Effects of Osteopathic T9–T10 Vertebral Manipulation in Tonsillitis: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Treatment Protocols
2.4. Evaluations
2.5. Statistical Analysis
3. Results
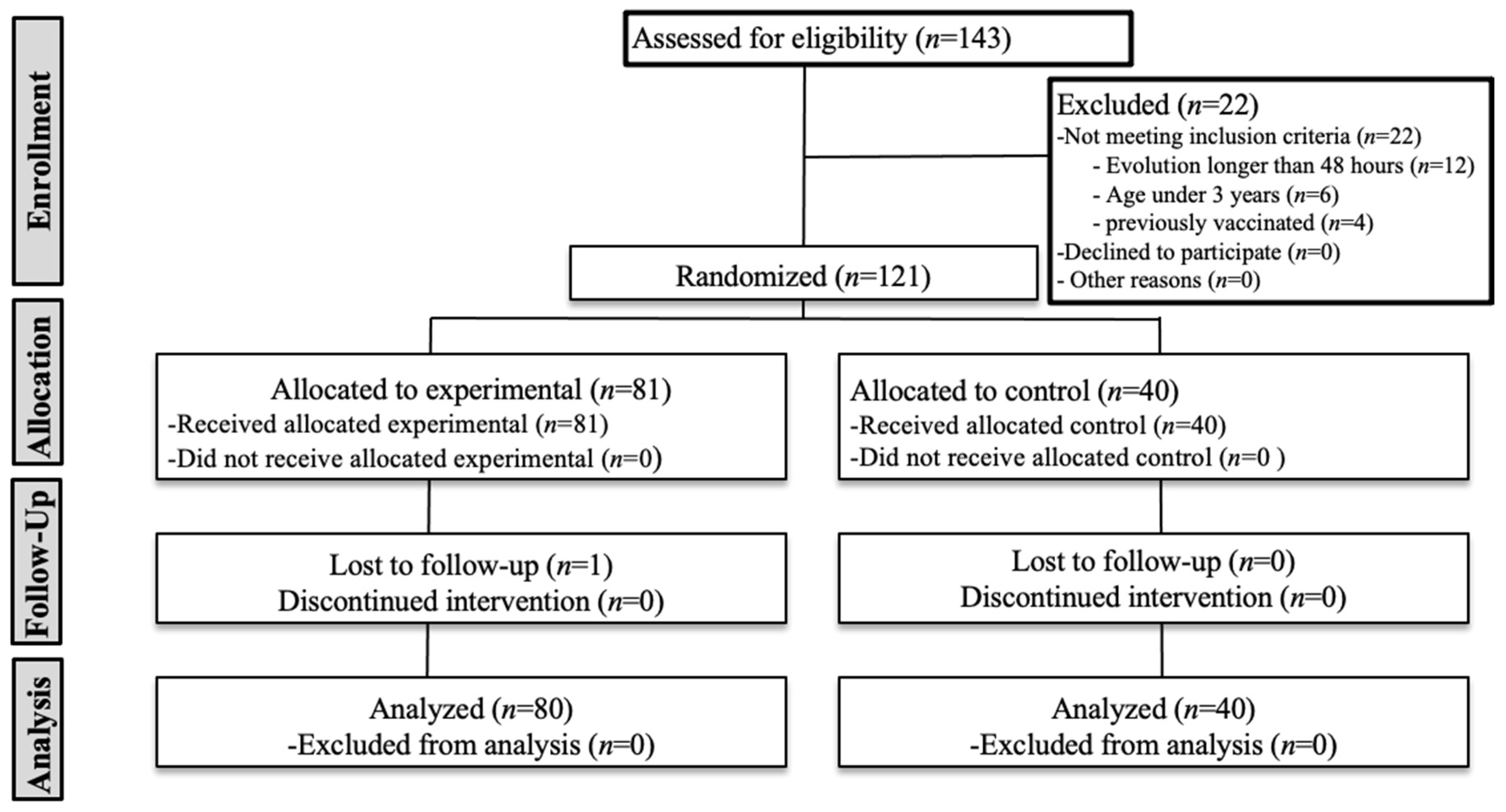
3.1. Sample
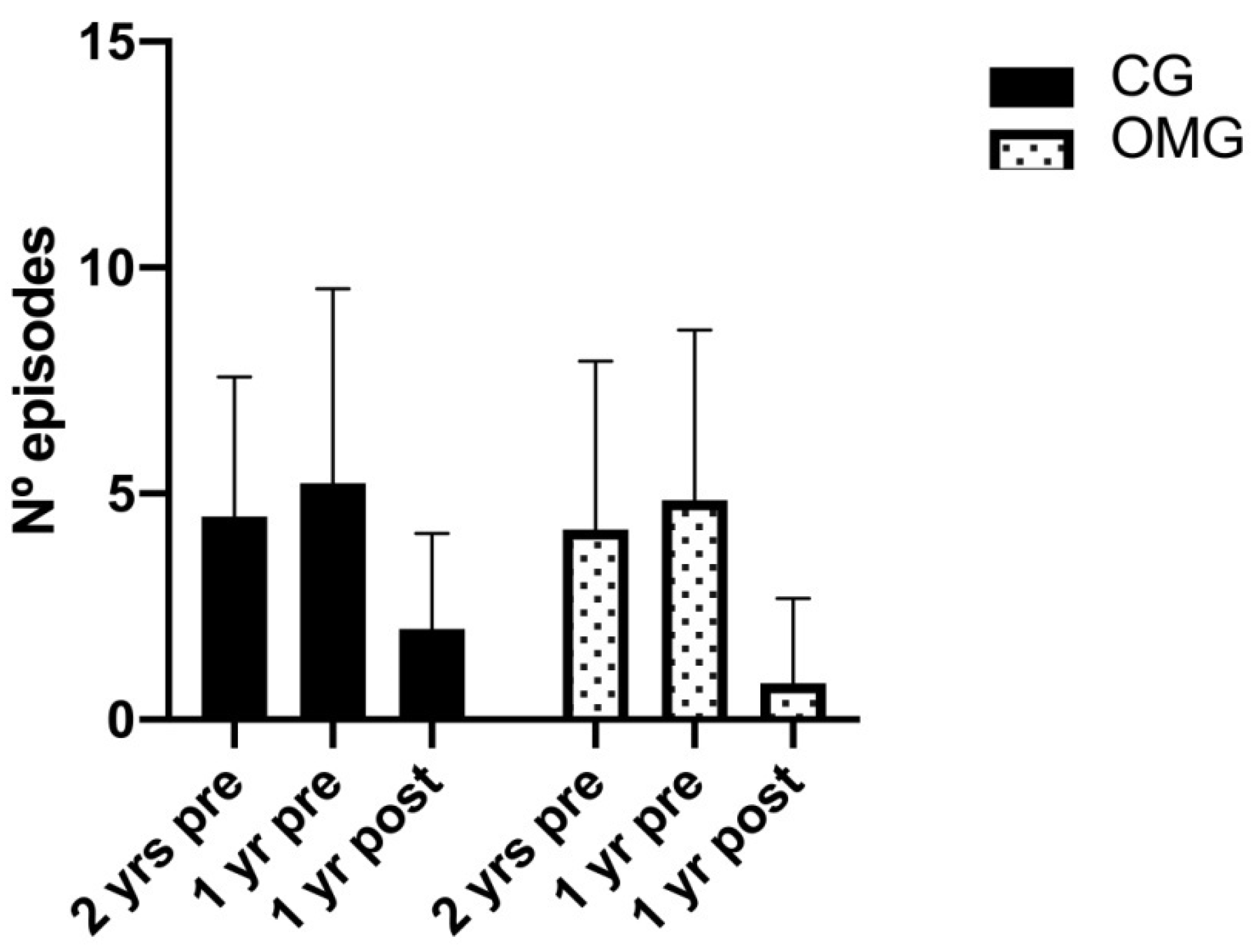
3.2. Intervention Effects on Tonsillitis
4. Discussion
5. Conclusions
6. Declaration
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Windfuhr, J.P.; Toepfner, N.; Steffen, G.; Waldfahrer, F.; Berner, R. Clinical practice guideline: Tonsillitis I. diagnostics and nonsurgical management. Eur. Arch. Otorhinolaryngol. 2016, 273, 973–987. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Archer, S.M.; Ishman, S.L.; Rosenfeld, R.M.; Coles, S.; Finestone, S.A.; Friedman, N.R.; Giordano, T.; Hildrew, D.M.; Kim, T.W.; et al. Clinical practice guideline: Tonsillectomy in children (update). Otolaryngol. Head Neck Surg. 2019, 160, S1–S42. [Google Scholar] [CrossRef]
- Spinks, A.; Glasziou, P.P.; Del Mar, C.B. Antibiotics for sore throat. Cochrane Database Syst. Rev. 2013, 2013, CD000023. [Google Scholar] [CrossRef]
- Munck, H.; Jørgensen, A.W.; Klug, T.E. Antibiotics for recurrent acute pharyngo-tonsillitis: Systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1221–1230. [Google Scholar] [CrossRef]
- Windfuhr, J.P.; Toepfner, N.; Steffen, G.; Waldfahrer, F.; Berner, R. Clinical practice guideline: Tonsillitis II. Surgical management. Eur. Arch. Otorhinolaryngol. 2016, 273, 989–1009. [Google Scholar] [CrossRef]
- Mitchell, R.B.; Archer, S.M.; Ishman, S.L.; Rosenfeld, R.M.; Coles, S.; Finestone, S.A.; Friedman, N.R.; Giordano, T.; Hildrew, D.M.; Kim, T.W.; et al. Clinical practice guideline: Tonsillectomy in children (Update)—Executive summary. Otolaryngol. Head Neck Surg. 2019, 160, 187–205. [Google Scholar] [CrossRef]
- Lee, H.S.; Yoon, H.Y.; Jin, H.J.; Hwang, S.H. The safety and efficacy of powered intracapsular tonsillectomy in children: A meta-analysis. Laryngoscope 2018, 128, 732–744. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, S.H.; Lee, E.J.; Yoon, Y.J. Can intracapsular tonsillectomy be an alternative to classical tonsillectomy? A meta-analysis. Otolaryngol. Head Neck Surg. 2017, 157, 178–189. [Google Scholar] [CrossRef]
- Burton, M.J.; Glasziou, P.P.; Chong, L.Y.; Venekamp, R.P. Tonsillectomy or adenotonsillectomy versus non-surgical treatment for chronic/recurrent acute tonsillitis. Cochrane Database Syst. Rev. 2014, 2014, CD001802. [Google Scholar] [CrossRef]
