Thiol-Based Probe Linker with Antifouling Properties for Aptasensor Development
Abstract
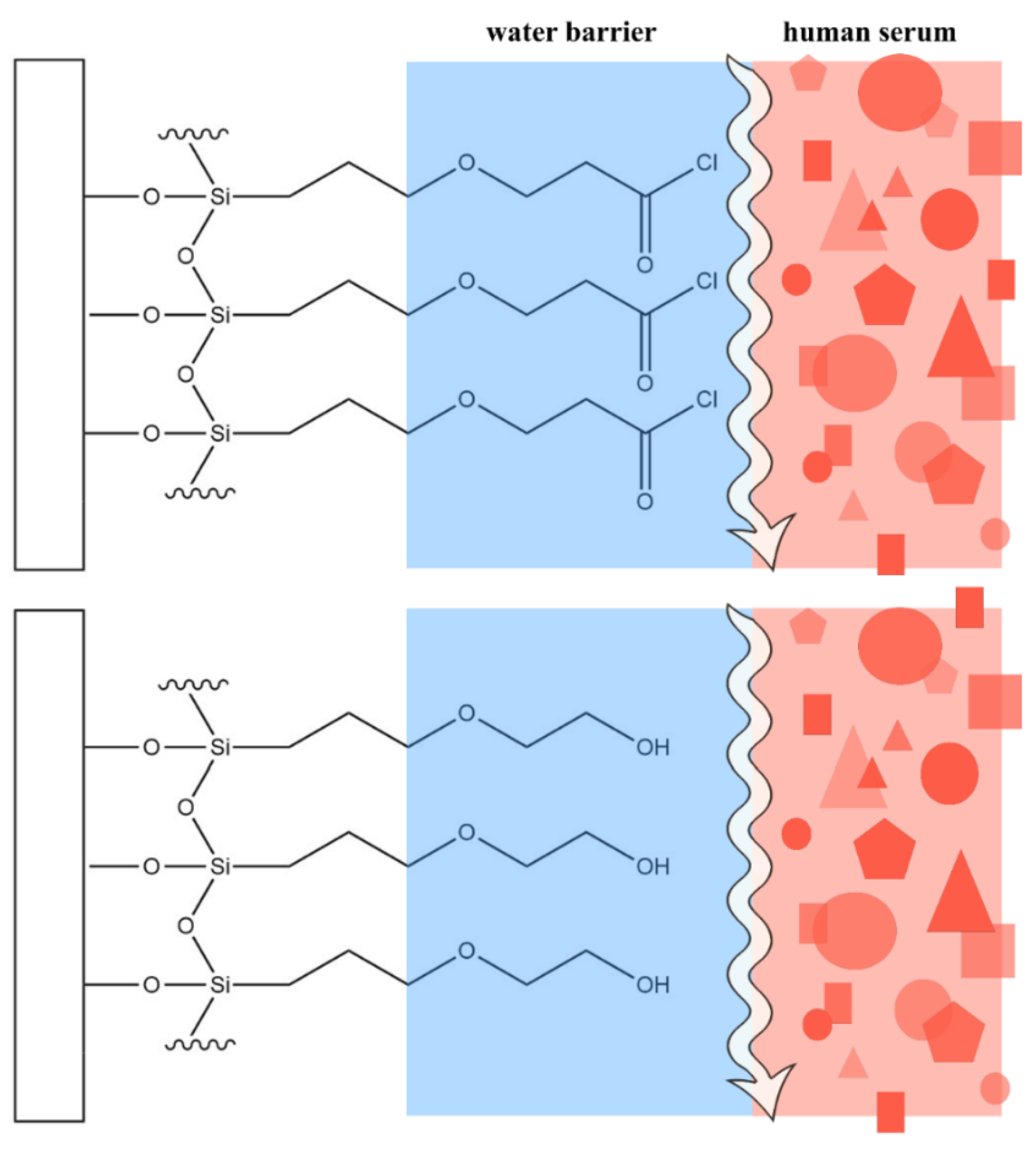
1. Introduction
2. Materials and Methods
2.1. Materials
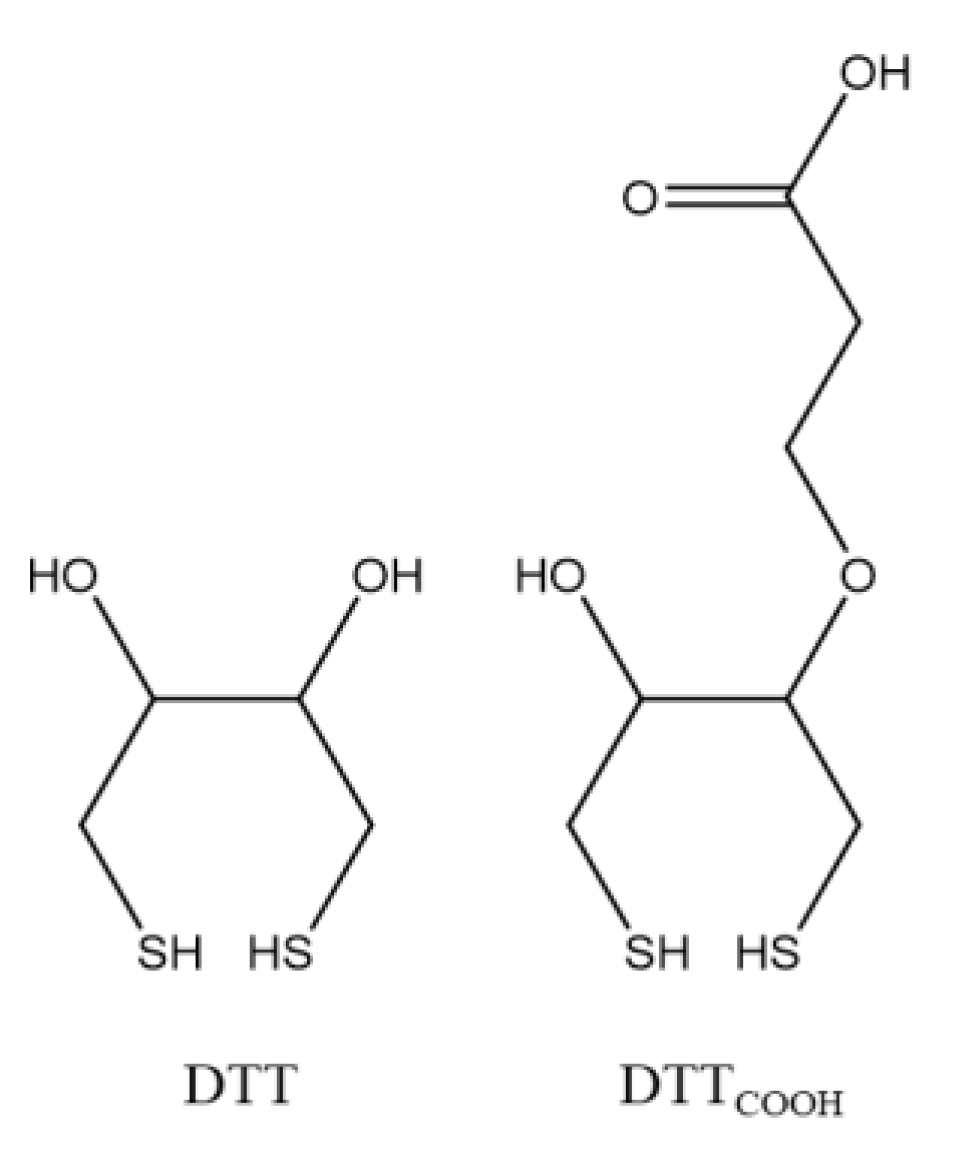
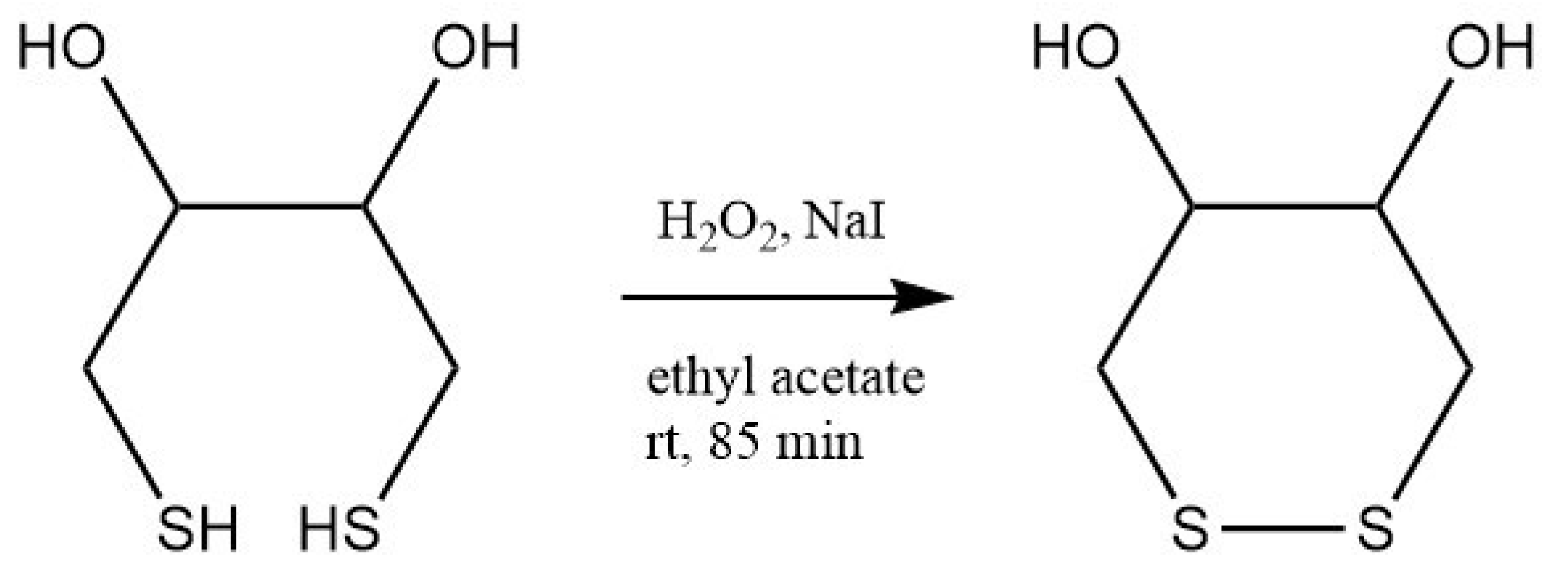
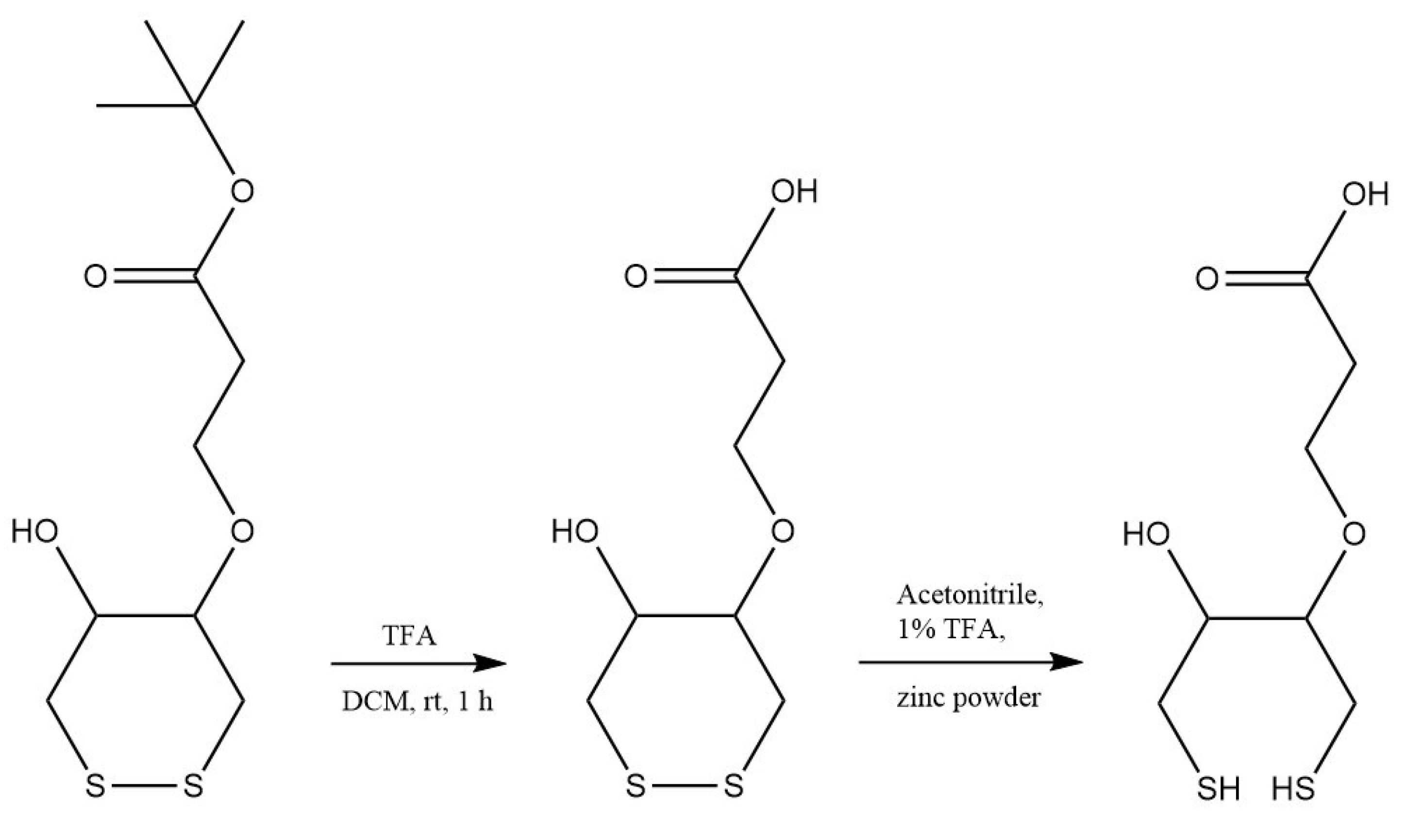
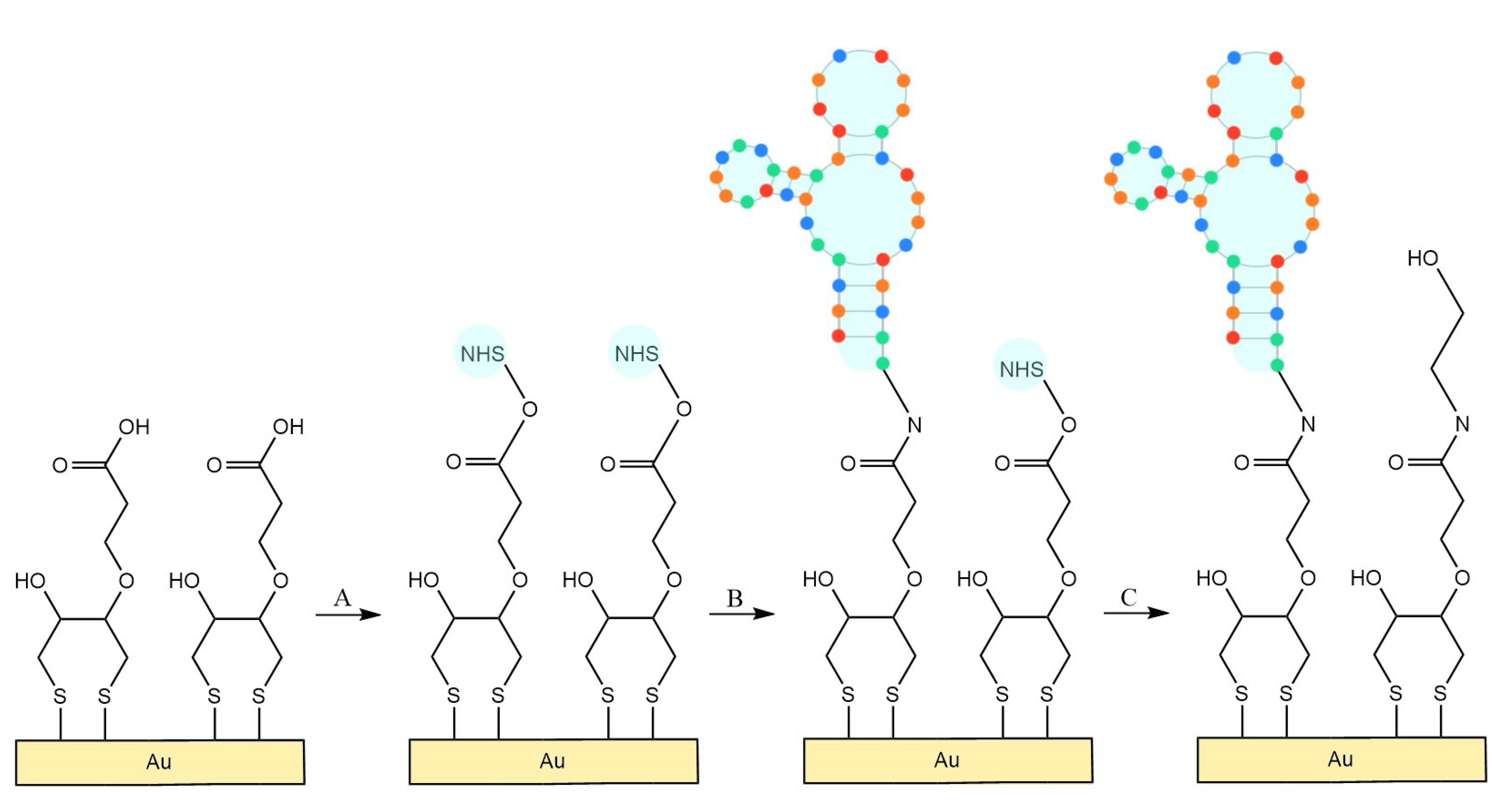
2.2. Synthesis of 3-Dithiothreitol Propanoic Acid (DTTCOOH) Linker
2.3. Aptamer Stock Solution Preparation
2.4. Cleaning and Surface Modification of QCM Crystals
2.5. Contact Angle Goniometry (CAG)
2.6. QCM Set-Up and Measurements
2.7. QCM Data Analysis
2.8. Electrochemical Measurements
3. Results and Discussion
3.1. Contact Angle Goniometry Analysis
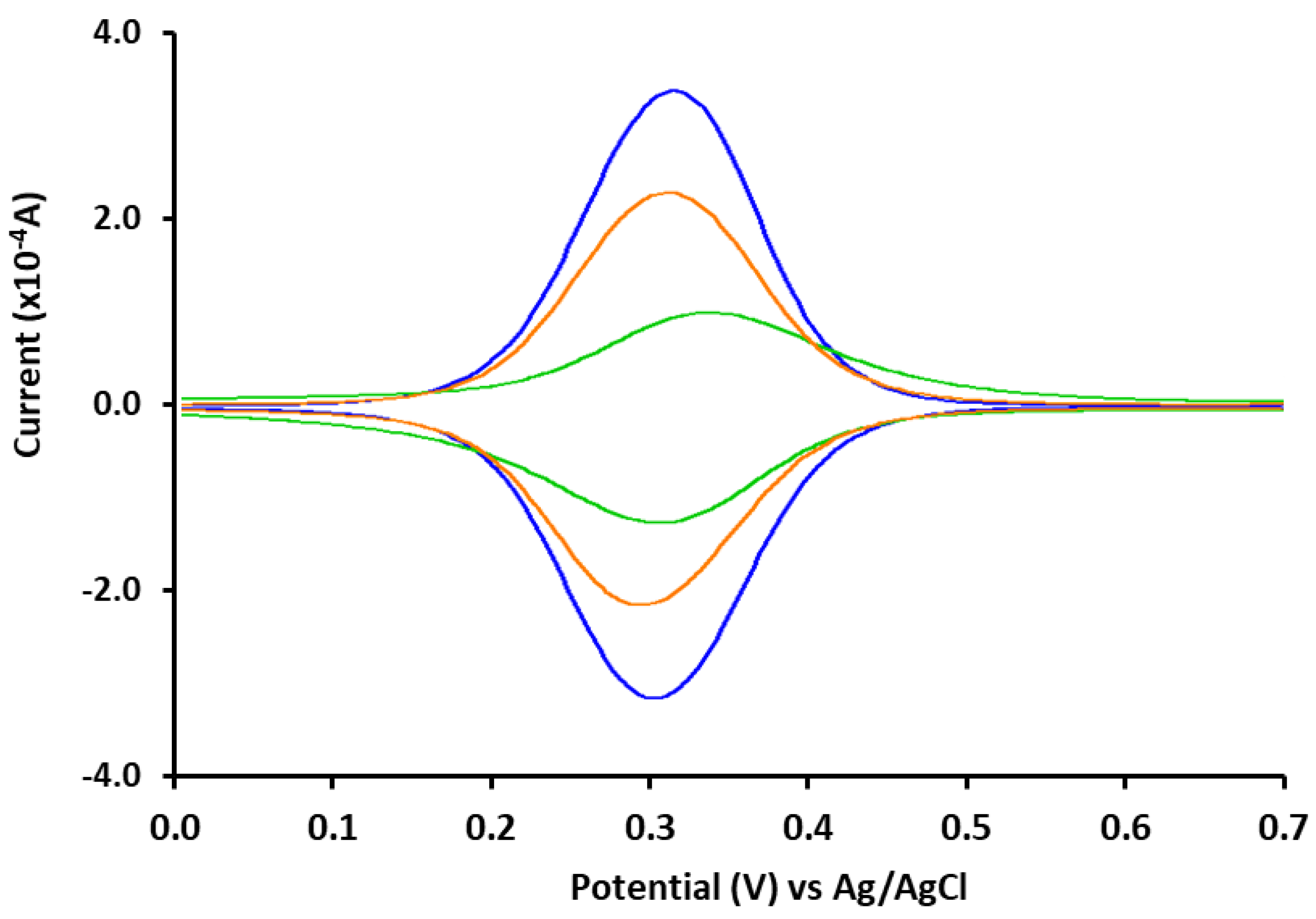
3.2. Electrochemical Characterization of the Aptasensor
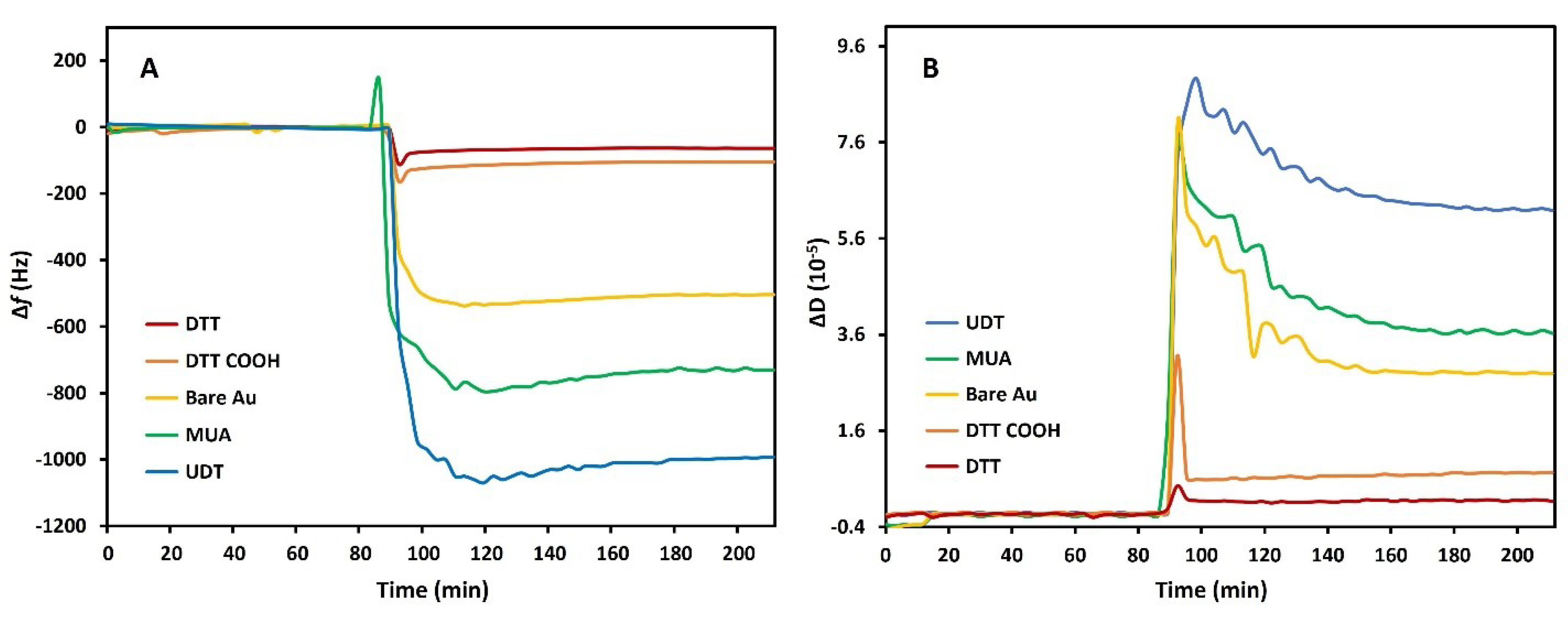
3.3. Serum Antifouling Test with Quartz Crystal Microbalance
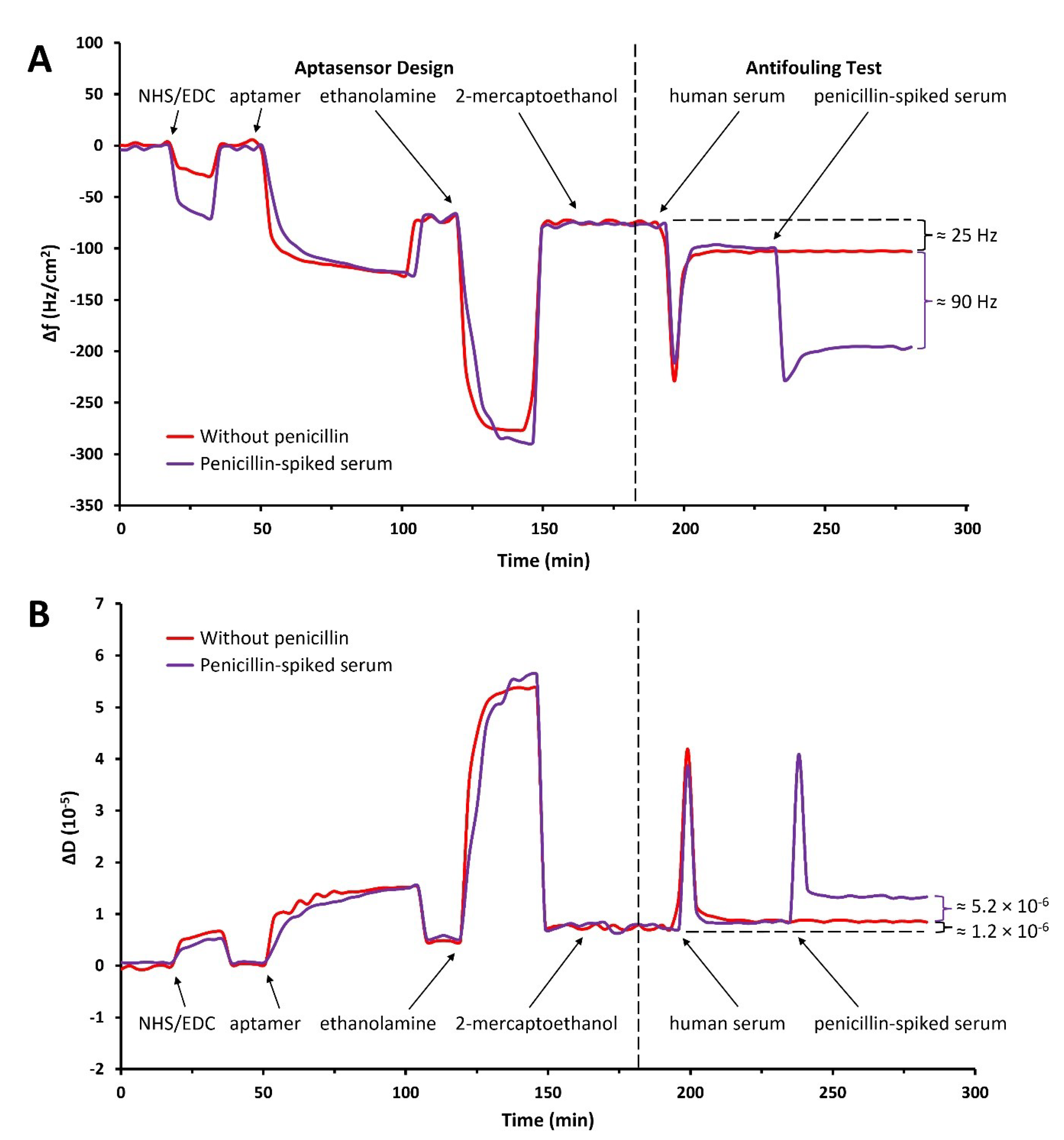
3.4. Antifouling Test of the Aptasensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spagnolo, S.; De La Franier, B.; Hianik, T.; Thompson, M. Surface probe linker with tandem anti-fouling properties for application in biosensor technology. Biosensors 2020, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Kuralay, F.; Lobo-Castañón, M.J.; Bartošík, M.; Vyavahare, K.; Paleček, E.; Haake, D.A.; Wang, J. Ternary