Sudden Death from Primary Cerebral Melanoma: Clinical Signs and Pathological Observations
Abstract
1. Introduction
2. Materials and Methods
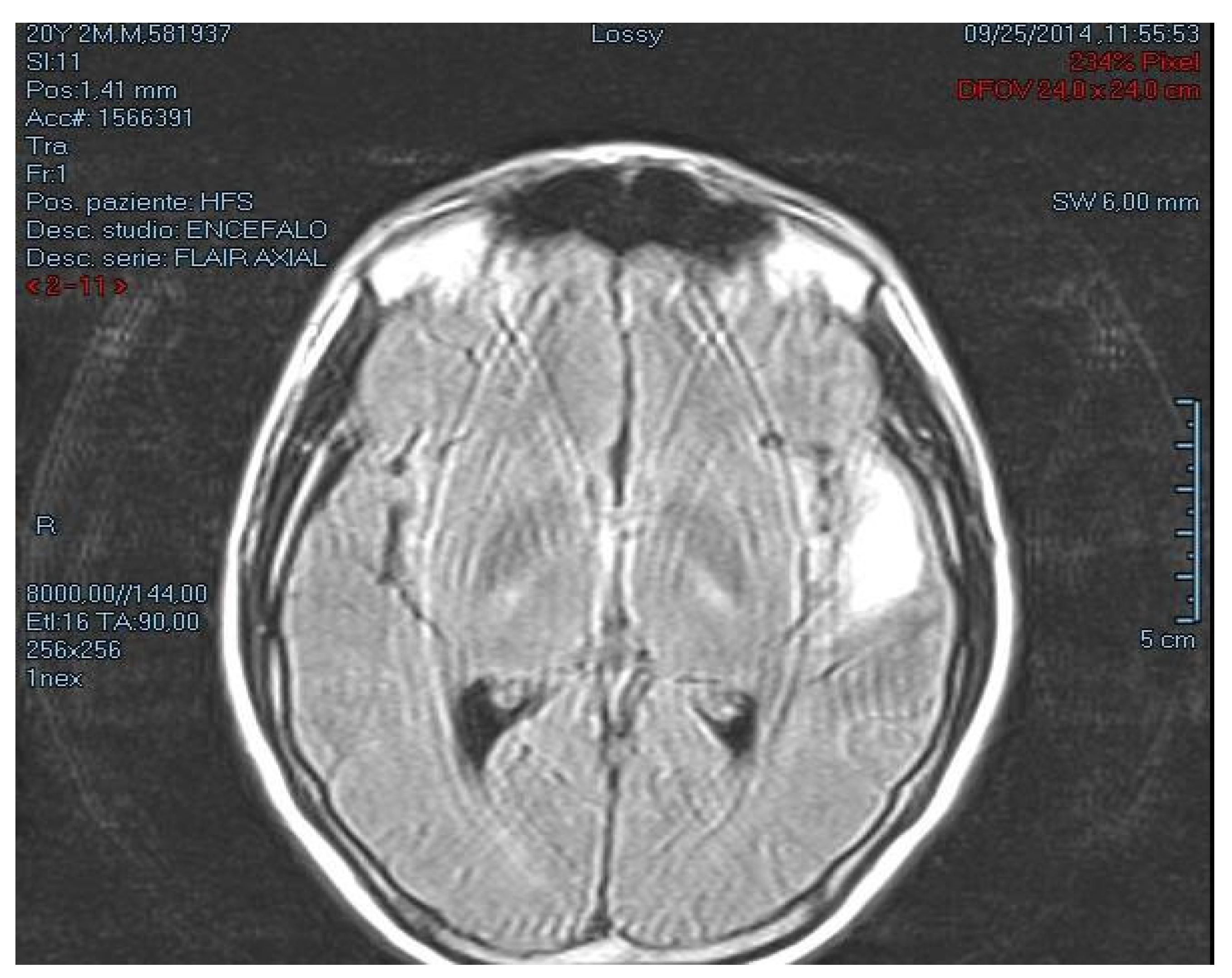
2.1. Case Description
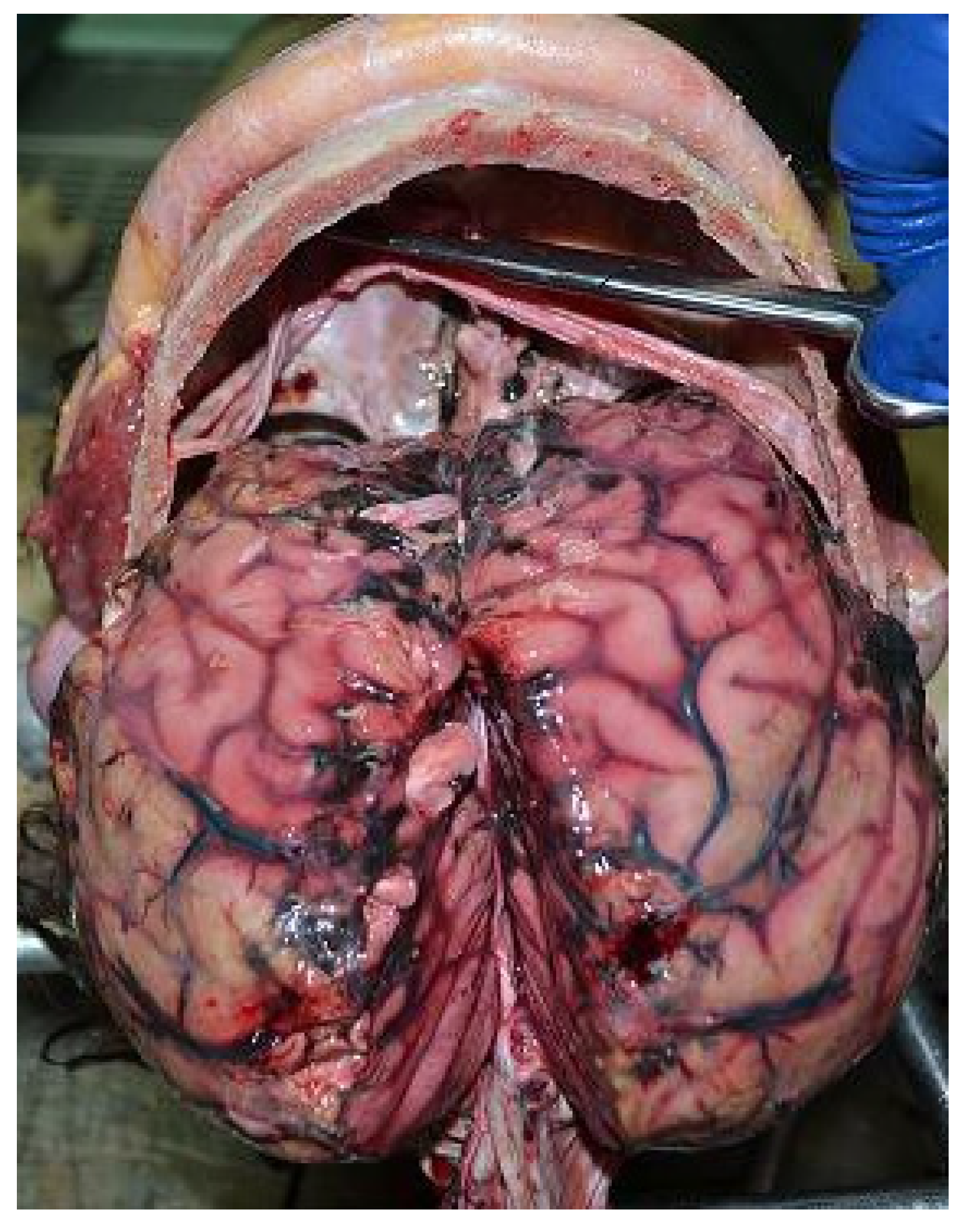
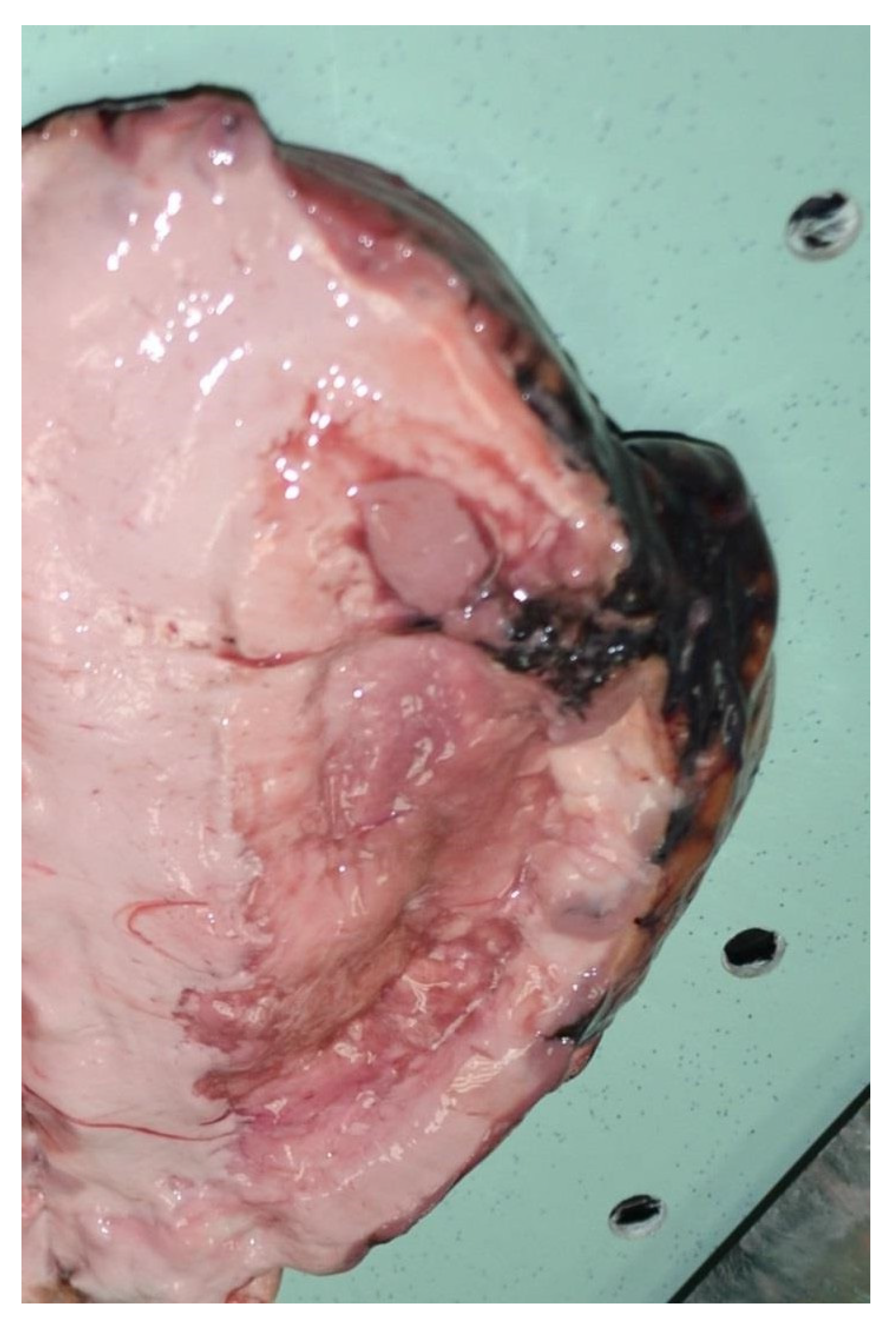
2.2. Autopsy Findings
2.3. Histological Analysis
3. Systematic Review
3.1. Inclusion and Exclusion Criteria
3.2. Quality Assessment and Data Extraction
3.3. Characteristics of Eligible Studies
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethical Approval and Consent to Participate
References
- Back, M.; Graham, D.I. Sudden unexplained death in adults caused by intracranial pathology. J. Clin. Pathol. 2002, 55, 44–50. [Google Scholar]
- Eberhart, C.G.; Morrison, A.; Gyure, K.A.; Frazier, J.; Smialek, J.E.; Troncoso, J.C. Drecreasing incidence of sudden death due to undiagnosed primary central nervous system tumors. Arch. Pathol. Lab. Med. 2001, 125, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Matschke, J.; Tsokos, M. Sudden unexpected death due to undiagnosed glioblastoma: Report of three cases and review of the literature. Int. J. Leg. Med. 2005, 119, 280–284. [Google Scholar] [CrossRef]
- Gleckman, A.M.; Smith, T.W. Unexpected death from primary posterior fossa tumors. Am. J. Forensic Med. Pathol. 1998, 19, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Sutton, J.T.; Cummings, P.M.; Ross, G.W.; Lopes, M.B. Sudden death of a 7-year-old boy due to undiagnosed glioblastoma. Am. J. Forensic Med. Pathol. 2010, 31, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Riezzo, I.; Zamparese, R.; Neri, M.; De Stefano, F.; Parente, R.; Pomara, C.; Turillazzi, E.; Ventura, F.; Fineschi, V. Sudden, unexpected death due to glioblastoma: Report of three fatal cases and review of literature. Diagn. Pathol. 2013, 8, 73. [Google Scholar] [CrossRef]
- Manousaki, M.; Papadaki, H.; Papavdi, A.; Kranioti, E.F.; Mylonakis, P.; Varakis, S.; Michalodimitrakis, M. Sudden unexpected death from oligodendroglioma: A case report and review of the literature. Am. J. Forensic Med. Pathol. 2011, 32, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Vougiouklakis, T.; Mitselou, A.; Agnantis, N.J. Sudden death due to primary intracranial neoplasm—A forensic autopsy study. Anticancer Res. 2006, 26, 2463–2466. [Google Scholar] [PubMed]
- Harrison, W.T.; Bouldin, T.; Buckley, A.; Estrada, J.; McLondon, R.; Jansen, K. Sudden unexpected death in a child from anaplastic ependymoma. Am. J. Forensic Med. Pathol. 2019, 40, 275–278. [Google Scholar] [CrossRef] [PubMed]
- Sidlo, J.; Sidlova, H. Sudden and unexpected death due to intracranial sellar extramedullary plasmacytoma. J. Forensic Leg. Med. 2019, 61, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Cuoco, J.A.; Rogers, C.M.; Busch, C.M.; Benko, M.J.; Apfel, L.S.; Elias, Z. Postexercise death due to hemorrhagic colloid cyst of third ventricle: Case report and literature review. World Neurosurg. 2019, 123, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Turillazzi, E.; Bello, S.; Neri, M.; Riezzo, I.; Fineschi, V. Colloid cyst of the third ventricle, hypothalamus, and heart: A dangerous link for sudden death. Diagn. Pathol. 2012, 7, 144–148. [Google Scholar] [CrossRef]
