Gold Nanostar-Based Sensitive Catechol Plasmonic Colorimetric Sensing Platform with Ultra-Wide Detection Range
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Synthesis of PVP-Coated Au Seeds
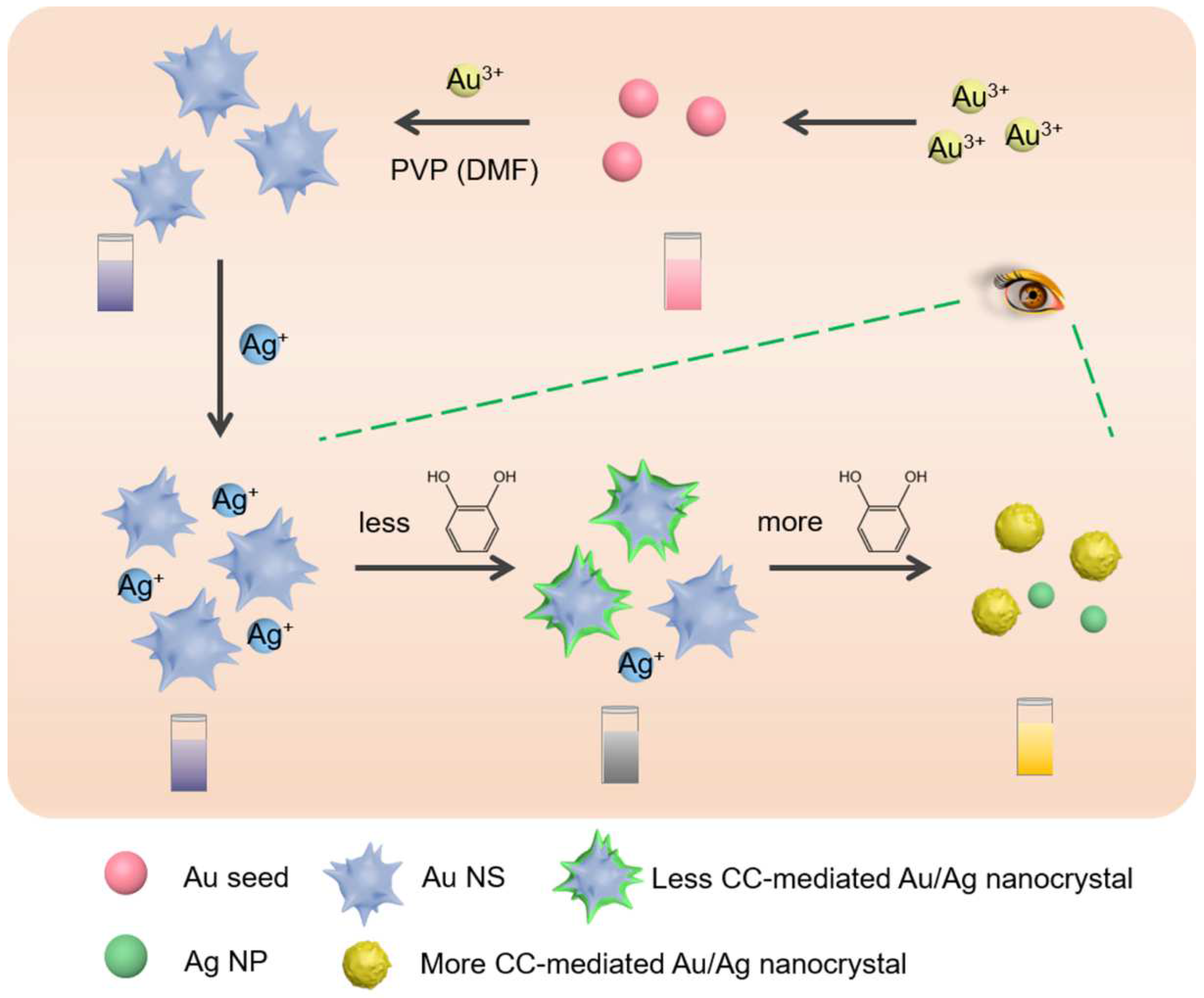
2.3. Preparation of Au NSs
2.4. Construction of Au NSs-Based CC Plasmonic Colorimetric Sensing Platform
2.5. Application of the Colorimetric Sensor in Real Samples
3. Results and Discussion
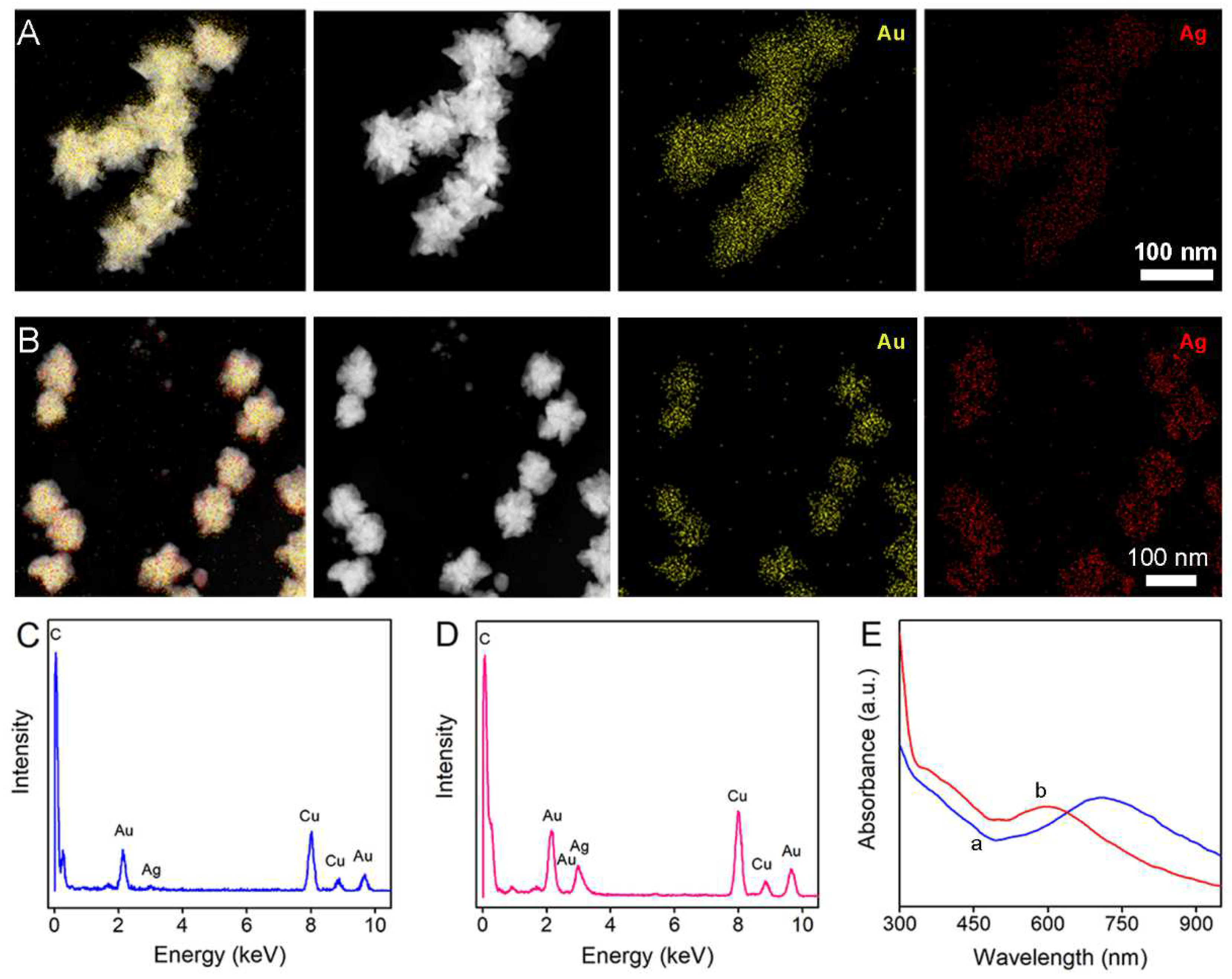
3.1. Characteristics of Au Seeds, Au NSs, and Au/Ag Nanocrystal
3.2. Feasibility of the Au NSs-Based Ultrasensitive CC Plasmonic Colorimetric Sensing
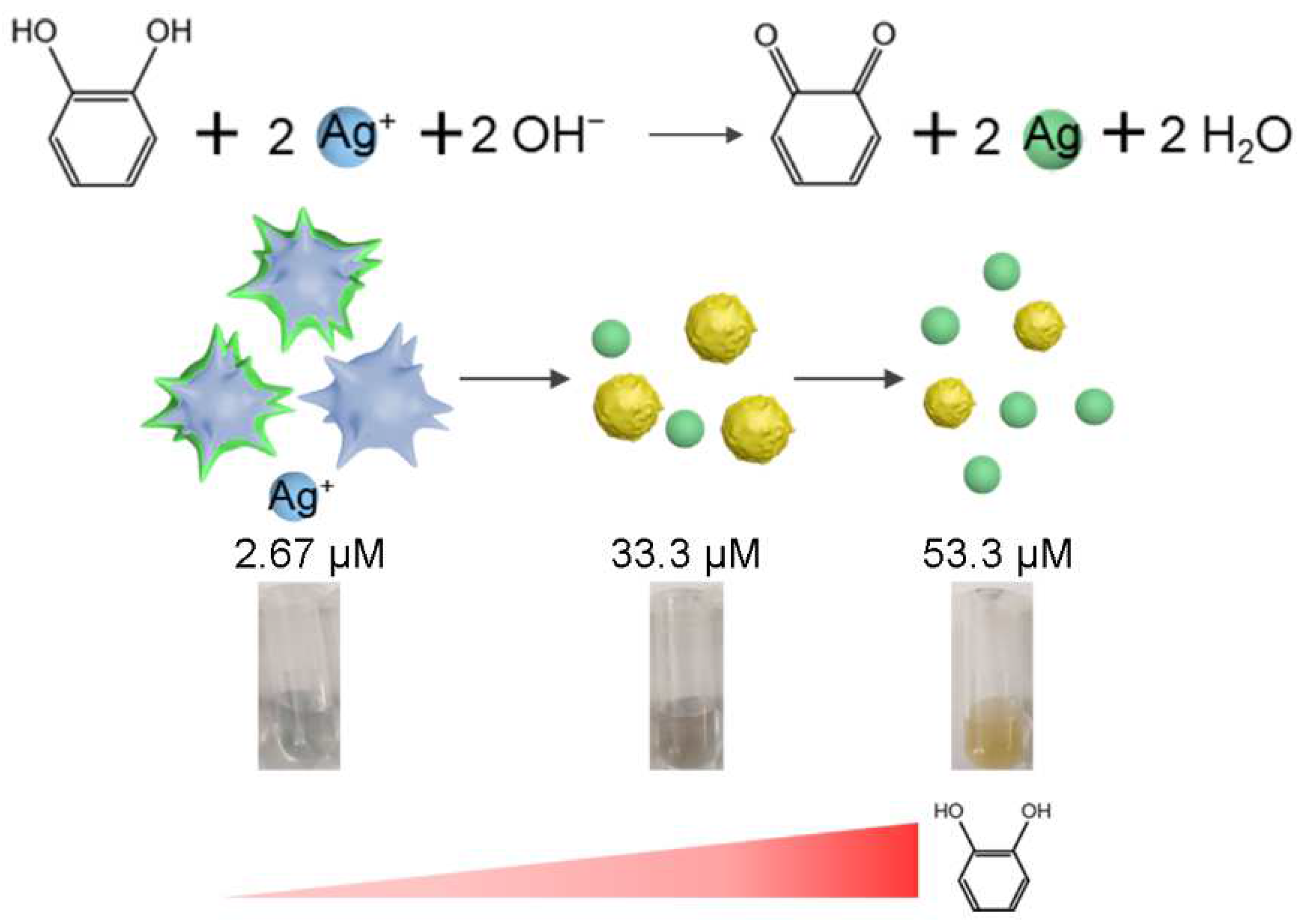
3.3. Mechanism of the Proposed Chemical Sensor
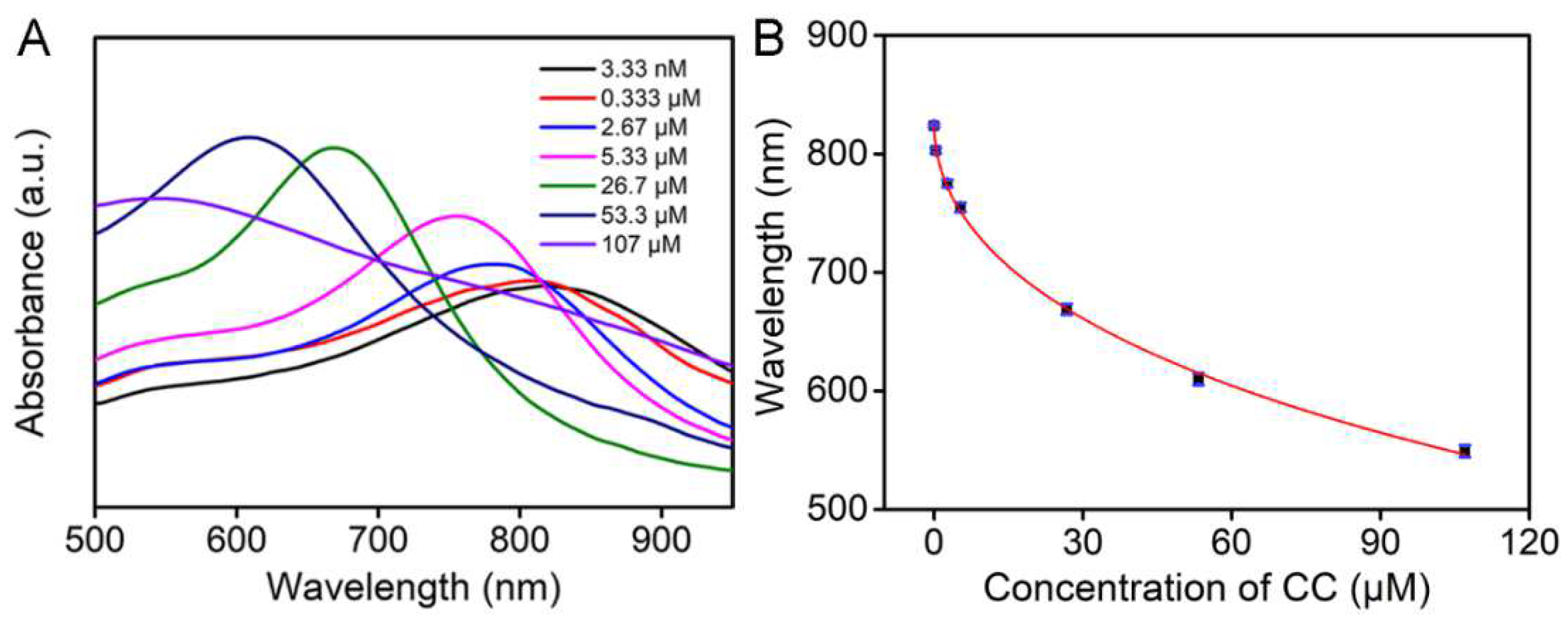
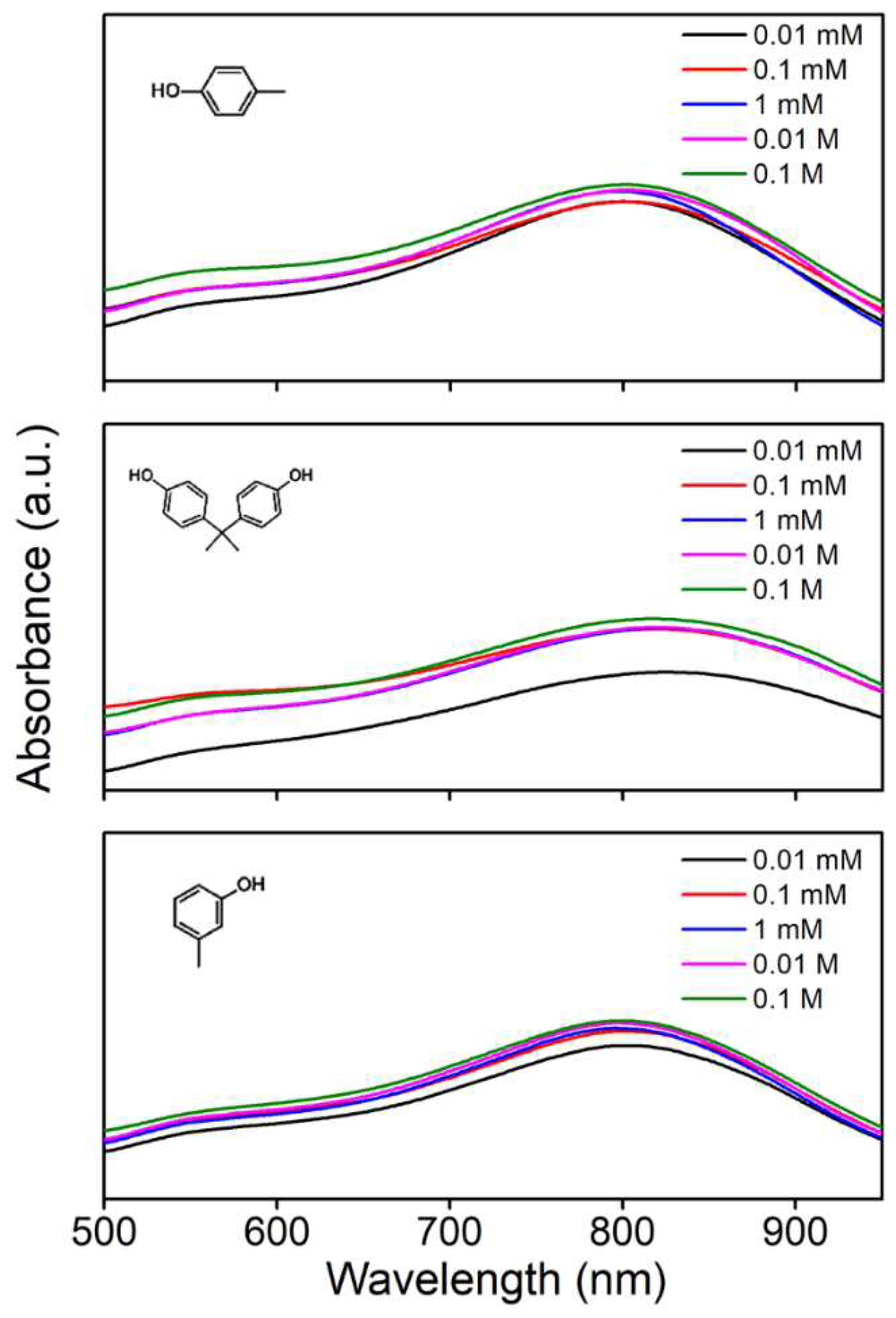
3.4. Analytical Performance Evaluation of the Sensor
3.5. Real Sample Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.F.; Wu, Y.Y.; Yan, X.P. Room-Temperature Phosphorescent Discrimination of Catechol from Resorcinol and Hydroquinone Based on Sodium Tripolyphosphate Capped Mn-Doped Zns Quantum Dots. Anal. Chem. 2013, 85, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, T.; Aziz, A.; Ashraf, G.; Xu, Y.; Li, G.; Zhang, T.; Asif, M.; Xiao, F.; Liu, H. Engineering MOFs Derived Metal Oxide Nanohybrids: Towards Electrochemical Sensing of Catechol in Tea Samples. Food Chem. 2022, 395, 133642. [Google Scholar] [CrossRef] [PubMed]
- Marrubini, G.; Calleri, E.; Coccini, T.; Castoldi, A.F.; Manzo, L. Direct Analysis of Phenol, Catechol and Hydroquinone in Human Urine by Coupled-Column HPLC with Fluorimetric Detection. Chromatographia 2005, 62, 25–31. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Biswas, S.; Zhu, F.; Zhan, J.; Wang, G.; Tung, C.H.; Wang, Y. Surface-Enhanced Raman Scattering of Phenols and Catechols by a Molecular Analogue of Titanium Dioxide. Anal. Chem. 2020, 92, 5929–5936. [Google Scholar] [CrossRef]
