Assessing the Relationship between Helicobacter pylori and Chronic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
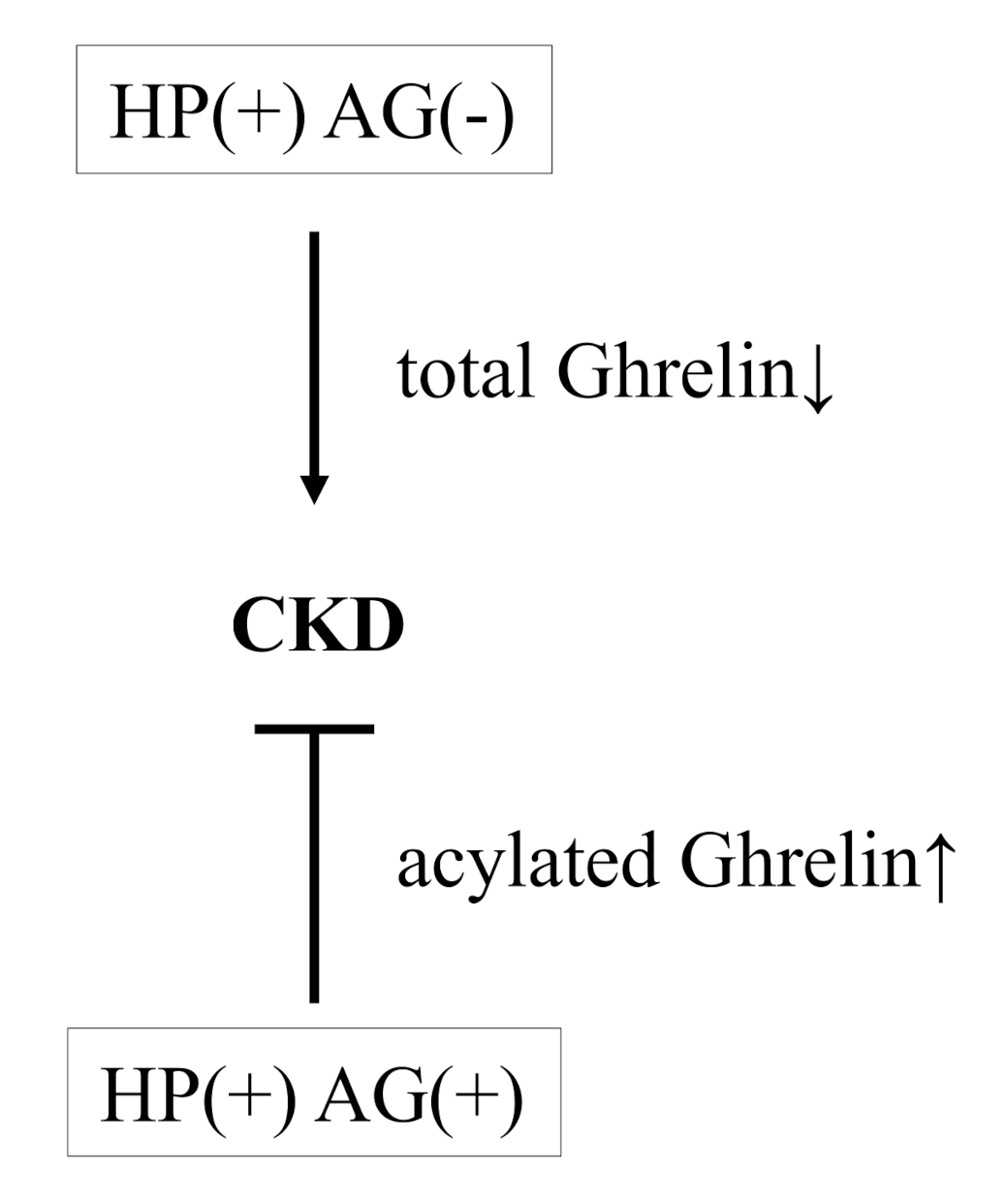
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takashima, T.; Adachi, K.; Kawamura, A.; Yuki, M.; Fujishiro, H.; Rumi, M.A.; Ishihara, S.; Watanabe, M.; Kinoshita, Y. Cardiovascular risk factors in subjects with Helicobacter pylori infection. Helicobacter 2002, 7, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Vijayvergiya, R.; Vadivelu, R. Role of Helicobacter pylori infection in pathogenesis of atherosclerosis. World J. Cardiol. 2015, 7, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Senmaru, T.; Fukui, M.; Tanaka, M.; Kuroda, M.; Yamazaki, M.; Oda, Y.; Naito, Y.; Hasegawa, G.; Toda, H.; Yoshikawa, T.; et al. Atrophic gastritis is associated with coronary artery disease. J. Clin. Biochem. Nutr. 2012, 51, 39–41. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gunji, T.; Matsuhashi, N.; Sato, H.; Fujibayashi, K.; Okumura, M.; Sasabe, N.; Urabe, A. Helicobacter pylori infection is significantly associated with metabolic syndrome in the Japanese population. Am. J. Gastroenterol. 2008, 103, 3005–3010. [Google Scholar] [CrossRef] [PubMed]
- Satoh, H.; Saijo, Y.; Yoshioka, E.; Tsutsui, H. Helicobacter Pylori infection is a significant risk for modified lipid profile in Japanese male subjects. J. Atheroscler. Thromb. 2010, 17, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Imai, J.; Yamada, T.; Saito, T.; Ishigaki, Y.; Hinokio, Y.; Kotake, H.; Oka, Y.; Katagiri, H. Eradication of insulin resistance. Lancet 2009, 374, 264. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Deretzi, G. The association between Helicobacter pylori infection and insulin resistance: A systematic review. Helicobacter 2011, 16, 79–88. [Google Scholar] [CrossRef]
- Waluga, M.; Kukla, M.; Zorniak, M.; Bacik, A.; Kotulski, R. From the stomach to other organs: Helicobacter pylori and the liver. World J. Hepatol. 2015, 7, 2136–2146. [Google Scholar] [CrossRef]
