An AIEE Active Anthracene-Based Nanoprobe for Zn2+ and Tyrosine Detection Validated by Bioimaging Studies
Abstract
:1. Introduction
2. Materials and Methods
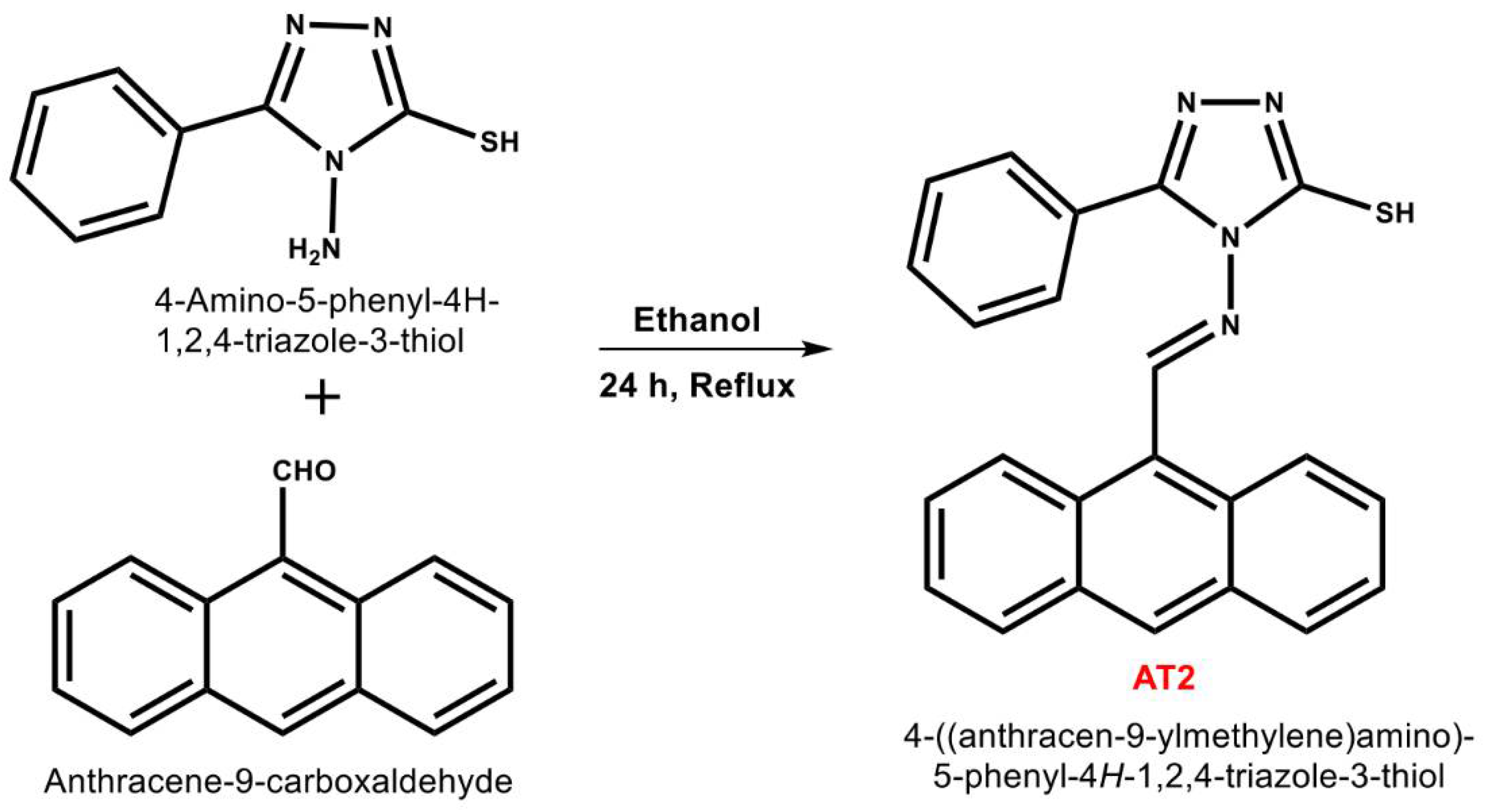
2.1. Synthesis of AT2 [3,15]
2.2. Computational Methods
2.3. MTT Assay
2.4. Procedure for Live Cell and Zebra Fish Imaging of AT2 [15]
2.4.1. Cellular Imaging of AIEE
2.4.2. Cellular Imaging of Zn2+ and Tyrosine
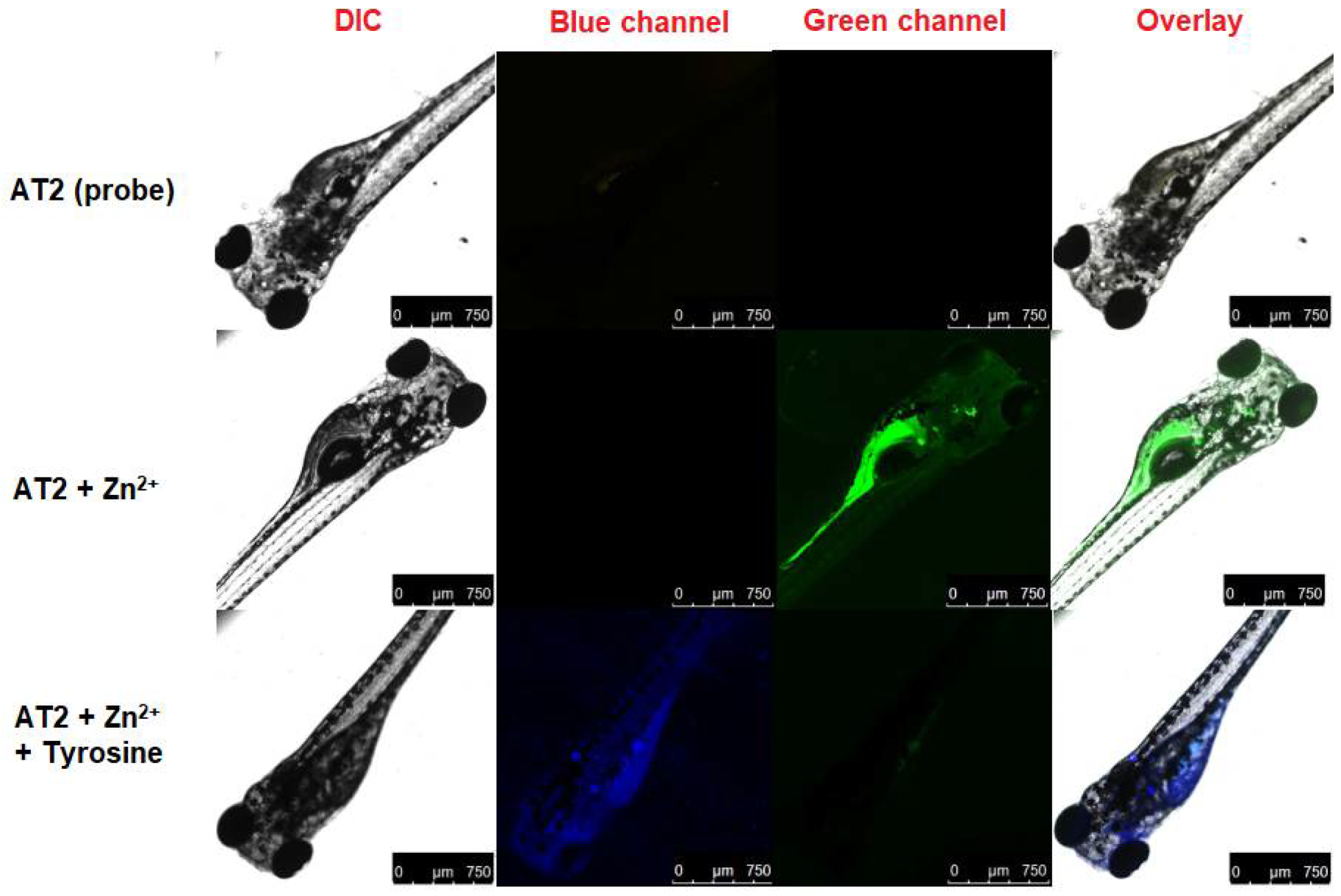
2.4.3. Zebrafish Imaging Studies
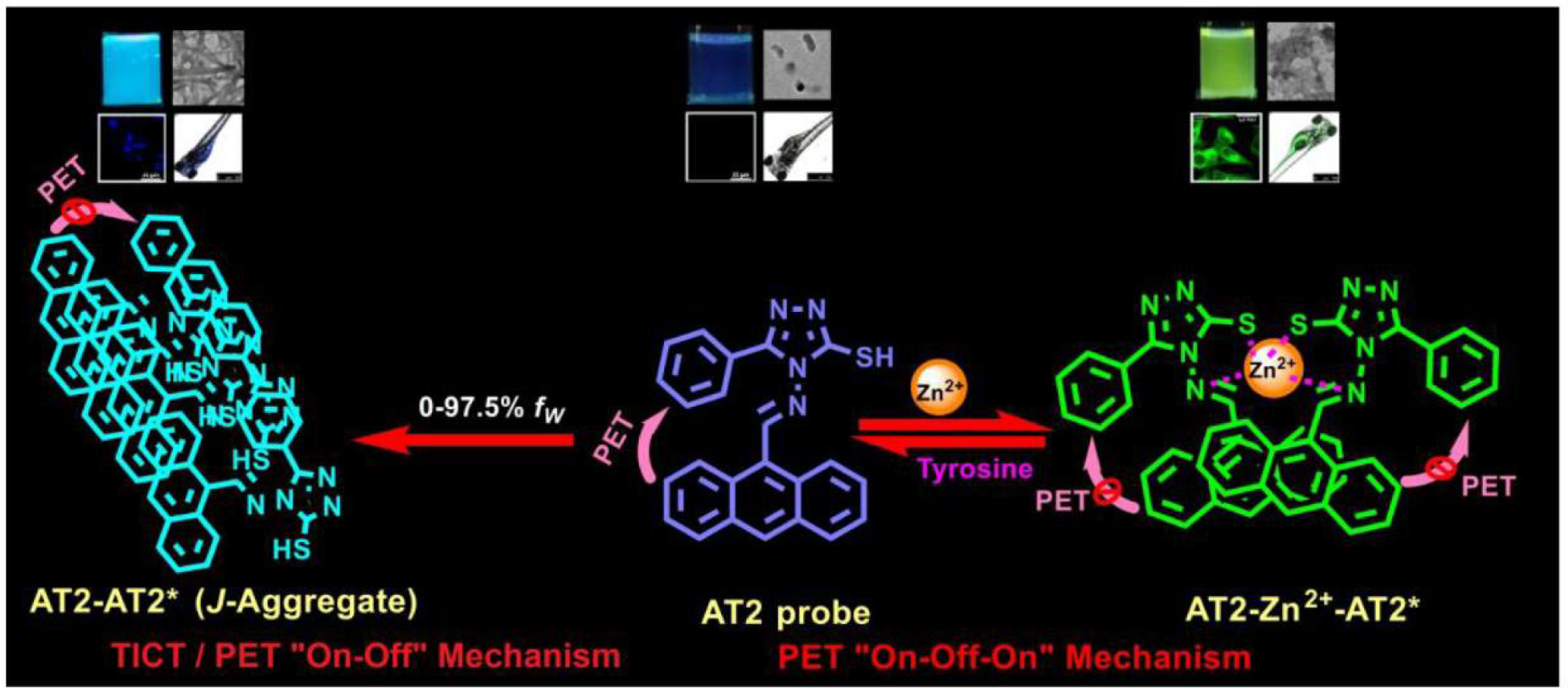
3. Results and Discussion
3.1. Synthesis and Characterization of AT2
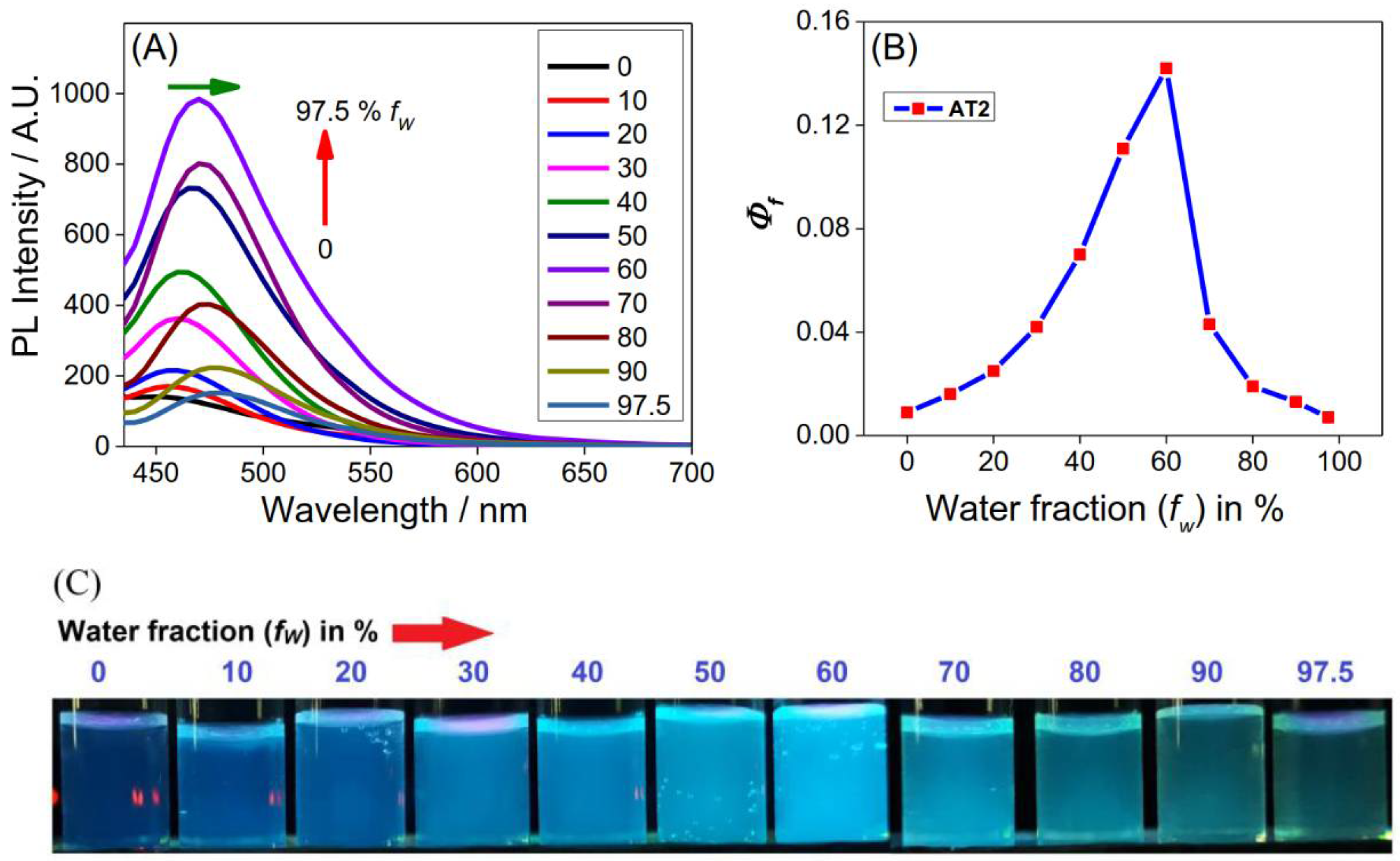
3.2. AIEE of AT2
3.3. Optimum Conditions for AT2-Based Sensors
3.4. Primary Sensory Results of AT2
3.5. Interference Studies and Detection Limits
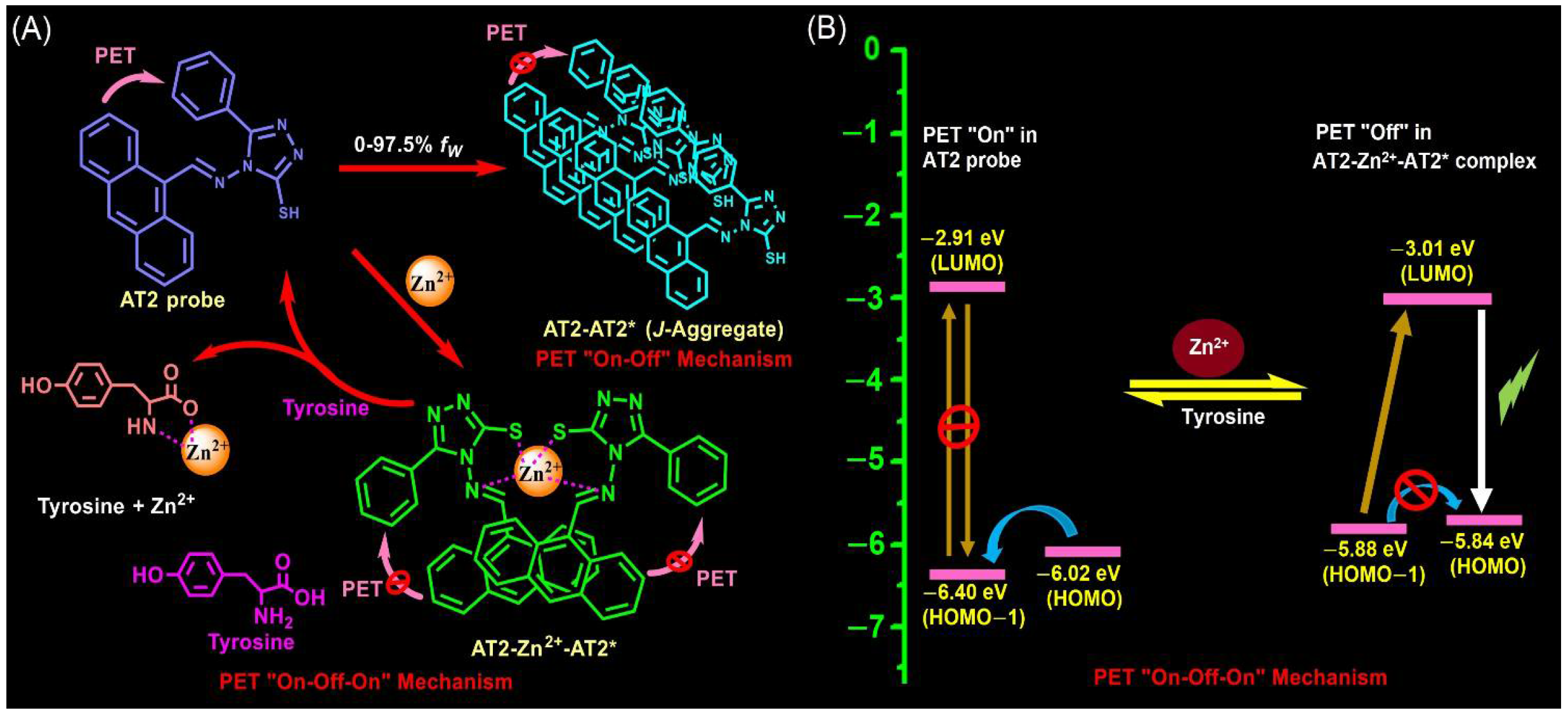
3.6. TRPL, pH Effect and Stoichiometry
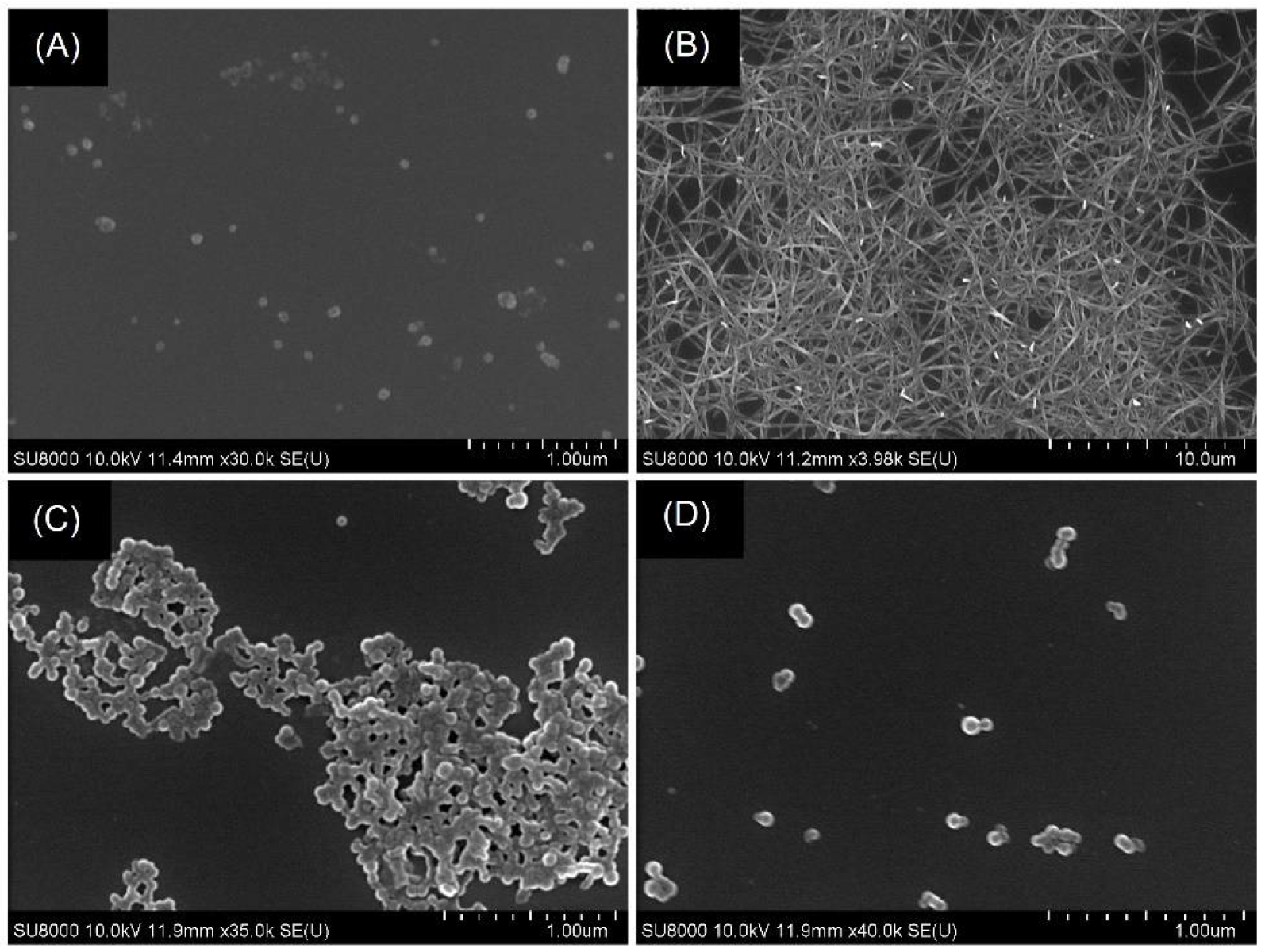
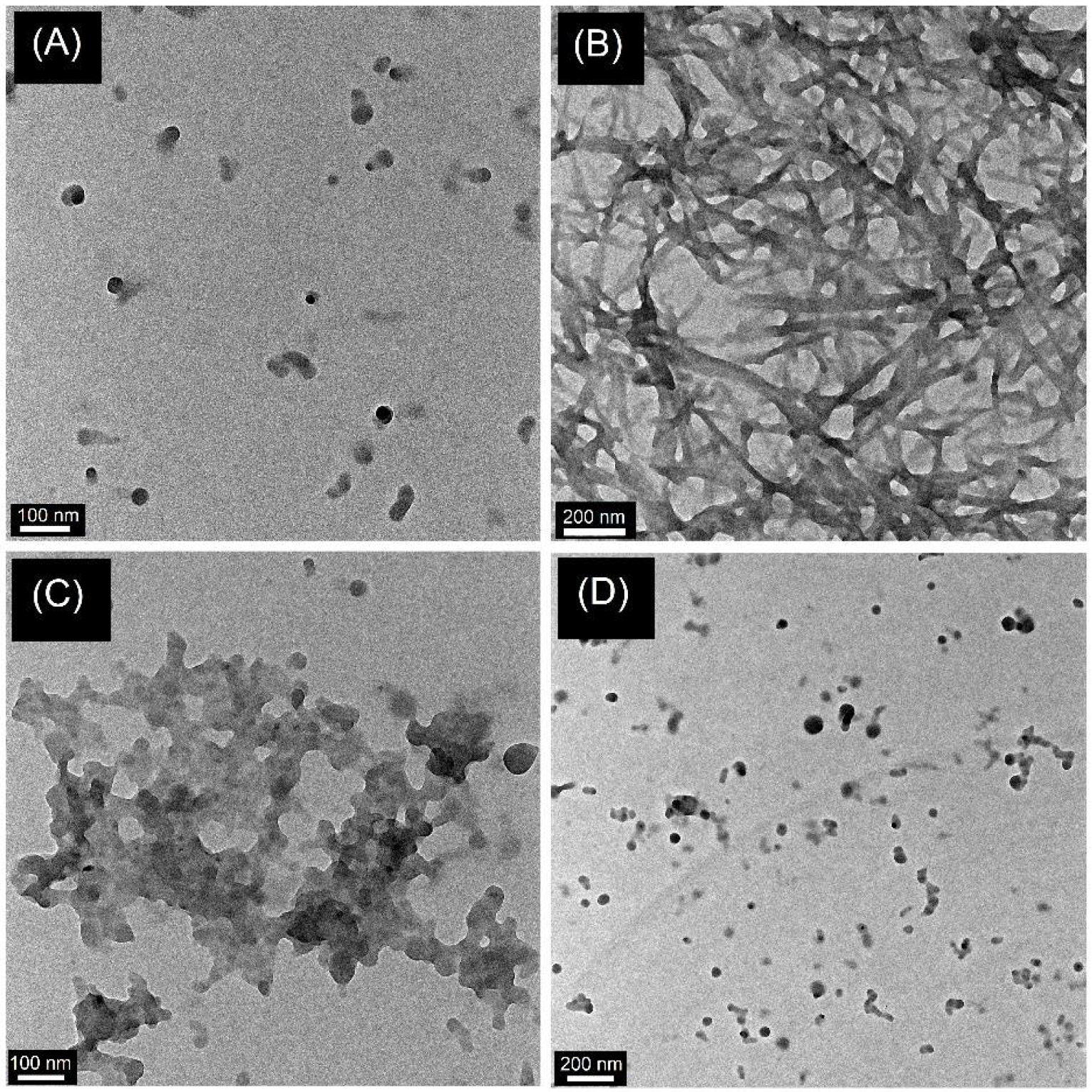
3.7. Association Constants and Nano-Morphological Interrogations
3.8. 1H-NMR Titrations and DFT Optimizations on Sensors
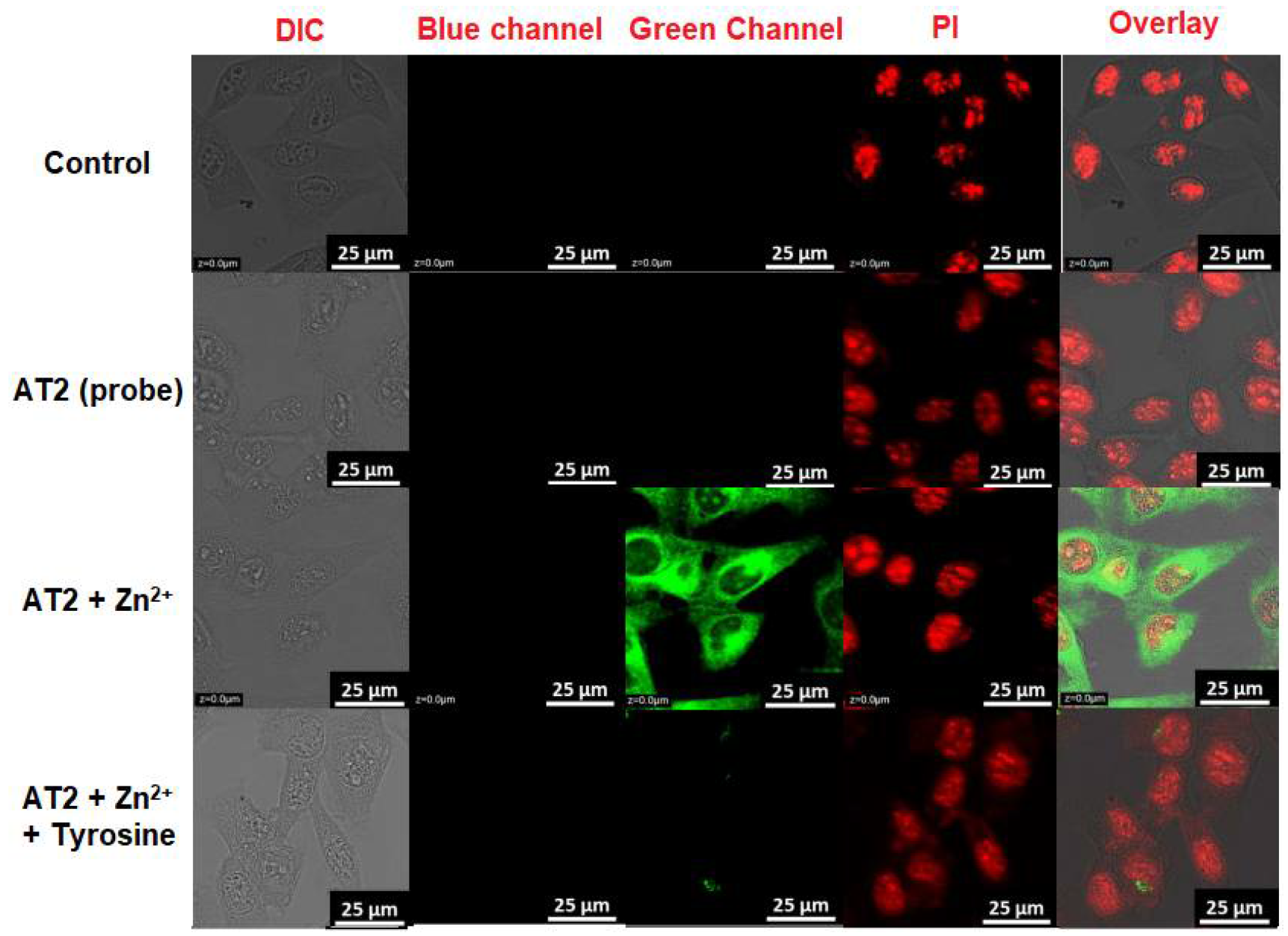
3.9. MTT Assay and Bioimaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harathi, J.; Thenmozhi, K. AIE-Active Schiff Base Compounds as Fluorescent Probes for the Highly Sensitive and Selective Detection of Fe3+ Ions. Mater. Chem. Front. 2020, 4, 1471–1482. [Google Scholar] [CrossRef]
- Jia, J.; Zhao, H. A Multi-Responsive AIE-Active Tetraphenylethylene-Functioned Salicylaldehyde-Based Schiff Base for Reversible Mechanofluorochromism and Zn2+ and CO32− Detection. Org. Electron. 2019, 73, 55–61. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Srinivasadesikan, V.; Lin, C.-M.; Sun, K.W.; Ko, F.-H.; Lin, M.-C.; Lin, H.-C. Novel Pyrene Containing Monomeric and Dimeric Supramolecular AIEE Active Nano-Probes Utilized in Selective “Off–On” Trivalent Metal and Highly Acidic pH Sensing with Live Cell Applications. J. Mater. Chem. C 2016, 4, 2056–2071. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.-W. Pyrene-Based AIE Active Materials for Bioimaging and Theranostics Applications. Biosensors 2022, 12, 550. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Lu, L.; Song, W.; Wang, X.; Sun, T.; Zhu, J.; Wang, J. AIE-Active Schiff Base Compounds as Fluorescent Probe for the Highly Sensitive and Selective Detection of Al3+ Ions. J. Lumin. 2021, 233, 117911. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, D.-X.; Zhu, L.-N.; Lan, Y.-L.; Cheng, M.; Zhang, L.-M.; Chu, J.-Q.; Li, X.-Z.; Kong, D.-M. Dinuclear HgII Tetracarbene Complex-Triggered Aggregation-Induced Emission for Rapid and Selective Sensing of Hg2+ and Organomercury Species. Chem. Sci. 2019, 10, 4220–4226. [Google Scholar] [CrossRef]