- Gitto, L.; Bolino, G.; Cina, S.J. Sudden unexpected deaths due to intracranial meningioma: Presentation of six fatal cases, review of the literature and a discussion of the mechanism of death. J. Forensic Sci. 2018, 63, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Durao, H.C.; Veiga, G.M.; Pedro, N.; Goncalves, M.M.; Pedrosa, F. Sudden death associated with melanoma brain metastates. J. Pathol. Nepal 2018, 8, 1412–1415. [Google Scholar]
- Glitza, I.C.; Heimberger, A.B.; Sulman, E.P.; Davies, M.A. Prognostic factors for survival in melanoma patients with brain metastates. In Brain Metastates from Primary Tumors; Hayat, M., Ed.; Academic Press: New York, NY, USA, 2016; pp. 267–292. [Google Scholar]
- Suranagi, V.V.; Maste, P.; Malur, P.R. Primary intracranial malignant melanoma: A rare case with review of literature. Asian J. Neurosurg. 2015, 10, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Tosaka, M.; Tamura, M.; Oriuchi, N. Cerebrospinal fluid immunocytochemical analysis and neuroimaging in the diagnosis of primary leptomenigeal melanoma: Case report. J. Neurosurg. 2001, 94, 528–532. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Aissaoui, A.; Mosrati, M.A.; Moussa, A.; Belhaj, M.; Bougattas, M.; Zakhama, A.; Chadly, A. Sudden death and primary leptomeningeal melanocytosis: A case report with an autopsy diagnosis. Am. J. Forensic Med. Pathol. 2015, 36, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, A.; Meteoglu, I.; Tataroglu, C.; Akyol, A. Primary diffuse leptomeningeal oligodendrogliomatosis causing sudden death. J. Neurooncol. 2007, 81, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Olar, A.; Fuller, C. A Pediatric Case of Diffuse Glioma Diagnosed at Autopsy. Acad. Forensic Pathol. 2017, 7, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Havlik, D.M.; Becher, M.W.; Nolte, K.B. Sudden death due to primary diffuse leptomeningeal gliomatosis. J. Forensic Sci. 2001, 46, 392–395. [Google Scholar] [CrossRef] [PubMed]
- DiMaio, S.M.; DiMaio, V.J.; Kirkpatrick, J.B. Sudden, unexpected deaths due to primary intracranial neoplasms. Am. J. Forensic Med. Pathol. 1980, 1, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.; Sng, I. A case of sudden death from primary intracranial germinoma complicated by microvascular disease of the heart. Forensic Sci. Int. 2003, 137, 1–5. [Google Scholar] [CrossRef]
- Shiferaw, K.; Pizzolato, G.P.; Perret, G.; La Harpe, R. Sudden, unexpected death due to undiagnosed frontal glioblastoma in a schizophrenic patient. Forensic Sci. Int. 2006, 158, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Matturri, L.; Ottaviani, G.; Rossi, L. Sudden and unexpected infant death due to an hemangioendothelioma located in the medulla oblongata. Adv. Clin. Pathol. 1999, 3, 29–33. [Google Scholar]
