Traditional versus Microsphere Embolization for Hepatocellular Carcinoma: An Effectiveness Evaluation Using Data Mining
Abstract
:1. Introduction
- Stage 0 (very early stage)
- ○
- ECOG PS 0
- ○
- Child-Pugh A
- Stages A, B and C (early, intermediate, and advanced, respectively)
- ○
- ECOG PS 0–2
- ○
- Child-Pugh A to C
- Stage D (end-stage)
- ○
- ECOG PS > 2
- ○
- Child-Pugh C
2. Methods and Materials
2.1. ID3 Algorithm
2.2. C4.5 (J48) Algorithm
3. Results
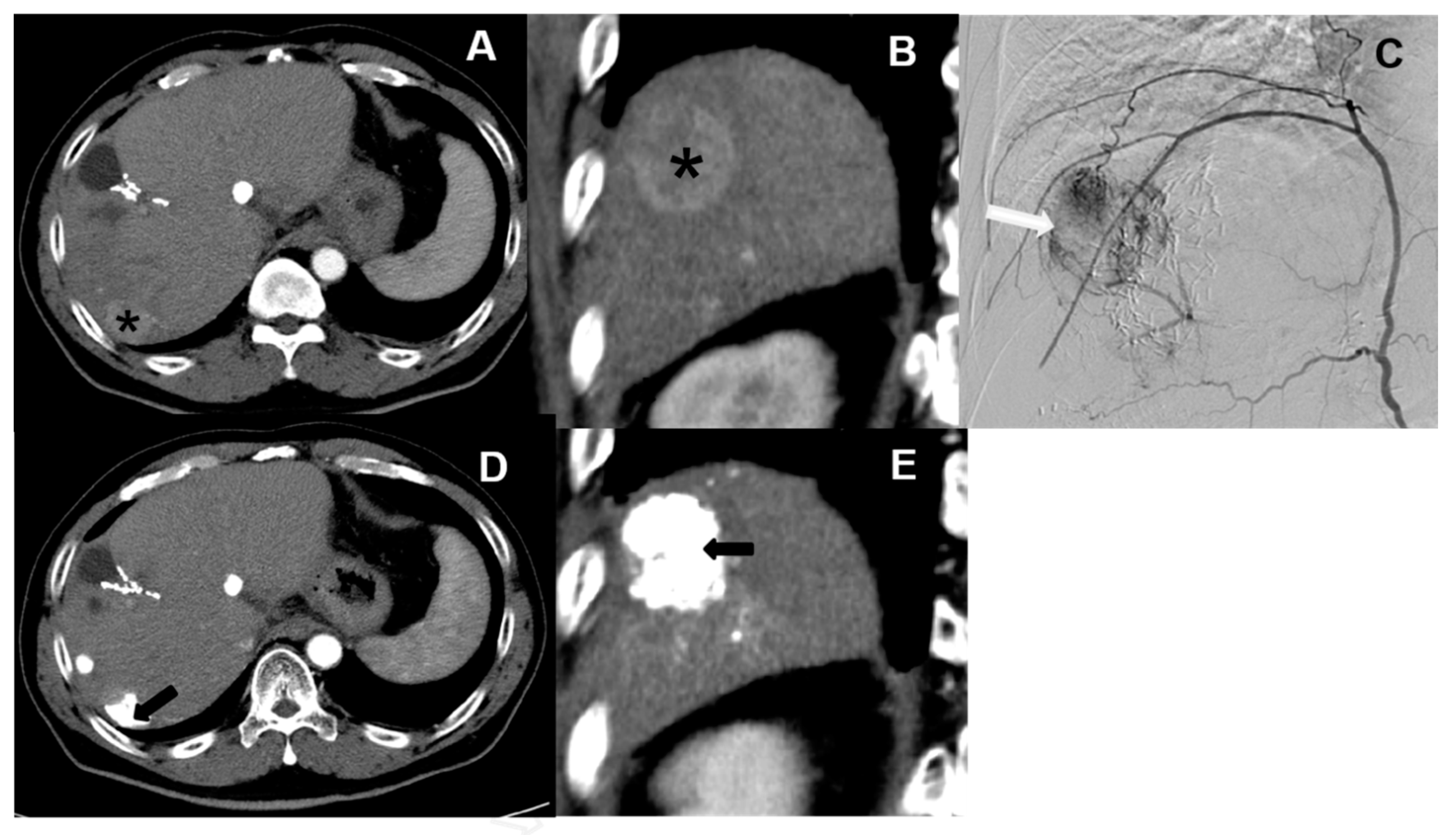
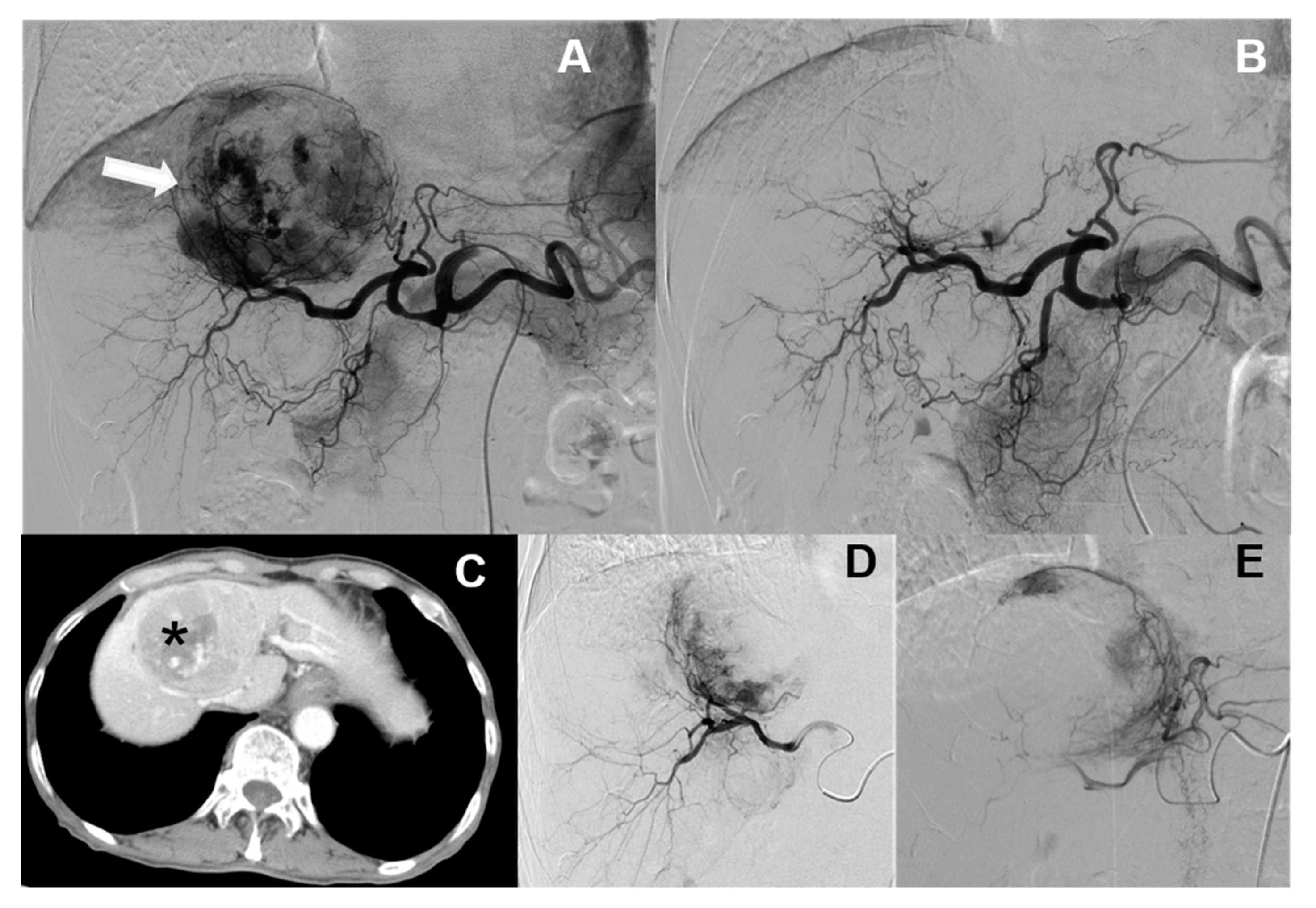
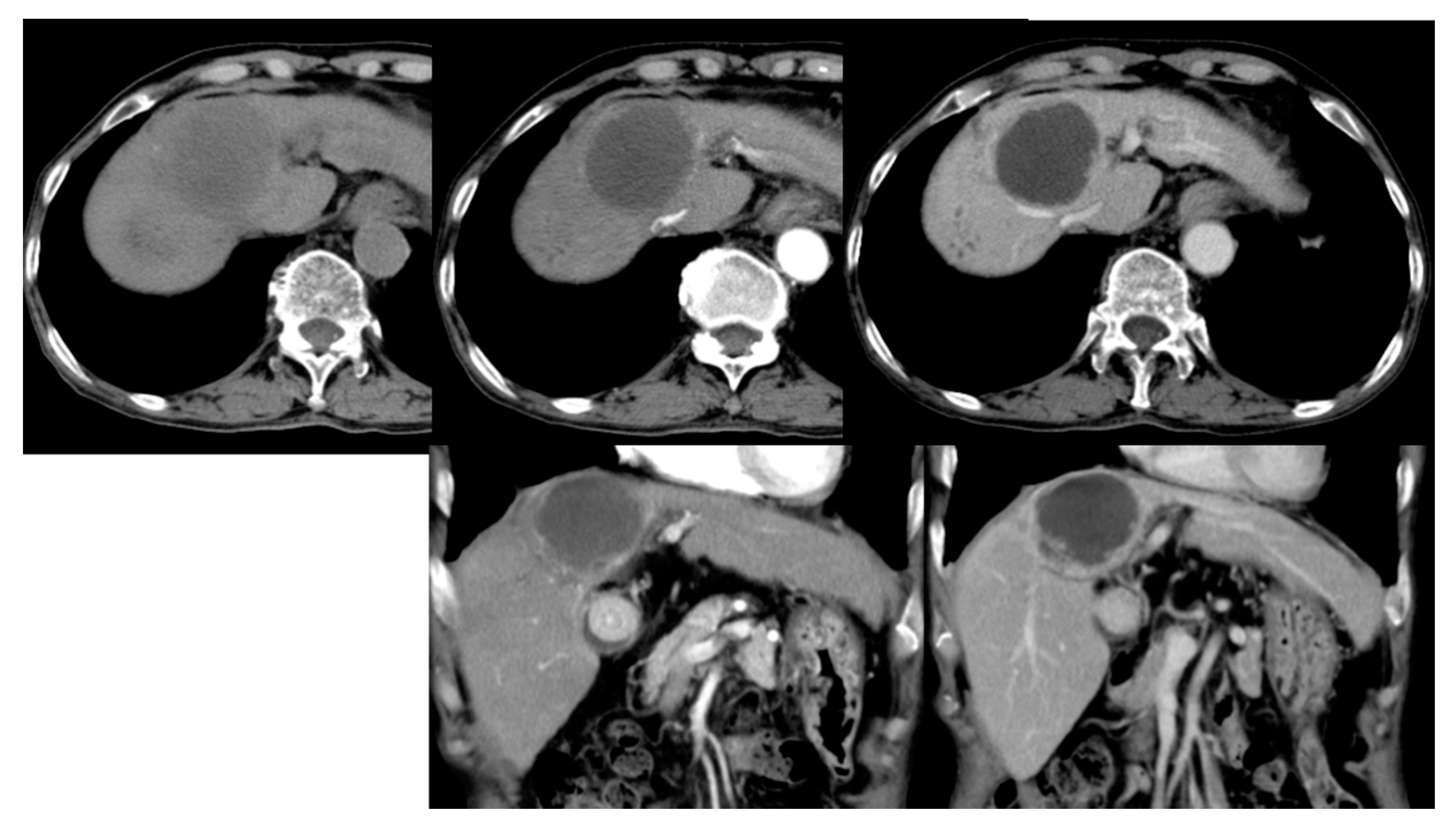
3.1. Baseline Comparison of cTACE and DC Bead TACE
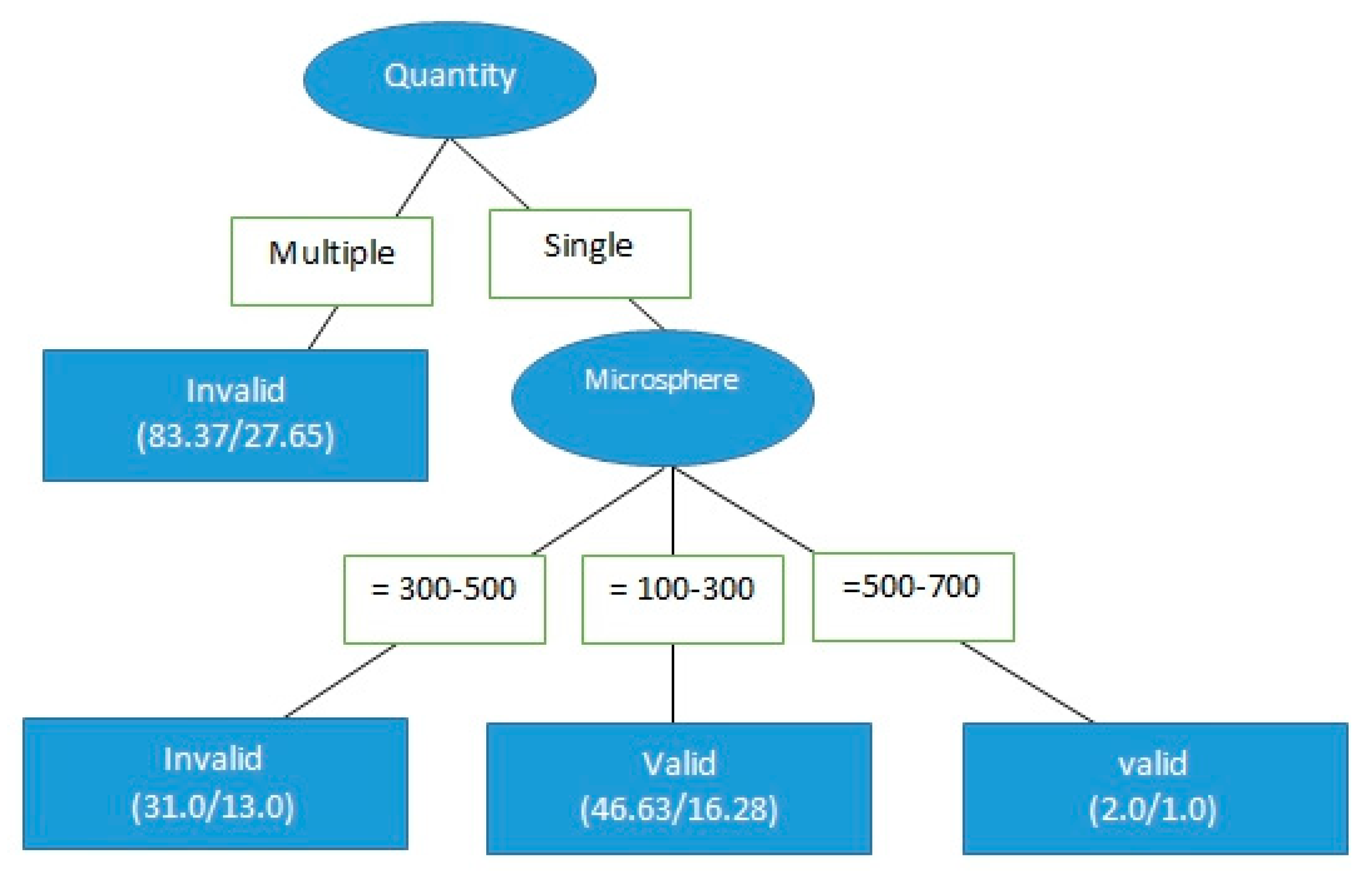
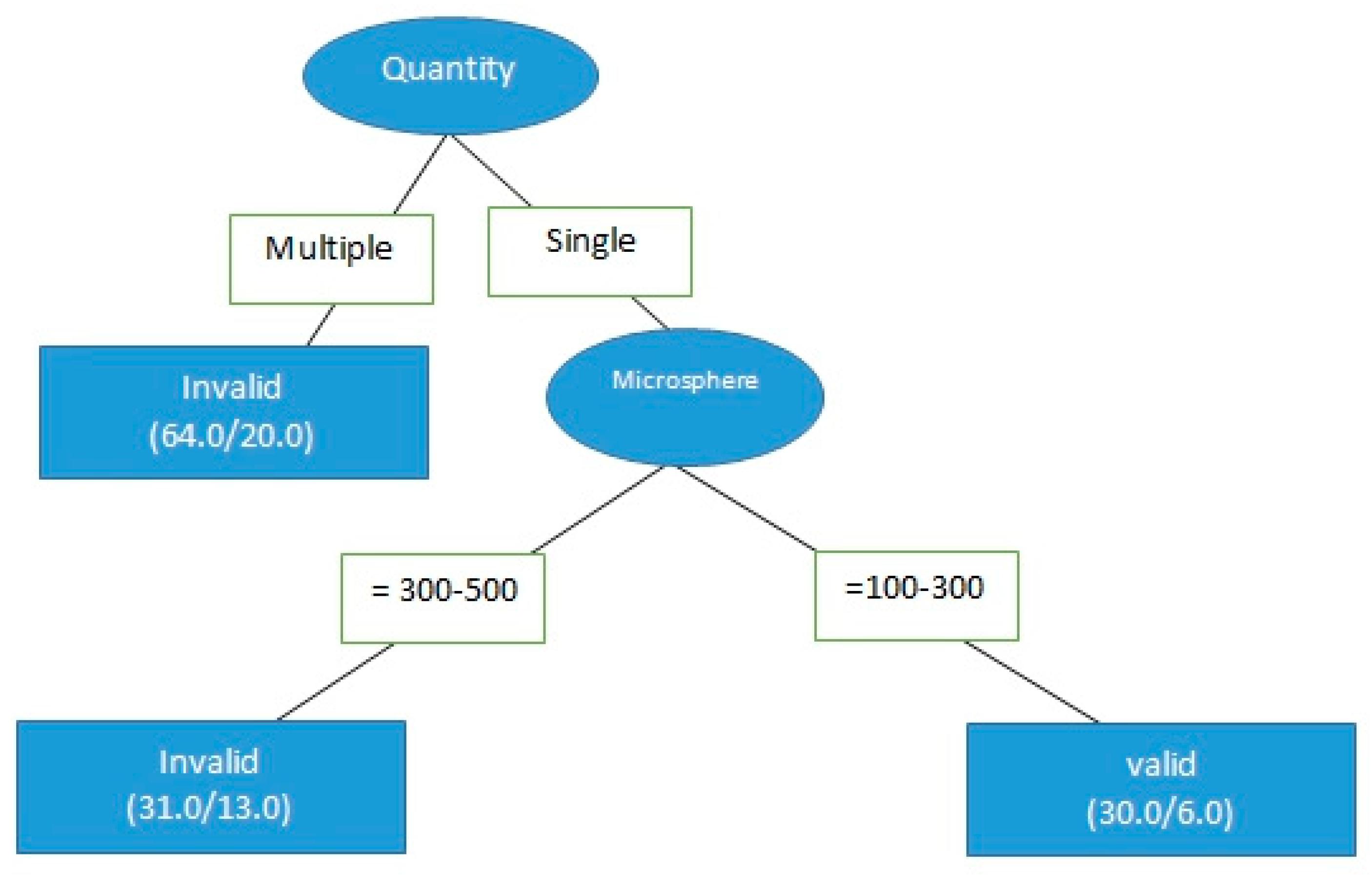
3.2. cTACE Limitations and Applicable Segments
4. Discussion
4.1. Baseline Comparison of cTACE and DC Bead TACE
4.2. cTACE Limitations and Applicable Segments
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Ethical Approval
References
- Planas, R.; Ballesté, B.; Alvarez-Gonzalez, M.A.; Rivera, M.; Montoliu, S.; Galeras, J.A.; Santos, J.; Coll, S.; Morillas, R.M.; Solà, R. Natural history of decompensated hepatitis C virus-related cirrhosis. A study of 200 patients. J. Hepatol. 2004, 40, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.C.; Jang, B.; Song, M.J. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma. World J. Gastroenterol. 2016, 22, 8853–8861. [Google Scholar] [CrossRef] [PubMed]