- Neupane, L.N.; Oh, E.-T.; Park, H.J.; Lee, K.-H. Selective and Sensitive Detection of Heavy Metal Ions in 100% Aqueous Solution and Cells with a Fluorescence Chemosensor Based on Peptide Using Aggregation-Induced Emission. Anal. Chem. 2016, 88, 3333–3340. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.-W.; Kang, Y.; Wu, M.; Liang, Q.-F.; Zheng, J.-Q.; Zheng, X.-J.; Jin, L.-P. ESIPT-AIE Active Schiff Base Based On 2-(2′-hydroxyphenyl)benzo-thiazole Applied as Multi-Functional Fluorescent Chemosensors. Dalton Trans. 2021, 50, 3916–3922. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, S.-J.; Cao, W.; Wang, Z.; Ma, Y. ESIPT-Active 8-Hydroxyquinoline-Based Fluorescence Sensor for Zn(II) Detection and Aggregation-Induced Emission of the Zn(II) Complex. ACS Omega 2022, 7, 18017–18026. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.; Shellaiah, M.; Srinivasadesikan, V.; Lin, C.-C.; Ko, F.-H.; Sun, K.W.; Lin, M.-C. Novel Anthracene- and Pyridine-Containing Schiff Base Probe for Selective “Off–On” Fluorescent Determination of Cu2+ Ions Towards Live Cell Application. New J. Chem. 2016, 40, 6101–6108. [Google Scholar] [CrossRef]
- Li, Y.; Zhong, H.; Huang, Y.; Zhao, R. Recent Advances in AIEgens for Metal Ion Biosensing and Bioimaging. Molecules 2019, 24, 4593. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, Y.; Nie, J.; Wei, J.; Miao, B.; Zhao, Y.; Zhang, L.; Ni, Z. Multifunctional AIE-ESIPT Dual Mechanism Tetraphenylethene-Based Schiff Base for Inkless Rewritable Paper and A Colorimetric/Fluorescent Dual-Channel Zn2+ Sensor. Mater. Chem. Front. 2021, 5, 347–354. [Google Scholar] [CrossRef]
- Han, T.; Gu, X.; Lam, J.W.Y.; Leung, A.C.S.; Kwok, R.T.K.; Han, T.; Tong, B.; Shi, J.; Dong, Y.; Tang, B.Z. Diaminomaleonitrile-Based Schiff Bases: Aggregation-Enhanced Emission, Red Fluorescence, Mechanochromism and Bioimaging Applications. J. Mater. Chem. C 2016, 4, 10430–10434. [Google Scholar] [CrossRef]
- Rullyani, C.; Shellaiah, M.; Ramesh, M.; Lin, H.-C.; Chu, C.-W. Pyrene-SH Functionalized OTFT for Detection of Hg2+ Ions in Aquatic Environments. Org. Electron. 2019, 69, 275–280. [Google Scholar] [CrossRef]
