Hyperbaric Oxygen Therapy Does Not Have a Negative Impact on Bone Signaling Pathways in Humans
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
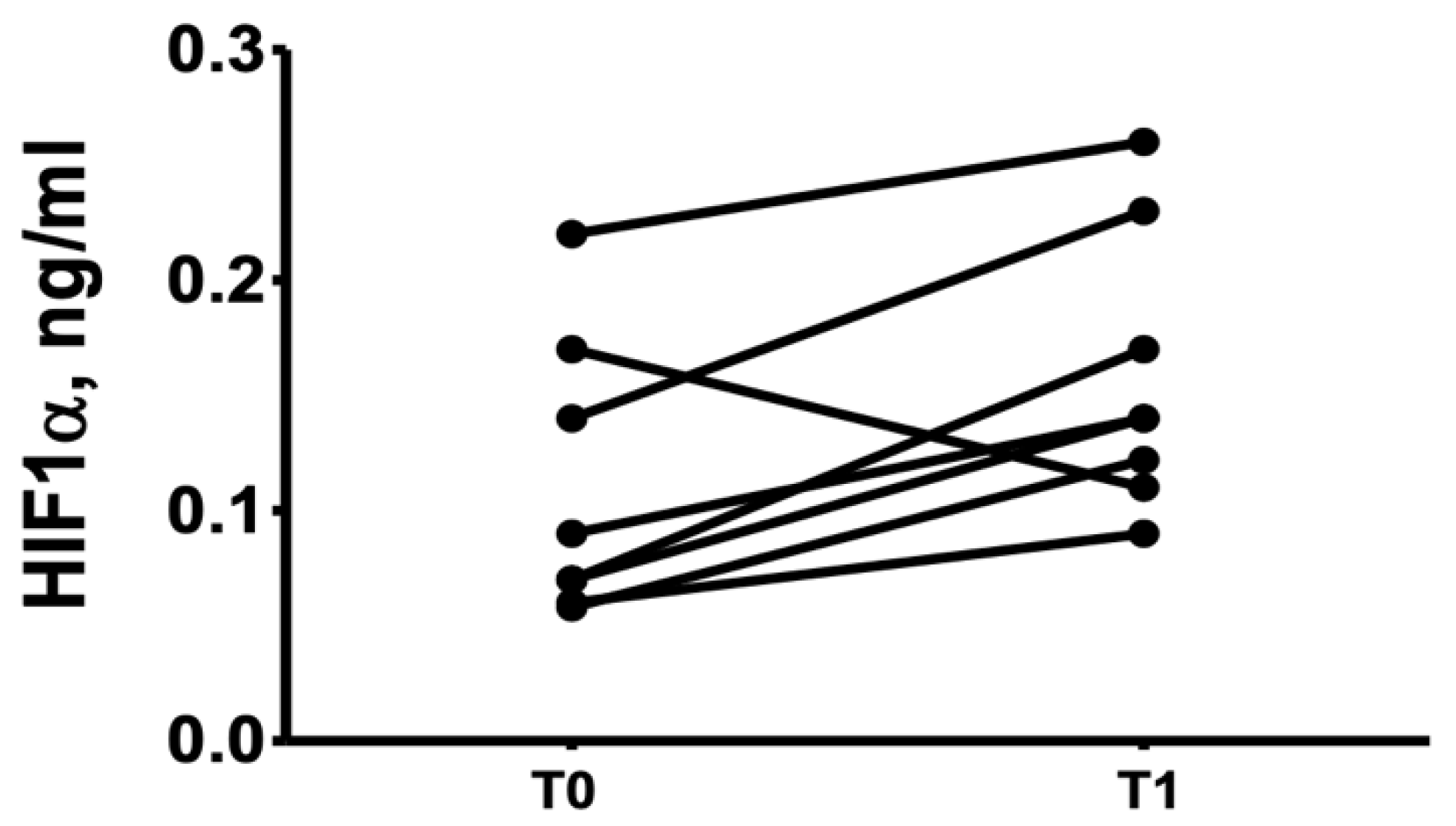
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Annu. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Uehara, S.; Udagawa, N.; Takahashi, J.N. Regulation of bone metabolism by Wnt signals. J. Biochem. 2016, 159, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Kobayashi, Y.; Koide, M.; Uehara, S.; Okamoto, M.; Ishihara, A.; Kayama, T.; Saito, M.; Marumo, K. The Regulation of Bone Metabolism and Disorders by Wnt Signaling. Int. J. Mol. Sci. 2019, 20, 5525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado-Calle, J.; Sato, A.Y.; Bellido, T. Role and mechanism of action of sclerostin in bone. Bone 2017, 96, 29–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robling, A.G.; Bonewald, L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020, 82, 485–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabre, S.; Funck-Brentano, T.; Cohen-Solal, M. Anti-Sclerostin Antibodies in Osteoporosis and Other Bone Diseases. J. Clin. Med. 2020, 9, 3439. [Google Scholar] [CrossRef]
- Matsumoto, T.; Endo, I. RANKL as a target for the treatment of osteoporosis. J. Bone Miner. Metab. 2021, 39, 91–105. [Google Scholar] [CrossRef]
- Watson, E.C.; Adams, R.H. Biology of Bone: The Vasculature of the Skeletal System. Cold Spring Harb. Perspect. Med. 2018, 8, a031559. [Google Scholar] [CrossRef] [PubMed]
- Yellowley, C.E.; Genetos, D.C. Hypoxia signaling in the skeleton: Implications for bone health. Curr. Osteoporos. Rep. 2019, 17, 26–35. [Google Scholar] [CrossRef]
- Utting, J.C.; Flanagan, A.M.; Brandao-Burch, A.; Orriss, I.R.; Arnett, T.R. Hypoxia stimulates osteoclast formation from human peripheral blood. Cell Biochem. Funct. 2010, 28, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Al Hadi, H.; Smerdon, G.R.; Fox, S.W. Hyperbaric oxygen therapy suppresses osteoclast formation and bone resorption. J. Orthop. Res. 2013, 31, 1839–1844. [Google Scholar] [CrossRef]
- Lin, S.S.; Ueng, S.W.; Niu, C.C.; Yuan, L.J.; Yang, C.Y.; Chen, W.J.; Lee, M.S.; Chen, J.K. Effects of hyperbaric oxygen on the osteogenic differentiation of mesenchymal stem cells. BMC Musculoskelet. Disord. 2014, 15, 56. [Google Scholar] [CrossRef] [Green Version]
- Ortega, M.A.; Fraile-Martinez, O.; García-Montero, C.; Callejón-Peláez, E.; Sáez, M.A.; Álvarez-Mon, M.A.; García-Honduvilla, N.; Monserrat, J.; Álvarez-Mon, M.; Bujan, J.; et al. General Overview on the Hyperbaric Oxygen Therapy: Applications, Mechanisms and Translational Opportunities. Medicina 2021, 57, 864. [Google Scholar] [CrossRef] [PubMed]