- Alho, O.-P.; Koivunen, P.; Penna, T.; Teppo, H.; Koskela, M.; Luotonen, J. Tonsillectomy versus watchful waiting in recurrent streptococcal pharyngitis in adults: Randomised controlled trial. Br. Med. J. 2007, 334, 939–941. [Google Scholar] [CrossRef]
- Andreou, N.; Hadjisymeou, S.; Panesar, J. Does tonsillectomy improve quality of life in adults? A systematic literature review. J. Laryngol. Otol. 2013, 127, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Koskenkorva, T.; Koivunen, P.; Koskela, M.; Niemela, O.; Kristo, A.; Alho, O.-P. Short-term outcomes of tonsillectomy in adult patients with recurrent pharyngitis: A randomized controlled trial. Can. Med Assoc. J. 2013, 185, E331–E336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Burton, M.J.; Pollard, A.J.; Ramsden, J.D.; Chong, L.Y.; Venekamp, R.P. Tonsillectomy for periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis syndrome (PFAPA). Cochrane Database Syst. Rev. 2014, 11, CD008669. [Google Scholar] [CrossRef] [PubMed]
- Byars, S.G.; Stearns, S.C.; Boomsma, J.J. Association of long-term risk of respiratory, allergic, and infectious diseases with removal of adenoids and tonsils in childhood. JAMA Otolaryngol. Head Neck Surg. 2018, 144, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Senska, G.; Atay, H.; Pütter, C.; Dost, P. Long-term results from tonsillectomy in adults. Dtsch. Aerzteblatt Int. 2015, 112, 849–855. [Google Scholar] [CrossRef]
- Greig, S.R. Current perspectives on the role of tonsillectomy. J. Paediatr. Child Health. 2017, 53, 1065–1070. [Google Scholar] [CrossRef]
- Amoils, M.; Chang, K.W.; Saynina, O.; Wise, P.H.; Honkanen, A. Postoperative complications in pediatric tonsillectomy and adenoidectomy in ambulatory vs inpatient settings. JAMA Otolaryngol. Head Neck Surg. 2016, 142, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Surgical Operations and Procedures Performed in Hospitals by ICD-9-CM. Available online: https://ec.europa.eu/eurostat/statistics-explained/pdfscache/37391.pdf (accessed on 28 January 2021).
- Huang, C.-G.; Huang, Y.-S.; Chen, N.-H.; Li, H.-Y.; Fang, T.-J.; Hsu, J.-F.; Lai, H.-C.; Chen, J.-Y.; Lee, L.-A. Relationships among and predictive values of obesity, inflammation markers, and disease severity in pediatric patients with obstructive sleep apnea before and after adenotonsillectomy. J. Clin. Med. 2020, 9, 579. [Google Scholar] [CrossRef]