- Song, D.S.; Choi, J.Y.; Yoo, S.H.; Kim, H.Y.; Song, M.J.; Bae, S.H.; Yoon, S.K.; Chun, H.J.; Gil Choi, B.; Lee, H.G. DC Bead Transarterial Chemoembolization Is Effective in Hepatocellular Carcinoma Refractory to Conventional Transarteral Chemoembolization: A Pilot Study. Gut Liver 2013, 7, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashrafi, K.; Tang, Y.; Britton, H.; Domenge, O.; Blino, D.; Bushby, A.J.; Shuturminska, K.; Hartog, M.D.; Radaelli, A.; Negussie, A.H.; et al. Characterization of a novel intrinsically radiopaque Drug-eluting Bead for image-guided therapy: DC Bead LUMI™. J. Control Release 2017, 250, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Lammer, J.; Malagari, K.; Vogl, T.; Pilleul, F.; Denys, A.; Watkinson, A.; Pitton, M.; Sergent, G.; Pfammatter, T.; Terraz, S.; et al. Prospective Randomized Study of Doxorubicin-Eluting-Bead Embolization in the Treatment of Hepatocellular Carcinoma: Results of the PRECISION V Study. Cardiovasc. Interv. Radiol. 2009, 33, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muggia, F.; Braly, P.S.; Brady, M.F.; Sutton, G.; Niemann, T.H.; Lentz, S.L.; Alvarez, R.D.; Kucera, P.R.; Small, J.M. Phase III Randomized Study of Cisplatin versus Paclitaxel Versus Cisplatin and Paclitaxel in Patients with Suboptimal Stage III or IV Ovarian Cancer: A Gynecologic Oncology Group Study. J. Clin. Oncol. 