- Shellaiah, M.; Chen, Y.-T.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. Pyrene-Based AIEE Active Nanoprobe for Zn2+ and Tyrosine Detection Demonstrated by DFT, Bioimaging, and Organic Thin-Film Transistor. ACS Appl. Mater. Interfaces 2021, 13, 28610–28626. [Google Scholar] [CrossRef] [PubMed]
- Okazawa, Y.; Kondo, K.; Akita, M.; Yoshizawa, M. Polyaromatic Nanocapsules Displaying Aggregation-Induced Enhanced Emissions in Water. J. Am. Chem. Soc. 2015, 137, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, J.; Li, P.; Tang, B.; James, T.D. Two-Photon Small-Molecule Fluorescence-Based Agents for Sensing, Imaging, and Therapy within Biological Systems. Chem. Soc. Rev. 2021, 50, 702–734. [Google Scholar] [CrossRef]
- Bresolí-Obach, R.; Nos, J.; Mora, M.; Sagristà, M.L.; Ruiz-González, R.; Nonell, S. Anthracene-Based Fluorescent Nanoprobes for Singlet Oxygen Detection in Biological Media. Methods 2016, 109, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ayyavoo, K.; Velusamy, P. Pyrene Based Materials as Fluorescent Probes in Chemical and Biological Fields. New J. Chem. 2021, 45, 10997–11017. [Google Scholar] [CrossRef]
- Wu, X.; Mao, Y.; Wang, D.; Huang, Q.; Yin, Q.; Zheng, M.; Hu, Q.; Wang, H. Designing A Colorimetric Sensor Containing Nitrogen and Oxygen Atoms for Uranyl Ions Identification: Chromatic Mechanism, Binding Feature and on-Site Application. Sens. Actuators B 2020, 307, 127681. [Google Scholar] [CrossRef]
- Shellaiah, M.; Wu, Y.-H.; Lin, H.-C. Simple Pyridyl-Salicylimine-Based Fluorescence “Turn-On” Sensors for Distinct Detections of Zn2+, Al3+ and OH− Ions in Mixed Aqueous Media. Analyst 2013, 138, 2931–2942. [Google Scholar] [CrossRef]
- Uzun, K.; Sayın, S.; Tamer, Ö.; Çevik, U. Comparison of Charge Transport and Opto-Electronic Properties of Pyrene and Anthracene Derivatives for OLED Applications. J. Mol. Model. 2021, 27, 174. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, D.; Antony, B.; Tudorovskaya, M.; Tennyson, J. Electron Scattering Cross Sections for Anthracene and Pyrene. J. Phys. Chem. A 2020, 124, 7088–7100. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Wu, Y.-H.; Singh, A.; Ramakrishnam Raju, M.V.; Lin, H.-C. Novel Pyrene- and Anthracene-Based Schiff Base Derivatives as Cu2+ and Fe3+ Fluorescence Turn-on Sensors and for Aggregation Induced Emissions. J. Mater. Chem. A 2013, 1, 1310–1318. [Google Scholar] [CrossRef]