- Templier, L.; Rossi, C.; Miguez, M.; Pérez, J.D.L.C.; Curto, A.; Albaladejo, A.; Vich, M.L. Combined surgical and orthodontic treatments in children with OSA: A systematic review. J. Clin. Med. 2020, 9, 2387. [Google Scholar] [CrossRef]
- Lewit, K. Manipulative Therapy in Rehabilitation of the Locomotor System, 3rd ed.; Butterworth Heinemann: Oxford, UK, 1999; pp. 21–23, 282–283. [Google Scholar]
- Kovanur-Sampath, K.; Mani, R.; Cotter, J.; Gisselman, A.S.; Tumilty, S. Changes in biochemical markers following spinal manipulation-a systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2017, 29, 120–131. [Google Scholar] [CrossRef]
- Plaza-Manzano, G.; Molina-Ortega, F.; Lomas-Vega, R.; Martínez-Amat, A.; Achalandabaso-Ochoa, A.; Hita-Contreras, F. Changes in biochemical markers of pain perception and stress response after spinal manipulation. J. Orthop. Sports Phys. Ther. 2014, 44, 231–239. [Google Scholar] [CrossRef]
- Nielsen, S.M.; Tarp, S.; Christensen, R.; Bliddal, H.; Klokker, L.; Henriksen, M. The risk associated with spinal manipulation: An overview of reviews. Syst. Rev. 2017, 6, 64. [Google Scholar] [CrossRef]
- Gorrell, L.M.; Engel, R.M.; Brown, B.; Lystad, R.P. The reporting of adverse events following spinal manipulation in randomized clinical trials—A systematic review. Spine J. 2016, 16, 1143–1151. [Google Scholar] [CrossRef]
- Kranenburg, H.; Schmitt, M.; Puentedura, E.; Luijckx, G.; van der Schans, C. Adverse events associated with the use of cervical spine manipulation or mobilization and patient characteristics: A systematic review. Musculoskelet. Sci. Pract. 2017, 28, 32–38. [Google Scholar] [CrossRef]
- Carnes, D.; Mars, T.S.; Mullinger, B.; Froud, R.; Underwood, M. Adverse events and manual therapy: A systematic review. Man. Ther. 2010, 15, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, A.; Coupland, R.E. The innervation of the adrenal gland. IV. Innervation of the rat adrenal medulla from birth to old age. A descriptive and quantitative morphometric and biochemical study of the innervation of chromaffin cells and adrenal medullary neurons in Wistar rats. J. Anat. 1990, 169, 209–236. [Google Scholar] [PubMed]
- Kesse, W.K.; Parker, T.L.; Coupland, R.E. The innervation of the adrenal gland. I. The source of pre- and postganglionic nerve fibres to the rat adrenal gland. J. Anat. 1988, 157, 33–41. [Google Scholar] [PubMed]
