The Effect of Trunk Stability Training Based on Visual Feedback on Trunk Stability, Balance, and Upper Limb Function in Stroke Patients: A Randomized Control Trial
Abstract
1. Introduction
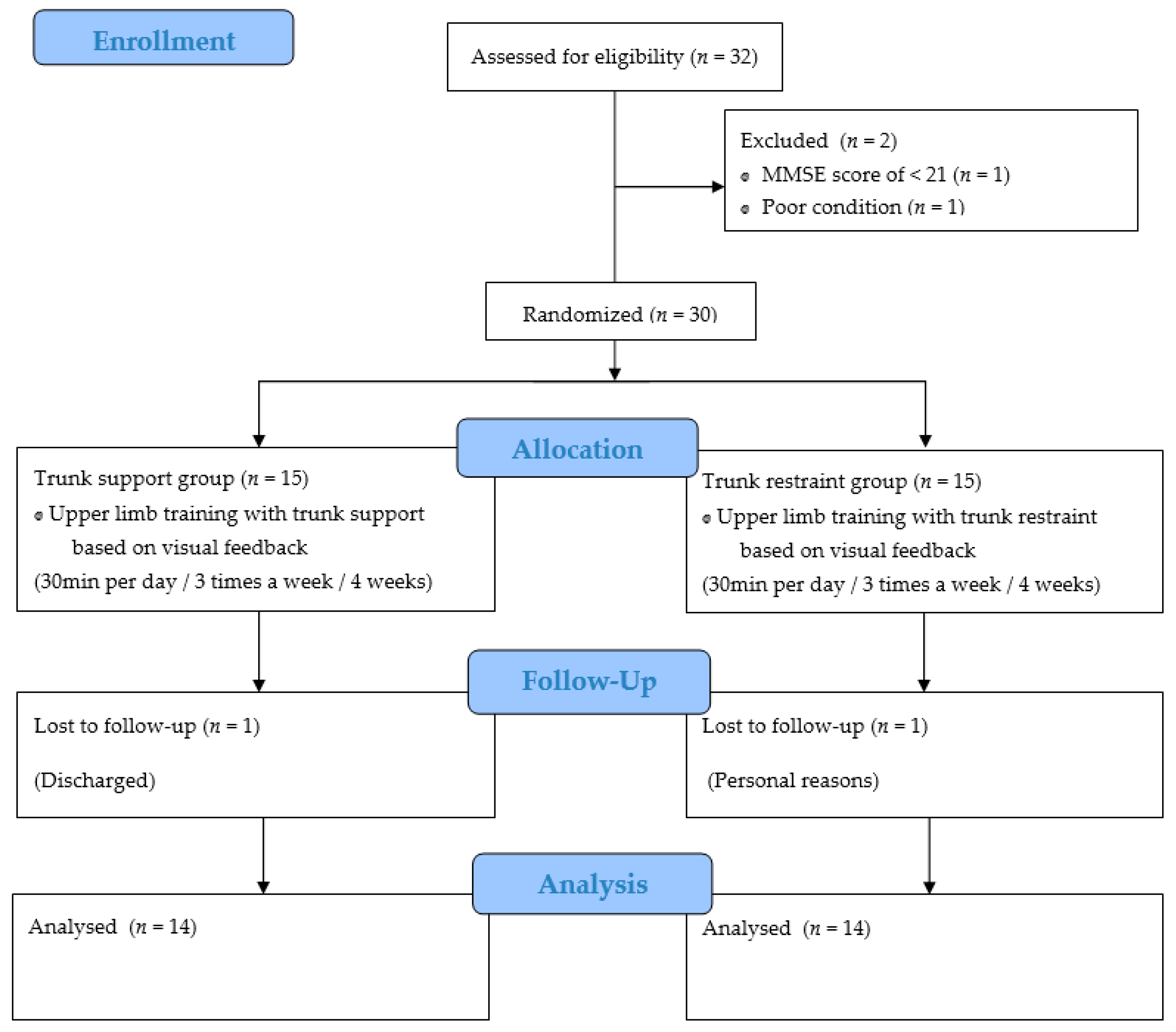
2. Materials and Methods
2.1. Subjects
2.2. Experimental Procedure
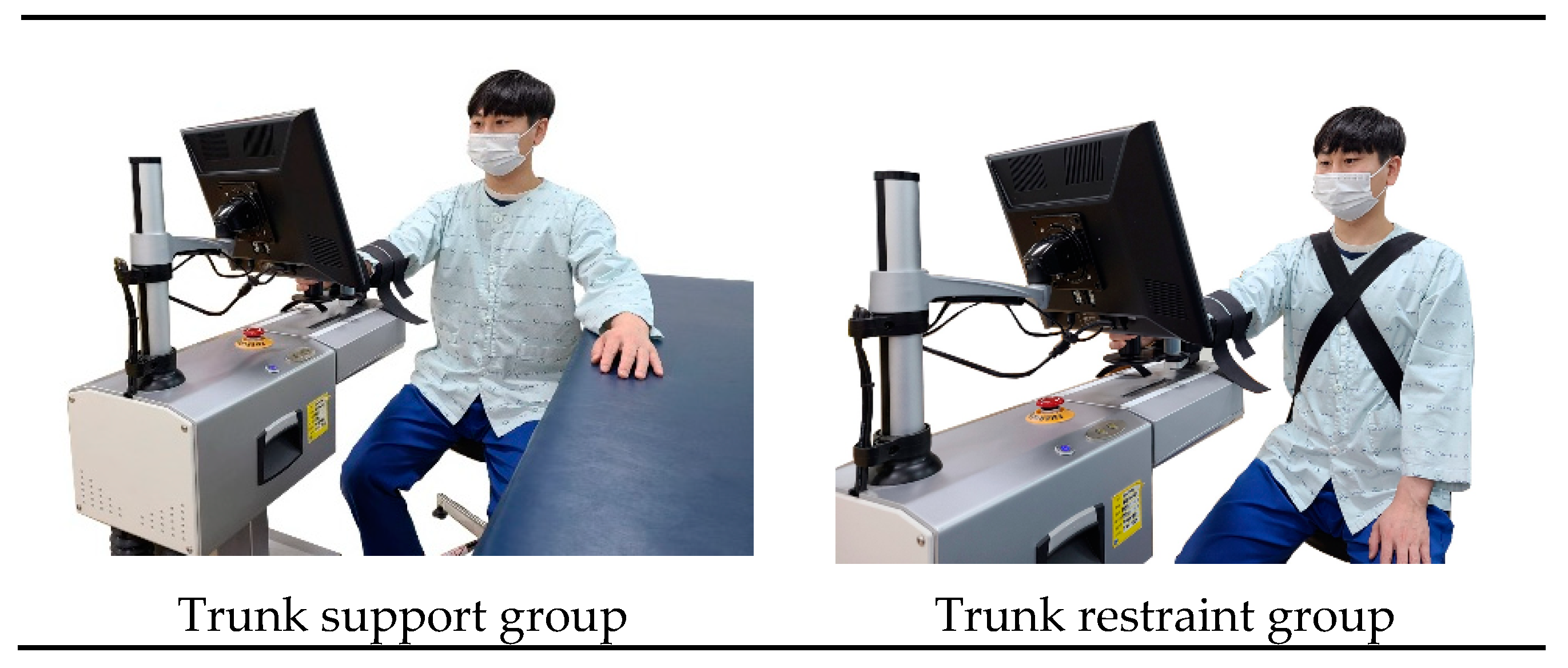
2.3. Training Program
2.4. Outcome Measures
2.5. Data Analysis
3. Results
3.1. Trunk Stability
3.2. Balance
3.3. Upper Limb Function
4. Discussion
4.1. Trunk Stability
4.2. Balance
4.3. Upper Limb Function
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobkin, B.H. Rehabilitation after stroke. N. Engl. J. Med. 2005, 352, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Franck, J.A.; Smeets, R.; Seelen, H.A.M. Changes in arm-hand function and arm-hand skill performance in patients after stroke during and after rehabilitation. PLoS ONE 2017, 12, e0179453. [Google Scholar] [CrossRef] [PubMed]
- Graef, P.; Michaelsen, S.M.; Dadalt, M.L.R.; Rodrigues, D.A.M.S.; Pereira, F.; Pagnussat, A.S. Effects of functional and analytical strength training on upper-extremity activity after stroke: A randomized controlled trial. Braz. J. Phys. 2016, 20, 543–552. [Google Scholar] [CrossRef]
- Rensink, M.; Schuurmans, M.; Lindeman, E.; Hafsteinsdóttir, T. Task-oriented training in rehabilitation after stroke: Systematic review. J. Adv. Nurs. 2009, 65, 737–754. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; Wegen, E.E.H.; Meskers, C.G.M.; Kwakkel, G. Effects of Robot-assisted therapy for the upper limb after stroke. Neurorehabil. Neural Repair 2017, 31, 107–121. [Google Scholar] [CrossRef]
- Lauber, B.; Keller, M. Improving motor performance: Selected aspects of augmented feedback in exercise and health. Eur. J. Sport Sci. 2014, 14, 36–43. [Google Scholar] [CrossRef]
- Brunner, I.; Skouen, J.S.; Hofstad, H.; Aßmuss, J.; Becker, F.; Pallesen, H.; Thijs, L.; Verheyden, G. Is upper limb virtual reality training more intensive than conventional training for patients in the subacute phase after stroke? An analysis of treatment intensity and content. BMC Neurol. 2016, 16, 219. [Google Scholar] [CrossRef] [PubMed]