monolayers as DNA recognition interfaces for direct and sensitive electrochemical detection in untreated clinical samples. Biosens. Bioelectron. 2011, 26, 3577–3583. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef]
- Del Grosso, C.A.; Leng, C.; Zhang, K.; Hung, H.-C.; Jiang, S.; Chen, Z.; Wilker, J.J. Surface hydration for antifouling and bio-adhesion. Chem. Sci. 2020, 11, 10367–10377. [Google Scholar] [CrossRef]
- Pawlowska, N.M.; Fritzsche, H.; Blaszykowski, C.; Sheikh, S.; Vezvaie, M.; Thompson, M. Probing the hydration of ultrathin antifouling organosilane adlayers using neutron reflectometry. Langmuir 2014, 30, 1199–1203. [Google Scholar] [CrossRef]
- Sheikh, S.; Blaszykowski, C.; Nolan, R.; Thompson, D.; Thompson, M. On the hydration of subnanometric antifouling organosilane adlayers: A molecular dynamics simulation. J. Colloid Interface Sci. 2015, 437, 197–204. [Google Scholar] [CrossRef]
- Avci, C.; Sheikh, S.; Blaszykowski, C.; Thompson, M. Critical role of surface hydration on the dynamics of serum adsorption studied with monoethylene glycol adlayers on gold. Chem. Commun. 2012, 49, 466–468. [Google Scholar] [CrossRef]