- Mohamed, A.A.; Parker, T.L.; Coupland, R.E. The innervation of the adrenal gland. II. The source of spinal afferent nerve fibres to the guinea-pig adrenal gland. J. Anat. 1988, 160, 51–58. [Google Scholar]
- Testud, L. Tratado de Anatomía Humana (Tomo III); Salvat: Barcelona, Spain, 1978; p. 1131. [Google Scholar]
- Malakasioti, G.; Alexopoulos, E.I.; Varlami, V.; Chaidas, K.; Liakos, N.; Gourgoulianis, K.; Kaditis, A.G. Low morning serum cortisol levels in children with tonsillar hypertrophy and moderate-to-severe OSA. Sleep 2013, 36, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; De Kloet, E.R.; Dijk, D.-J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The functional and clinical significance of the 24-h rhythm of circulating glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef]
- Lightman, S.L.; Birnie, M.T.; Conway-Campbell, B.L. Dynamics of ACTH and cortisol secretion and implications for disease. Endocr. Rev. 2020, 1–21. [Google Scholar] [CrossRef]
- Sampath, K.K.; Katare, R.; Tumilty, S. Stress axis and osteopathy: A dual hormone approach. Int. J. Osteopat. Med. 2019, 1–7. [Google Scholar] [CrossRef]
- Sampath, K.K.; Botnmark, E.; Mani, R.; Cotter, J.D.; Katare, R.; Munasinghe, P.E.; Tumilty, S. Neuroendocrine response following a thoracic spinal manipulation in healthy men. J. Orthop. Sports Phys. Ther. 2017, 47, 617–627. [Google Scholar] [CrossRef]
- Luceño Mardones, A. Tratamiento de la amigdalitis mediante manipulación osteopática de las vértebras T8 a T12 hipomóviles. Rev. Científica Ter. Man. Osteopat. 2004, 17, 13–32. [Google Scholar]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, C.; Peraza Cruces, K.R.; Rodrigáñez Riesco, L.; García-Vela, J.; Sanz-Fernández, R. Immunomodulatory effect of Polypodium leucotomos (Anapsos) in child palatine tonsil model. Int. J. Pediatr. Otorhinolaryngol. 2018, 107, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Fisher, L.R.; Alvar, B.A.; Maher, S.F.; Cleland, J.A. Short-term effects of thoracic spine thrust manipulation, exercise, and education in individuals with low back pain: A randomized controlled trial. J. Orthop. Sports Phys. Ther. 2020, 50, 24–32. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.F.; Liebano, R.E.; Costa, L.D.C.M.; Rissato, L.L.; Costa, L.O.P. Immediate effects of region-specific and non-region-specific spinal manipulative therapy in patients with chronic low back pain: A randomized controlled trial. Phys. Ther. 2013, 93, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Hartman, L. Handbook of Osteopathic Technique, 3rd ed.; Stanley Thornes: Cheltenham, UK, 1997; pp. 116–130. [Google Scholar]