- Wei, X.; Liu, J.; Xia, G.-J.; Deng, J.; Sun, P.; Chruma, J.J.; Wu, W.; Yang, C.; Wang, Y.-G.; Huang, Z. Enantioselective Photoinduced Cyclodimerization of A Prochiral Anthracene Derivative Adsorbed on Helical Metal Nanostructures. Nature Chem. 2020, 12, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Song, J.; Li, M.; Yang, Y.; Gu, W.; Xu, X.; Xu, H.; Wang, S. A Highly Specific and Sensitive Turn-On Fluorescence Probe for Hypochlorite Detection Based on Anthracene Fluorophore and Its Bioimaging Applications. Dyes Pig. 2019, 161, 172–181. [Google Scholar] [CrossRef]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- Hara, T.; Takeda, T.-a.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological Roles of Zinc Transporters: Molecular and Genetic Importance in Zinc Homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Biological Inorganic Chemistry of Zinc Ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Jurowski, K.; Szewczyk, B.; Nowak, G.; Piekoszewski, W. Biological Consequences of Zinc Deficiency in the Pathomechanisms of Selected Diseases. J. Biol. Inorg. Chem. 2014, 19, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Liu, M.-Y.; Zhong, X.; Wei, M.-J. Decreased Circulating Zinc Levels in Parkinson’s Disease: A Meta-Analysis Study. Sci. Rep. 2017, 7, 3902. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, C. Development of A Method for the Detection of Zinc in Brassica Oleracea using Solid Phase Extraction and Size-Exclusion Chromatography Inductively Coupled Plasma Mass Spectrometry (SEC-ICP-MS). MethodsX 2021, 8, 101428. [Google Scholar] [CrossRef] [PubMed]
- Sharanov, P.Y.; Volkov, D.S.; Alov, N.V. Quantification of Elements in Copper–Zinc Ores at Micro- and Macro-Levels by Total Reflection X-Ray Fluorescence and Inductively Coupled Plasma Atomic Emission Spectrometry. Anal. Methods 2019, 11, 3750–3756. [Google Scholar] [CrossRef]
- Rajabi, M.; Asemipour, S.; Barfi, B.; Jamali, M.R.; Behzad, M. Ultrasound-Assisted Ionic Liquid Based Dispersive Liquid–Liquid Microextraction and Flame Atomic Absorption Spectrometry of Cobalt, Copper, and Zinc in Environmental Water Samples. J. Mol. Liq. 2014, 194, 166–171. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Diamond-Based Electrodes for Detection of Metal Ions and Anions. Nanomaterials 2022, 12, 64. [Google Scholar] [CrossRef]