2000, 18, 106. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Liang, R.; Qiu, Y.; Lv, Y.; Zhang, J.; Qin, G.; Yuan, C.; Liu, Z.; Li, Y.; Zou, D.; et al. Expression and gene regulation network of RBM8A in hepatocellular carcinoma based on data mining. Aging 2019, 11, 423–447. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Liao, M.; Xu, W.; Yao, R.; Liao, W. Data mining of the expression and regulatory role of BCAT1 in hepatocellular carcinoma. Oncol. Lett. 2019, 18, 5879–5888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ye, J.; Huang, H.; Du, X. Mining featured biomarkers associated with vascular invasion in HCC by bioinformatics analysis with TCGA RNA sequencing data. Biomed. Pharmacother. 2019, 118, 109274. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Fu, S.; Li, Y.; Li, T.; Mei, P.; Feng, L.; Cai, L.; Cheng, Y.; Zhou, C.; Tang, Y.; et al. TCGA and ESTIMATE data mining to identify potential prognostic biomarkers in HCC patients. Aging 2020, 12, 21544–21558. [Google Scholar] [CrossRef] [PubMed]
- Omran, D.A.E.H.; Awad, A.H.; Mabrouk, M.; Soliman, A.F.; Aziz, A.O.A. Application of Data Mining Techniques to Explore Predictors of HCC in Egyptian Patients with HCV-related Chronic Liver Disease. Asian Pac. J. Cancer Prev. 2015, 16, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Jiang, Y.-Y.; Xiao, K.; Huang, X.-Q.; Wang, J.; Chen, S.