Clinical Findings among Patients with Respiratory Symptoms Related to Moisture Damage Exposure at the Workplace—The SAMDAW Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
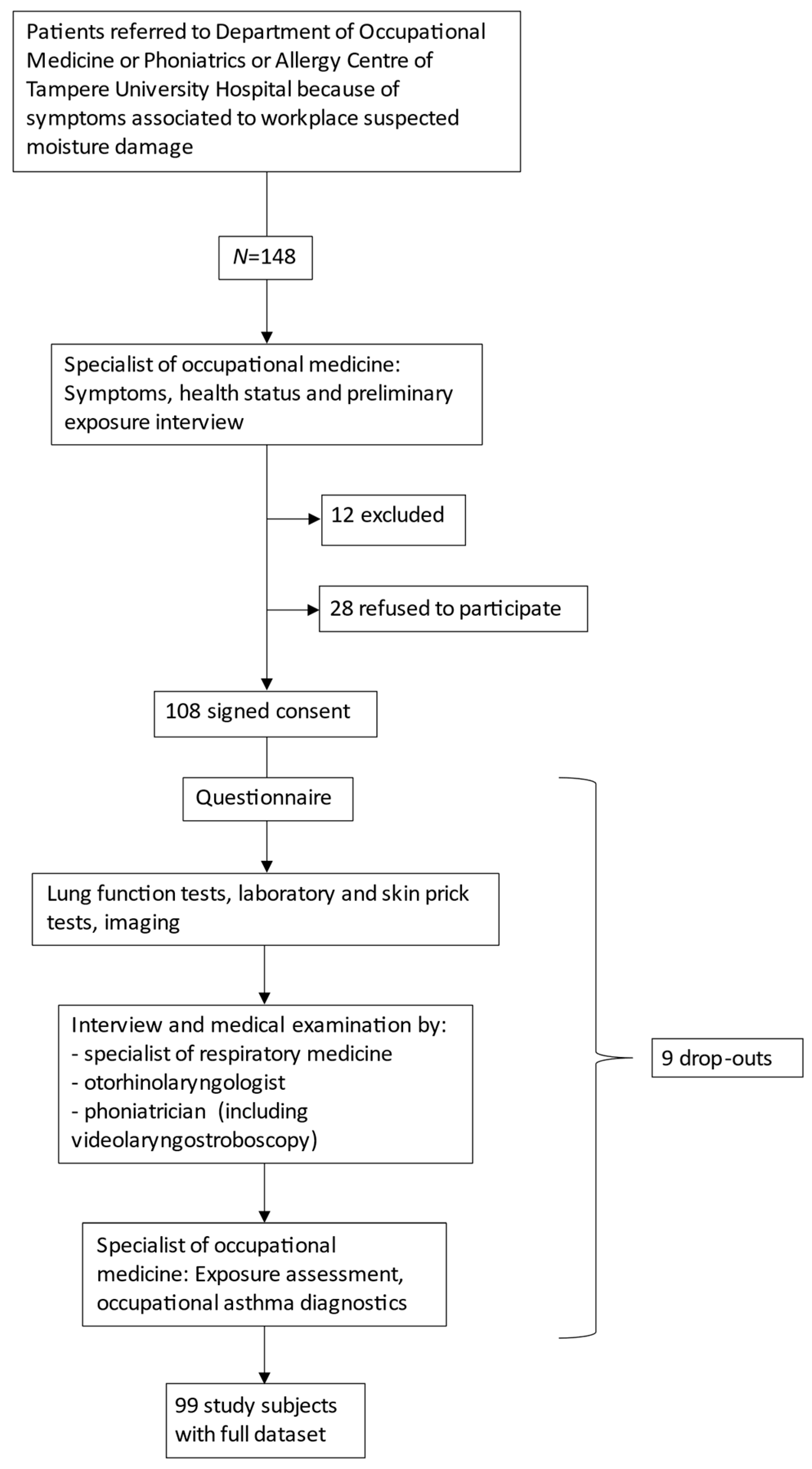
3.1. Study Patients
3.2. Clinical Findings of Respiratory Medicine Specialists
3.3. Clinical Findings of Otorhinolaryngology Specialist
3.4. Clinical Findings of Phoniatrician
3.5. Results of Allergy Tests
3.6. Non-Participant Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Guidelines for Indoor Air Quality: Dampness and Mould; WHO Regional Office for Europe: Copenhagen, Denmark, 2009; p. 228. Available online: http://www.euro.who.int/__data/assets/pdf_file/0017/43325/E92645.pdf (accessed on 14 June 2014).