- Spagnolo, S.; De La Franier, B.; Davoudian, K.; Hianik, T.; Thompson, M. Detection of E. coli bacteria in milk by an acoustic wave aptasensor with an anti-fouling coating. Sensors 2022, 22, 1853. [Google Scholar] [CrossRef]
- Chávez, M.; Sánchez-Obrero, G.; Madueño, R.; Sevilla, J.M.; Blázquez, M.; Pineda, T. Characterization of a self-assembled monolayer of O-(2-Mercaptoethyl)-O′-Methyl-Hexa(ethylene Glycol) (EG7-SAM) on gold electrodes. J. Electroanal. Chem. 2021, 880, 114892. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; de Oliveira Pedro, R.; Carr, O.; Melendez, M.E.; Gobbi, A.L.; de Oliveira Piazzetta, M.H.; Carvalho, A.L.; Reis, R.M.; Miranda, P.B.; Oliveira, O.N., Jr. Influence of the molecular orientation and ionization of self-assembled monolayers in biosensors: Application to genosensors of prostate cancer antigen 3. J. Phys. Chem. C 2021, 125, 498–506. [Google Scholar] [CrossRef]
- Hianik, T. Advances in electrochemical and acoustic aptamer-based biosensors and immunosensors in diagnostics of leukemia. Biosensors 2021, 11, 177. [Google Scholar] [CrossRef]
- Bertok, T.; Dosekova, E.; Belicky, S.; Holazova, A.; Lorencova, L.; Mislovicova, D.; Paprckova, D.; Vikartovska, A.; Plicka, R.; Krejci, J.; et al. Mixed zwitterion-based self-assembled monolayer interface for impedimetric glycomic analyses of human IgG samples in an array format. Langmuir 2016, 32, 7070–7078. [Google Scholar] [CrossRef]