- Le Corre, F.; Rageot, E. Manipulaciones Vertebrales; Masson: Barcelona, Spain, 1990; pp. 143–147. [Google Scholar]
- Maigne, R. Manipulaciones Columna Vertebral y Extremidades; Norma: Madrid, Spain, 1997; p. 54. [Google Scholar]
- Brueton, V.C.; Tierney, J.; Stenning, S.; Harding, S.; Meredith, S.; Nazareth, I.; Rait, G. Strategies to improve retention in randomised trials. Cochrane Database Syst. Rev. 2013, MR000032–126. [Google Scholar] [CrossRef]
- Elnicki, D.M.; The TELI Group; Ogden, P.; Flannery, M.; Hannis, M.; Cykert, S. Telephone medicine for internists. J. Gen. Intern. Med. 2000, 15, 337–343. [Google Scholar] [CrossRef]
- Downes, M.J.; Mervin, M.C.; Byrnes, J.M.; Scuffham, P.A. Telephone consultations for general practice: A systematic review. Syst. Rev. 2017, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Flodgren, G.; Rachas, A.; Farmer, A.J.; Inzitari, M.; Shepperd, S. Interactive telemedicine: Effects on professional practice and health care outcomes. Cochrane Database Syst. Rev. 2015, 2015, CD002098. [Google Scholar] [CrossRef]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 3rd ed.; Sage Publication: London, UK, 2013. [Google Scholar]
- Fernández-Domínguez, J.C.; Escobio-Prieto, I.; Sesé-Abad, A.; Jiménez-López, R.; Romero-Franco, N.; Oliva-Pascual-Vaca, Á. Health sciences—Evidence based practice questionnaire (HS-EBP): Normative data and differential profiles in spanish osteopathic professionals. Int. J. Environ. Res. Public Health 2020, 17, 8454. [Google Scholar] [CrossRef] [PubMed]
- Capper, R.; Canter, R.J. How well do parents recognize the difference between tonsillitis and other sore throats? Clin. Otolaryngol. 2001, 26, 458–464. [Google Scholar] [CrossRef]
- McCambridge, J.; Witton, J.; Elbourne, D.R. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014, 67, 267–277. [Google Scholar] [CrossRef]
- Georgalas, C.C.; Tolley, N.S.; Narula, P.A. Tonsillitis. BMJ Clin Evid. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Lildholdt, T.; Doessing, H.; Lyster, M.; Outzen, K. The natural history of recurrent acute tonsillitis and a clinical trial of azithromycin for antibiotic prophylaxis. Clin. Otolaryngol. Allied Sci. 2003, 28, 371–373. [Google Scholar] [CrossRef]