- Hurraß, J.; Heinzow, B.; Aurbach, U.; Bergmann, K.-C.; Bufe, A.; Buzina, W.; Cornely, O.A.; Engelhart, S.; Fischer, G.; Gabrio, T.; et al. Medical diagnostics for indoor mold exposure. Int. J. Hyg. Environ. Health 2017, 220, 305–328. [Google Scholar] [CrossRef]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: A review of the epidemiologic evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Pekkanen, J.; Hyvärinen, A.; Haverinen-Shaughnessy, U.; Korppi, M.; Putus, T.; Nevalainen, A. Moisture damage and childhood asthma: A population-based incident case-control study. Eur. Respir. J. 2007, 29, 509–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karvonen, A.M.; Hyvarinen, A.; Korppi, M.; Haverinen-Shaughnessy, U.; Renz, H.; Pfefferle, P.I.; Remes, S.; Genuneit, J.; Pekkanen, J. Moisture Damage and Asthma: A Birth Cohort Study. Pediatrics 2015, 135, e598–e606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borràs-Santos, A.; Jacobs, J.H.; Täubel, M.; Haverinen-Shaughnessy, U.; Krop, E.J.; Huttunen, K.; Hirvonen, M.-R.; Pekkanen, J.; Heederik, D.J.J.; Zock, J.-P.; et al. Dampness and mould in schools and respiratory symptoms in children: The HITEA study. Occup. Environ. Med. 2013, 70, 681–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingham, T.; Keall, M.; Jones, B.; Aldridge, D.R.T.; Dowell, A.C.; Davies, C.; Crane, J.; Draper, J.B.; Bailey, L.O.; Viggers, H.; et al. Damp mouldy housing and early childhood hospital admissions for acute respiratory infection: A case control study. Thorax 2019, 74, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisk, W.J.; Chan, W.R.; Johnson, A.L. Does dampness and mold in schools affect health? Results of a meta-analysis. Indoor Air 2019, 29, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Karvala, K.; Toskala, E.; Luukkonen, R.; Uitti, J.; Lappalainen, S.; Nordman, H. Prolonged exposure to damp and moldy workplaces and new-onset asthma. Int. Arch. Occup. Environ. Health 2011, 84, 713–721. [Google Scholar] [CrossRef]
- Caillaud, D.; Leynaert, B.; Keirsbulck, M.; Nadif, R. Mould ANSES Working Group. Indoor mould exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies. Eur. Respir. Rev. 2018, 27, 170137. [Google Scholar] [CrossRef] [Green Version]
- Graff, P.; Bryngelsson, I.-L.; Fredrikson, M.; Flodin, U. Adult onset asthma in non-allergic women working in dampness damaged buildings: A retrospective cohort study. Am. J. Ind. Med. 2019, 62, 357–363. [Google Scholar] [CrossRef]
- Kurth, L.; Virji, M.A.; Storey, E.; Framberg, S.; Kallio, C.; Fink, J.; Laney, A.S. Current asthma and asthma-like symptoms among workers at a Veterans Administration Medical Center. Int. J. Hyg. Environ. Health 2017, 220, 1325–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karvala, K.; Nordman, H.; Luukkonen, R.; Nykyri, E.; Lappalainen, S.; Hannu, T.; Toskala, E. Occupational rhinitis in damp and moldy workplaces. Am. J. Rhinol. 2008, 22, 457–462. [Google Scholar] [CrossRef]
- Wang, J.; Pindus, M.; Janson, C.; Sigsgaard, T.; Kim, J.-L.; Holm, M.; Sommar, J.; Orru, H.; Gislason, T.; Johannessen, A.; et al. Dampness, mould, onset and remission of adult respiratory symptoms, asthma and rhinitis. Eur. Respir. J. 2019, 53, 1801921. [Google Scholar] [CrossRef] [PubMed]