- Wu, J.; Campuzano, S.; Halford, C.; Haake, D.A.; Wang, J. Ternary surface monolayers for ultrasensitive (zeptomole) amperometric detection of nucleic acid hybridization without signal amplification. Anal. Chem. 2010, 82, 8830–8837. [Google Scholar] [CrossRef]
- Spagnolo, S.; Muckley, E.S.; Ivanov, I.N.; Hianik, T. Analysis of trypsin activity at β-casein layers formed on hydrophobic surfaces using a multiharmonic acoustic method. Analyst 2022, 147, 461–470. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Carmagnola, I.; Ciardelli, G. Quartz crystal microbalance with dissipation monitoring: A powerful method to predict the in vivo behavior of bioengineered surfaces. Front. Bioeng. Biotechnol. 2018, 6, 158. [Google Scholar] [CrossRef]
- Spagnolo, S.; Muckley, E.S.; Ivanov, I.N.; Hianik, T. Application of multiharmonic QCM-D for detection of plasmin at hydrophobic surfaces modified by β-casein. Chemosensors 2022, 10, 143. [Google Scholar] [CrossRef]
- Tatarko, M.; Spagnolo, S.; Oravczová, V.; Süle, J.; Hun, M.; Hucker, A.; Hianik, T. Changes of viscoelastic properties of aptamer-based sensing layers following interaction with Listeria innocua. Sensors 2021, 21, 5585. [Google Scholar] [CrossRef]
