A New Chemosensor Based on a Luminescent Complex for the Investigation of Some Organophosphorus Pesticides in Environmental Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
2.3. Solution Preparation
2.4. Binding Affinity Calculation
2.5. Preparation of Water Samples
3. Results and Discussions
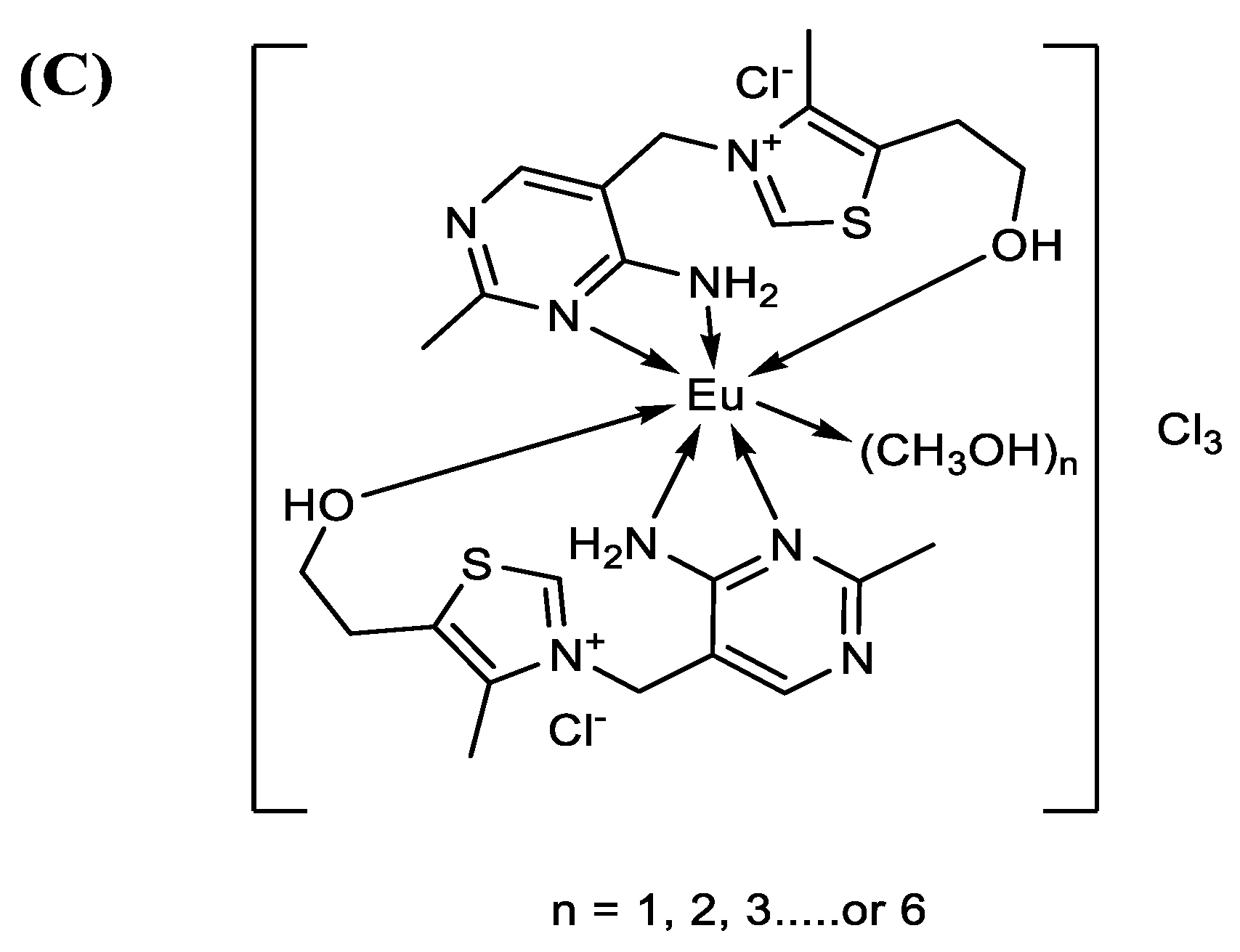
3.1. The Spectroscopy of Europium (III)–(vitB1)2 Complex
3.1.1. UV-Vis Absorption Spectroscopy
3.1.2. Luminescence Spectroscopy
3.1.3. Molar Ratio Method
3.2. Emission Spectra of Europiumn(III)–(vitB1)2 Complex with Various OPPs
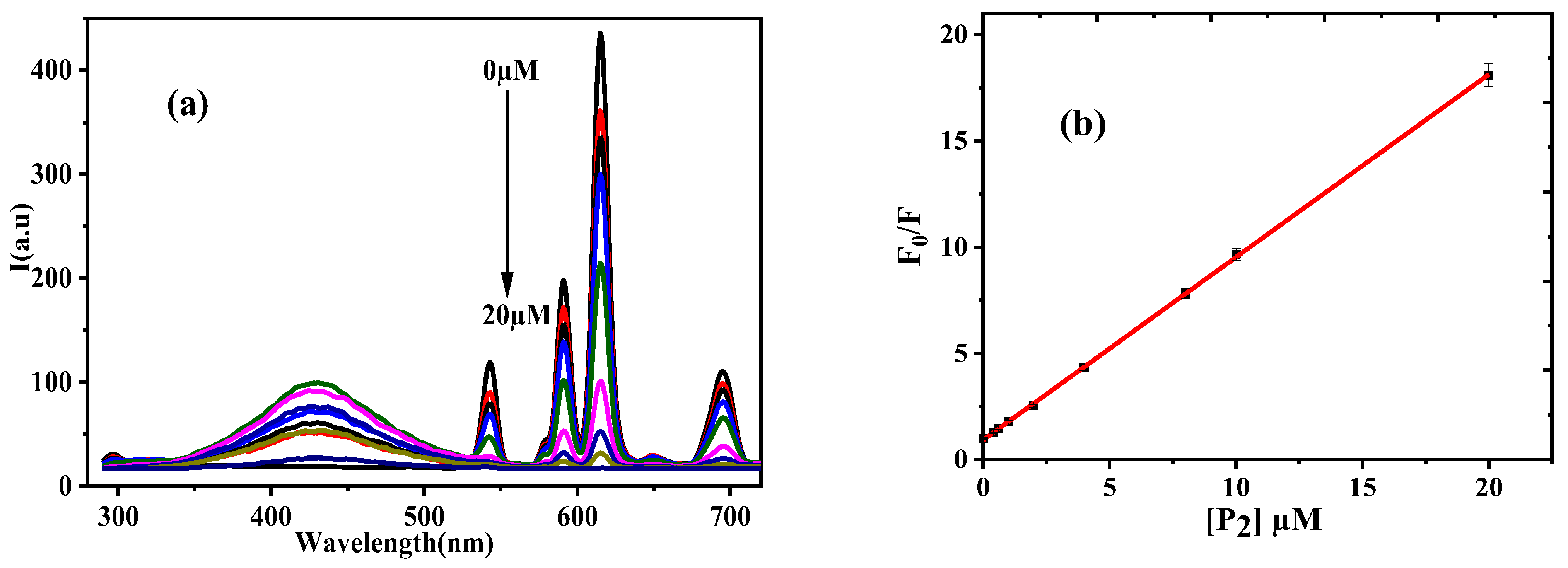
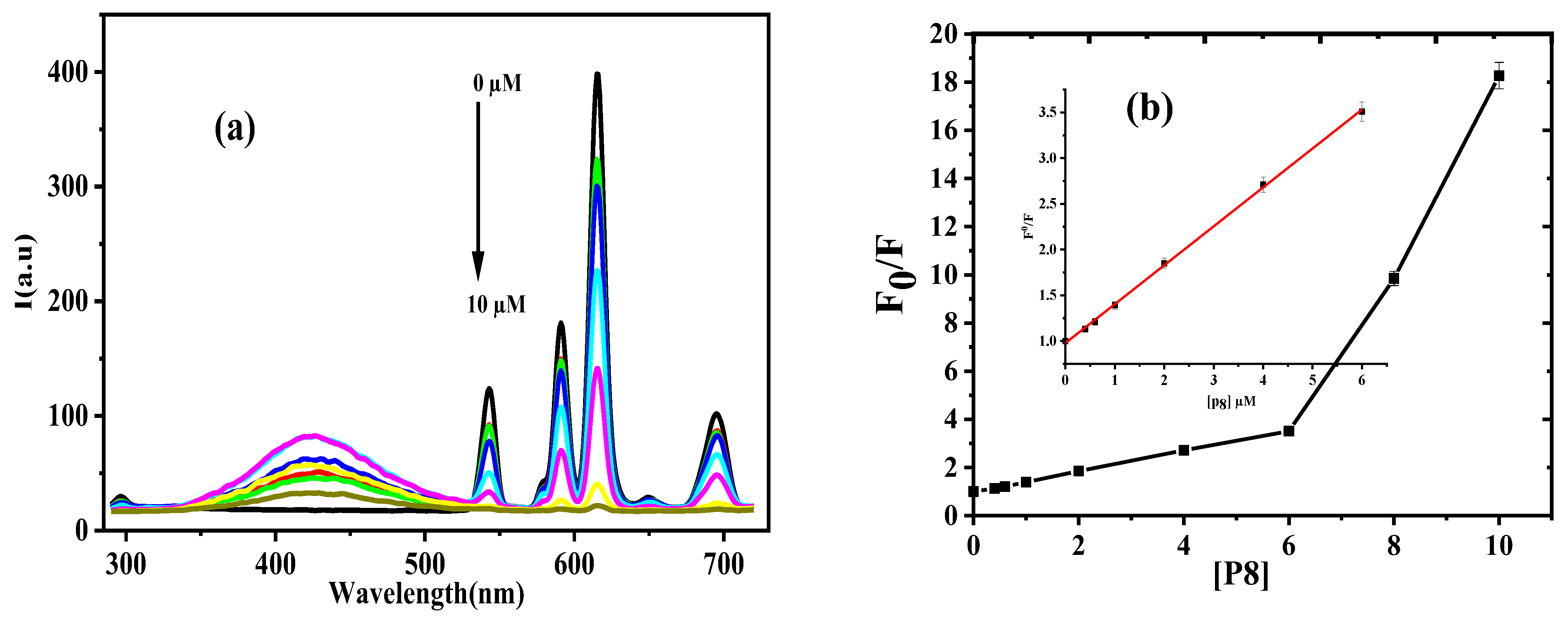
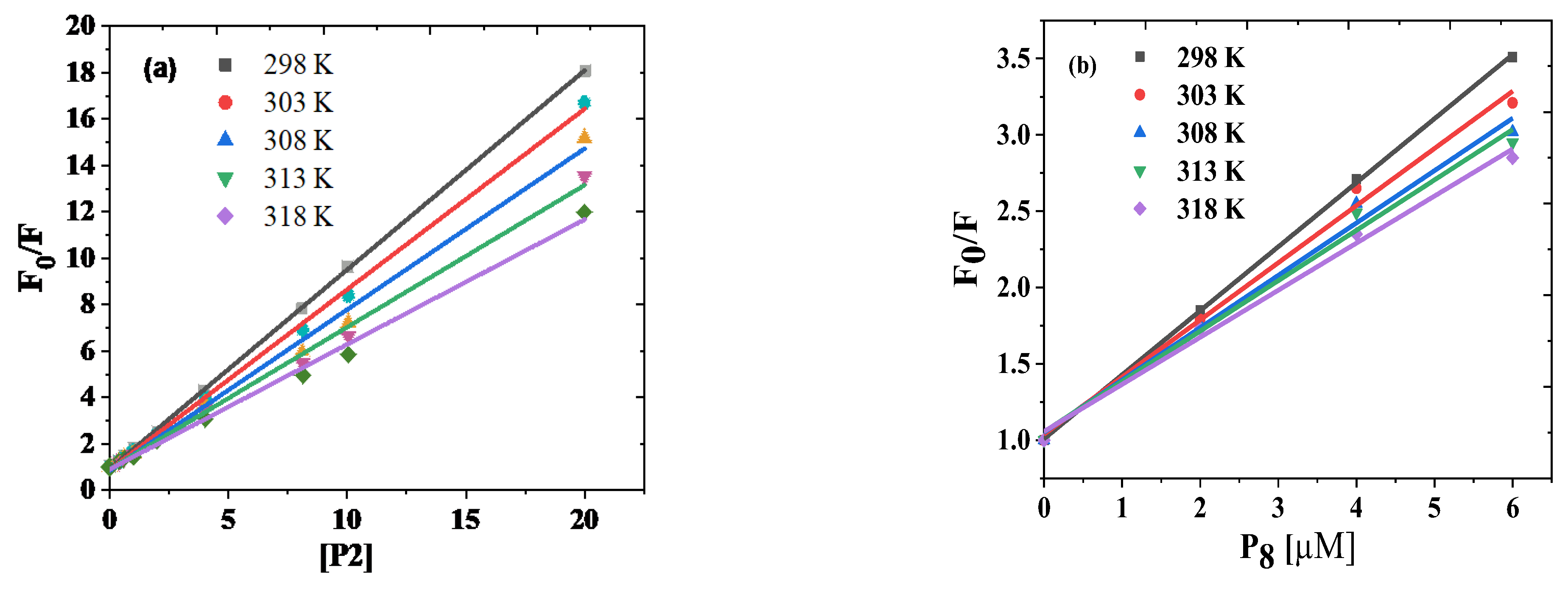
3.2.1. Calibration Data and Investigation of Quenching Mechanism
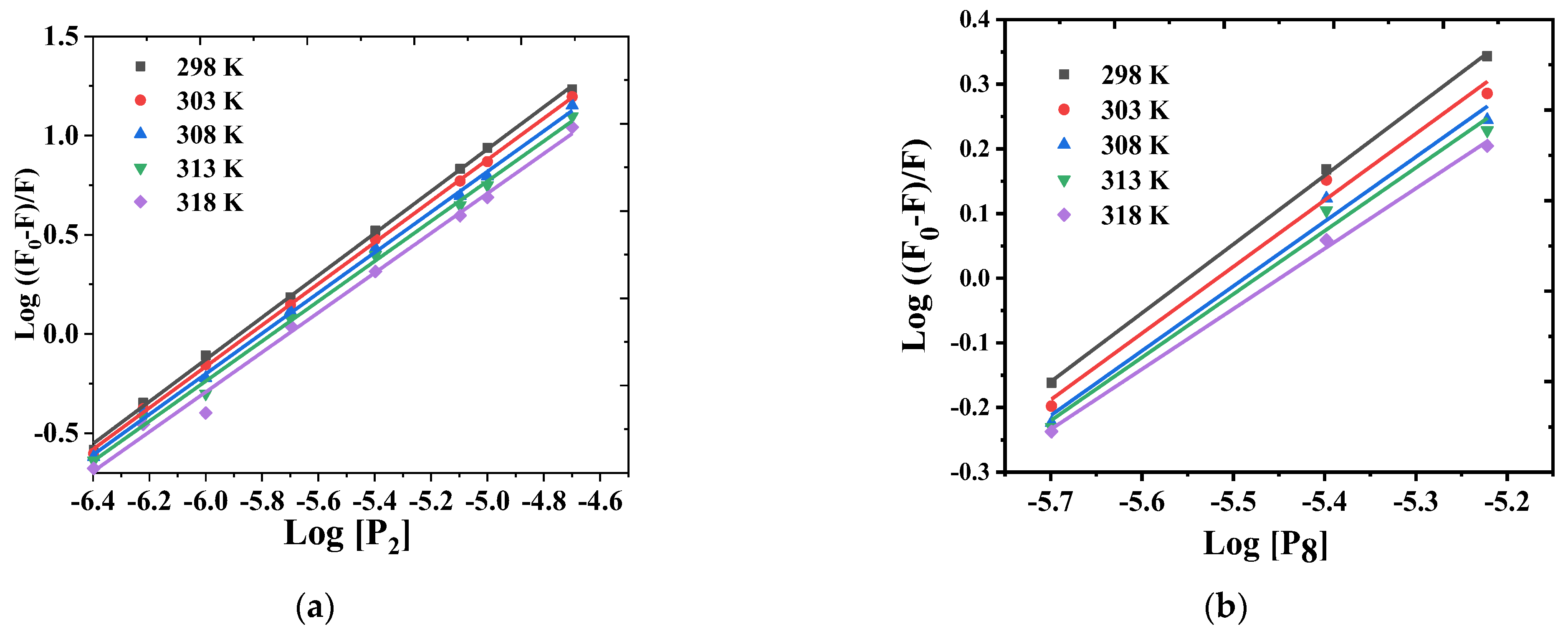
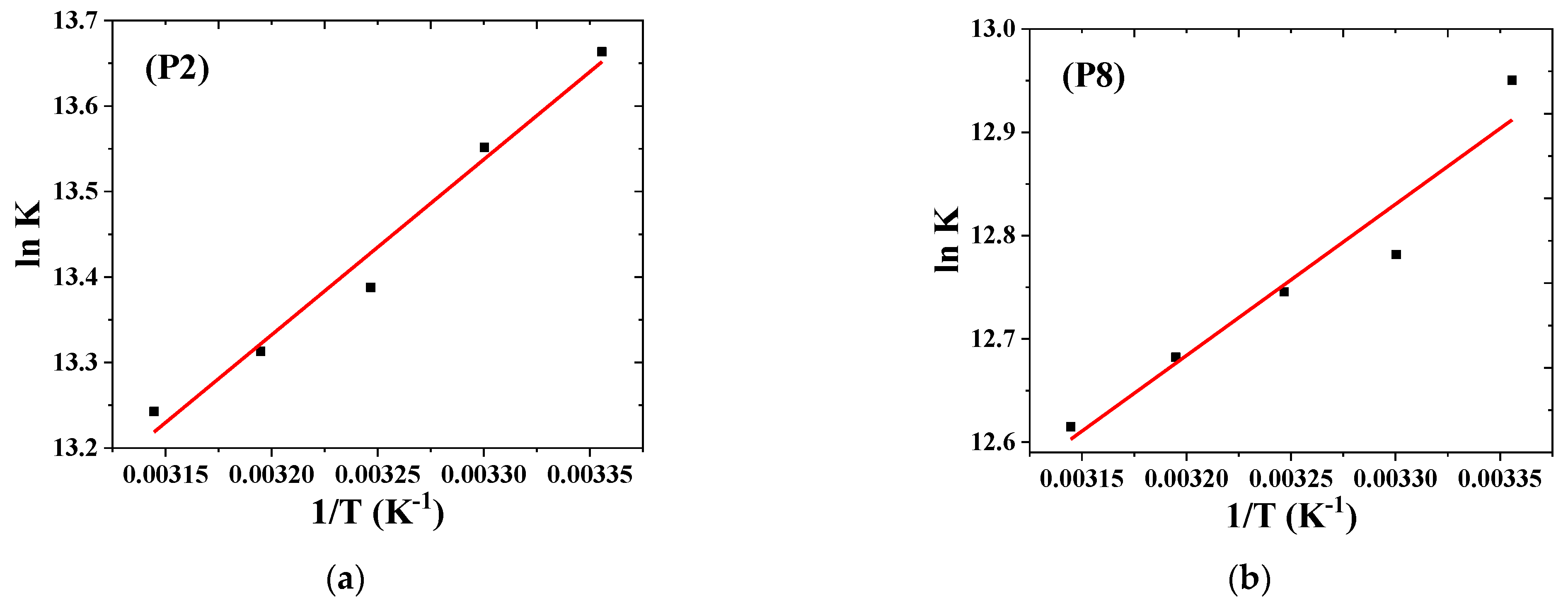
3.2.2. Stoichiometry and Binding Constant
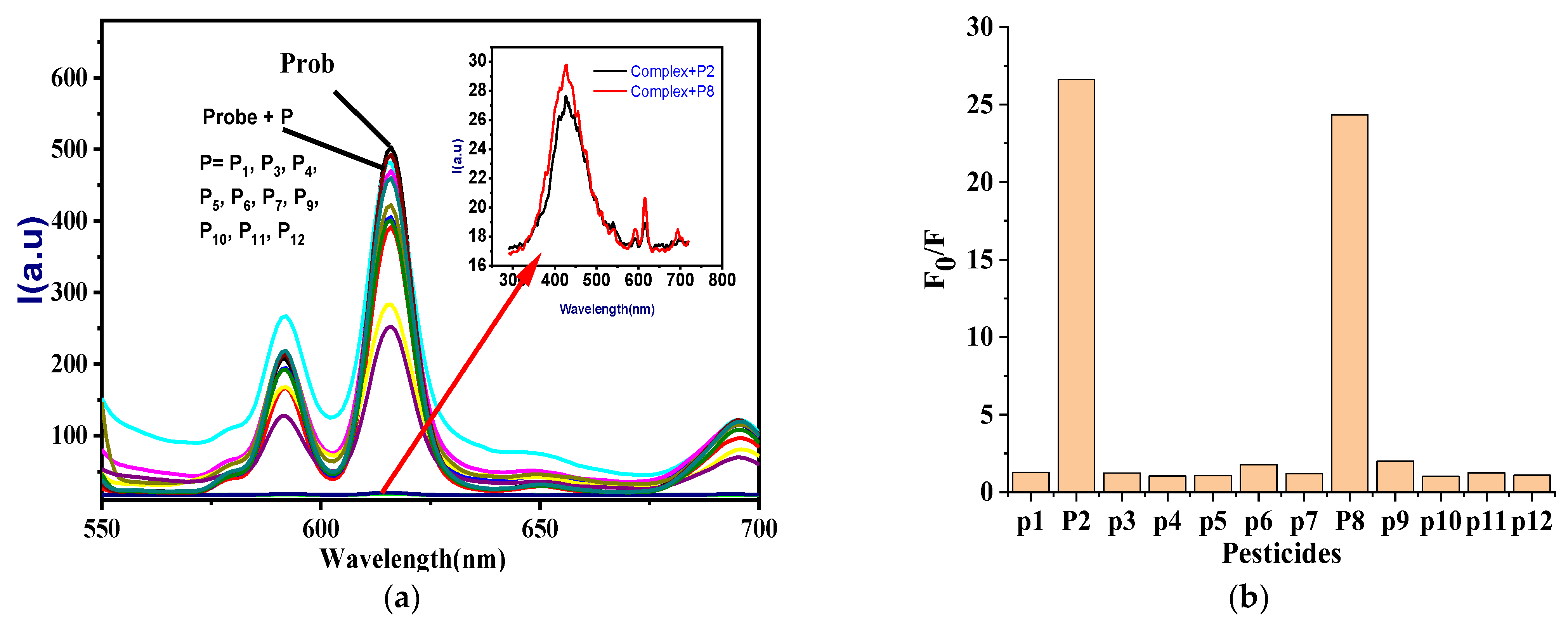
3.2.3. Selectivity
3.2.4. Effect of Interference
4. Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R.; Li, N.; Sun, J.; Gao, F. Colorimetric and Phosphorimetric Dual-Signaling Strategy Mediated by Inner Filter Effect for Highly Sensitive Assay of Organophosphorus Pesticides. J. Agric. Food Chem. 2015, 63, 8947–8954. [Google Scholar] [CrossRef]
- Cooper, J.; Dobson, H. The benefits of pesticides to mankind and the environment. Crop Prot. 2007, 26, 1337–1348. [Google Scholar] [CrossRef]
- van der Werf, H.M. Assessing the impact of pesticides on the environment. Agric. Ecosyst. Environ. 1996, 60, 81–96. [Google Scholar] [CrossRef]
- Bayrami, M.; Hashemi, T.; Malekirad, A.A.; Ashayeri, H.; Faraji, F.; Abdollahi, M. Electroencephalogram, Cognitive State, Psychological Disorders, Clinical Symptom, and Oxidative Stress in Horticulture Farmers Exposed to Organophosphate Pesticides. Toxicol. Ind. Health 2012, 28, 90–96. [Google Scholar] [CrossRef] [PubMed]
- McCauley, L.A.; Anger, W.K.; Keifer, M.; Langley, R.; Robson, M.G.; Rohlman, D. Studying Health Outcomes in Farmworker Populations Exposed to Pesticides. Environ. Health Perspect. 2006, 114, 953–960. [Google Scholar] [CrossRef]