- Kielb, C.; Lin, S.; Muscatiello, N.; Hord, W.; Rogers-Harrington, J.; Healy, J. Building-related health symptoms and classroom indoor air quality: A survey of school teachers in New York State. Indoor Air 2015, 25, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.S.; Victor, J.C.; Guan, J.; Aaron, S.D.; To, T. Pulmonary function testing in the diagnosis of asthma: A population study. Chest 2012, 141, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Aaron, S.D.; Vandemheen, K.L.; FitzGerald, J.M.; Ainslie, M.; Gupta, S.; Lemière, C.; Field, S.K.; Mclvor, A.; Hernandez, P.; Mayers, I.; et al. Reevaluation of Diagnosis in Adults With Physician-Diagnosed Asthma. JAMA 2017, 317, 269. [Google Scholar] [CrossRef]
- Cox-Ganser, J.M.; White, S.K.; Jones, R.; Hilsbos, K.; Storey, E.; Enright, P.L.; Rao, C.Y.; Kreiss, K. Respiratory Morbidity in Office Workers in a Water-Damaged Building. Environ. Health Perspect. 2005, 113, 485–490. [Google Scholar] [CrossRef] [Green Version]
- White, S.K.; Cox-Ganser, J.M.; Benaise, L.G.; Kreiss, K. Work-related peak flow and asthma symptoms in a damp building. Occup. Med. 2013, 63, 287–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Famokunwa, B.; Walsted, E.S.; Hull, J.H. Assessing laryngeal function and hypersensitivity. Pulm. Pharmacol. Ther. 2019, 56, 108–115. [Google Scholar] [CrossRef]
- Perkner, J.J.; Fennelly, K.P.; Balkissoon, R.; Bartelson, B.B.; Ruttenber, A.J.; Wood, R.P.; Newman, L. Irritant-Associated Vocal Cord Dysfunction. J. Occup. Environ. Med. 1998, 40, 136–143. [Google Scholar] [CrossRef]
- Hoy, R.F.; Ribeiro, M.; Anderson, J.; Tarlo, S.M. Work-associated irritable larynx syndrome. Occup. Med. 2010, 60, 546–551. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.A. Work-associated irritable larynx syndrome. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 150–155. [Google Scholar] [CrossRef]
- Cummings, K.J.; Fink, J.N.; Vasudev, M.; Piacitelli, C.; Kreiss, K. Vocal Cord Dysfunction Related to Water-Damaged Buildings. J. Allergy Clin. Immunol. Pract. 2013, 1, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, J.; Karvala, K.; Kauppi, P.; Komulainen, J.; Lampi, J.; Lampi, P. Current care guideline: Patient exposed to moisture damage. Duodecim 2016, 133, 513–514. [Google Scholar]
- Lampi, J.; Hyvärinen, A.; Erhola, M.; Haahtela, T.; Haukipuro, K.; Haverinen-Shaughnessy, U.; Jalkanen, K.; Karvala, K.; Lappalainen, S.; Reijula, K.; et al. Healthy people in healthy premises: The Finnish Indoor Air and Health Programme 2018–2028. Clin. Transl. Allergy 2020, 10, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nynäs, P.; Vilpas, S.; Kankare, E.; Karjalainen, J.; Lehtimäki, L.; Numminen, J.; Tikkakoski, A.; Kleemola, L.; Uitti, J. Observational cross-sectional study on Symptoms Associated to Moisture DAmage at Workplace: The SAMDAW study protocol. BMJ Open 2019, 9, e026485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology 2012, 23, 1–298. [Google Scholar]
- Lund, V.J.; Mackay, I.S. Staging in rhinosinusitus. Rhinology 1993, 31, 183–184. [Google Scholar]
- Morris, M.J.; Christopher, K.L. Diagnostic criteria for the classification of vocal cord dysfunction. Chest 2010, 138, 1213–1223. [Google Scholar] [CrossRef]