- Muckley, E.S.; Collins, L.; Srijanto, B.R.; Ivanov, I.N. Machine learning-enabled correlation and modeling of multimodal response of thin film to environment on macro and nanoscale using Lab-on-a-Crystal. Adv. Funct. Mater. 2020, 30, 1908010. [Google Scholar] [CrossRef]
- Yoon, S.M.; Cho, N.J.; Kanazawa, K. Analyzing spur-distorted impedance spectra for the QCM. J. Sens. 2009, 2009, 259746. [Google Scholar] [CrossRef]
- Mann, M.A.; Bottomley, L.A. Cyclic square wave voltammetry of surface-confined quasireversible electron transfer reactions. Langmuir 2015, 31, 9511–9520. [Google Scholar] [CrossRef]
- Zina, F.; Nooredeen, N.M.; Azzouzi, S.; Ali, M.B.; Abbas, M.N.; Errachid, A. Novel sensitive impedimetric microsensor for phosphate detection based on a novel copper phthalocyanine derivative. Anal. Lett. 2018, 51, 371–386. [Google Scholar] [CrossRef]
- Mattiuzzi, A.; Troian-Gautier, L.; Mertens, J.; Reniers, F.; Bergamini, J.-F.; Lenne, Q.; Lagrost, C.; Jabin, I. Robust hydrophobic gold, glass and polypropylene surfaces obtained through a nanometric covalently bound organic layer. RSC Adv. 2020, 10, 13553–13561. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Moore, E. Electrochemical immunosensor modified with self-assembled monolayer of 11-mercaptoundecanoic acid on gold electrodes for detection of benzo[a]pyrene in water. Analyst 2012, 137, 5839–5844. [Google Scholar] [CrossRef] [PubMed]