- Piña, S.; Candia-Onfray, C.; Hassan, N.; Jara-Ulloa, P.; Contreras, D.; Salazar, R. Glassy Carbon Electrode Modified with C/Au Nanostructured Materials for Simultaneous Determination of Hydroquinone and Catechol in Water Matrices. Chemosensors 2021, 9, 88. [Google Scholar] [CrossRef]
- de Sá, A.C.; Barbosa, S.C.; Raymundo-Pereira, P.A.; Wilson, D.; Shimizu, F.M.; Raposo, M.; Oliveira, O.N., Jr. Flexible Carbon Electrodes for Electrochemical Detection of Bisphenol-A, Hydroquinone and Catechol in Water Samples. Chemosensors 2020, 8, 103. [Google Scholar]
- Huang, R.; Liao, D.; Chen, S.; Yu, J.; Jiang, X. A Strategy for Effective Electrochemical Detection of Hydroquinone and Catechol: Decoration of Alkalization-Intercalated Ti3C2 with MOF-Derived N-Doped Porous Carbon. Sens. Actuators B Chem. 2020, 320, 128386. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, A.Y.; Huang, Y.Y.; He, X.; Xie, X.F.; He, B.; Yang, J.H.; Wang, X.Y. Off-on fluorescent switching of boron-doped carbon quantum dots for ultrasensitive sensing of catechol and glutathione. Carbon 2020, 162, 234–244. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Cioffi, N. Novel Polyethylene Oxide Coatings Implementing Ultra-Stable Laser-Ablated Silver Nanoparticles. Appl. Surf. Sci. 2020, 507, 145156. [Google Scholar] [CrossRef]
- Rodriguez-Lorenzo, L.; de la Rica, R.; Alvarez-Puebla, R.A.; Liz-Marzan, L.M.; Stevens, M.M. Plasmonic Nanosensors with Inverse Sensitivity by Means of Enzyme-Guided Crystal Growth. Nat. Mater. 2012, 11, 604–607. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Li, J.; Zhu, Q.; Wang, Z.; Dai, Z. Enhancing Photoelectric Response of an Au@Ag/AgI Schottky Contact through Regulation of Localized Surface Plasmon Resonance. J. Am. Chem. Soc. 2021, 143, 13478–13482. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Yang, K.; Wei, J.; Xu, M.; Sun, C.; Ding, Y.; Yuan, Z.; Wang, S.; Wang, R. Supramolecular Vesicles Based on Gold Nanorods for Precise Control of Gene Therapy and Deferred Photothermal Therapy. CCS Chem. 2022, 4, 1745–1757. [Google Scholar] [CrossRef]