- Matsumoto, H.; Yamamoto, K. A case of sudden death by undiagnosed glioblastoma multiforme. Nihon Hoigaku Zasshi Jpn. J. Leg. Med. 1993, 47, 336–339. [Google Scholar]
- Prahlow, J.A.; Teot, L.A.; Lantz, P.E.; Stanton, C.A. Sudden death in epilepsy due to an isolated subependymal giant cell astrocytoma of the septum pellucidum. Am. J. Forensic Med. Pathol. 1995, 16, 30–37. [Google Scholar] [CrossRef]
- Shields, L.B.; Balko, M.G.; Hunsaker, J.C. Sudden and unexpected death from pituitary tumor apoplexy. J. Forensic Sci. 2012, 57, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Reyes, R.; Dragovic, L.; Eriksson, A. Sudden unexpected death resulting from previously nonsymptomatic subependymoma. Am. J. Forensic Med. Pathol. 2002, 23, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Frost, J.L.; Schochet, S.S., Jr. Sudden, unexpected death in a 5-year-old boy with an unusual primary intracranial neoplasm. Ganglioglioma of the medulla. Am. J. Forensic Med. Pathol. 1987, 8, 148–152. [Google Scholar] [CrossRef]
- Virchow, R. Pigment und diffuse melanoses der arachnoides. Virchows Arch. 1859, 16, 180–182. [Google Scholar] [CrossRef]
- Tang, K.; Kong, X.; Mao, G.; Quin, M.; Znov, H.; Zhou, L.; Nie, Q.; Xu, Y.; Du, S. Primary cerebral malignant melanoma. A case report with literature review. Medicine 2017, 96, e5805. [Google Scholar] [CrossRef]
- Liubinas, S.V.; Maartens, N.; Drummond, K.J. Primary melanocytic neoplasm of the central nervous system. J. Clin. Neurosci. 2010, 17, 1227–1232. [Google Scholar] [CrossRef]
- Hayward, R.D. Malignant melanoma and cerebral nervous system. A guide for classification based on the clinical findings. J. Neurol. Neurosurg. Psychiatry 1976, 39, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Gempt, J.; Buchmann, N.; Grams, A.E. Black brain: Transformation of a melanocytoma with diffuse melanocytosis into a primary cerebral melanoma. J. Neurooncol. 2011, 102, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Bydon, A.; Guttierez, J.A.; Mahmood, A. Meningeal melanocytoma: An aggressive course for a benign tumor. J. Neurooncol. 2003, 64, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Rades, D.; Schild, S.E. Dose responde relationship for fractionated irradiation in the treatment of spinal meningeal melanocytomas: A review of the literature. J. Neurooncol. 2006, 77, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Troya-Castilla, M.; Rocha-Romero, S.; Crochon-Gonzales, Y.; Marquez-Rivas, F.J. Primary cerebral malignant melanoma in insular region with extracranial metastasis: Case report and review literature. World J. Surg. Oncol. 2016, 14, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Brachmann, D.E.; Doherty, J.K. CPA melanoma: Diagnosis and management. Otol. Neurotol. 2007, 28, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, L.; Alapatt, J.; Govindan-Sreekumar, T. Primary cerebellopontine angle melanoma: A case report and review. Turk. Neurosurg. 2012, 22, 469–474. [Google Scholar] [CrossRef]