- Shiri, S.; Feintuch, U.; Lorber-Haddad, A.; Moreh, E.; Twito, D.; Tuchner-Arieli, M.; Meiner, Z. Novel virtual reality system integrating online self-face viewing and mirror visual feedback for stroke rehabilitation: Rationale and feasibility. Top Stroke Rehabil. 2012, 19, 277–286. [Google Scholar] [CrossRef]
- Kumru, H.; Albu, S.; Pelayo, R.; Rothwell, J.; Opisso, E.; Leon, D.; Soler, D.; Tormos, J.M. Motor cortex plasticity during unilateral finger movement with mirror visual feedback. Neural Plast. 2016, 2016, 6087896. [Google Scholar] [CrossRef][Green Version]
- Wee, S.K.; Hughes, A.M.; Warner, M.; Burridge, J.H. Trunk restraint to promote upper extremity recovery in stroke patients: A systematic review and meta-analysis. Neurorehabil. Neural Repair 2014, 28, 660–677. [Google Scholar] [CrossRef]
- Chan, I.H.; Fong, K.N.K.; Chan, D.Y.L.; Wang, A.Q.L.; Cheng, E.K.N.; Chau, P.H.Y.; Chow, K.K.Y.; Cheung, H.K.Y. Effects of arm weight support training to promote recovery of upper limb function for subacute patients after stroke with different levels of arm impairments. Biomed. Res. Int. 2016, 2016, 9346374. [Google Scholar] [CrossRef] [PubMed]
- Kanekar, N.; Aruin, A.S. Aging and balance control in response to external perturbations: Role of anticipatory and compensatory postural mechanisms. Age 2014, 36, 9621. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.C.; Nascimento, L.R.; Michaelsen, S.M.; Polese, J.C.; Pereira, N.D.; Teixeira-Salmela, L.F. Influences of hand dominance on the maintenance of benefits after home-based modified constraint-induced movement therapy in individuals with stroke. Braz. J. Phys. 2014, 18, 435–444. [Google Scholar] [CrossRef]
- Schrafl-Altermatt, M.; Dietz, V. Neural coupling of cooperative hand movements after stroke: Role of ipsilateral afference. Ann. Clin. Transl. Neurol. 2016, 3, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Brauer, S.G.; Hayward, K.S.; Carson, R.G.; Cresswell, A.G. Ruth N Barker The efficacy of SMART Arm training early after stroke for stroke survivors with severe upper limb disability: A protocol for a randomised controlled trial. BMC Neurol. 2013, 13, 71. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Silva, C.C.; Ferreira, S.; Silva, C.; Oliveira, N.; Santos, R.; Vilas-Boas, J.P.; Correia, M.V. Anticipatory postural adjustments during sitting reach movement in post-stroke subjects. J. Electromyogr. Kinesiol. 2014, 24, 165–171. [Google Scholar] [CrossRef]