- Wilson, C.; Tisdell, C. Why Farmers Continue to Use Pesticides despite Environmental, Health and Sustainability Costs. Ecol. Econ. 2001, 39, 449–462. [Google Scholar] [CrossRef]
- Hu, T.T.; Lu, C.M.; Li, H.; Zhang, Z.X.; Zhao, Y.H.; Li, J. Determination of Eleven Organophosphorus Pesticide Residues in Textiles by Using HPLC-HRMS. Anal. Sci. 2017, 33, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Zheng, S.; Yan, R.; Lian, Y. Determination of Organophosphorus Pesticides Using Solid-Phase Extraction Followed by Gas Chromatography-Mass Spectrometry. J. Chromatogr. Sci. 2022, 60, 1–6. [Google Scholar] [CrossRef]
- Boneva, I.; Yaneva, S.; Danalev, D. Development and Validation of Method for Determination of Organophosphorus Pesticides Traces in Liver Sample by GC-MS/MS-Ion Trap. Acta Chromatogr. 2021, 33, 188–194. [Google Scholar] [CrossRef]
- Moinfar, S.; Khodayari, A.; Abdulrahman, S.S.; Aghaei, A.; Sohrabnezhad, S.; Jamil, L.A. Development of a SPE/GC–MS Method for the Determination of Organophosphorus Pesticides in Food Samples Using Syringe Filters Packed by GNP/MIL-101(Cr) Nanocomposite. Food Chem. 2022, 371, 130997. [Google Scholar] [CrossRef]
- de Souza, D.; Machado, S.A.S. Electroanalytical Method for Determination of the Pesticide Dichlorvos Using Gold-Disk Microelectrodes. Anal. Bioanal. Chem. 2005, 382, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, C. Contemporary voltammetric techniques and its application to pesticide analysis: A review. Mater. Today: Proc. 2020, 37, 3231–3240. [Google Scholar] [CrossRef]
- Chen, G.; Wang, P. Electroanalytical Methods for Detecting Pesticides in Agricultural Products: A Review and Recent Developments. Int. J. Electrochem. Sci. 2020, 15, 2700–2712. [Google Scholar] [CrossRef]
- Roda, A.; Rauch, P.; Ferri, E.; Girotti, S.; Ghini, S.; Carrea, G.; Bovara, R. Chemiluminescent Flow Sensor for the Determination of Paraoxon and Aldicarb Pesticides. Anal. Chim. Acta 1994, 294, 35–42. [Google Scholar] [CrossRef]