- Lu, J.; Qin, Y.; Wu, Y.; Meng, M.; Yan, Y.; Li, C. Recent Advances in Ion-Imprinted Membranes: Separation and Detection Via Ion-Selective Recognition. Environ. Sci. Water Res. Technol. 2019, 5, 1626–1653. [Google Scholar] [CrossRef]
- Xu, Z.; Yoon, J.; Spring, D.R. Fluorescent Chemosensors for Zn2+. Chem. Soc. Rev. 2010, 39, 1996–2006. [Google Scholar] [CrossRef]
- Liu, P.; Han, X.-F.; Wu, W.-N.; Wang, Y.; Fan, Y.-C.; Zhao, X.-L.; Xu, Z.-H. A Water Soluble Hydrazone Probe for Subsequent Fluorescent Detection of Zn2+ and S2− in Neat Aqueous Solution and Imaging in Mitochondria of Living Cells. J. Mol. Struct. 2022, 1249, 131629. [Google Scholar] [CrossRef]
- Koide, S.; Sidhu, S.S. The Importance of Being Tyrosine: Lessons in Molecular Recognition from Minimalist Synthetic Binding Proteins. ACS Chem. Biol. 2009, 4, 325–334. [Google Scholar] [CrossRef]
- Boni, C.; Laudanna, C.; Sorio, C. A Comprehensive Review of Receptor-Type Tyrosine-Protein Phosphatase Gamma (PTPRG) Role in Health and Non-Neoplastic Disease. Biomolecules 2022, 12, 84. [Google Scholar] [CrossRef]
- Dinu, A.; Apetrei, C. A Review of Sensors and Biosensors Modified with Conducting Polymers and Molecularly Imprinted Polymers Used in Electrochemical Detection of Amino Acids: Phenylalanine, Tyrosine, and Tryptophan. Int. J. Mol. Sci. 2022, 23, 1218. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.K.; Upadhyay, R.K.; Kumar, D.; Bano, D.; Chandra, S.; Jit, S.; Hasan, S.H. Synthesis of Green Fluorescent Carbon Quantum Dots from the Latex of Ficus Benghalensis for the Detection of Tyrosine and Fabrication of Schottky Barrier Diode. New J. Chem. 2021, 45, 12549–12556. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Yeh, Y.-C. Detection of Tyrosine and Monitoring Tyrosinase Activity using An Enzyme Cascade-Triggered Colorimetric Reaction. RSC Adv. 2020, 10, 29745–29750. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.-Q.; Liu, G.; Ma, G.; Nan, Z.; Wang, W. Phosphodiesters Quaternary Ammonium Nanoparticles as Label-Free Light Scattering Probe for Turn-Off Detection of Tyrosine. Spectrochim. Acta A 2019, 208, 1–6. [Google Scholar] [CrossRef]
- Bellomo, E.; Abro, A.; Hogstrand, C.; Maret, W.; Domene, C. Role of Zinc and Magnesium Ions in the Modulation of Phosphoryl Transfer in Protein Tyrosine Phosphatase 1B. J. Am. Chem. Soc. 2018, 140, 4446–4454. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Wang, X.-C.; Liu, R. Zinc in Regulating Protein Kinases and Phosphatases in Neurodegenerative Diseases. Biomolecules 2022, 12, 785. [Google Scholar] [CrossRef]