-Y. Candidate lncRNA–miRNA–mRNA network in predicting hepatocarcinogenesis with cirrhosis: An integrated bioinformatics analysis. J. Cancer Res. Clin. Oncol. 2019, 146, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Shimose, S.; Kawaguchi, T.; Tanaka, M.; Iwamoto, H.; Miyazaki, K.; Moriyama, E.; Suzuki, H.; Niizeki, T.; Shirono, T.; Nakano, M.; et al. Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: A multicenter cohort study using data mining analysis. Oncol. Lett. 2020, 20, 2257–2265. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-M.; Lin, L.-S.; Liu, T.-P. Sorafenib Inhibits Ribonucleotide Reductase Regulatory Subunit M2 (RRM2) in Hepatocellular Carcinoma Cells. Biomolecules 2020, 10, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, L.H.; Litière, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1—Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joerger, A.C.; Fersht, A.R. Structure–function–rescue: The diverse nature of common p53 cancer mutants. Oncogene 2007, 26, 2226–2242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela, M.; Real, M.I.; Burrel, M.; Forner, A.; Sala, M.; Brunet, M.; Ayuso, C.; Castells, L.; Montañá, X.; Llovet, J.M.; et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics. J. Hepatol. 2007, 46, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T. An introduction to ROC analysis. Pattern Recognit. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]