- Xu, Z.L.; Sun, W.J.; Yang, J.Y.; Jiang, Y.M.; Campbell, K.; Shen, Y.D.; Lei, H.T.; Zeng, D.P.; Wang, H.; Sun, Y.M. Development of a Solid-Phase Extraction Coupling Chemiluminescent Enzyme Immunoassay for Determination of Organophosphorus Pesticides in Environmental Water Samples. J. Agric. Food Chem. 2012, 60, 2069–2075. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, S.; Bhattacharyya, R.; Banerjee, D. A Novel Fluorescence Based Assay for the Detection of Organophosphorus Pesticide Exposed Cholinesterase Activity Using 1-Naphthyl Acetate. Biochimie 2019, 160, 100–112. [Google Scholar] [CrossRef]
- Luo, Q.J.; Li, Z.G.; Lai, J.H.; Li, F.Q.; Qiu, P.; Wang, X.L. An On-off-on Gold Nanocluster-Based Fluorescent Probe for Sensitive Detection of Organophosphorus Pesticides. RSC Adv. 2017, 7, 55199–55205. [Google Scholar] [CrossRef]
- Hemmilä, I.; Laitala, V. Progress in Lanthanides as Luminescent Probes. J. Fluoresc. 2005, 15, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.-C.; Kuo, C.-C.; Chen, B.-Y.; Cho, C.-J.; Hung, C.-C.; Chen, W.-C.; Borsali, R. RGB-Switchable Porous Electrospun Nanofiber Chemoprobe-Filter Prepared from Multifunctional Copolymers for Versatile Sensing of PH and Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 16381–16396. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Perspectives in Supramolecular Chemistry—From Molecular Recognition towards Molecular Information Processing and Self-Organization. Angew. Chem. Int. Ed. Engl. 1990, 29, 1304–1319. [Google Scholar] [CrossRef]
- Azab, H.A.; Khairy, G.M.; Kamel, R.M. Time-Resolved Fluorescence Sensing of Pesticides Chlorpyrifos, Crotoxyphos and Endosulfan by the Luminescent Eu(III)-8-Allyl-3-Carboxycoumarin Probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Hussein, B.H.M.; Khairy, G.M.; Kamel, R.M. Fluorescence Sensing of Phosdrin Pesticide by the Luminescent Eu(III)- and Tb(III)-Bis(Coumarin-3-Carboxylic Acid) Probes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 158, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Azab, H.A.; Khairy, G.M.; Abd El-Ghany, N.; Ahmed, M.A. A New Luminescent Bio-Probe of Europium(III)-Complex for Sensing Some Biomolecules and CT-DNA. J. Photochem. Photobiol. A Chem. 2019, 374, 1–9. [Google Scholar] [CrossRef]