- Deng, X.; Liang, S.; Cai, X.; Huang, S.; Cheng, Z.; Shi, Y.; Pang, M.; Ma, P.; Lin, J. Yolk–Shell Structured Au Nanostar@Metal–Organic Framework for Synergistic Chemo-Photothermal Therapy in the Second Near-Infrared Window. Nano Lett. 2019, 19, 6772–6780. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Wang, W.; Zhao, T.; Cui, Y.; Liu, P.; Xu, S.; Luo, X. More Symmetrical "Hot Spots" Ensure Stronger Plasmon-Enhanced Fluorescence: From Au Nanorods to Nanostars. Anal. Chem. 2021, 93, 2480–2489. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Ji, L.; Xu, M.; Bai, B.; Wan, X.; Hou, D.; Qiao, Z.Y.; Wang, H.; Zhang, J. Hybrid Plasmonic Nanodumbbells Engineering for Multi-Intensified Second Near-Infrared Light Induced Photodynamic Therapy. ACS Nano 2021, 15, 8694–8705. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Leis, A.; Torreggiani, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Hollow Au/Ag Nanostars Displaying Broad Plasmonic Resonance and High Surface-Enhanced Raman Sensitivity. Nanoscale 2015, 7, 13629–13637. [Google Scholar] [CrossRef]
- Wang, L.; Patskovsky, S.; Gauthier-Soumis, B.; Meunier, M. Porous Au-Ag Nanoparticles from Galvanic Replacement Applied as Single-Particle SERS Probe for Quantitative Monitoring. Small 2022, 18, 2105209. [Google Scholar] [CrossRef]
- Kołątaj, K.; Krajczewski, J.; Kudelski, A. Plasmonic Nanoparticles for Environmental Analysis. Environ. Chem. Lett. 2020, 18, 529–542. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Liu, J.; Xiong, Y.; Zheng, J.; Liu, Y.; Tang, Z. Core-Shell Noble-Metal@Metal-Organic-Framework Nanoparticles with Highly Selective Sensing Property. Angew. Chem. Int. Ed. 2013, 52, 3741–3745. [Google Scholar] [CrossRef]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.F.; Patra, H.K. A Repertoire of Biomedical Applications of Noble Metal Nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef]