- Bellomo, E.; Massarotti, A.; Hogstrand, C.; Maret, W. Zinc Ions Modulate Protein Tyrosine Phosphatase 1B Activity. Metallomics 2014, 6, 1229–1239. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Venkatesan, P.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Modified Diamond Nanoparticles Applied in Cellular Imaging and Hg2+ Ions Detection. Appl. Surf. Sci. 2019, 465, 340–350. [Google Scholar] [CrossRef]
- Shellaiah, M.; Awasthi, K.; Chandran, S.; Aazaad, B.; Sun, K.W.; Ohta, N.; Wu, S.-P.; Lin, M.-C. Methylammonium Tin Tribromide Quantum Dots for Heavy Metal Ion Detection and Cellular Imaging. ACS Appl. Nano Mater. 2022, 5, 2859–2874. [Google Scholar] [CrossRef]
- Awasthi, K.; Chang, F.-L.; Hsieh, P.-Y.; Hsu, H.-Y.; Ohta, N. Characterization of Endogenous Fluorescence in Nonsmall Lung Cancerous Cells: A Comparison with Nonmalignant Lung Normal Cells. J. Biophoton. 2020, 13, e201960210. [Google Scholar] [CrossRef] [PubMed]
- Shellaiah, M.; Chen, T.H.; Simon, T.; Li, L.-C.; Sun, K.W.; Ko, F.-H. An Affordable Wet Chemical Route to Grow Conducting Hybrid Graphite-Diamond Nanowires: Demonstration by A Single Nanowire Device. Sci. Rep. 2017, 7, 11243. [Google Scholar] [CrossRef]
- Shellaiah, M.; Thirumalaivasan, N.; Sun, K.W.; Wu, S.-P. A pH Cooperative Strategy for Enhanced Colorimetric Sensing of Cr(III) Ions using Biocompatible L-Glutamic Acid Stabilized Gold Nanoparticles. Microchem. J. 2021, 160, 105754. [Google Scholar] [CrossRef]
- Shellaiah, M.; Rajan, Y.C.; Balu, P.; Murugan, A. A Pyrene Based Schiff Base Probe for Selective Fluorescence Turn-On Detection of Hg2+ Ions with Live Cell application. New J. Chem. 2015, 39, 2523–2531. [Google Scholar] [CrossRef]
- Shellaiah, M.; Sun, K.W. Review on Sensing Applications of Perovskite Nanomaterials. Chemosensors 2020, 8, 55. [Google Scholar] [CrossRef]
- Shellaiah, M.; Simon, T.; Thirumalaivasan, N.; Sun, K.W.; Ko, F.-H.; Wu, S.-P. Cysteamine-Capped Gold-Copper Nanoclusters for Fluorometric Determination and Imaging of Chromium(VI) and Dopamine. Microchim. Acta 2019, 186, 788. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.-F.; Zeng, C.; Hu, L.; Liang, X.-J. Ultrasensitive Tyrosinase-Activated Turn-On Near-Infrared Fluorescent Probe with a Rationally Designed Urea Bond for Selective Imaging and Photodamage to Melanoma Cells. Anal. Chem. 2018, 90, 3666–3669. [Google Scholar] [CrossRef]