- Azab, H.A.; Khairy, G.M.; Abd El-Ghany, N.; Ahmed, M.A. Time-Resolved Fluorescence Sensing of N-Acetyl Amino Acids, Nucleobases, Nucleotides and DNA by the Luminescent Tb (III)-8-Alkyl-2-Oxo-2H-Chromene-3-Carbaldehyde Probe. J. Lumin. 2016, 176, 181–192. [Google Scholar] [CrossRef]
- Leonard, J.P.; Jensen, P.; McCabe, T.; O’Brien, J.E.; Peacock, R.D.; Kruger, P.E.; Gunnlaugsson, T. Self-Assembly of Chiral Luminescent Lanthanide Coordination Bundles. J. Am. Chem. Soc. 2007, 129, 10986–10987. [Google Scholar] [CrossRef]
- Anwar, Z.M.; Ibrahim, I.A.; Kamel, R.M.; Abdel-Salam, E.T.; El-Asfoury, M.H. New Highly Sensitive and Selective Fluorescent Terbium Complex for the Detection of Aluminium Ions. J. Mol. Struct. 2018, 1154, 272–279. [Google Scholar] [CrossRef]
- Qu, L.-L.; Liu, Y.-Y.; He, S.-H.; Chen, J.-Q.; Liang, Y.; Li, H.-T. Highly Selective and Sensitive Surface Enhanced Raman Scattering Nanosensors for Detection of Hydrogen Peroxide in Living Cells. Biosens. Bioelectron. 2016, 77, 292–298. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer: New York, NY, USA, 1983; p. 454. [Google Scholar]
- Kazakov, V.P.; Voloshin, A.I.; Ostakhov, S.S.; Shavaleev, N.M. The Anomalous Influence of Water on the Intensity and Lifetime of Fluorescence in Tris(Benzoyltrifluoroacetonate)Europium(III). Mendeleev Commun. 1998, 8, 47–49. [Google Scholar] [CrossRef]
- Wang, S.; Cao, T.; Yan, H.; Li, Y.; Lu, J.; Ma, R.; Li, D.; Dou, J.; Bai, J. Functionalization of Microporous Lanthanide-Based Metal–Organic Frameworks by Dicarboxylate Ligands with Methyl-Substituted Thieno[2,3- b ]Thiophene Groups: Sensing Activities and Magnetic Properties. Inorg. Chem. 2016, 55, 5139–5151. [Google Scholar] [CrossRef]
- Leonard, J.P.; Nolan, C.B.; Stomeo, F.; Gunnlaugsson, T. Photochemistry and Photophysics of Coordination Compounds: Lanthanides. In Photochemistry and Photophysics of Coordination Compounds II; Springer: Berlin/Heidelberg, Germany; pp. 1–43.
- Lakowicz, J.R. Quenching of Fluorescence. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 1999; pp. 237–265. [Google Scholar]
- Kamel, R.M. Facile Luminescent Sensing Trace Levels of Pesticides Azinphos Ethyl, Diazinon, Chlorfenviphos and Isofenphos. Inorg. Chem. Commun. 2020, 111, 107662. [Google Scholar] [CrossRef]