- Belafsky, P.C.; Postma, G.N.; Reulbach, T.R.; Holland, B.W.; Koufman, J.A. Muscle tension dysphonia as a sign of underlying glottal insufficiency. Otolaryngol. Head Neck Surg. 2002, 127, 448–451. [Google Scholar] [CrossRef]
- Christensen, P.M.; Heimdal, J.-H.; Christopher, K.L.; Bucca, C.; Cantarella, G.; Friedrich, G.; Halvorsen, T.; Herth, F.; Jung, H.; Morris, M.J.; et al. ERS/ELS/ACCP 2013 international consensus conference nomenclature on inducible laryngeal obstructions. Eur. Respir. Rev. 2015, 24, 445–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzerling, L.; Mari, A.; Bergmann, K.-C.; Bresciani, M.; Burbach, G.; Darsow, U.; Durham, S.; Fokkens, W.; Gjomarkaj, M.; Haahtela, T.; et al. The skin prick test—European standards. Clin. Transl. Allergy 2013, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Ilmarinen, P.; Tuomisto, L.E.; Niemelä, O.; Tommola, M.; Haanpää, J.; Kankaanranta, H. Cluster Analysis on Longitudinal Data of Patients with Adult-Onset Asthma. J. Allergy Clin. Immunol. Pract. 2017, 5, 967–978.e3. [Google Scholar] [CrossRef]
- Vandenplas, O.; Wiszniewska, M.; Raulf, M.; De Blay, F.; Gerth van Wijk, R.; Moscato, G.; Nemery, B.; Pala, G.; Quirce, S.; Sastre, J.; et al. EAACI position paper: Irritant-induced asthma. Allergy 2014, 69, 1141–1153. [Google Scholar] [CrossRef] [Green Version]
- Eerikäinen, J.; Nynäs, P.; Uitti, J. Subacute hypersensitivity pneumonitis after occupational mold exposure. Duodecim 2013, 129, 972–975. [Google Scholar] [PubMed]
- Selman, M.; Pardo, A.; King, T.E. Hypersensitivity Pneumonitis. Am. J. Respir. Crit. Care Med. 2012, 186, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Ilomäki, I.; Leppänen, K.; Kleemola, L.; Tyrmi, J.; Laukkanen, A.-M.; Vilkman, E. Relationships between self-evaluations of voice and working conditions, background factors, and phoniatric findings in female teachers. Logop. Phoniatr. Vocol. 2009, 34, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; An, J.; Won, H.K.; Kang, Y.; Kwon, H.S.; Kim, T.B.; Cho, Y.S.; Moon, H.B.; Song, W.J.; Hull, J.H. Prevalence and impact of comorbid laryngeal dysfunction in asthma: A systematic review and meta-analysis. J. Allergy Clin. Immunol. 2020, 145, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.; Rammage, L.; Emami, A.J. The irritable larynx syndrome. J. Voice 1999, 13, 447–455. [Google Scholar] [CrossRef]
- Idrees, M.; FitzGerald, J.M. Vocal cord dysfunction in bronchial asthma. A review article. J. Asthma 2015, 52, 327–335. [Google Scholar] [CrossRef]
- Lyberg-Åhlander, V.; Rydell, R.; Fredlund, P.; Magnusson, C.; Wilén, S. Prevalence of Voice Disorders in the General Population, Based on the Stockholm Public Health Cohort. J. Voice 2019, 33, 900–905. [Google Scholar] [CrossRef]
- Spantideas, N.; Drosou, E.; Karatsis, A.; Assimakopoulos, D. Voice disorders in the general greek population and in patients with laryngopharyngeal reflux. Prevalence and risk factors. J. Voice 2015, 29, 389.e27–389.e32. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, A.-M.; Ilomäki, I.; Leppänen, K.; Vilkman, E. Acoustic measures and self-reports of vocal fatigue by female teachers. J. Voice 2008, 22, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Trinite, B. Epidemiology of Voice Disorders in Latvian School Teachers. J. Voice 2017, 31, 508.e1–508.e9. [Google Scholar] [CrossRef] [PubMed]
