 10, no. 11: 443. https://doi.org/10.3390/chemosensors10110443
APA StyleZhu, L., Zhou, Q., Shao, W., Wei, Z., & Wang, J. (2022). A Sensitive Co-MOF/CNTs/SiO2 Composite Based Electrode for Determination of Gallic Acid. Chemosensors, 10(11), 443. https://doi.org/10.3390/chemosensors10110443