| Prediction | ||||
|---|---|---|---|---|
| Correct | Error | |||
| Real | Correct | True Positive (TP) | False Negative (FN) | TP + FN = A |
| Error | False Positive (FP) | True Negative (TN) | FP + TN = B | |
| TP + FP = C | FN + TN = D | A + B = C + D = E | ||
| cTACE vs. DC Bead Crosstab | |||||||
|---|---|---|---|---|---|---|---|
| cTACE | DC Bead | ||||||
| Valid/Invalid | Invalid | Number | 64 | 78 | |||
| Within the cTACE and DC bead treatments | 31.80% | 54.54% | |||||
| Overall percentage | 45.10% | 54.90% | |||||
| Valid | Number | 137 | 65 | ||||
| Within cTACE vs. DC bead treatments | 68.20% | 45.60% | |||||
| Overall percentage | 67.80% | 32.20% | |||||
| Chi-Square Test | |||||||
| Degrees of | Asymptotic | Precise | Precise | ||||
| Value | Freedom | Significant | Significant | Significant | |||
| (two-tail) | (two-tail) | (single-tail) | |||||
| Pearson chi-square | 17.770 a | 1 | <0.001 | ||||
| Continuity correction b | 16.845 | 1 | <0.001 | ||||
| Approximate ratio | 17.793 | 1 | <0.001 | ||||
| Fisher’s accurate verification | <0.001 | <0.001 | |||||
| Linear connection | 17.718 | 1 | <0.001 | ||||
| The valid observation number | 344 | ||||||
| Variable | Class Valid |
|---|---|
| Hepatitis = B | 0.8758 |
| Hepatitis = C | 1.1042 |
| Hepatitis = non-B non-C | 0.9071 |
| Hepatitis = B + C | 1.3726 |
| BCLC stage = B | 0.9389 |
| BCLC stage = C | 1.2431 |