- Memar, M.Y.; Yekani, M.; Alizadeh, N.; Baghi, H.B. Hyperbaric oxygen therapy: Antimicrobial mechanisms and clinical application for infections. Biomed. Pharmacother. 2019, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Ceponis, P.; Keilman, C.; Guerry, C.; Freiberger, J. Hyperbaric oxygen therapy and osteonecrosis. Oral Dis. 2017, 23, 141–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paderno, E.; Zanon, V.; Vezzani, G.; Giacon, T.A.; Enrico, T.L.B.; Camporesi, M.; Bosco, G. Evidence-Supported HBO Therapy in Femoral Head Necrosis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 2888. [Google Scholar] [CrossRef]
- Ververidis, A.N.; Paraskevopoulos, K.; Keskinis, A.; Ververidis, N.A.; Moustafa, R.M.; Tilkeridis, K. Bone marrow edema syndrome/transient osteoporosis of the hip joint and management with the utilization of hyperbaric oxygen therapy. J. Orthop. 2020, 22, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Hannah, S.S.; McFadden, S.; McNeilly, A.; McClean, C. “Take My Bone Away?” Hypoxia and bone: A narrative review. J. Cell Physiol. 2021, 236, 721–740. [Google Scholar] [CrossRef]
- Fratantonio, D.; Virgili, F.; Zucchi, A.; Lambrechts, K.; Latronico, T.; Lafère, P.; Germonpré, P.; Balestra, C. Increasing Oxygen Partial Pressures Induce a Distinct Transcriptional Response in Human PBMC: A Pilot Study on the “Normobaric Oxygen Paradox”. Int. J. Mol. Sci. 2021, 5, 458. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Arnett, T.R. Acidosis, hypoxia and bone. Arch. Biochem. Biophys. 2010, 503, 103–109. [Google Scholar] [CrossRef]
- Schipani, E.; Maes, C.; Carmeliet, G.; Semenza, G.L. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J. Bone Miner. Res. 2009, 24, 1347–1353. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Chaekyun Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef] [Green Version]
- Vladana Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H. Discovery of the RANKL/RANK/OPG system. J. Bone Miner. Metab. 2021, 39, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xiao, C.; Du, Y.; Liu, S.; Dum, Y.; Li, S. Effect of hypoxia on the expression of RANKL/OPG in human periodontal ligament cells in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 12929–12935. [Google Scholar] [PubMed]
- Silva, M.L.; Tasso, L.; Azambuja, A.A.; Figueiredo, M.A.; Gonçalves Salum, F.G.; Duval da Silva, V.; Cherubini, K. Effect of hyperbaric oxygen therapy on tooth extraction sites in rats subjected to bisphosphonate therapy-histomorphometric and immunohistochemical analysis. Clin. Oral Investig. 2017, 21, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, G.; Quartesan, S.; Cancellara, P.; Camporesi, E.; Mangar, D.; Bernasek, T.; Dalvi, P.; Yang, Z.; Paoli, A.; Rizzato, A.; et al. Hyperbaric oxygen therapy modulates serum OPG/RANKL in femoral head necrosis patients. J. Enzym. Inhib. Med. Chem. 2017, 32, 707–711. [Google Scholar] [CrossRef]