- Dadashi, A.; Hosseinzadeh, N. High seroprevalence of anti-Helicobacter pylori antibodies in patients with ventilator-associated pneumonia. J. Res. Med. Sci. 2018, 23, 79. [Google Scholar] [CrossRef]
- Mizuno, S.; Matsui, D.; Watanabe, I.; Ozaki, E.; Kuriyama, N.; Watanabe, Y. Serologically Determined Gastric Mucosal Condition Is a Predictive Factor for Osteoporosis in Japanese Men. Dig. Dis. Sci. 2015, 60, 2063–2069. [Google Scholar] [CrossRef]
- Lin, S.Y.; Lin, C.L.; Liu, J.H.; Yang, Y.F.; Huang, C.C.; Kao, C.H. Association between Helicobacter pylori infection and the subsequent risk of end-stage renal disease: A nationwide population-based cohort study. Int. J. Clin. Pract. 2015, 69, 604–610. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Hu, H.Y. Lower Helicobacter pylori infection rate in chronic kidney disease and end-stage renal disease patients with peptic ulcer disease. J. Chin. Med Assoc. JCMA 2014, 77, 354–359. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wakai, K.; Hamajima, N.; Okada, R.; Naito, M.; Morita, E.; Hishida, A.; Kawai, S.; Nishio, K.; Yin, G.; Asai, Y.; et al. Profile of Participants and Genotype Distributions of 108 Polymorphisms in a Cross-Sectional Study of Associations of Genotypes With Lifestyle and Clinical Factors: A Project in the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study. J. Epidemiol. 2011, 21, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Matsui, D.; Kuriyama, N.; Ozaki, E.; Tanaka, K.; Oze, I.; Hamajima, N.; Wakai, K.; Okada, R.; Arisawa, K.; et al. Genetic variants of SLC17A1 are associated with cholesterol homeostasis and hyperhomocysteinaemia in Japanese men. Sci. Rep. 2015, 5, 15888. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2009, 53, 982–992. [Google Scholar] [CrossRef]
- Miki, K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels—“ABC method”. Proc. Jpn. Acad. Ser. B 2011, 87, 405–414. [Google Scholar] [CrossRef]
- Ohata, H.; Kitauchi, S.; Yoshimura, N.; Mugitani, K.; Iwane, M.; Nakamura, H.; Yoshikawa, A.; Yanaoka, K.; Arii, K.; Tamai, H.; et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 2004, 109, 138–143. [Google Scholar] [CrossRef]