| BCLC stage = A | 0.9672 |
| BCLC stage = D | 1.7284 |
| Quantity = single | 2.8719 |
| Size | 0.9137 |
| Microsphere = 300–500 | 0.2855 |
| Microsphere = 300–500 + 500–700 | 1.5049 |
| Microsphere = 100–300 | 0.5364 |
| Microsphere = 500–700 | 0.4341 |
| Microsphere = 100–300 + 500–700 | 9.99 × 1019 |
| Microsphere = 100–300 + 300–500 | 46,749,492.226 |
| Microsphere = 0 | 1.0669 |
| New vs. old treatments | 1.0669 |
| Tumor Size vs. Effectiveness Crosstab | |||||||
|---|---|---|---|---|---|---|---|
| ≤9.3 cm | >9.3 cm | ||||||
| Invalid | Number | 51 | 13 | ||||
| Within size tumor 9.3 | 27.70% | 76.50% | |||||
| Valid/ | Overall percentage | 79.70% | 20.30% | ||||
| Invalid | Valid | Number | 133 | 4 | |||
| Within size tumor 9.3 | 72.30% | 23.50% | |||||
| Overall percentage | 97.10% | 2.90% | |||||
| Chi-Squared Test | |||||||
| Degrees of | Asymptotic | Precise | Precise | ||||
| Value | Freedom | Significant | Significant | Significant | |||
| (two-tail) | (two-tail) | (single-tail) | |||||
| Pearson chi-square | 17.044 a | 1 | <0.001 | ||||
| Continuity correction b | 14.871 | 1 | <0.001 | ||||
| Approximate ratio | 15.749 | 1 | <0.001 | ||||
| Fisher’s accurate verification | <0.001 | <0.001 | |||||
| Linear connection | 16.959 | 1 | <0.001 | ||||
| The valid observation number | 201 | ||||||
| Tumor Number Crosstab | ||||||
|---|---|---|---|---|---|---|
| Single | Multiple | |||||