- Azab, H.A.; Ibrahim, I.A.; Hassan, N.; Abbas, A.M.; Darwish, H.M. Synthesis and Photo-Physical Properties of Novel Tb(III) -[Ethyl-4-Hydroxy-1-(4-Methoxyphenyl)−2-Quinolinone-3-Carboxylate] Complex and Luminescence Sensitivity towards Malathion and Crotoxyphos Pesticides. J. Lumin. 2017, 192, 376–384. [Google Scholar] [CrossRef]
- Azab, H.A.; Kamel, R.M. Sensitive and Selective Fluorescent Chemosensor for the Detection of Some Organophosphorus Pesticides Using Luminescent Eu(III) Complex. J. Photochem. Photobiol. A Chem. 2016, 321, 33–40. [Google Scholar] [CrossRef]
- Azab, H.A.; Kamel, R.M. Binding of Chlorfenvinphos and Malathion with DNA and Their Detection Using New Sensitive Luminescent Tb(III) Complex Probe. J. Lumin. 2016, 170, 671–678. [Google Scholar] [CrossRef]
- Azab, H.A.; Orabi, A.S.; Abbas, A.M. New Polymerizable Luminescence Probe for Detection of Chlorfenvinphos and Dichlorvos Pesticides. J. Lumin. 2015, 167, 360–370. [Google Scholar] [CrossRef]
- Azab, H.A.; Anwar, Z.M.; Rizk, M.A.; Khairy, G.M.; El-Asfoury, M.H. Determination of Organophosphorus Pesticides in Water Samples by Using a New Sensitive Luminescent Probe of Eu (III) Complex. J. Lumin. 2015, 157, 371–382. [Google Scholar] [CrossRef]
- Azab, H.A.; Duerkop, A.; Anwar, Z.M.; Hussein, B.H.M.; Rizk, M.A.; Amin, T. Luminescence Recognition of Different Organophosphorus Pesticides by the Luminescent Eu(III)–Pyridine-2,6-Dicarboxylic Acid Probe. Anal. Chim. Acta 2013, 759, 81–91. [Google Scholar] [CrossRef]
- Azab, H.A.; El-Korashy, S.A.; Anwar, Z.M.; Khairy, G.M.; Duerkop, A. Reactivity of a Luminescent “off–on” Pyrylium Dye toward Various Classes of Amines and Its Use in a Fluorescence Sensor Microtiter Plate for Environmental Samples. J. Photochem. Photobiol. A Chem. 2012, 243, 41–46. [Google Scholar] [CrossRef]
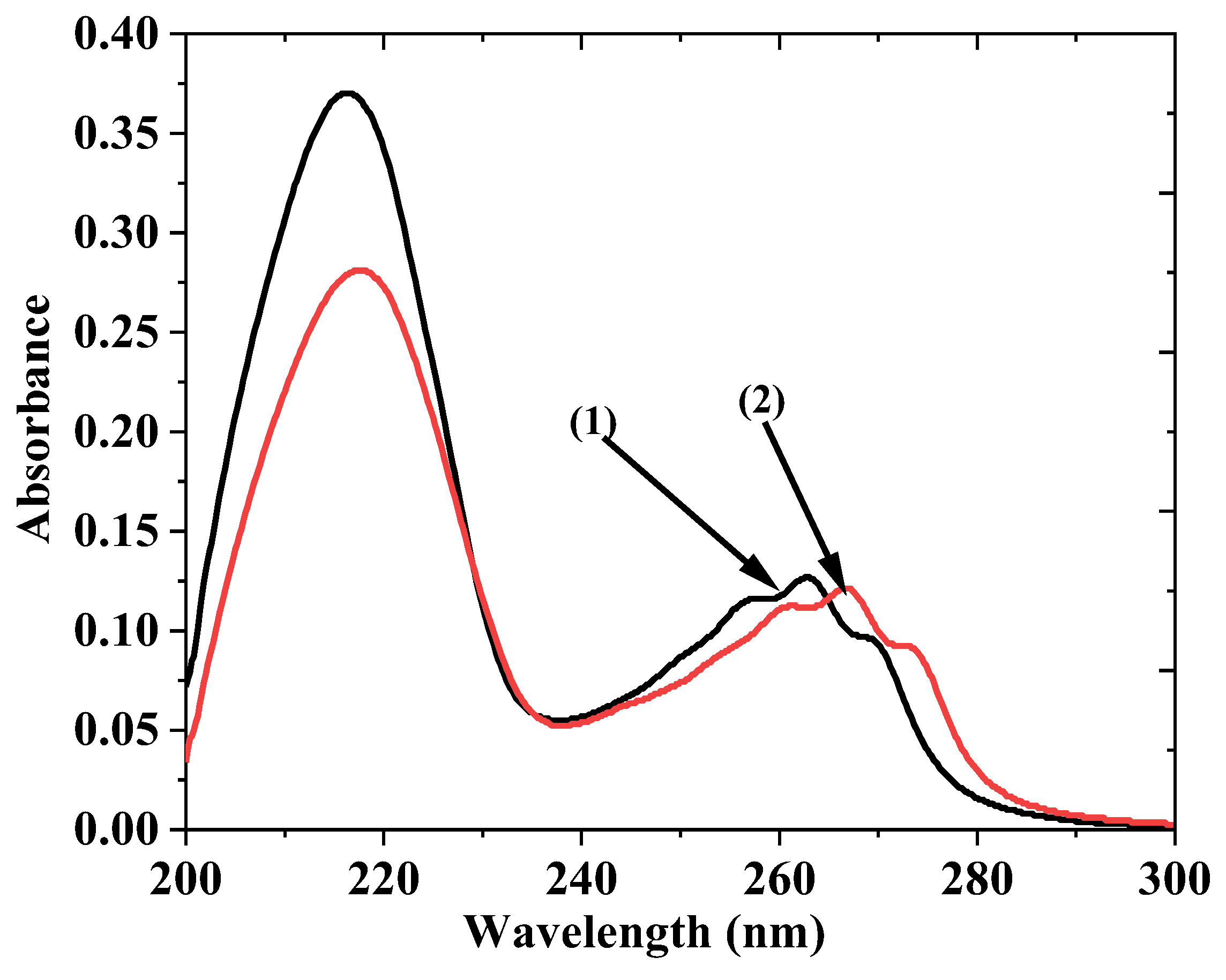
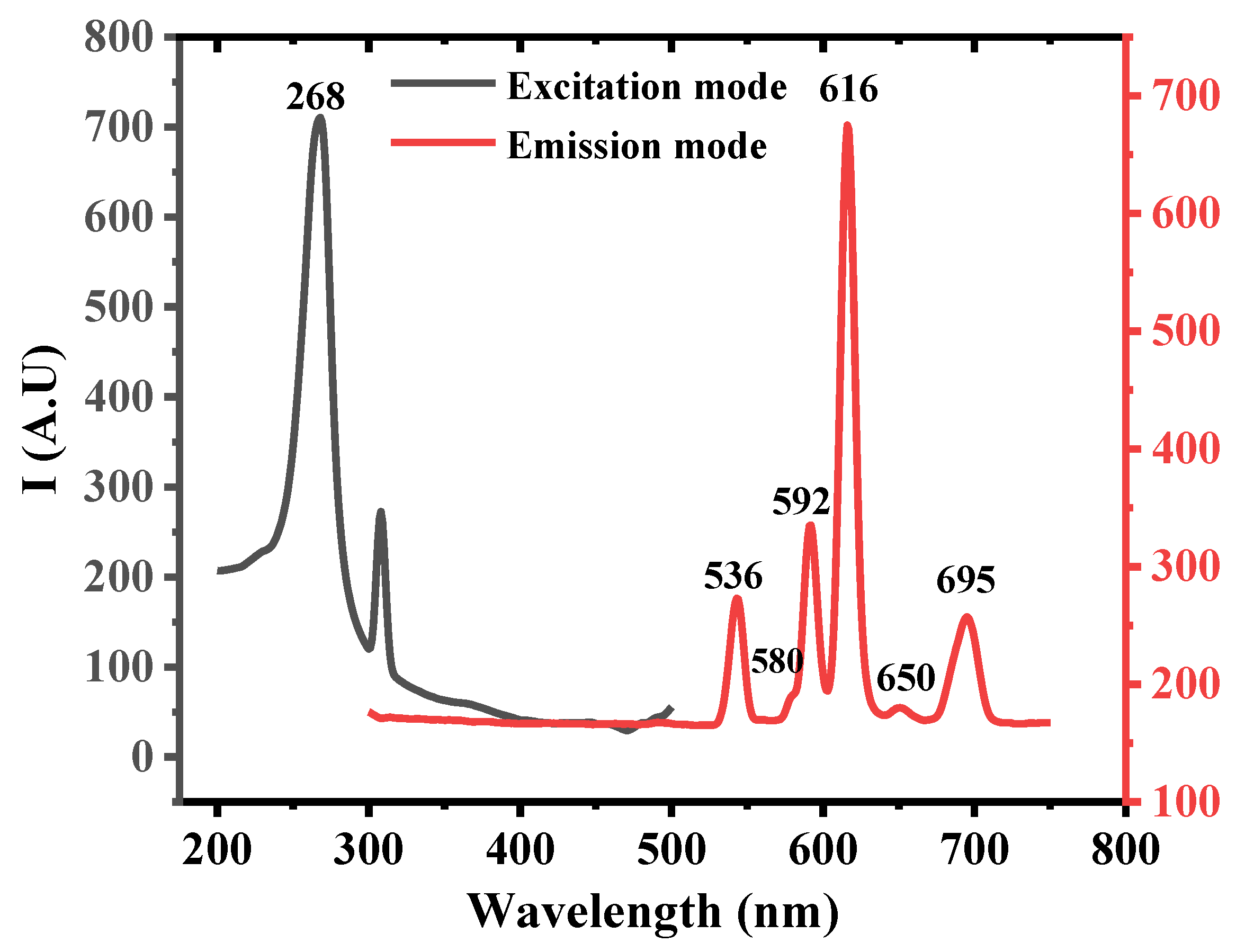

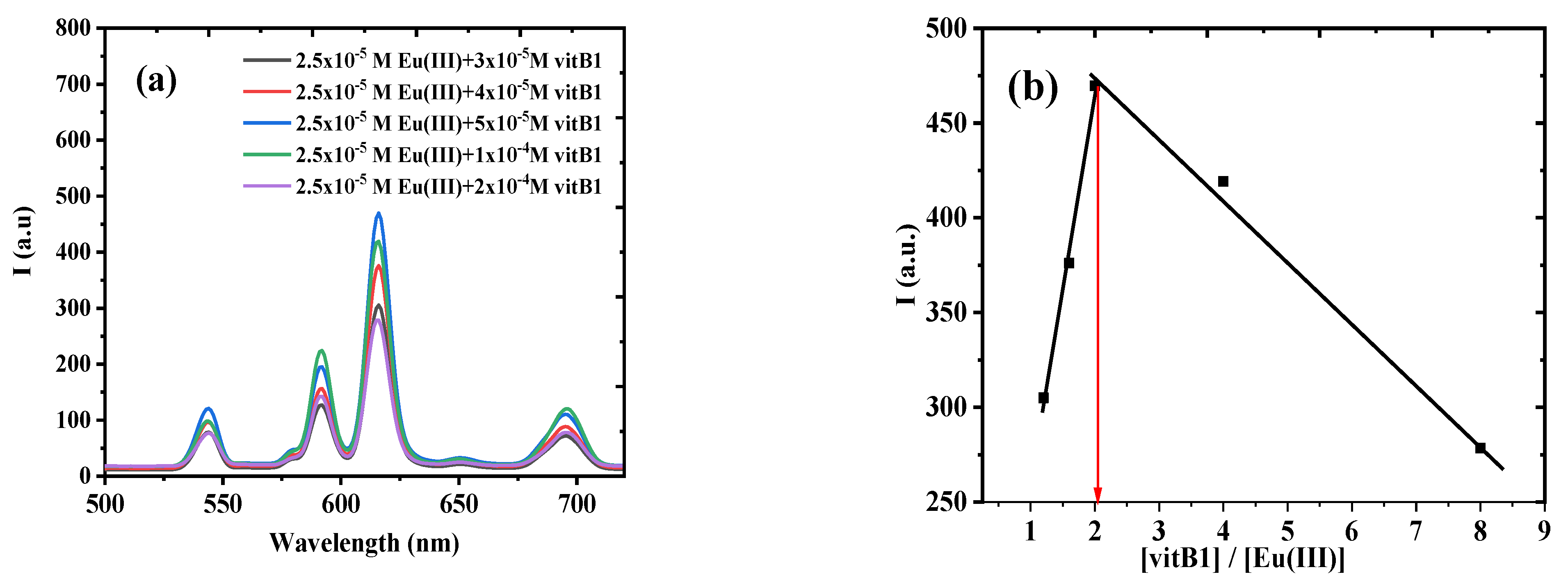
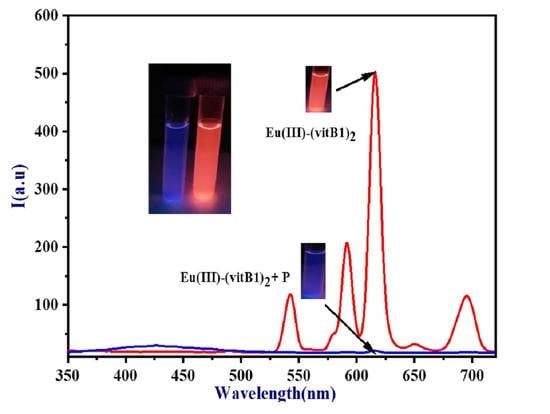
| Pesticides | Temp. (K) | R2 | Stern–Volmer Quenching Constant K (L mol−1) × 105 | SD |
|---|---|---|---|---|