- Watabe, H.; Mitsushima, T.; Yamaji, Y.; Okamoto, M.; Wada, R.; Kokubo, T.; Doi, H.; Yoshida, H.; Kawabe, T.; Omata, M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: A prospective endoscopic cohort study. Gut 2005, 54, 764–768. [Google Scholar] [CrossRef]
- Kawai, T.; Kawakami, K.; Kudo, T.; Ogiahara, S.; Handa, Y.; Moriyasu, F. A new serum antibody test kit (E plate) for evaluation of Helicobacter pylori eradication. Intern. Med. 2002, 41, 780–783. [Google Scholar] [CrossRef][Green Version]
- Wijarnpreecha, K.; Thongprayoon, C.; Nissaisorakarn, P.; Jaruvongvanich, V.; Nakkala, K.; Rajapakse, R.; Cheungpasitporn, W. Association of Helicobacter pylori with Chronic Kidney Diseases: A Meta-Analysis. Dig. Dis. Sci. 2017, 62, 2045–2052. [Google Scholar] [CrossRef]
- Gu, M.; Xiao, S.; Pan, X.; Zhang, G. Helicobacter pylori Infection in Dialysis Patients: A Meta-Analysis. Gastroenterol. Res. Pract. 2013, 2013, 785892. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.P.; Bang, C.S.; Lee, J.J.; Baik, G.H. Helicobacter pylori Infection in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Gut Liver 2019, 13, 628–641. [Google Scholar] [CrossRef] [PubMed]
- Sayehmiri, K.; Abangah, G.; Kalvandi, G.; Tavan, H.; Aazami, S. Prevalence of peptic ulcer in Iran: Systematic review and meta-analysis methods. J. Res. Med. Sci. 2018, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Glassock, R.J.S.; Rule, A.D. Aging and the Kidneys: Anatomy, Physiology and Consequences for Defining Chronic Kidney Disease. Nephron 2016, 134, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer 2017, 20, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.D.; Nogueiras, R.; Andermann, M.L.; Andrews, Z.B.; Anker, S.D.; Argente, J.; Batterham, R.L.; Benoit, S.C.; Bowers, C.Y.; Broglio, F.; et al. Ghrelin. Mol. Metab. 2015, 4, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Hosoda, H.; Kojima, M.; Matsuo, H.; Kangawa, K. Ghrelin and des-acyl ghrelin: Two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000, 279, 909–913. [Google Scholar] [CrossRef]