- John, A.; Benny, L.; Cherian, A.R.; Narahari, S.Y.; Varghese, A.; Hegde, G. Electrochemical Sensors Using Conducting Polymer/Noble Metal Nanoparticle Nanocomposites for the Detection of Various Analytes: A Review. J. Nanostructure Chem. 2021, 11, 1–31. [Google Scholar] [CrossRef]
- Gu, Y.; Song, J.; Li, M.-X.; Zhang, T.-T.; Zhao, W.; Xu, J.-J.; Liu, M.; Chen, H.-Y. Ultrasensitive microRNA assay via surface plasmon resonance responses of Au@Ag nanorods etching. Anal. Chem. 2017, 89, 10585–10591. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Sha, J.; Li, Z.; Wang, W.; Zhang, H. High Affinity Truncated Aptamers for Ultra-Sensitive Colorimetric Detection of Bisphenol a with Label-Free Aptasensor. Food Chem. 2020, 317, 126459. [Google Scholar] [CrossRef]
- Chang, L.; Besteiro, L.V.; Sun, J.; Santiago, E.Y.; Gray, S.K.; Wang, Z.; Govorov, A.O. Electronic Structure of the Plasmons in Metal Nanocrystals: Fundamental Limitations for the Energy Efficiency of Hot Electron Generation. ACS Energy Lett. 2019, 4, 2552–2568. [Google Scholar] [CrossRef]
- Li, D.; Wieckowska, A.; Willner, I. Optical Analysis of Hg2+ Ions by Oligonucleotide-Gold-Nanoparticle Hybrids and DNA-Based Machines. Angew. Chem. Int. Ed. 2008, 47, 3927–3931. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, Y.; Zeng, T.; Aleisa, R.; Qiu, Z.; Chen, Y.; Huang, J.; Wang, D.; Yan, Z.; Yin, Y. Anisotropic Plasmonic Nanostructures for Colorimetric Sensing. Nano Today 2020, 32, 100855. [Google Scholar] [CrossRef]
- Liu, B.; Zhuang, J.; Wei, G. Recent Advances in the Design of Colorimetric Sensors for Environmental Monitoring. Environ. Sci.-Nano 2020, 7, 2195–2213. [Google Scholar] [CrossRef]
- Soh, J.H.; Chan, H.-M.; Ying, J.Y. Strategies for Developing Sensitive and Specific Nanoparticle-Based Lateral Flow Assays as Point-of-Care Diagnostic Device. Nano Today 2020, 30, 100831. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Chen, Z.; Wang, X.; Choo, J.; Chen, L. Plasmonic Colorimetric Sensors Based on Etching and Growth of Noble Metal Nanoparticles: Strategies and Applications. Biosens. Bioelectron. 2018, 114, 52–65. [Google Scholar] [CrossRef]