- Kochana, J.; Starzec, K.; Wieczorek, M.; Knihnicki, P.; Góra, M.; Rokicińska, A.; Kościelniak, P.; Kuśtrowski, P. Study on self-assembled monolayer of functionalized thiol on gold electrode forming capacitive sensor for chromium(VI) determination. J. Solid State Electrochem. 2019, 23, 1463–1472. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, M.; He, H. Alkyl chain dependent alkanethiol self-assembled adsorption dynamics. Surf. Rev. Lett. 2015, 22, 1550004. [Google Scholar] [CrossRef]
- Thébault, P.; Ammoun, M.; Boudjemaa, R.; Ouvrard, A.; Steenkeste, K.; Bourguignon, B.; Fontaine-Aupart, M.-P. Surface functionalization strategy to enhance the antibacterial effect of nisin Z peptide. Surf. Interfaces 2022, 30, 101822. [Google Scholar] [CrossRef]
- MacDairmid, A.R.; Gallagher, M.C.; Banks, J.T. Structure of dithiothreitol monolayers on Au(111). J. Phys. Chem. B 2003, 107, 9789–9792. [Google Scholar] [CrossRef]
- Nuzzo, R.G.; Allara, D.L. Adsorption of bifunctional organic disulfides on gold surfaces. J. Am. Chem. Soc. 1983, 105, 4481–4483. [Google Scholar] [CrossRef]