- Cerritelli, F.; Verzella, M.; Cicchitti, L.; D’Alessandro, G.; Vanacore, N. The paradox of sham therapy and placebo effect in osteopathy: A systematic review. Medicine 2016, 95, e4728. [Google Scholar] [CrossRef]
- Bishop, F.L.; Coghlan, B.; Geraghty, A.W.; Everitt, H.; Little, P.; Holmes, M.M.; Seretis, D.; Lewith, G. What techniques might be used to harness placebo effects in non-malignant pain? A literature review and survey to develop a taxonomy. BMJ Open 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Benedetti, F. Placebo and the new physiology of the doctor-patient relationship. Physiol. Rev. 2013, 93, 1207–1246. [Google Scholar] [CrossRef]
- Mora, R.; Salami, A.; Mora, F.; Cordone, M.P.; Ottoboni, S.; Passali, G.C.; Barbieri, M. Efficacy of cefpodoxime in the prophylaxis of recurrent pharyngotonsillitis. Int. J. Pediatr. Otorhinolaryngol. 2003, 67, S225–S228. [Google Scholar] [CrossRef]
- Sanders, A.E.; Slade, G.D.; Fillingim, R.B.; Ohrbach, R.; Arbes, S.J.; Tchivileva, I.E. Effect of treatment expectation on placebo response and analgesic efficacy: A secondary aim in a randomized clinical trial. JAMA Netw. Open 2020, 3, e202907. [Google Scholar] [CrossRef]
- Olsson, B. Effect of patients’ expectations on recovery from acute tonsillitis. Fam. Pr. 1989, 6, 188–192. [Google Scholar] [CrossRef]
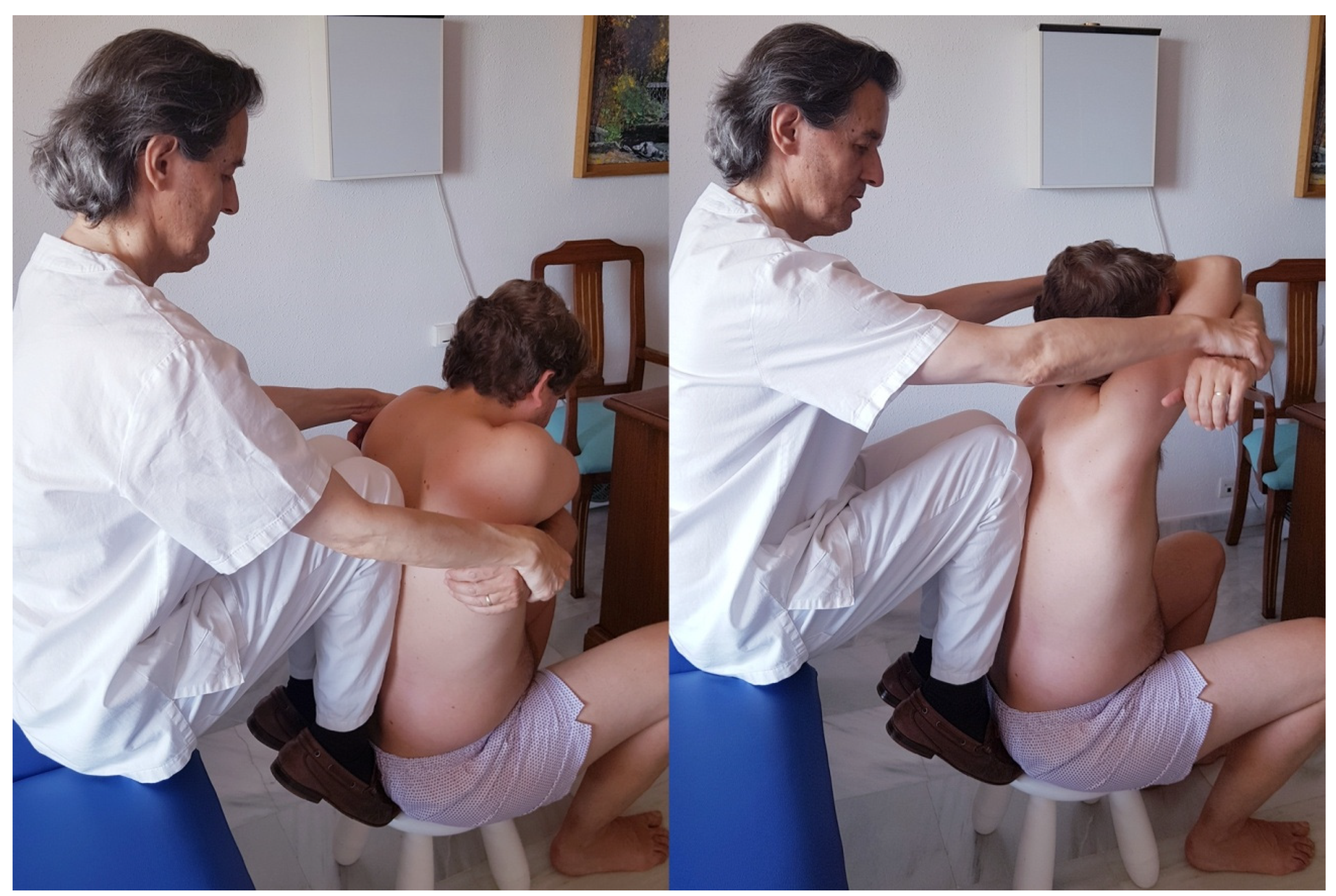
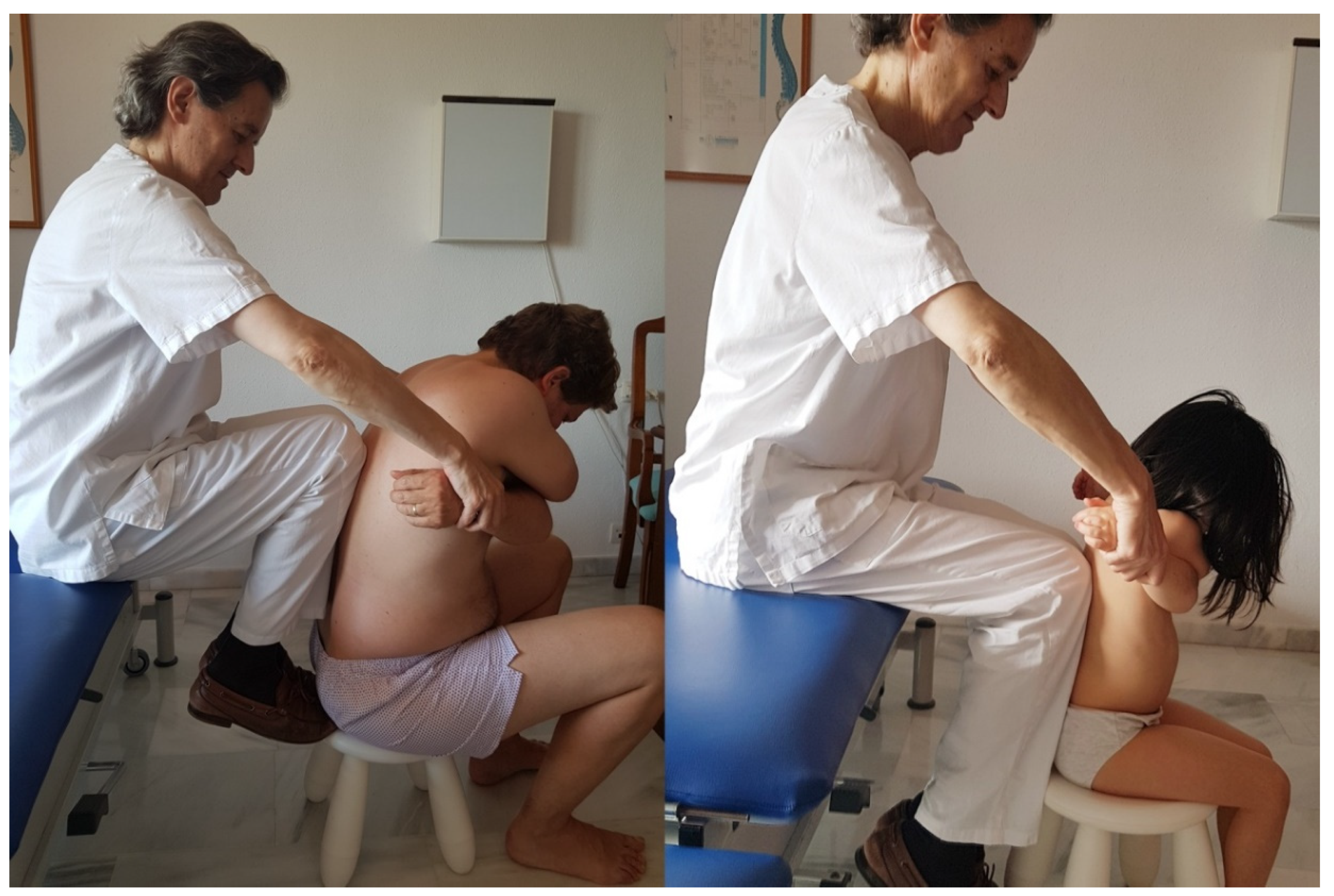
| Control Control Group | Osteopathic Manipulative Group | |||||
|---|---|---|---|---|---|---|
| Characteristics | Mean/n | SD/% | Mean/n | SD/% | p-Value | |
| Age (years) (n = 120) | 25.30 | 15.11 | 22.64 | 14.73 | 0.439 | |
| N° episodes 2 years before (n = 116) | 4.75 | 3.95 | 4.21 | 3.61 | 0.435 | |
| N° episodes 1 year before (n = 120) | 5.45 | 4.13 | 5.16 | 3.89 | 0.781 | |
| Sex (n =120) | Male | 17 | 42.5% | 33 | 41.3% | 0.896 |
| Female | 23 | 57.5% | 47 | 58.8% | ||
| Fever (n =120) | No (Temperature <37.5 °C) | 10 | 25.0% | 21 | 26.3% | 0.883 |
| Yes (Temperature ≥37.5 °C) | 30 | 75.0% | 59 | 73.8% | ||
| Pain or odinophagy (n = 119) | No | 0 | 0.0% | 4 | 5.1% | 0.148 |
| Yes | 40 | 100.0% | 75 | 94.9% | ||
| Cough currently (n = 118) | No | 22 | 55.0% | 32 | 41.0% | 0.149 |
| Yes | 18 | 45.0% | 46 | 49.0% | ||
| Levels of tonsilar hypertrophy (n = 115) | The pillars do not protrude | 1 | 2.6% | 5 | 6.7% | 0.760 |
| Occupy less than 25% of the space | 9 | 23.1% | 16 | 21.3% | ||