- Hosoda, H.; Kojima, M.; Matsuo, H.; Kangawa, K. Purification and characterization of rat des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J. Biol. Chem. 2000, 275, 21995–22000. [Google Scholar] [CrossRef]
- Cheung, W.W.; Mak, R.H. Ghrelin in chronic kidney disease. Int. J. Pept. 2010, 2010. [Google Scholar] [CrossRef]
- Gunta, S.S.; Mak, R.H. Ghrelin and leptin pathophysiology in chronic kidney disease. Pediatr. Nephrol. 2013, 28, 611–616. [Google Scholar] [CrossRef]
- Paoluzi, O.A.; Blanco del, V.G.; Caruso, R.; Monteleone, I.; Monteleone, G.; Pallone, F. Impairment of ghrelin synthesis in Helicobacter pylori-colonized stomach: New clues for the pathogenesis of H. pylori-related gastric inflammation. World J. Gastroenterol. 2014, 20, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Ueno, H.; Saenko, V.A.; Mondal, M.S.; Nishi, Y.; Kawano, N.; Ohnita, K.; Mizuta, Y.; Ohtsuru, A.; Yamashita, S.; et al. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am. J. Gastroenterol. 2005, 100, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Campana, D.; Nori, F.; Pagotto, U.; De Iasio, R.; Morselli-Labate, A.M.; Pasquali, R.; Corinaldesi, R.; Tomassetti, P. Plasma acylated ghrelin levels are higher in patients with chronic atrophic gastritis. Clin. Endocrinol. 2007, 67, 761–766. [Google Scholar] [CrossRef] [PubMed]
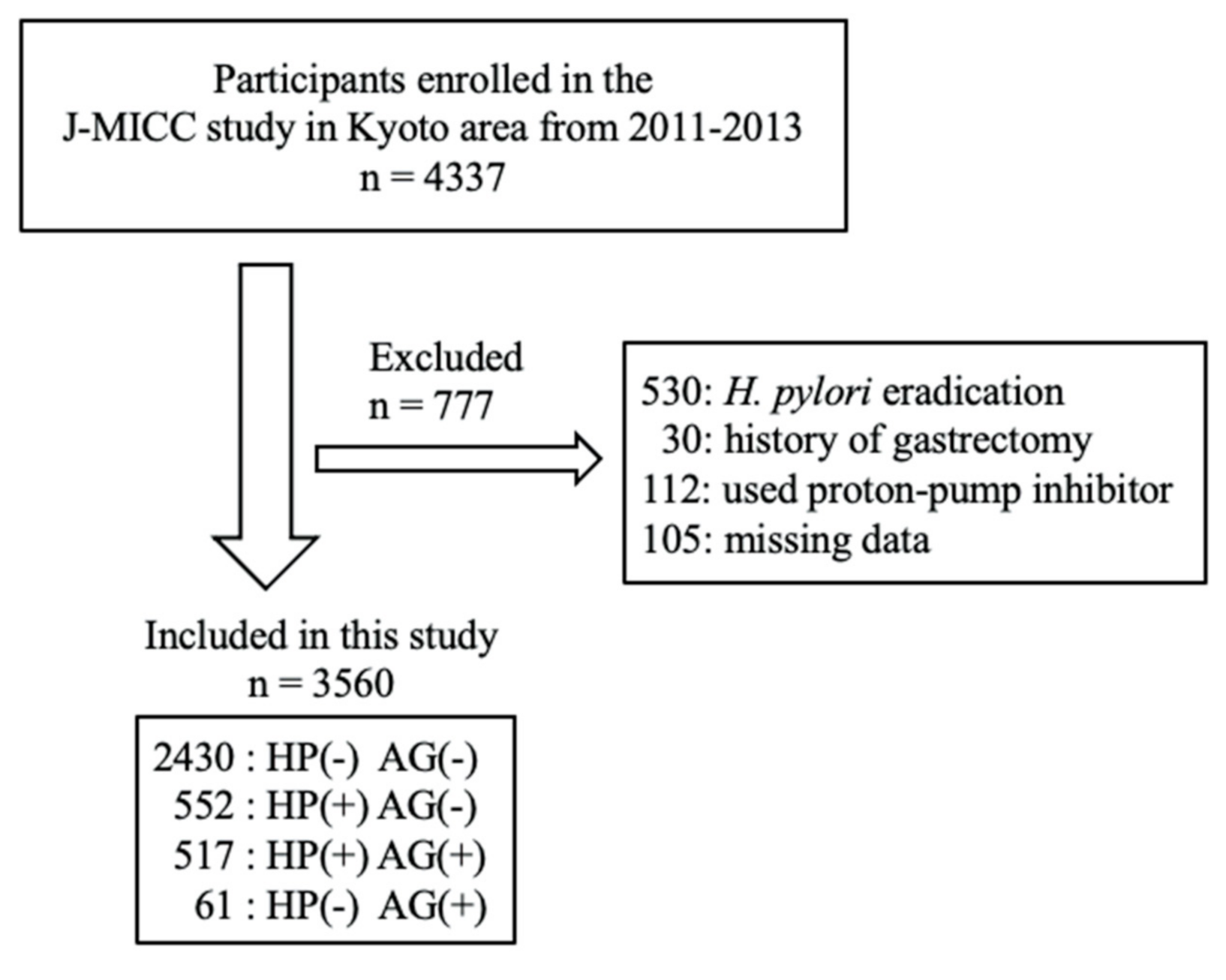
| Variable | HP (−) AG (−) | HP (+) AG (−) | HP (+) AG (+) | HP (−) AG (+) | |||||
|---|---|---|---|---|---|---|---|---|---|
| n = 2430 | n = 552 | n = 517 | n = 61 | p-Value | |||||
| Mean (n) | SD (%) | Mean (n) | SD (%) | Mean (n) | SD (%) | Mean (n) | SD (%) | ||
| Sex (male) | (744) | (30.6) | (200) | (36.2) | (160) | (30.9) | (23) | (37.7) | 0.053 |
| Age (years) | 50.0 | 10.1 | 54.4 | 9.80 | 57.7 | 8.86 | 57.5 | 9.80 | <0.001 |
| BMI (kg/m2) | 21.9 | 3.06 | 22.4 | 3.24 | 22.1 | 3.08 | 22.2 | 3.64 | 0.004 |
| Systolic blood pressure (mmHg) | 130 | 20.3 | 135 | 20.8 | 137 | 21.6 | 132 | 21.0 | <0.001 |
| Diastolic blood pressure (mmHg) | 77.9 | 11.8 | 79.9 | 12.1 | 80.3 | 11.5 | 77.8 | 13.1 | <0.001 |
| Total cholesterol (mg/dL) | 213 | 37.2 | 219 | 37.3 | 218 | 36.3 | 216 | 34.2 | 0.002 |