- Alawad, A.; Istamboulié, G.; Calas-Blanchard, C.; Noguer, T. A reagentless aptasensor based on intrinsic aptamer redox activity for the detection of tetracycline in water. Sens. Actuators B Chem. 2019, 288, 141–146. [Google Scholar] [CrossRef]
- Paleček, E.; Bartošík, M. Electrochemistry of nucleic acids. Chem. Rev. 2012, 112, 3427–3481. [Google Scholar] [CrossRef]
- Wang, H.-S.; Ju, H.-X.; Chen, H.-Y. Simultaneous determination of guanine and adenine in DNA using an electrochemically pretreated glassy carbon electrode. Anal. Chim. Acta 2002, 461, 243–250. [Google Scholar] [CrossRef]
- Kerman, K.; Morita, Y.; Takamura, Y.; Ozsoz, M.; Tamiya, E. DNA-directed attachment of carbon nanotubes for enhanced label-free electrochemical detection of DNA hybridization. Electroanalysis 2004, 16, 1667–1672. [Google Scholar] [CrossRef]
- Urmann, K.; Modrejewski, J.; Scheper, T.; Walter, J.-G. Aptamer-modified nanomaterials: Principles and applications. BioNanoMaterials 2017, 18, 20160012. [Google Scholar] [CrossRef]
- Tan, S.Y.; Acquah, C.; Tan, S.Y.; Ongkudon, C.M.; Danquah, M.K. Characterisation of charge distribution and stability of aptamer-thrombin binding interaction. Process. Biochem. 2017, 60, 42–51. [Google Scholar] [CrossRef]




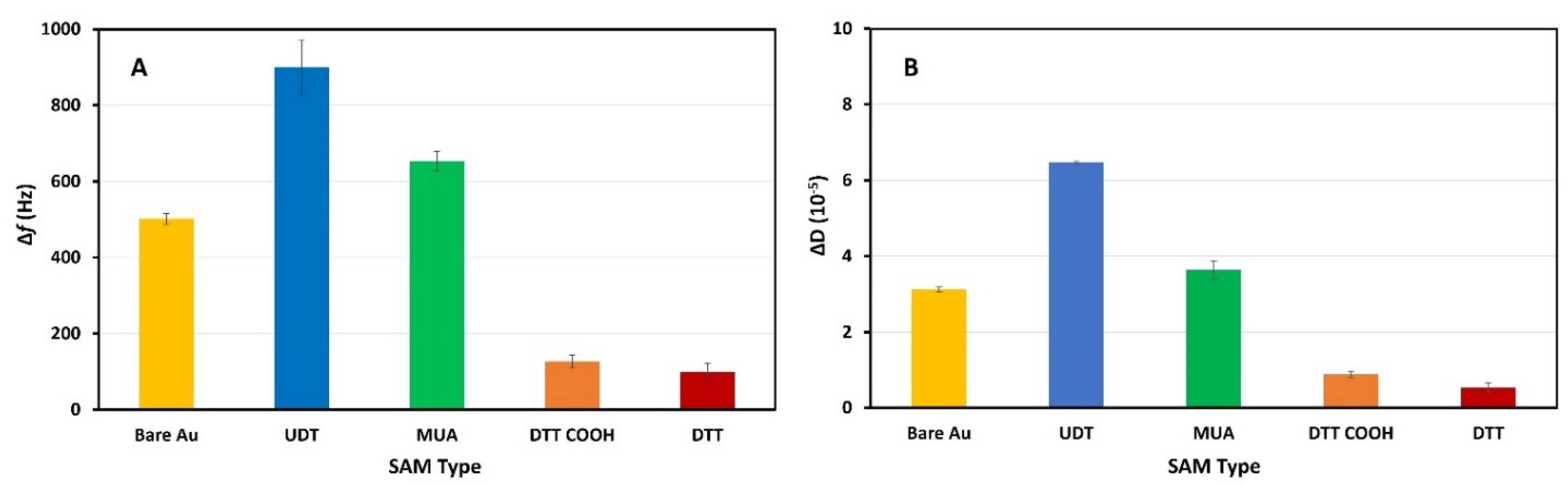
| SAM | Avg. Δf ± St. Dv. MILOS (Hz) | % Fouling Relative to MILOSDTTCOOH | Avg. ΔD ± St. Dv. MILOS (10−5) |