- Tang, C.-Y.; Wu, M.; Zhao, D.; Edwards, D.; McVicar, A.; Luo, Y.; Zhu, G.; Wang, Y.; Zhou, H.-D.; Chen, W.; et al. Runx1 is a central regulator of osteogenesis for bone homeostasis by orchestrating BMP and WNT signaling pathways. PLoS Genet. 2021, 17, e1009233. [Google Scholar] [CrossRef] [PubMed]
- Stegen, S.; Stockmans, I.; Moermans, K.; Thienpont, B.; Maxwell, P.H.; Carmeliet, P.; Carmeliet, G. Osteocytic oxygen sensing controls bone mass through epigenetic regulation of sclerostin. Nat. Commun. 2018, 9, 2557. [Google Scholar] [CrossRef]
- Weivoda, M.M.; Youssef, S.J.; Oursler, M.J. Sclerostin expression and functions beyond the osteocyte. Bone 2017, 96, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Genetos, D.C.; Toupadakis, C.A.; Raheja, L.F.; Wong, A.; Papanicolaou, S.E.; Fyhrie, D.F.; Loots, G.G.; Yellowley, C.E. Hypoxia Decreases Sclerostin Expression and Increases Wnt Signaling in Osteoblasts. J. Cell Biochem. 2010, 110, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Janjić, K.; Cvikl, B.; Kurzmann, C.; Moritz, A.; Agis, H. Do hypoxia and L-mimosine modulate sclerostin and dickkopf-1 production in human dental pulp-derived cells? Insights from monolayer, spheroid and tooth slice cultures. BMC Oral Health 2018, 18, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmón-González, Z.; Anchuelo, J.; Borregán, J.C.; Real, A.; Sañudo, C.; García, C.; Pérez, M.I.; Riancho, J.A.; Valero, C. Influencia del oxígeno a alta concentración en cámara hiperbárica sobre el metabolismo óseo. Rev. Osteoporos. Metab. Miner. 2020, 12, 28–31. [Google Scholar] [CrossRef]
- Takemura, A.; Paola, P.D.; Egawa, T.; Teshigawara, R.; Hayashi, T.; Ishihara, A. Effects of mild hyperbaric oxygen on osteoporosis induced by hindlimb unloading in rats. J. Bone Miner. Metab. 2020, 38, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Schupbach, D.; Comeau-Gauthier, M.; Harvey, E.; Merle, G. Wnt modulation in bone healing. Bone 2020, 138, 115491. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Guo, J.; Liu, J.; Xie, Y.; Li, X.; Jiang, H.; Wang, J.; Peng, Z.; Wang, J.; Wang, S.; et al. Hypoxia-induced CREB cooperates MMSET to modify chromatin and promote DKK1 expression in multiple mieloma. Oncogene 2021, 40, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Mulawarmanti, D.; Parisihni, K.; Widyastuti, W. The Impact of Hyperbaric Oxygen Therapy on Serum C-Reactive Protein Levels, Osteoprotegerin Expression, and Osteoclast Numbers in Induced-Periodontitis Diabetic Rats. Eur. J. Dent. 2020, 14, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Vezzani, G.; Iezzi, M.; Rizzato, A.; Quartesan, S.; Mangar, D.; Camporesi, E.M.; Paganini, M.; Bosco, G. Effects of hyperbaric oxygen exposure on mobilization of endothelial progenitor cells in healthy volunteers. Acta Med. Mediterr. 2017, 33, 801–805. [Google Scholar]
- Adamopoulos, I.E. Inflammation in bone physiology and pathology. Curr. Opin. Rheumatol. 2018, 30, 59–64. [Google Scholar] [CrossRef]