| Triglyceride (mg/dL) | 128 | 102 | 146 | 102 | 129 | 90.8 | 128 | 78.4 | 0.001 |
| HDL-cholesterol (mg/dL) | 72.2 | 19.4 | 68.4 | 18.2 | 69.6 | 18.6 | 71.5 | 20.1 | <0.001 |
| LDL-cholesterol (mg/dL) | 120 | 31.6 | 126 | 31.4 | 126 | 31.6 | 122 | 25.7 | <0.001 |
| Glucose (mg/dL) | 92.3 | 20.2 | 94.3 | 24.4 | 95.3 | 22.2 | 96.0 | 25.7 | 0.009 |
| Hemoglobin (g/dL) | 13.6 | 1.41 | 13.7 | 1.40 | 13.5 | 1.33 | 13.6 | 1.71 | 0.035 |
| Hemoglobin A1C (%) | 5.37 | 0.42 | 5.46 | 0.58 | 5.48 | 0.49 | 5.49 | 0.55 | <0.001 |
| Uric acid (mg/dL) | 5.19 | 2.29 | 5.30 | 2.32 | 5.25 | 2.50 | 5.59 | 3.03 | 0.456 |
| BUN (mg/dL) | 13.9 | 4.04 | 14.7 | 4.16 | 14.2 | 4.27 | 14.4 | 6.32 | <0.001 |
| Creatinine (mg/dL) | 0.69 | 0.15 | 0.72 | 0.18 | 0.67 | 0.14 | 0.84 | 1.31 | <0.001 |
| eGFR (mL/min/1.73 m2) | 79.9 | 13.9 | 76.1 | 14.5 | 79.0 | 13.8 | 78.8 | 17.0 | <0.001 |
| PG I (ng/mL) | 49.1 | 19.8 | 81.9 | 44.6 | 44.0 | 17.2 | 25.9 | 16.6 | <0.001 |
| PG II (ng/mL) | 9.28 | 3.75 | 27.3 | 16.1 | 23.1 | 8.60 | 13.5 | 6.87 | <0.001 |
| PG I/II ratio | 5.44 | 1.28 | 3.40 | 1.36 | 1.92 | 0.63 | 1.90 | 0.84 | <0.001 |
| METs (h/day) | 12.4 | 10.3 | 13.2 | 10.6 | 14.1 | 10.6 | 16.6 | 12.6 | <0.001 |
| Current smokers | (269) | (11.1) | (68) | (12.3) | (58) | (11.2) | (10) | (16.4) | 0.522 |
| Current drinkers | (1484) | (61.1) | (324) | (58.7) | (281) | (54.4) | (34) | (55.7) | 0.034 |
| Hypertension | (794) | (32.7) | (232) | (42.0) | (244) | (47.2) | (22) | (36.1) | <0.001 |
| Diabetes mellitus | (58) | (2.4) | (28) | (5.1) | (29) | (5.6) | (4) | (6.6) | <0.001 |
| Dyslipidemia | (790) | (32.5) | (242) | (43.8) | (229) | (44.3) | (21) | (34.4) | <0.001 |
| Anemia | (248) | (10.2) | (51) | (9.2) | (65) | (12.6) | (8) | (13.1) | 0.265 |
| Myocardial infarction and/or stenocardia | (33) | (1.4) | (11) | (2.0) | (8) | (1.5) | (1) | (1.6) | 0.738 |
| Stroke | (22) | (0.9) | (4) | (0.7) | (6) | (1.2) | (1) | (1.6) | 0.825 |
| Year | HP (−) AG (−) | HP (+) AG (−) | HP (+) AG (+) | HP (−) AG (+) | ||||
|---|---|---|---|---|---|---|---|---|
| CKD | CKD | CKD | CKD | |||||
| (−) | (+) | (−) | (+) | (−) | (+) | (−) | (+) | |