- Arantes, M.; Castro, A.F.; Romao, H.; Meireles, P.; Garcia, R.; Honavar, M.; Vaz Ar Resende, M. Primary pineal malignant melanoma: Case report and literature review. Clin. Neurosurg. 2011, 113, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Greco Castro, S.; Soffietti, R.; Bradac, G.B.; Boldorini, R. Primitive cerebral melanoma: Case report and review of the literature. Surg. Neurol. 2001, 55, 163–168. [Google Scholar] [CrossRef]
- Quillo-Olvera, J.; Uribe-Olalde, J.S.; Alcantara-Gomez, L.A.; Rejon-Perez, J.D.; Palomera-Gomez, H.G. Primary malignant melanoma of the central nervous system: A diagnostic challenge. Cir. Cir. 2015, 83, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Wadasadawala, T.; Trivedi, S.; Gupta, T.; Epari, S.; Jalari, R. The diagnostic dilemma of primary central nervous system melanoma. J. Clin. Neurosci. 2010, 17, 1014–1017. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.H.; Wang, L.C.; Lee, E.J. Primary intracranial melanoma. J. Cancer Res. Pract. 2017, 4, 23–26. [Google Scholar] [CrossRef]
- Berzero, G.; Diamanti, L.; Di Stefano, A.L.; Bini, P.; Franciotta, D.; Imarisio, I.; Pedrazzoli, P.; Magrassi, L.; Morbini, P.; Farina, L.M.; et al. Meningeal Melanomatosis: A challenge for timely diagnosis. BioMed Res. Int. 2015, 2015, 6. [Google Scholar] [CrossRef]
- Gleissner, B.; Chamberlain, M.C. Neoplastic meningitis. Lancet Neurol. 2006, 5, 443–452. [Google Scholar] [CrossRef]
- Sarnast, A.H.; Mujtaba, B.; Bhat, A.R.; Kirmani, A.R.; Tanki, H.N. A unique case of primary intracranial melanoma. Asian J. Neurosurg. 2018, 13, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.A.; Liu, P.; Mcintyre, S.; Kim, K.B.; Papadopoulos, N.; Hwu, W.J.; Bedikian, A. Prognostic factors for survival in melanoma patients with brain metastates. Cancer 2011, 117, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Hwu, W.J.; Panageas, K.S. Brain and leptomenigeal metastates from cutaneous melanoma: Survival outcomes based on clinical features. Neurol. Oncol. 2008, 10, 199–207. [Google Scholar] [CrossRef]
- Cohen, J.V.; Tawbi, H.; Margolin, K.A.; Amravadi, R.; Bosenberg, M.; Brastianos, P.K.; Chiang, V.L.; De Groot, J.; Glitza, I.C.; Herlin, M. Melanoma central nervous system metastates: Current approaches, challenges, and opportunities. Pigment Cell Melanoma Res. 2016, 29, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.V.; Alomari, A.K.; Vortmeyer, A.O.; Jilaveanu, L.B.; Gold-Berg, S.B.; Mahajan, A.; Chiang, V.L.; Kluger, H.M. Melanoma brain metastasis pseudoprogression after pembrolizumab treatment. Cancer Immunol. Res. 2016, 4, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Braeuer, R.R.; Watson, I.R.; Wu, C.S.; Mobley, A.K.; Kamiya, T.; Shoshan, E.; Bar-Eli, M. Why is melanoma so metastatic? Pigment Cell Melanoma Res. 2013, 27, 19–36. [Google Scholar] [CrossRef]
- Rodriguez y Baena, R.; Gaetani, P.; Danova, M. Primary solitary intracranial melanoma: Case report and review of the literature. Surg. Neurol. 1992, 38, 26–37. [Google Scholar] [CrossRef]