- Jung, E.; Shim, I.; An, J.; Ji, M.S.; Jangili, P.; Chi, S.-G.; Kim, J.S. Phenylthiourea-Conjugated BODIPY as an Efficient Photosensitizer for Tyrosinase-Positive Melanoma-Targeted Photodynamic Therapy. ACS Appl. Bio Mater. 2021, 4, 2120–2127. [Google Scholar] [CrossRef]
- Savastano, M.; Fiaschi, M.; Ferraro, G.; Gratteri, P.; Mariani, P.; Bianchi, A.; Bazzicalupi, C. Sensing Zn2+ in Aqueous Solution with a Fluorescent Scorpiand Macrocyclic Ligand Decorated with an Anthra-cene Bearing Tail. Molecules 2020, 25, 1355. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Noh, J.Y.; Hwang, I.H.; Kang, J.; Kim, J.; Kim, C. An Anthracene-Based Fluorescent Chemosensor for Zn2+. Tetrahed. Lett. 2013, 54, 2415–2418. [Google Scholar] [CrossRef]
- Nguyen, M.-H.; Nguyen, T.-N.; Do, D.-Q.; Nguyen, H.-H.; Phung, Q.-M.; Thirumalaivasan, N.; Wu, S.-P.; Dinh, T.-H. A Highly Selective Fluorescent Anthracene-Based Chemosensor for Imaging Zn2+ in Liv-ing Cells and Zebrafish. Inorg. Chem. Commun. 2020, 115, 107882. [Google Scholar] [CrossRef]
- Ghosh Dastidar, D.; Mukherjee, P.; Ghosh, D.; Banerjee, D. Carbon Quantum Dots Prepared from Onion Extract as Fluorescence Turn-On Probes for Selective Estimation of Zn2+ in Blood Plasma. Colloids Surf. A 2021, 611, 125781. [Google Scholar] [CrossRef]
- Wang, B.; Liang, Z.; Tan, H.; Duan, W.; Luo, M. Red-Emission Carbon Dots-Quercetin Systems as Ratiometric Fluorescent Nanoprobes Towards Zn2+ and Adenosine Triphosphate. Microchim. Acta 2020, 187, 345. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ma, Y.; Duan, H.; Jiang, F.; Deng, W.; Ren, X. Fluorescent/SERS Dual-Sensing and Imaging of Intracellular Zn2+. Anal. Chim. Acta 2018, 1038, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Ly, N.H.; Kim, J.S.; Jung, H.S.; Joo, S.-W. A Selective Triarylmethine-Based Spectroscopic Probe for Zn2+ Ion Monitoring. Dyes Pig. 2019, 171, 107721. [Google Scholar] [CrossRef]
- Fu, J.; Yao, K.; Li, B.; Mei, H.; Chang, Y.; Xu, K. Coumarin-Based Colorimetric-Fluorescent Sensors for the Sequential Detection of Zn2+ Ion and Phosphate Anions and Applications in Cell Imaging. Spectrochim. Acta A 2020, 228, 117790. [Google Scholar] [CrossRef]
- Dervin, S.; Ganguly, P.; Dahiya, R.S. Disposable Electrochemical Sensor Using Graphene Oxide–Chitosan Modified Carbon-Based Electrodes for the Detection of Tyrosine. IEEE Sensors J. 2021, 21, 26226–26233. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Z.; Zhang, L.; Tian, Y. Electrochemical Detection of Tyrosinase in Cell Lysates at Functionalized Nanochannels via Amplifying of Ionic Current Response. Electroanalysis 2022, 34, 1021–1026. [Google Scholar] [CrossRef]