- Persson, C.U.; Hansson, P.O.; Danielsson, A.; Sunnerhagen, K.S. A validation study using a modified version of Postural Assessment Scale for Stroke Patients: Postural Stroke Study in Gothenburg (POSTGOT). J. Neuroeng. Rehabil. 2011, 8, 57. [Google Scholar] [CrossRef]
- Merchán-Baeza, J.A.; González-Sánchez, M.; Cuesta-Vargas, A.I. Reliability in the parameterization of the functional reach test in elderly stroke patients: A pilot study. Biomed Res. Int. 2014, 2014, 637671. [Google Scholar] [CrossRef] [PubMed]
- Kolber, M.J.; Vega, F.; Widmayer, K.; Cheng, M.S.S. The reliability and minimal detectable change of shoulder mobility measurements using a digital inclinometer. Physiother Theory Pr. 2011, 27, 176–184. [Google Scholar] [CrossRef]
- Pandian, S.; Arya, K.N. Motor impairment of the ipsilesional body side in poststroke subjects. J. Bodyw. Mov. 2013, 17, 495–503. [Google Scholar] [CrossRef]
- Pandian, S.; Arya, K.N.; Kumar, D. Effect of motor training involving the less-affected side (MTLA) in post-stroke subjects: A pilot randomized controlled trial. Top Stroke Rehabil. 2015, 22, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.Y.; Huang, Y.H.; Chou, L.W.; Wu, S.C.; Wang, R.Y.; Lin, L.C. Effects of primary caregiver participation in vestibular rehabilitation for unilateral neglect patients with right hemispheric stroke: A randomized controlled trial. Neuropsychiatr. Dis. Treat 2013, 9, 477–484. [Google Scholar] [CrossRef]
- Kalron, A.; Fonkatz, I.; Frid, L.; Baransi, H.; Achiron, A. The effect of balance training on postural control in people with multiple sclerosis using the CAREN virtual reality system: A pilot randomized controlled trial. J. Neuroeng. Rehabil. 2016, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Aruin, A.S. Enhancing Anticipatory Postural Adjustments: A Novel Approach to Balance Rehabilitation. J. Nov. Physiother 2016, 6, e144. [Google Scholar] [CrossRef] [PubMed]