- Li, J.; Kong, C.; Liu, Q.; Chen, Z. Colorimetric Ultrasensitive Detection of DNA Based on the Intensity of Gold Nanoparticles with Dark-Field Microscopy. Analyst 2018, 143, 4051–4056. [Google Scholar] [CrossRef]
- Choi, J.H.; Lim, J.; Shin, M.; Paek, S.H.; Choi, J.W. CRISPR-Cas12a-Based Nucleic Acid Amplification-Free DNA Biosensor via Au Nanoparticle-Assisted Metal-Enhanced Fluorescence and Colorimetric Analysis. Nano Lett. 2021, 21, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, J.; Xu, X.; Chen, X.; Wang, J. Protein Corona-Triggered Catalytic Inhibition of Insufficient POSS Polymer-Caged Gold Nanoparticles for Sensitive Colorimetric Detection of Metallothioneins. Anal. Chem. 2020, 92, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yu, Q.; Wang, H.; Zhu, W.; Liu, J.; Wang, Z. A General Scattering Proximity Immunoassay with the Formation of Dimer of Gold Nanoparticle. Talanta 2021, 233, 122515. [Google Scholar] [CrossRef]
- Liu, X.; He, F.; Zhang, F.; Zhang, Z.; Huang, Z.; Liu, J. Dopamine and Melamine Binding to Gold Nanoparticles Dominates Their Aptamer-Based Label-Free Colorimetric Sensing. Anal. Chem. 2020, 92, 9370–9378. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Zhang, S.; Li, M.; Tu, J.; Lu, X. Achieving Ultrasensitive Point-of-Care Assay for Mercury Ions with a Triple-Mode Strategy Based on the Mercury-Triggered Dual-Enzyme Mimetic Activities of Au/WO3 Hierarchical Hollow Nanoflowers. ACS Appl. Mater. Interfaces 2022, 14, 9442–9453. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhou, B.; Li, M.; Shen, M.; Shi, X. Colorimetric Detection of Cr3+ Ions in Aqueous Solution Using Poly(γ-Glutamic Acid)-Stabilized Gold Nanoparticles. Anal. Methods 2020, 12, 3145–3150. [Google Scholar] [CrossRef]