| Invalid | Number | 24 | 44 | |||
| Within number of tumors | 39.30% | 68.80% | ||||
| Valid/ | Overall percentage | 35.30% | 64.70% | |||
| Invalid | Valid | Number | 37 | 20 | ||
| Within number of tumors | 60.70% | 31.30% | ||||
| Overall percentage | 64.90% | 35.10% | ||||
| Chi-Square Test | ||||||
| Degrees of | Asymptotic | Precise | Precise | |||
| Value | Freedom | Significant | Significant | Significant | ||
| (two-tail) | (two-tail) | (single-tail) | ||||
| Pearson chi-square | 10.887 a | 1 | 0.001 | |||
| Continuity correction b | 9.734 | 1 | 0.002 | |||
| Approximate ratio | 11.046 | 1 | 0.001 | |||
| Fisher’s accurate verification | 0.001 | 0.001 | ||||
| Linear connection | 10.8 | 1 | 0.001 | |||
| The valid observation number | 125 | |||||
| Effectiveness Crosstab | ||||||
|---|---|---|---|---|---|---|
| Invalid | Effective | |||||
| Number | 6 | 24 | ||||
| 100–300 μm | Within the size of microsphere | 25.00% | 64.90% | |||
| microsphere | Overall percentage | 20.00% | 80.00% | |||
| size | Number | 18 | 13 | |||
| 300–500 μm | Within the size of microsphere | 75.00% | 35.10% | |||
| Overall percentage | 58.10% | 41.90% | ||||
| Chi-Square Test | ||||||
| Degree of | Asymptotic | Precise | Precise | |||
| Value | Freedom | Significant | Significant | Significant | ||
| (two-tail) | (two-tail) | (single-tail) | ||||
| Pearson chi-square | 9.256 a | 1 | 0.002 | |||