- Balakrishnan, R.; Porag, R.; Asif, D.S. Primary intracranial melanoma with early leptomeningeal spread: A case report and treatment options available. Case Rep. Oncol. Med. 2015, 29, 3802. [Google Scholar] [CrossRef]
- Garbacz, T.; Osuchowski, M.; Bartosik-Psujek, H. Primary diffuse meningeal melanomatosis—A rare form of meningeal melanoma: Case report. BMC Neurol. 2019, 19, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Cabral, A.; Santos, G.; Madeira, N. Melanoma brain metastates presenting as delirium: A case report. Arch Clin. Psychiatry 2017, 44, 53–54. [Google Scholar]
- Lin, B.; Yang, H.; Qu, L.; Li, Y.; Yu, J. Primary meningeal melanocytoma of the anterior cranial fossa: A case report and review of the literature. World J. Surg. Oncol. 2012, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Samuels, M.A. The brain-heart connection. Circulation 2007, 116, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.M.; Schwartz, E.S.; Younkin, D.; Myers, S.R. Atypical Syncope in a child due to a colloid of the third ventricle. Pediatr. Neurol. 2011, 45, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeov, S.I.; Rimoldi, D.; Iseli, C. Exome sequencing identifies recurrent somatic MAP2K1 and MAP2K2 mutations in melanoma. Nat. Genet. 2012, 44, 133–139. [Google Scholar] [CrossRef]
- Stark, M.S.; Woods, S.L.; Gartside, M.G. Frequent somatic mutations in MAP3K5 and MAP3K9 in metastatic melanoma identified by exome sequencing. Nat. Genet. 2012, 44, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kusters-Vandevelde, H.V.N.; Kusters, B.; Van Engen-Van Grusven, A.C.H.; Groenen, P.J.T.A.; Wesseling, P.; Blokx, W.A.M. Primary melanocytic tumors of the central nervous system: A review with focus on molecular aspects. Brain Pathol. 2015, 25, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Yang, G.; Wang, Y.; Yuan, T.; Gao, Y.; Dong, L. Leptomeningeal metastases from a primary central nervous system melanoma: A case report and literature review. World J. Surg. Oncol. 2014, 12, 265–272. [Google Scholar] [CrossRef]
- Ma, Y.; Gui, Q.; Lang, S. Intracranial malignant melanoma: A report of 7 cases. Oncol. Lett. 2015, 10, 2171–2175. [Google Scholar] [CrossRef] [PubMed]
- Isiklar, I.; Leeds, N.E.; Fuller, G.N.; Kumar, A.J. Intracranial metastatic melanoma: Correlation between MR imaging characteristics and melanin content. Am. J. Roentgenol. 1995, 165, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Hsu, Y.I.; Ho, Y.S. Intracranial meningeal melanocytoma. CT and MRI. Neuroradiology 1997, 39, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ahn, B.C.; Hwang, S.W.; Cho, S.K.; Kim, H.W.; Lee, S.W.; Hwang, J.H.; Lee, J. F-18 fluorodeoxyglucose PET/CT and post hoc PET/MRI in a case of primary meningeal melanomatosis. Korean J. Radiol. 2013, 14, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Young, G.J.; Bi, W.L.; Wu, W.W.; Johanns, T.M.; Dung, G.P.; Dun, I.F. Management of intracranial melanomas in the era of precision medicine. Oncotarget 2017, 8, 89326–89347. [Google Scholar] [CrossRef][Green Version]