- De Baets, L.; Deun, S.V.; Monari, D.; Jaspers, E. Three-dimensional kinematics of the scapula and trunk, and associated scapular muscle timing in individuals with stroke. Hum. Mov. Sci. 2016, 48, 82–90. [Google Scholar] [CrossRef]
- Haruyama, K.; Kawakami, M.; Otsuka, T. Effect of core stability training on trunk function, standing balance, and mobility in stroke patients. Neurorehabil. Neural Repair 2017, 31, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Cabanas-Valdés, R.; Bagur-Calafat, C.; Girabent-Farrés, M.; Caballero-Gómez, F.M.; Hernández-Valiño, M.; Cuchí, G.U. The effect of additional core stability exercises on improving dynamic sitting balance and trunk control for subacute stroke patients: A randomized controlled trial. Clin. Rehabil. 2016, 30, 1024–1033. [Google Scholar] [CrossRef]
- Lee, N.G.; You, J.S.H.; Yi, C.H.; Jeon, H.S.; Choi, B.S.; Lee, D.R.; Park, J.M.; Lee, T.H.; Ryu, I.T.; Yoon, H.S. Best core stabilization for anticipatory postural adjustment and falls in hemiparetic stroke. Arch. Phys. Med. Rehabil. 2018, 99, 2168–2174. [Google Scholar] [CrossRef]
- Merkert, J.; Nieczaj, S.B.R.; Steinhagen-Thiessen, E.; Eckardt, R. Combined whole body vibration and balance training using Vibrosphere®: Improvement of trunk stability, muscle tone, and postural control in stroke patients during early geriatric rehabilitation. Z Gerontol. Geriatr. 2011, 44, 256–261. [Google Scholar] [CrossRef]
- Torres-Oviedo, G.; Macpherson, J.M.; Ting, L.H. Muscle synergy organization is robust across a variety of postural perturbations. J. Neurophysiol. 2006, 96, 1530–1546. [Google Scholar] [CrossRef]
- Clark, D.J.; Ting, L.H.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J. Neurophysiol. 2010, 103, 844–857. [Google Scholar] [CrossRef]
- Ajiboye, A.B.; Weir, R.F. Muscle synergies as a predictive framework for the EMG patterns of new hand postures. J. Neural Eng. 2009, 6, 036004. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Rymer, W.Z.; Beer, R.F. Robustness of muscle synergies underlying three-dimensional force generation at the hand in healthy humans. J. Neurophysiol. 2012, 107, 2123–2142. [Google Scholar] [CrossRef] [PubMed]