| Occupy 25–49% of the space | 12 | 30.8% | 20 | 26.7% | ||
| Occupy 50–74% | 14 | 35.9% | 31 | 41.3% | ||
| Occupy >75% | 3 | 7.7% | 3 | 4.0% | ||
| Mucus days before the episode (n = 119) | No | 16 | 40.0% | 28 | 35.4% | 0.627 |
| Yes | 24 | 60.0% | 51 | 64.6% | ||
| Pain or infection of ears (n = 119) | No | 25 | 64.1% | 47 | 58.8% | 0.575 |
| Yes | 14 | 35.9% | 33 | 42.3% | ||
| Usual nasal voice (n = 119) | No | 32 | 80.0% | 58 | 73.4% | 0.429 |
| Yes | 8 | 20.0% | 21 | 26.6% | ||
| Nasal voice in the episode (n = 119) | No | 6 | 15.0% | 11 | 13.9% | 0.874 |
| Yes | 34 | 85.0% | 68 | 86.1% | ||
| Snore usually (n = 117) | No | 22 | 56.4% | 56 | 71.8% | 0.096 |
| Yes | 17 | 43.6% | 22 | 29.2% | ||
| Snore during the episode (n = 116) | No | 11 | 28.2% | 30 | 39.0% | 0.252 |
| Yes | 28 | 71.8% | 47 | 61.0% | ||
| Take prescribed antibiotics (n = 120) | No | 18 | 45.0% | 37 | 46.3% | 0.897 |
| Yes | 22 | 55.0% | 43 | 53.8% | ||
| Take prescribed NSAID (n = 119) | No | 6 | 15.0% | 22 | 27.8% | 0.119 |
| Yes | 34 | 85.0% | 57 | 72.2% | ||
| Take prescribed paracetamol (n = 120) | No | 15 | 37.5% | 38 | 47.5% | 0.298 |
| Yes | 25 | 62.5% | 42 | 52.5% | ||
| Pultous tonsilitis (n = 117) | No | 24 | 61.5% | 49 | 62.8% | 0.893 |
| Yes | 15 | 38.5% | 29 | 37.2% | ||
| Palpable adenitis over 2 cm (n = 116) | No | 5 | 13.2% | 21 | 26.9% | 0.095 |
| Yes | 33 | 86.8% | 57 | 73.1% | ||
| Station (n = 120) | Spring | 8 | 20.0% | 13 | 16.3% | 0.673 |
| Summer | 8 | 20.0% | 12 | 15.0% | ||
| Autumn | 9 | 22.5% | 26 | 32.5% | ||
| Winter | 15 | 37.5% | 29 | 36.3% | ||
| Results 1st Week | Results 1st Week Grouped | |||||
|---|---|---|---|---|---|---|
| Group | E | G | M | P | E + G (Resolution < 48 h) | M + P (Resolution ≥ 48 h) |
| CG | 5 (12.5%) | 12 (30.0%) | 14 (35.0%) | 9 (22.5%) | 17 (42.5%) | 23 (57.5%) |
| EG | 20 (25.0%) | 31 (38.75%) | 11 (13.75%) | 18 (22.5%) | 51 (63.75%) | 29 (36.25%) |
| p value | χ2 (3) = 8.35, p = 0.039, V = 0.264 | χ2 (1) = 4.90, p = 0.022, V = 0.202 | ||||
| Children | ||||||
| CG | 4 (30.7%) | 4 (30.7%) | 4 (30.7%) | 1 (7.7%) | 8 (61.5%) | 5 (38.5%) |
| EG | 9 (31%) | 7 (24.1%) | 5 (17.2%) | 8 (27.6%) | 16 (55.2%) | 13 (44.8%) |
| p value | χ2 (3) = 2.57, p = 0.462 | χ2 (1) = 0.15, p = 0.700 | ||||
| Adults | ||||||
| CG | 1 (3.7%) | 8 (29.6%) | 10 (37%) | 8 (29.6%) | 9 (33.3%) | 18 (66.6%) |
| EG | 11 (21.5%) | 24 (47%) | 6 (11.7%) | 10 (19.6%) | 35 (68.6%) | 16 (31.4%) |