| Chlorfenvinphos | 298 | 0.9998 | 8.61 | 0.22 |
| 303 | 0.9985 | 7.85 | 0.25 | |
| 308 | 0.9939 | 6.93 | 0.3.44 | |
| 313 | 0.9953 | 6.15 | 0534 | |
| 318 | 0.9949 | 5.42 | 0.117 | |
| Malathion | 298 | 0.9998 | 4.21 | 0.235 |
| 303 | 0.9964 | 3.82 | 0.085 | |
| 308 | 0.9943 | 3.41 | 0.034 | |
| 313 | 0.9945 | 3.33 | 0.016 | |
| 318 | 0.9965 | 3.10 | 0.018 |
| Parameters | P2 | P8 |
|---|---|---|
| Regression equation | F0/F = 8600 X + 0.92 | F0/F = 4300 X + 0.98 |
| Slope | 8600 | 4300 |
| Intercept | 0.92 | 0.98 |
| R2 | 0.9933 | 0.9993 |
| Accuracy (n = 8) | 100.61 ± 2.74 | 101.88 ± 1.99 |
| Correlation coefficient (r) | 0.9998 | 0.99926 |
| Linear range | 0.95–20 µM | 0.36–6 µM |
| SE of intercept | 0.03358 | 0.01359 |
| SD of intercept | 0.095 | 0.036 |
| LOQ | 0.95 µM | 0.36 µM |
| LOD | 0.31 µM | 0.12 µM |
| Chemosensors | Detection Limit | Other Pesticides Sensitive to the Probe | Optimum Condition for Detection | Ref. |
|---|---|---|---|---|
| Eu(III)–(vitB1)2 complex | 0.31 and 0.12 µM for chlorfenvinphos and malathion, respectively | ----- | In methanol | This work |
| Eu–bathophenanthroline (batho) probe | 1.46 µM for chlorfenvinphos | Azinphos-ethyl, diazinon, and isofenphos | In 50% (v/v) acetonitrile–water mixture at pH = 6 | [33] |
| Tb(III)-[ethyl-4-hydroxy-1-(4-methoxyphenyl)−2-quinolinone-3-carboxylate] complex | 0.94 and 2.68 μM for malathion in ethanol and water, respectively | Crotoxyphos | In water or ethanol | [34] |
| Europium-o-(4-methoxy benzoyl) benzoic acid [o-(4-anisoyl)] complex | 2.82 µM for chlorfenvinphos | Azinphos-ethyl, diazinon, and isofenphos | In ethanol–water (5:5 v/v) solution | [35] |
| Terbium-N(acetoacetyl)-3-allyl-2hydroxybenzaldehyde hydrazone complex | 4.53 and 9.59 μ M for chlorfenvinphos and malathion, respectively | ------ | In ethanol | [36] |
| Europium-(allyl-3-carboxycoumarin) complex | ------- | Chlorpyrifos, endosulfan, and crotoxyphos | In ethanol | [21] |
| Tb(III)-(3-allyl-2-hydroxybenzoic acid)3 complex | 1.9 μM for chlorfenvinphos | Dichlorvos | In methanol | [37] |
| Eu(III)–TAN-1,10 phenanthroline | 0.64 μM for malathion | Endosulfan, heptachlor, and chlorpyrifos | In HEPS buffer (pH = 7.5) | [38] |