- Li, Y.; Cai, N.; Wang, M.; Na, W.; Shi, F.; Su, X. Fluorometric Detection of Tyrosine and Cysteine Using Graphene Quantum dots. RSC Adv. 2016, 6, 33197–33204. [Google Scholar] [CrossRef]
- Zou, H.-Y.; Lu, X.-Y.; Kong, F.-Y.; Wang, Z.-X.; Li, H.-Y.; Fang, H.-L.; Wang, W. A Voltammetric Sensor Based on Reduced Graphene Oxide-Hemin-Ag Nanocomposites for Sensitive Determination of Tyrosine. RSC Adv. 2020, 10, 28026–28031. [Google Scholar] [CrossRef] [PubMed]




| Composition | HOMO (eV) | LUMO (eV) | Band Gap (eV) | λabs (nm) | λem (nm) | Φf | τ (ns) |
|---|---|---|---|---|---|---|---|
| Probe AT2 | −6.02 | −2.91 | 3.11 | 396 | 456 | 0.008 a | 0.1001 |
| AT2@60% fW | ND | ND | ND | 407 | 470 | 0.142 a | 0.2021 |
| AT2 + Zn2+ | −5.84 b | −3.01 b | 2.83 b | 401 | 520 | 0.103 a | 0.2329 |
| −5.69 c | −2.64 c | 3.05c | |||||
| AT2 + Zn2+ + Tyr | ND | ND | ND | 396 | 520 | 0.011 a | 0.1003 |
| Tyr + Zn2+ | −5.47 d | −2.70 d | 2.77d | ND | ND | ND | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shellaiah, M.; Thirumalaivasan, N.; Aazaad, B.; Awasthi, K.; Sun, K.W.; Wu, S.-P.; Lin, M.-C.; Ohta, N. An AIEE Active Anthracene-Based Nanoprobe for Zn2+ and Tyrosine Detection Validated by Bioimaging Studies. Chemosensors 2022, 10, 381. https://doi.org/10.3390/chemosensors10100381
Shellaiah M, Thirumalaivasan N, Aazaad B, Awasthi K, Sun KW, Wu S-P, Lin M-C, Ohta N. An AIEE Active Anthracene-Based Nanoprobe for Zn2+ and Tyrosine Detection Validated by Bioimaging Studies. Chemosensors. 2022; 10(10):381. https://doi.org/10.3390/chemosensors10100381
Chicago/Turabian StyleShellaiah, Muthaiah, Natesan Thirumalaivasan, Basheer Aazaad, Kamlesh Awasthi, Kien Wen Sun, Shu-Pao Wu, Ming-Chang Lin, and Nobuhiro Ohta. 2022. "An AIEE Active Anthracene-Based Nanoprobe for Zn2+ and Tyrosine Detection Validated by Bioimaging Studies" Chemosensors 10, no. 10: 381. https://doi.org/10.3390/chemosensors10100381
APA StyleShellaiah, M., Thirumalaivasan, N., Aazaad, B., Awasthi, K., Sun, K. W., Wu, S.-P., Lin, M.-C., & Ohta, N. (2022). An AIEE Active Anthracene-Based Nanoprobe for Zn2+ and Tyrosine Detection Validated by Bioimaging Studies. Chemosensors, 10(10), 381. https://doi.org/10.3390/chemosensors10100381