- Yu, Y.; Naik, S.S.; Oh, Y.; Theerthagiri, J.; Lee, S.J.; Choi, M.Y. Lignin-Mediated Green Synthesis of Functionalized Gold Nanoparticles Via Pulsed Laser Technique for Selective Colorimetric Detection of Lead Ions in Aqueous Media. J. Hazard. Mater. 2021, 420, 126585. [Google Scholar] [CrossRef]
- Zhuang, Z.; Zhang, C.; Yu, Z.; Liu, W.; Zhong, Y.; Zhang, J.; Xu, Z. Turn-on Colorimetric Detection of Hydroquinone Based on Au/CuO Nanocomposite Nanozyme. Mikrochim. Acta 2022, 189, 293. [Google Scholar] [CrossRef]
- Choi, H.; Kang, T.; Um, K.; Kim, J.; Lee, K. Reduction of Silver Ions in Gold Nanoparticle Suspension for Detection of Dihydroxybenzene Isomers. Colloid. Surf. A 2014, 459, 120–127. [Google Scholar] [CrossRef]
- Guo, L.; Xu, Y.; Ferhan, A.R.; Chen, G.; Kim, D.H. Oriented Gold Nanoparticle Aggregation for Colorimetric Sensors with Surprisingly High Analytical Figures of Merit. J. Am. Chem. Soc. 2013, 135, 12338–12345. [Google Scholar] [CrossRef]
- Xianyu, Y.; Lin, Y.; Chen, Q.; Belessiotis-Richards, A.; Stevens, M.M.; Thomas, M.R. Iodide-Mediated Rapid and Sensitive Surface Etching of Gold Nanostars for Biosensing. Angew. Chem. Int. Ed. 2021, 60, 9891–9896. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Guerrero, Y.; Romaguera-Barcelay, Y.; Moreira, F.T.C.; Brito, W.R.; Fortunato, E.; Sales, M.G.F. Poly(Thionine)-Modified Screen-Printed Electrodes for CA 19-9 Detection and Its Properties in Raman Spectroscopy. Chemosensors 2022, 10, 92. [Google Scholar] [CrossRef]
- Cui, X.; Wei, T.; Hao, M.; Qi, Q.; Wang, H.; Dai, Z. Highly Sensitive and Selective Colorimetric Sensor for Thiocyanate Based on Electrochemical Oxidation-Assisted Complexation Reaction with Gold Nanostars Etching. J. Hazard. Mater. 2020, 391, 122217. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, Y. Preparation of Aptamer-Linked Gold Nanoparticle Purple Aggregates for Colorimetric Sensing of Analytes. Nat. Protoc. 2006, 1, 246–252. [Google Scholar] [CrossRef]