- Muceli, S.; Boye, A.T.; d’Avella, A.; Farina, D. Identifying representative synergy matrices for describing muscular activation patterns during multidirectional reaching in the horizontal plane. J. Neurophysiol. 2010, 103, 1532–1542. [Google Scholar] [CrossRef]
- Ellis, M.D.; Acosta, A.M.; Yao, J.; Dewald, J.P.A. Position-dependent torque coupling and associated muscle activation in the hemiparetic upper extremity. Exp. Brain Res. 2007, 176, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.; Rymer, W.Z.; Perreault, E.J.; Yoo, S.B.; Beer, R.F. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J. Neurophysiol. 2013, 109, 768–781. [Google Scholar] [CrossRef] [PubMed]
- Houwink, A.; Steenbergen, B.; Prange, G.B.; Buurke, J.H.; Geurts, A.C.H. Upper-limb motor control in patients after stroke: Attentional demands and the potential beneficial effects of arm support. Hum. Mov. Sci. 2013, 32, 377–387. [Google Scholar] [CrossRef]
| Characteristics | Trunk Support Group (n = 14) | Trunk Restraint Group (n = 14) | t | p/x2 |
|---|---|---|---|---|
| Sex (male/female) | 12/2 | 12/2 | 0.000 | 1.000 |
| Ages (years) | 62.64 ± 9.24 | 65.93 ± 11.96 | −0.813 | 0.579 |
| Height (cm) | 165.29 ± 7.46 | 170.93 ± 7.95 | −1.935 | 0.946 |
| Weight (kg) | 64.71 ± 9.94 | 65.86 ± 11.41 | −0.283 | 0.611 |
| Affected side (left/right) | 8/6 | 8/6 | 0.000 | 1.000 |
| Post-stroke duration (months) | 30.57 ± 15.47 | 29.43 ± 17.81 | 0.181 | 0.413 |
| Body mass index | 23.57 ± 2.25 | 22.36 ± 2.65 | 1.305 | 0.674 |
| K-MMSE (score) | 25.57 ± 3.54 | 26.50 ± 3.05 | −0.742 | 0.626 |
| Trunk Stability | Trunk Support Group (n = 14) | Trunk Restraint Group (n = 14) | t | p/x2 |
|---|---|---|---|---|
| PASS (score) | ||||
| Pre-test | 25.29 ± 6.37 | 26.07 ± 4.74 | ||
| Post-test | 28.00 ± 5.60 | 27.36 ± 4.39 | ||
| Pre-post | 2.71 ± 1.20 | 1.29 ± 1.06 | 3.319 | 0.003 |
| t (p) | −8.432 (0.00) | −4.500 (0.01) |
| Balance | Trunk Support Group (n = 14) | Trunk Restraint Group (n = 14) | t | p/x2 |
|---|---|---|---|---|
| FRT (score) | ||||
| Pre-test | 14.80 ± 4.18 | 14.86 ± 3.85 | ||
| Post-test | 16.88 ± 4.00 | 16.00 ± 3.80 | ||