| Eu(III)–pyridine-2,6-dicarboxylic acid probe | 2.5 and 0.55 μM for malathion and chlorfenvinphos | Azinphos | In 0.10 volume fraction ethanol–water mixture at pH 7.5 (HEPES buffer) | [39] |
| Pesticides | Temp. (K) | Ka (L. mol−1) × 105 | SD | n | R2 | ∆H0 (kJ mol−1) | ∆S0 (J mol−1 K−1) | ∆G0 (kJ mol−1) |
|---|---|---|---|---|---|---|---|---|
| Chlorfenvinphos | 298 | 8.59 | 0.25 | 1.06 | 0.9990 | −17.09 ± 1.47 | 56.29 ± 4.77 | −33.85 |
| 303 | 7.68 | 0.22 | 1.04 | 0.9995 | −34.14 | |||
| 308 | 6.52 | 0.02 | 1.02 | 0.9984 | −34.28 | |||
| 313 | 6.05 | 0.03 | 1.00 | 0.9964 | −34.64 | |||
| 318 | 5.64 | 0.03 | 1.00 | 0.9924 | −35.01 | |||
| Malathion | 298 | 4.21 | 0.03 | 1.06 | 0.9987 | −12.18 ± 1.85 | 66.43 ± 6.01 | −32.09 |
| 303 | 3.54 | 0.02 | 1.03 | 0.9790 | −32.19 | |||
| 308 | 3.43 | 0.01 | 1.00 | 0.9710 | −32.64 | |||
| 313 | 3.22 | 0.05 | 0.98 | 0.9760 | −33.00 | |||
| 318 | 3.01 | 0.01 | 0.93 | 0.9961 | −33.35 |
| Interfering Species | Tolerance (µM) | |
|---|---|---|
| Chlorfenvinphos (P2) | Malathion (P8) | |
| Co2+ | 15 | 25 |
| Cd2+ | 48 | 60 |
| CO32- | 50 | 200 |
| Cu2+ | 16 | 100 |
| K+ | 150 | 50 |
| Na+ | 20 | 30 |
| NH4+ | 40 | 60 |
| Ni2+ | 10 | 20 |
| NO3− | 25 | 20 |
| Pb2+ | 200 | 205 |
| PO43− | 30 | 150 |
| Azinphos-ethyl, chlorpyrifos, crotoxyphos, diazinon, dichlorvos, isofenphos, paraoxon-ethyl, phosdrin, endosulfan, and heptachlor | 100 | 100 |
| Pesticide (P) | Type of Water Sample | Equation for Regression | r2 | RDS (%) | Added [µM] | Calculated [µM] | Recovery (%) |
|---|---|---|---|---|---|---|---|
| Chlorfenvinphos (P2) | Tap water | F0/F = 0.89 + 4500 (P2) | 0.99321 | 2.33 | 2.00 | 2.01 | 100.50 |
| 2.00 | 1.92 | 96.00 | |||||
| 2.00 | 2.10 | 105.00 | |||||
| 4.00 | 4.27 | 106.75 | |||||
| 4.00 | 4.13 | 103.25 | |||||
| 4.00 | 4.03 | 100.75 | |||||
| River water | F0/F = 0.91 + 4200 (P2) | 0.99459 | 2.21 | 2.00 | 2.04 | 102.00 | |
| 2.00 | 1.84 | 92.00 | |||||
| 2.00 | 2.18 | 109.00 | |||||
| 4.00 | 4.24 | 101.2 | |||||
| 4.00 | 4.05 | 101.25 | |||||
| 4.00 | 3.85 | 96.25 | |||||
| Wastewater | F0/F = 0.88 + 4320 (P2) | 0.99593 | 2.37 | 2.00 | 2.13 | 105.50 | |