|---|---|---|---|
| Bare Au | 501.7 ± 28.9 | 75 | 3.1 ± 0.2 |
| UDT | 900.4 ± 141.4 | 86 | 6.5 ± 0.1 |
| MUA | 653.3 ± 50.3 | 81 | 3.6 ± 0.5 |
| DTTCOOH | 126.9 ± 33.9 | - | 0.9 ± 0.2 |
| DTT | 99.7 ± 42.4 | −21 | 0.5 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spagnolo, S.; Davoudian, K.; Ahmadi, S.; Chan, E.; Hianik, T.; Thompson, M. Thiol-Based Probe Linker with Antifouling Properties for Aptasensor Development. Chemosensors 2022, 10, 435. https://doi.org/10.3390/chemosensors10100435
Spagnolo S, Davoudian K, Ahmadi S, Chan E, Hianik T, Thompson M. Thiol-Based Probe Linker with Antifouling Properties for Aptasensor Development. Chemosensors. 2022; 10(10):435. https://doi.org/10.3390/chemosensors10100435
Chicago/Turabian StyleSpagnolo, Sandro, Katharina Davoudian, Soha Ahmadi, Edmund Chan, Tibor Hianik, and Michael Thompson. 2022. "Thiol-Based Probe Linker with Antifouling Properties for Aptasensor Development" Chemosensors 10, no. 10: 435. https://doi.org/10.3390/chemosensors10100435
APA StyleSpagnolo, S., Davoudian, K., Ahmadi, S., Chan, E., Hianik, T., & Thompson, M. (2022). Thiol-Based Probe Linker with Antifouling Properties for Aptasensor Development. Chemosensors, 10(10), 435. https://doi.org/10.3390/chemosensors10100435