- Pazos-Perez, N.; Guerrini, L.; Alvarez-Puebla, R.A. Plasmon Tunability of Gold Nanostars at the Tip Apexes. ACS Omega 2018, 3, 17173–17179. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.L.; Baker, G.A. Bespoke Nanostars: Synthetic Strategies, Tactics, and Uses of Tailored Branched Gold Nanoparticles. Nanoscale Adv. 2021, 3, 3980–4004. [Google Scholar] [CrossRef]
- Shameli, K.; Ahmad, M.B.; Jazayeri, S.D.; Shabanzadeh, P.; Sangpour, P.; Jahangirian, H.; Gharayebi, Y. Investigation of Antibacterial Properties Silver Nanoparticles Prepared via Green Method. Chem. Cent. J. 2012, 6, 73. [Google Scholar] [CrossRef]
- Liu, X.; Yang, J.; Cheng, J.; Xu, Y.; Chen, W.; Li, Y. Facile Preparation of Four-in-One Nanozyme Catalytic Platform and the Application in Selective Detection of Catechol and Hydroquinone. Sens. Actuator. B Chem. 2021, 337, 129763. [Google Scholar] [CrossRef]
- Zhao, X.-E.; Zuo, Y.-N.; Xia, Y.; Sun, J.; Zhu, S.; Xu, G. Multifunctional NH2-Cu-MOF Based Ratiometric Fluorescence Assay for Discriminating Catechol from Its Isomers. Sens. Actuator. B Chem. 2022, 371, 132548. [Google Scholar] [CrossRef]
- Yin, D.; Liu, J.; Bo, X.; Guo, L. Cobalt-Iron Selenides Embedded in Porous Carbon Nanofibers for Simultaneous Electrochemical Detection of Trace of Hydroquinone, Catechol and Resorcinol. Anal. Chim. Acta 2020, 1093, 35–42. [Google Scholar] [CrossRef]
- Lounasvuori, M.M.; Kelly, D.; Foord, J.S. Carbon Black as Low-Cost Alternative for Electrochemical Sensing of Phenolic Compounds. Carbon 2018, 129, 252–257. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, Q.; Qiao, J.; Ye, B.; Li, G. Simultaneous Detection of Bisphenol A and Bisphenol S with High Sensitivity Based on A New Electrochemical Sensor. J. Electroanal. Chem. 2019, 854, 113541. [Google Scholar] [CrossRef]