| Pre-post | 2.07 ± 0.83 | 1.13 ± 0.65 | 3.327 | 0.003 |
| t (p) | −9.356 (0.000) | −6.456 (0.000) |
| Upper Limb Function | Trunk Support Group (n = 14) | Trunk Restraint Group (n = 14) | t | p/x2 |
|---|---|---|---|---|
| ROM (°) (shoulder flexion) | ||||
| Pre-test | 139.29 ± 25.50 | 138.21 ± 23.10 | ||
| Post-test | 142.14 ± 24.59 | 140.29 ± 21.19 | ||
| Pre-post | 2.86 ± 2.28 | 2.07 ± 2.12 | 0.942 | 0.355 |
| t (p) | −4.684 (0.000) | −3.640 (0.03) | ||
| MMT (score) (triceps brachii) | ||||
| Pre-test | 3.21 ± 1.29 | 3.46 ± 1.08 | ||
| Post-test | 3.67 ± 0.84 | 3.71 ± 0.91 | ||
| Pre-post | 0.46 ± 0.57 | 0.25 ± 0.32 | 1.221 | 0.233 |
| t (p) | −3.045 (0.009) | −3.640 (0.03) | ||
| FMA-upper limb (score) | ||||
| Pre-test | 40.71 ± 12.95 | 44.57 ± 12.10 | ||
| Post-test | 44.71 ± 12.05 | 45.93 ± 11.44 | ||
| Pre-post | 4.00 ± 1.79 | 1.36 ± 1.21 | 4.577 | 0.000 |
| t (p) | −8.327 (0.00) | −4.177 (0.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.-H.; Chung, E.-J.; Lee, J.; Lee, S.-H.; Lee, B.-H. The Effect of Trunk Stability Training Based on Visual Feedback on Trunk Stability, Balance, and Upper Limb Function in Stroke Patients: A Randomized Control Trial. Healthcare 2021, 9, 532. https://doi.org/10.3390/healthcare9050532
Yang S-H, Chung E-J, Lee J, Lee S-H, Lee B-H. The Effect of Trunk Stability Training Based on Visual Feedback on Trunk Stability, Balance, and Upper Limb Function in Stroke Patients: A Randomized Control Trial. Healthcare. 2021; 9(5):532. https://doi.org/10.3390/healthcare9050532
Chicago/Turabian StyleYang, Seok-Hui, Eun-Jung Chung, Jin Lee, Su-Hyun Lee, and Byoung-Hee Lee. 2021. "The Effect of Trunk Stability Training Based on Visual Feedback on Trunk Stability, Balance, and Upper Limb Function in Stroke Patients: A Randomized Control Trial" Healthcare 9, no. 5: 532. https://doi.org/10.3390/healthcare9050532
APA StyleYang, S.-H., Chung, E.-J., Lee, J., Lee, S.-H., & Lee, B.-H. (2021). The Effect of Trunk Stability Training Based on Visual Feedback on Trunk Stability, Balance, and Upper Limb Function in Stroke Patients: A Randomized Control Trial. Healthcare, 9(5), 532. https://doi.org/10.3390/healthcare9050532