| 2.00 | 2.08 | 104.00 | |||||
| 2.00 | 1.97 | 98.50 | |||||
| 4.00 | 4.32 | 108.00 | |||||
| 4.00 | 4.11 | 102.75 | |||||
| 4.00 | 4.04 | 101.00 | |||||
| Malathion (P8) | Tap water | F0/F = 0.93 + 8600 (P8) | 0.99489 | 1.78 | 5.00 | 4.23 | 84.60 |
| 5.00 | 4.52 | 90.4 | |||||
| 5.00 | 4.55 | 91.00 | |||||
| 10.00 | 9.41 | 94.10 | |||||
| 10.00 | 9.70 | 97.00 | |||||
| 10.00 | 9.79 | 97.90 | |||||
| River water | F0/F = 0.97 + 8700 (P8) | 0.99658 | 1.82 | 5.00 | 5.05 | 101.00 | |
| 5.00 | 5.10 | 102.00 | |||||
| 5.00 | 5.06 | 101.20 | |||||
| 10.00 | 9.59 | 95.90 | |||||
| 10.00 | 9.78 | 97.80 | |||||
| 10.00 | 9.77 | 97.70 | |||||
| Wastewater | F0/F = 0.89 + 8500 (P8) | 0.99875 | 1.62 | 5.00 | 5.30 | 106.00 | |
| 5.00 | 5.20 | 104.00 | |||||
| 5.00 | 5.12 | 102.4 | |||||
| 10.00 | 10.20 | 102.00 | |||||
| 10.00 | 10.20 | 102.00 | |||||
| 10.00 | 10.19 | 101.90 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamami, M.A.M.; Algethami, J.S.; Rizk, M.A.; Abbas, A.M.; Khairy, G.M. A New Chemosensor Based on a Luminescent Complex for the Investigation of Some Organophosphorus Pesticides in Environmental Samples. Chemosensors 2022, 10, 391. https://doi.org/10.3390/chemosensors10100391
Alhamami MAM, Algethami JS, Rizk MA, Abbas AM, Khairy GM. A New Chemosensor Based on a Luminescent Complex for the Investigation of Some Organophosphorus Pesticides in Environmental Samples. Chemosensors. 2022; 10(10):391. https://doi.org/10.3390/chemosensors10100391
Chicago/Turabian StyleAlhamami, Mohsen A. M., Jari S. Algethami, Moustafa A. Rizk, Abbas M. Abbas, and Gasser M. Khairy. 2022. "A New Chemosensor Based on a Luminescent Complex for the Investigation of Some Organophosphorus Pesticides in Environmental Samples" Chemosensors 10, no. 10: 391. https://doi.org/10.3390/chemosensors10100391
APA StyleAlhamami, M. A. M., Algethami, J. S., Rizk, M. A., Abbas, A. M., & Khairy, G. M. (2022). A New Chemosensor Based on a Luminescent Complex for the Investigation of Some Organophosphorus Pesticides in Environmental Samples. Chemosensors, 10(10), 391. https://doi.org/10.3390/chemosensors10100391