- Kircher, D.A.; Silvis, M.R.; Cho, J.H.; Holmen, S.L. Melanoma brain metastasis: Mechanism, models and medicine. Int. J. Mol. Sci. 2016, 17, 1468. [Google Scholar] [CrossRef]
- Abate-Daga, D.; Ramello, M.C.; Smalley, I.; Forsyth, P.A.; Smalley, K.S.M. The biology and therapeutic management of melanoma brain metastases. Biochem. Pharm. 2018, 153, 35–45. [Google Scholar] [CrossRef]
- Basnet, A.; Saad, N.; Benjamin, S. A case of vanishing brain metastasis in a melanoma patient on nivolumab. Anticancer Res. 2016, 36, 4795–4798. [Google Scholar] [CrossRef] [PubMed]
- Puyana, C.; Denyer, S.; Burch, T.; Bhimani, A.D.; McGuire, L.S.; Patel, A.S.; Mehta, A.I. Primary Malignant Melanoma of the Brain: A Population-Based Study. World Neurosurg. 2019, 130, 1091–1097. [Google Scholar] [CrossRef] [PubMed]



| Reference | Study Design | Primary Cerebral Tumor | Autopsy Findings | Cause of Death |
|---|---|---|---|---|
| Eberhart, C.G. [2] | Original Article | Astrocytoma (n.2) Schwannoma (n.1) Glioblastoma multiforme (n.4) Colloid cyst (n.2) Glioma (n.1) Pituitary adenoma (n.1) | Brain edema was shown in all cases. The microscopic study diagnosed the type-specific tumor. | Death was attributed in all cases to hydrocephalus and intracranial hypertension except for one case of glioblastoma multiforme in which death was attributed to massive tumor hemorrhage. |
| Matschke, J. [3] | Case Series | Glioblastoma multiform (n.3) | Gross examination of the brain showed numerous cystic spaces. Microscopic examination revealed polymorphic astrocytic cells. | Death was attributed to intracranial hypertension. |
| Gleckman, A.M. [4] | Case Series | Ganglioma (n.1) Astrocytoma (n.1) | Brain edema was shown in all cases. The microscopic study diagnosed the type-specific tumor. | Death was attributed to hydrocephalus and intracranial hypertension. |
| Sutton, J.T. [5] | Case Report | Glioblastoma multiform | Gross examination of the brain showed a hemorrhagic infiltration of the right lobe equal to 7 × 5 × 5 cm. Microscopic examination revealed hemorrhagic infiltration of the cortex with tumor invasion. | Death was due to an acute hemorrhage of the tumor. |
| Riezzo, I. [6] | Case Series | Glioblastoma multiforme (n.3) | Macroscopic findings of the brain were characterized by diffuse hypoxia/ischemia and edema of the brain tissue with extensive hemorrhagic infiltration and necrosis confirmed also on histological examination. | Death was attributed in all cases to hydrocephalus and intracranial hypertension. |
| Manousaki, M. [7] | Case Report | Oligodendroglioma | Brain edema with “fried-egg” cell tumor. | Death was due to hemorrhagic leakage into the cerebrospinal fluid |
| Vougiouklakis, T. [8] | Case Series | Glioblastoma multiforme (n.1) Astrocytoma WHO grade III (n.1) | The examination of the brain revealed flattening of the fissures with large hemorrhagic infarction in both cases. | Death was due to massive tumor hemorrhage. |