| 35–49 | 1262 | 23 | 179 | 5 | 97 | 2 | 13 | 0 |
| 98.2% | 1.8% | 97.3% | 2.7% | 98.0% | 2.0% | 100.0% | 0.0% | |
| 50–59 | 516 | 44 | 134 | 21 | 125 | 12 | 15 | 2 |
| 92.1% | 7.9% | 86.5% | 13.5% | 91.2% | 8.8% | 88.2% | 11.8% | |
| 60–69 | 502 | 83 | 173 | 40 | 263 | 18 | 26 | 5 |
| 85.8% | 14.2% | 81.2% | 18.8% | 93.6% | 6.4% | 83.9% | 16.1% | |
| CKD | OR † | 95% CI † | p-Value | OR †† | 95% CI †† | p-Value | OR § | 95% CI § | p-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (−) | (+) | ||||||||||
| HP (−) AG (−) | 2280 | 150 | Reference | Reference | Reference | ||||||
| HP (+) AG (−) | 486 | 66 | 1.465 | 1.066–2.012 | 0.018 | 1.439 | 1.046–1.979 | 0.025 | 1.443 | 1.047–1.989 | 0.025 |
| HP (+) AG (+) | 485 | 32 | 0.610 | 0.406–0.917 | 0.017 | 0.615 | 0.408–0.926 | 0.020 | 0.608 | 0.402–0.920 | 0.019 |
| HP (−) AG (+) | 54 | 7 | 1.126 | 0.492–2.573 | 0.779 | 1.134 | 0.492–2.614 | 0.768 | 1.076 | 0.456–2.539 | 0.867 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hata, K.; Koyama, T.; Ozaki, E.; Kuriyama, N.; Mizuno, S.; Matsui, D.; Watanabe, I.; Uehara, R.; Watanabe, Y. Assessing the Relationship between Helicobacter pylori and Chronic Kidney Disease. Healthcare 2021, 9, 162. https://doi.org/10.3390/healthcare9020162
Hata K, Koyama T, Ozaki E, Kuriyama N, Mizuno S, Matsui D, Watanabe I, Uehara R, Watanabe Y. Assessing the Relationship between Helicobacter pylori and Chronic Kidney Disease. Healthcare. 2021; 9(2):162. https://doi.org/10.3390/healthcare9020162
Chicago/Turabian StyleHata, Koichi, Teruhide Koyama, Etsuko Ozaki, Nagato Kuriyama, Shigeto Mizuno, Daisuke Matsui, Isao Watanabe, Ritei Uehara, and Yoshiyuki Watanabe. 2021. "Assessing the Relationship between Helicobacter pylori and Chronic Kidney Disease" Healthcare 9, no. 2: 162. https://doi.org/10.3390/healthcare9020162
APA StyleHata, K., Koyama, T., Ozaki, E., Kuriyama, N., Mizuno, S., Matsui, D., Watanabe, I., Uehara, R., & Watanabe, Y. (2021). Assessing the Relationship between Helicobacter pylori and Chronic Kidney Disease. Healthcare, 9(2), 162. https://doi.org/10.3390/healthcare9020162