- González-Costas, J.M.; Gómez-Fernández, S.; García, J.; González-Romero, E. Screen-Printed Electrodes-Based Technology: Environmental Application to Real Time Monitoring of Phenolic Degradation by Phytoremediation with Horseradish Roots. Sci. Total Environ. 2020, 744, 140782. [Google Scholar] [CrossRef] [PubMed]

| Analytical Method | Linear Range | Detection Limit | References |
|---|---|---|---|
| Colorimetry | 1 to 1000 μM | 0.35 μM | [48] |
| Fluorescence | 0.3 to 40 μM | 0.1 μM | [49] |
| Electrochemistry | 0.5 to 190 μM | 0.15 μM | [50] |
| Electrochemistry | 10 nM to 22 μM | 7.8 nM | [2] |
| Colorimetry | 3.33 nM to 107 μM | 1 nM | This Work |
| Samples | Spiked (μM) | Found (μM) | Recovery (%) ± SD, n = 5 |
|---|---|---|---|
| Tap Water | 0 | Undetected | − |
| 0.0100 | 0.0107 | 107 ± 5.03 | |
| 0.100 | 0.0980 | 98.0 ± 5.36 | |
| 10.0 | 9.65 | 96.5 ± 4.58 | |
| Industrial Wastewater (1000 Times Diluted) | 0 | 5.01 × 10−3 | − |
| 0.0100 | 0.0108 | 108 ± 6.08 | |
| 0.100 | 0.103 | 103 ± 5.78 | |
| 10.0 | 10.1 | 101 ± 5.69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Fang, T.; Liu, H.; Wei, T.; Dai, Z. Gold Nanostar-Based Sensitive Catechol Plasmonic Colorimetric Sensing Platform with Ultra-Wide Detection Range. Chemosensors 2022, 10, 439. https://doi.org/10.3390/chemosensors10110439
Wang H, Fang T, Liu H, Wei T, Dai Z. Gold Nanostar-Based Sensitive Catechol Plasmonic Colorimetric Sensing Platform with Ultra-Wide Detection Range. Chemosensors. 2022; 10(11):439. https://doi.org/10.3390/chemosensors10110439
Chicago/Turabian StyleWang, Huafeng, Ting Fang, Hua Liu, Tianxiang Wei, and Zhihui Dai. 2022. "Gold Nanostar-Based Sensitive Catechol Plasmonic Colorimetric Sensing Platform with Ultra-Wide Detection Range" Chemosensors 10, no. 11: 439. https://doi.org/10.3390/chemosensors10110439
APA StyleWang, H., Fang, T., Liu, H., Wei, T., & Dai, Z. (2022). Gold Nanostar-Based Sensitive Catechol Plasmonic Colorimetric Sensing Platform with Ultra-Wide Detection Range. Chemosensors, 10(11), 439. https://doi.org/10.3390/chemosensors10110439