| Age (yrs.), Mean (SD) | 58 (15) |
|---|---|
| Sex male, n (%) | 8 (40%) |
| Physical activity, n (%) | |
| -Sedentary (<2 h/week) | 2 (10%) |
| -Moderate (2–4 h/week) | 14 (70%) |
| -Vigorous (>4 h/week) | 4 (20%) |
| Alcohol consumption, n (%) | 4 (20%) |
| Smoker habit, n (%) | 2 (10%) |
| Charlson comorbidity index, n (%) | |
| -0 | 5 (25%) |
| -1–2 | 11 (55%) |
| -3–4 | 3 (15%) |
| ->4 | 1 (5%) |
| HBOT indication, n (%) | |
| -Anal fissure | 10 (50%) |
| -Proctitis | 5 (25%) |
| -Cystitis | 2 (10%) |
| -Radionecrosis | 3 (15%) |
| Tumor type, n (%) | |
| -Adenocarcinoma rectum/colon | 3 (30%) |
| -Carcinoma head and neck | 3 (30%) |
| -Carcinoma prostate | 2 (20%) |
| -Adenocarcinoma gynecological | 2 (20%) |
| Bone Biomarkers | Baseline (n = 20) | After HBOT (n = 20) | p-Value |
|---|---|---|---|
| OPG pg/mL | 154 (62) | 165 (64) | 0.73 |
| RANKL pmol/L | 352 (249) | 334 (228) | 0.50 |
| Sclerostin * ng/mL | 0.87 (1.38) | 0.73 (0.67) | 0.11 |
| DKK1 ng/ml | 2.88 (6.22) | 3.15 (8.01) | 0.39 |
| Bone Biomarkers | Baseline (n = 10) | After HBOT (n = 10) | p-Value |
|---|---|---|---|
| OPG pg/mL | 155 (78) | 173 (97) | 0.24 |
| RANKL pmol/L | 357 (202) | 343 (262) | 0.50 |
| Sclerostin * ng/mL | 1.16 (1.18) | 0.65 (1.28) | 0.18 |
| DKK1 ng/mL | 1.62 (3.03) | 1.81 (2.94) | 0.87 |
| Bone Biomarkers | Baseline (n = 10) | After HBOT (n = 10) | p-Value |
|---|---|---|---|
| OPG pg/mL | 148 (79) | 159 (82) | 0.12 |
| RANKL pmol/L | 332 (431) | 323 (252) | 0.47 |
| Sclerostin * ng/mL | 0.57 (1.58) | 0.83 (1.02) | 0.11 |
| DKK1 ng/mL | 3.35 (8.98) | 3.44 (8.12) | 0.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salmón-González, Z.; Anchuelo, J.; Borregán, J.C.; del Real, A.; Sañudo, C.; García-Unzueta, M.T.; Riancho, J.A.; Valero, C. Hyperbaric Oxygen Therapy Does Not Have a Negative Impact on Bone Signaling Pathways in Humans. Healthcare 2021, 9, 1714. https://doi.org/10.3390/healthcare9121714
Salmón-González Z, Anchuelo J, Borregán JC, del Real A, Sañudo C, García-Unzueta MT, Riancho JA, Valero C. Hyperbaric Oxygen Therapy Does Not Have a Negative Impact on Bone Signaling Pathways in Humans. Healthcare. 2021; 9(12):1714. https://doi.org/10.3390/healthcare9121714
Chicago/Turabian StyleSalmón-González, Zaida, Javier Anchuelo, Juan C. Borregán, Alvaro del Real, Carolina Sañudo, Maria Teresa García-Unzueta, José A. Riancho, and Carmen Valero. 2021. "Hyperbaric Oxygen Therapy Does Not Have a Negative Impact on Bone Signaling Pathways in Humans" Healthcare 9, no. 12: 1714. https://doi.org/10.3390/healthcare9121714
APA StyleSalmón-González, Z., Anchuelo, J., Borregán, J. C., del Real, A., Sañudo, C., García-Unzueta, M. T., Riancho, J. A., & Valero, C. (2021). Hyperbaric Oxygen Therapy Does Not Have a Negative Impact on Bone Signaling Pathways in Humans. Healthcare, 9(12), 1714. https://doi.org/10.3390/healthcare9121714