| Harrison, W.T. [9] | Case Report | Anaplastic Ependymoma | After formalin fixation, the brain showed a 7 × 6 × 6 cm necrotic cavity mass of the lateral ventricle. Microscopically, the tumor has been attributed to an anaplastic ependymoma with parenchyma characterized by fibrillary processes. | Death was attributed to hydrocephalus and intracranial hypertension. |
| Sidlo, J. [10] | Case Report | Sellar extramedullary plasmacytoma | After brain removal, the examination of the sella turcica showed an intrasellar tumor mass of 5.5 × 5.5 × 3 cm. Histopathological examination showed mature plasma cells with eccentrically positioned round nuclei. | Death was attributed to hydrocephalus and intracranial hypertension |
| Aissaoui, A. [19] | Case Report | Leptomeningeal Melanocytosis | A dark brown mass was present on the basal leptomeninges in the frontal areas. The brain was edematous. Microscopic analysis revealed the dark color of the tumor due to melanin pigments. | Death was attributed to hydrocephalus and intracranial hypertension |
| Ozkul, A. [20] | Case Report | Leptomeningeal oligodendrogliomatosis | Macroscopic examination revealed edema of the brain. H&E examination showed an invasion of tumor at the brain, cerebellum and spinal cord by plasmacytoid cells. | Death was attributed to hydrocephalus and intracranial hypertension. |
| Ross, J. [21] | Case Report | Glioma | The brain was characterized by diffuse hypoxia/ischemia and edema of the brain tissue. At H&E examination, a hyper cellularity of glial tumor cells was displayed. | Death was attributed to hydrocephalus and intracranial hypertension |
| Havlik, D.M. [22] | Case Report | Glioma | Macroscopic examination of the brain showed swelling of hemispheres; at H&E examination, pseudo rosettes and tumor cells were seen. | Death was attributed to intracranial hypertension. |
| DiMaio, S.M. [23] | Original Article | Colloid cyst (n.1) Oligodendroglioma (n.2) Glioblastoma multiforme (n.2) Astrocytoma WHO grade III (n.3) Medulloblastoma (n.1) Astrocytoma WHO grade II (n.4) Sarcoma (n.1) Teratoma cyst (n.1) Meningioma (n.1) Chromophobe adenoma (n.1) | Brain edema was shown in all cases. The microscopic study diagnosed the type-specific tumor. | Death was attributed in all cases to hydrocephalus and intracranial hypertension. |
| Lau, G. [24] | Case Report | Intracranial Germinoma | Macroscopic examination was unremarkable with a normal brain size and weight. At microscopic examination the pituitary gland displayed a massive tumor invasion with extensive peripheral lymphoid aggregates. | Death was due to a combination of acute hemorrhage of the tumor combined with microvascular disease of the heart. |
| Shiferaw, K. [25] | Case Report | Glioblastoma multiform | Macroscopic findings of the brain showed a tumor occupying both frontal lobes with extensive hemorrhagic infiltration and necrosis confirmed also on histological examination. | Death was attributed in all cases to hydrocephalus and intracranial hypertension. |