- De Brito Mota, A.F.; Giannini, S.P.P.; De Oliveira, I.B.; Paparelli, R.; Dornelas, R.; Ferreira, L.P. Voice Disorder and Burnout Syndrome in Teachers. J. Voice 2019, 33, 581.e7–581.e16. [Google Scholar] [CrossRef]
- Pallasaho, P.; Ronmark, E.; Haahtela, T.; Sovijarvi, A.R.A.; Lundback, B. Degree and clinical relevance of sensitization to common allergens among adults: A population study in Helsinki, Finland. Clin. Exp. Allergy 2006, 36, 503–509. [Google Scholar] [CrossRef]
- Rosati, M.G.; Peters, A.T. Relationships among allergic rhinitis, asthma, and chronic rhinosinusitis. Am. J. Rhinol. Allergy 2016, 30, 44. [Google Scholar] [CrossRef] [Green Version]
- Latgé, J.-P. Aspergillus fumigatus and Aspergillosis. Clin. Microbiol. Rev. 1999, 12, 310. [Google Scholar] [CrossRef] [Green Version]
- Denning, D.W.; Pashley, C.; Hartl, D.; Wardlaw, A.; Godet, C.; Del Giacco, S.; Delhaes, L.; Sergejeva, S. Fungal allergy in asthma-state of the art and research needs. Clin. Transl. Allergy 2014, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.; Corazza, F.; Chasseur, C.; Bladt, S.; Romano, M.; Huygen, K.; Denis, O.; Michel, O. Relationship between mold exposure, specific IgE sensitization, and clinical asthma. Ann. Allergy Asthma Immunol. 2018, 121, 333–339. [Google Scholar] [CrossRef]
- Sullivan, A.; Hunt, E.B.; Ward, C.; Lapthorne, S.; Eustace, J.A.; Fanning, L.J.; Plant, B.J.; O’Byrne, P.M.; MacSharry, J.A.; Murphy, D.M. The presence of Aspergillus fumigatus in asthmatic airways is not clearly related to clinical disease severity. Allergy 2019, 75, 1146–1154. [Google Scholar] [CrossRef]
- Watai, K.; Fukutomi, Y.; Hayashi, H.; Nakamura, Y.; Hamada, Y.; Tomita, Y.; Mistui, C.; Kamide, Y.; Sekiya, K.; Mori, A.; et al. De novo sensitization to Aspergillus fumigatus in adult asthma over a 10-year observation period. Allergy 2018, 73, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, M.S.; Ieromnimon, A.; Jaakkola, J.J.K. Are atopy and specific IgE to mites and molds important for adult asthma? J. Allergy Clin. Immunol. 2006, 117, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, J.; Hulkkonen, J.; Pessi, T.; Huhtala, H.; Nieminen, M.M.; Aromaa, A.; Klaukka, T.; Hurme, M. The IL1A genotype associates with atopy in nonasthmatic adults. J. Allergy Clin. Immunol. 2002, 110, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Salo, P.M.; Arbes, S.J.; Jaramillo, R.; Calatroni, A.; Weir, C.H.; Sever, M.L.; Hoppin, J.A.; Rose, K.M.; Liu, A.H.; Gergen, P.J.; et al. Prevalence of allergic sensitization in the United States: Results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J. Allergy Clin. Immunol. 2014, 134, 350–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadfield, E.; McKeever, T.M.; Scrivener, S.; Venn, A.; Lewis, S.A.; Britton, J. Increase in the prevalence of allergen skin sensitization in successive birth cohorts. J. Allergy Clin. Immunol. 2002, 109, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Reijula, K.; Ahonen, G.; Alenius, H.; Holopainen, R. Moisture Damage Issues in Buildings. Parliament of Finland. Available online: https://www.eduskunta.fi/FI/tietoaeduskunnasta/julkaisut/Documents/trvj_1+2012.pdf (accessed on 3 January 2013).