| p value | χ2 (3) = 11.23, p = 0.011, V = 0.38 | χ2 (1) = 8.94, p = 0.003, V = 0.34 | ||||
| Control Group (n = 35) | Osteopathic Manipulative Group (n = 66) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD (95% CI) | Mean | SD (95% CI) | p-Value (Time) | p-Value (Group Time) | p-Value (Time) | |
| Two years before | 4.49 | 3.09 (3.19–5.78) | 4.20 | 3.73 (3.25–5.14) | 0.721 | 0.247 | <0.001 |
| One year before | 5.23 | 4.30 (3.90–6.55) | 4.86 | 3.76 (3.89–5.83) | 0.660 | ||
| After intervention | 2 | 2.12 (1.33–2.66) | 0.80 | 1.88 (0.32–1.28) | 0.005 | ||
| Two years before vs. after intervention | −2.48 | 3.59 (−3.84–−1.12) | −3.39 | 3.15 (−4.38–−2.40) | CG p < 0.001; OMG p < 0.001 | ||
| One year before vs. after intervention | −3.22 | 3.52 (−4.55–−1.89) | −4.06 | 3.06 (−5.02–−3.09) | CG p < 0.001; OMG p < 0.001 | ||
| Control Group (n = 40) | Osteopathic Manual Group (n = 79) | |||||
|---|---|---|---|---|---|---|
| Count | % | Count | % | p Value (Exact Sig. 2-Sided) | Chi-Square | |
| Recurrence | 31 | 77.5% | 31 | 39.2% | <0.001 | χ2 (1) = 15.57 |
| No recurrence | 9 | 22.5% | 48 | 60.8% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luceño-Mardones, A.; Luceño-Rodríguez, I.; Rodríguez-López, E.S.; Oliva-Pascual-Vaca, J.; Rosety, I.; Oliva-Pascual-Vaca, Á. Effects of Osteopathic T9–T10 Vertebral Manipulation in Tonsillitis: A Randomized Clinical Trial. Healthcare 2021, 9, 394. https://doi.org/10.3390/healthcare9040394
Luceño-Mardones A, Luceño-Rodríguez I, Rodríguez-López ES, Oliva-Pascual-Vaca J, Rosety I, Oliva-Pascual-Vaca Á. Effects of Osteopathic T9–T10 Vertebral Manipulation in Tonsillitis: A Randomized Clinical Trial. Healthcare. 2021; 9(4):394. https://doi.org/10.3390/healthcare9040394
Chicago/Turabian StyleLuceño-Mardones, Agustín, Irene Luceño-Rodríguez, Elena Sonsoles Rodríguez-López, Jesús Oliva-Pascual-Vaca, Ignacio Rosety, and Ángel Oliva-Pascual-Vaca. 2021. "Effects of Osteopathic T9–T10 Vertebral Manipulation in Tonsillitis: A Randomized Clinical Trial" Healthcare 9, no. 4: 394. https://doi.org/10.3390/healthcare9040394
APA StyleLuceño-Mardones, A., Luceño-Rodríguez, I., Rodríguez-López, E. S., Oliva-Pascual-Vaca, J., Rosety, I., & Oliva-Pascual-Vaca, Á. (2021). Effects of Osteopathic T9–T10 Vertebral Manipulation in Tonsillitis: A Randomized Clinical Trial. Healthcare, 9(4), 394. https://doi.org/10.3390/healthcare9040394