| Matturri, L. [26] | Case Report | Hemangioendothelioma | Macroscopic and microscopic examination of the brain found a solid tumor inside the medulla oblongata. | Death was due to the impaired breathing control due to the location of the tumor. |
| Matsumoto, H. [27] | Case Report | Glioblastoma multiform | Macroscopic findings of the brain were characterized by diffuse hypoxia/ischemia and edema of the brain tissue with extensive hemorrhagic infiltration and necrosis confirmed also on histological examination. | Death was attributed in all cases to hydrocephalus and intracranial hypertension. |
| Prahlow, J.A. [28] | Case Report | Astrocytoma | Macroscopic examination of the brain showed a solid tumor of 1.2 cm. Microscopically, cells of various shapes with nuclei of different size, and microcalcifications of the parenchyma were shown. | Death was due to a seizure disorder related to the tumor combined with acute ethanol intoxication. |
| Shields, L.B. [29] | Case Report | Pituitary adenoma | The pituitary fossa showed the presence of a red-colored tumor with hemorrhagic infarction. The examination in H&E revealed the presence of tumor with hemorrhagic infiltration. | Death was attributed to intracranial hypertension. |
| Ortiz-Reyes, R. [30] | Case Report | Subependymoma | Gross examination of the brain showed bilateral ventricular dilatation and inside a tumor of 3 cm in diameter. Microscopic examination showed meningothelial tumoral cells. | Death was due to the impaired breathing control due to the location of the tumor. |
| Nelson, J. [31] | Case Report | Ganglioma of the medulla | Examination of the brain after fixation showed a medulla with a mass invading the cerebellum. Microscopic examination revealed two types of neoplastic cells: astrocytes and oligodendrocytes. | Death was attributed to intracranial hypertension. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maiellaro, A.; Perna, A.; Giugliano, P.; Esposito, M.; Vacchiano, G. Sudden Death from Primary Cerebral Melanoma: Clinical Signs and Pathological Observations. Healthcare 2021, 9, 341. https://doi.org/10.3390/healthcare9030341
Maiellaro A, Perna A, Giugliano P, Esposito M, Vacchiano G. Sudden Death from Primary Cerebral Melanoma: Clinical Signs and Pathological Observations. Healthcare. 2021; 9(3):341. https://doi.org/10.3390/healthcare9030341
Chicago/Turabian StyleMaiellaro, Alfonso, Antonio Perna, Pasquale Giugliano, Massimiliano Esposito, and Giuseppe Vacchiano. 2021. "Sudden Death from Primary Cerebral Melanoma: Clinical Signs and Pathological Observations" Healthcare 9, no. 3: 341. https://doi.org/10.3390/healthcare9030341
APA StyleMaiellaro, A., Perna, A., Giugliano, P., Esposito, M., & Vacchiano, G. (2021). Sudden Death from Primary Cerebral Melanoma: Clinical Signs and Pathological Observations. Healthcare, 9(3), 341. https://doi.org/10.3390/healthcare9030341