- The World Bank Data. Primary Education Teachers, Finland. 2019. Available online: https://data.worldbank.org/indicator/SE.PRM.TCHR.FE.ZS?locations=FI (accessed on 26 August 2019).
- Tehy (The Union of Health and Social Care Professionals in Finland). Statistics. Available online: https://www.tehy.fi/en/tehy-statistics (accessed on 26 June 2018).
- Reijula, K.; Sundman-Digert, C. Assessment of indoor air problems at work with a questionnaire. Occup. Environ. Med. 2004, 61, 33–38. [Google Scholar]
- National FinSote Survey. 2017. Available online: https://www.thl.fi/en/web/alcohol-tobacco-and-addictions/tobacco/smoking-in-finland (accessed on 26 June 2018).
| Asthma (N = 32) | No Asthma (N = 67) | All (N = 99) | |
|---|---|---|---|
| Organic or functional laryngeal finding | 12 (13% *) | 30 (31% *) | 42 (44% *) |
| Chronic rhinosinusitis | 4 (13% **) | 7 (10% **) | 11 (11% **) |
| Allergen | Positive Reactions (%) |
|---|---|
| 1. Birch | 20 |
| 2. Timothy | 23 |
| 3. Mugwort | 15 |
| 4. Horse | 5 |
| 5. Dog | 16 |
| 6. Cat | 10 |
| 7. Dermatophagoides pteronyssinus | 2 |
| 8. Latex | 0 |
| 9. Aspergillus fumigatus | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nynäs, P.; Vilpas, S.; Kankare, E.; Karjalainen, J.; Lehtimäki, L.; Numminen, J.; Tikkakoski, A.; Kleemola, L.; Uitti, J. Clinical Findings among Patients with Respiratory Symptoms Related to Moisture Damage Exposure at the Workplace—The SAMDAW Study. Healthcare 2021, 9, 1112. https://doi.org/10.3390/healthcare9091112
Nynäs P, Vilpas S, Kankare E, Karjalainen J, Lehtimäki L, Numminen J, Tikkakoski A, Kleemola L, Uitti J. Clinical Findings among Patients with Respiratory Symptoms Related to Moisture Damage Exposure at the Workplace—The SAMDAW Study. Healthcare. 2021; 9(9):1112. https://doi.org/10.3390/healthcare9091112
Chicago/Turabian StyleNynäs, Pia, Sarkku Vilpas, Elina Kankare, Jussi Karjalainen, Lauri Lehtimäki, Jura Numminen, Antti Tikkakoski, Leenamaija Kleemola, and Jukka Uitti. 2021. "Clinical Findings among Patients with Respiratory Symptoms Related to Moisture Damage Exposure at the Workplace—The SAMDAW Study" Healthcare 9, no. 9: 1112. https://doi.org/10.3390/healthcare9091112
APA StyleNynäs, P., Vilpas, S., Kankare, E., Karjalainen, J., Lehtimäki, L., Numminen, J., Tikkakoski, A., Kleemola, L., & Uitti, J. (2021). Clinical Findings among Patients with Respiratory Symptoms Related to Moisture Damage Exposure at the Workplace—The SAMDAW Study. Healthcare, 9(9), 1112. https://doi.org/10.3390/healthcare9091112