| Continuity correction b | 7.73 | 1 | 0.005 | |||
| Approximate ratio | 9.583 | 1 | 0.002 | |||
| Fisher’s accurate verification | 0.004 | 0.002 | ||||
| Linear connection | 3.105 | 1 | 0.003 | |||
| The valid observation number | 61 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, P.-Y.; Cheng, C.-Y.; Hon, J.-S.; Kuo, C.-D.; Yen, C.-L.; Chai, J.-W. Traditional versus Microsphere Embolization for Hepatocellular Carcinoma: An Effectiveness Evaluation Using Data Mining. Healthcare 2021, 9, 929. https://doi.org/10.3390/healthcare9080929
Chang P-Y, Cheng C-Y, Hon J-S, Kuo C-D, Yen C-L, Chai J-W. Traditional versus Microsphere Embolization for Hepatocellular Carcinoma: An Effectiveness Evaluation Using Data Mining. Healthcare. 2021; 9(8):929. https://doi.org/10.3390/healthcare9080929
Chicago/Turabian StyleChang, Pi-Yi, Chen-Yang Cheng, Jau-Shin Hon, Cheng-Ding Kuo, Chieh-Ling Yen, and Jyh-Wen Chai. 2021. "Traditional versus Microsphere Embolization for Hepatocellular Carcinoma: An Effectiveness Evaluation Using Data Mining" Healthcare 9, no. 8: 929. https://doi.org/10.3390/healthcare9080929
APA StyleChang, P.-Y., Cheng, C.-Y., Hon, J.-S., Kuo, C.-D., Yen, C.-L., & Chai, J.-W. (2021). Traditional versus Microsphere Embolization for Hepatocellular Carcinoma: An Effectiveness Evaluation Using Data Mining. Healthcare, 9(8), 929. https://doi.org/10.3390/healthcare9080929






