Assessments of Muscle Thickness and Tonicity of the Masseter and Sternocleidomastoid Muscles and Maximum Mouth Opening in Patients with Temporomandibular Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instrumentation
2.3. Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiter, S.; Goldsmith, C.; Emodi-Perlman, A.; Friedman-Rubin, P.; Winocur, E. Masticatory muscle disorders diagnostic criteria: The American Academy of Orofacial Pain versus the research diagnostic criteria/temporomandibular disorders (RDC/TMD). J. Oral Rehabil. 2012, 39, 941–947. [Google Scholar] [CrossRef]
- Gauer, R.L.; Semidey, M.J. Diagnosis and treatment of temporomandibular disorders. Am. Fam. Physician 2015, 91, 378–386. [Google Scholar] [PubMed]
- Del Vecchio, A.; Floravanti, M.; Boccassini, A.; Gaimari, G.; Vestri, A.; Di Paolo, C.; Romeo, U. Evaluation of the efficacy of a new low-level laser therapy home protocol in the treatment of temporomandibular joint disorder-related pain: A randomized, double-blind, placebo-controlled clinical trial. Cranio J. Craniomandib. Pract. 2021, 39, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.; Bhate, K.; Kumar, S.; Kshirsagar, K.; Jagtap, B.; Kakodkar, P. Comparative evaluation of low-level laser therapy and ultrasound heat therapy in reducing temporomandibular joint disorder pain. J. Dent. Anesth. Pain Med. 2019, 19, 289–294. [Google Scholar] [CrossRef]
- Abate, A.; Cavagnetto, D.; Rusconi, F.M.E.; Cressoni, P.; Esposito, L. Safety and Effects of the Rapid Maxillary Expander on Temporomandibular Joint in Subjects Affected by Juvenile Idiopathic Arthritis: A Retrospective Study. Children 2021, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Graff-Radford, S.B. Temporomandibular disorders and headache. Dent. Clin. N. Am. 2007, 51, 129–144. [Google Scholar] [CrossRef]
- Poveda Roda, R.; Díaz Fernández, J.M.; Hernández Bazán, S.; Jiménez Soriano, Y.; Margaix, M.; Sarrión, G. A review of temporomandibular joint disease (TMJD). Part II: Clinical and radiological semiology. Morbidity processes. Med. Oral Patol. Oral Y Cir. Bucal 2008, 13, E102–E109. [Google Scholar]
- Durham, J.; Steele, J.G.; Wassell, R.W.; Exley, C. Living with uncertainty: Temporomandibular disorders. J. Dent. Res. 2010, 89, 827–830. [Google Scholar] [CrossRef]
- Kim, M.E. A study on TMD and stress of first children. J. Korean Soc. Dent. Hyg. 2010, 10, 683–693. [Google Scholar]
- Pond, L.H.; Barghi, N.; Barnwell, G.M. Occlusion and chewing side preference. J. Prosthet. Dent. 1986, 55, 498–500. [Google Scholar] [CrossRef]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99–115. [Google Scholar] [CrossRef] [Green Version]
- McComas, A.J. Oro-facial muscles: Internal structure, function and ageing. Gerodontology 1998, 15, 3–14. [Google Scholar] [CrossRef]
- Guo, S.X.; Li, B.Y.; Zhang, Y.; Zhou, L.J.; Liu, L.; Widmalm, S.E.; Wang, M.Q. An electromyographic study on the sequential recruitment of bilateral sternocleidomastoid and masseter muscle activity during gum chewing. J. Oral Rehabil. 2017, 44, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Pizolato, R.A.; Gavião, M.B.; Berretin-Felix, G.; Sampaio, A.C.; Trindade Junior, A.S. Maximal bite force in young adults with temporomandibular disorders and bruxism. Braz. Oral Res. 2007, 21, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.J.; Gavião, M.B.; Bonjardim, L.R.; Castelo, P.M.; van der Bilt, A. Muscle thickness, bite force, and craniofacial dimensions in adolescents with signs and symptoms of temporomandibular dysfunction. Eur. J. Orthod. 2007, 29, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Strini, P.J.; Strini, P.J.; de Souza Barbosa, T.; Gavião, M.B. Assessment of thickness and function of masticatory and cervical muscles in adults with and without temporomandibular disorders. Arch. Oral Biol. 2013, 58, 1100–1108. [Google Scholar] [CrossRef]
- Strini, P.; Strini, P.; Barbosa, T.; Gavião, M.B. Effects of Head Posture on Cervical Muscle Thickness and Activity in Young Adults With and Without Temporomandibular Disorders. J. Musculoskelet. Pain 2014, 22, 89–98. [Google Scholar] [CrossRef]
- Kibana, Y.; Ishijima, T.; Hirai, T. Occlusal support and head posture. J. Oral Rehabil. 2002, 29, 58–63. [Google Scholar] [CrossRef]
- Pallegama, R.W.; Ranasinghe, A.W.; Weerasinghe, V.S.; Sitheeque, M.A. Influence of masticatory muscle pain on electromyographic activities of cervical muscles in patients with myogenous temporomandibular disorders. J. Oral Rehabil. 2004, 31, 423–429. [Google Scholar] [CrossRef]
- Cuccia, A.M.; Caradonna, C.; Caradonna, D. Manual therapy of the mandibular accessory ligaments for the management of temporomandibular joint disorders. J. Am. Osteopath. Assoc. 2011, 111, 102–112. [Google Scholar]
- Shamim, T. The psychosomatic disorders pertaining to dental practice with revised working type classification. Korean J. Pain 2014, 27, 16–22. [Google Scholar] [CrossRef]
- Jang, J.Y.; Kwon, J.S.; Lee, D.H.; Bae, J.H.; Kim, S.T. Clinical Signs and Subjective Symptoms of Temporomandibular Disorders in Instrumentalists. Yonsei Med. J. 2016, 57, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- McNeill, C.; Danzig, W.M.; Farrar, W.B.; Gelb, H.; Lerman, M.D.; Moffett, B.C.; Pertes, R.; Solberg, W.K.; Weinberg, L.A. Position paper of the American Academy of Craniomandibular Disorders. Craniomandibular (TMJ) disorders—The state of the art. J. Prosthet. Dent. 1980, 44, 434–437. [Google Scholar] [CrossRef]
- Wadhwa, S.; Kapila, S. TMJ disorders: Future innovations in diagnostics and therapeutics. J. Dent. Educ. 2008, 72, 930–947. [Google Scholar] [CrossRef] [PubMed]
- Emshoff, R.; Bertram, S.; Strobl, H. Ultrasonographic cross-sectional characteristics of muscles of the head and neck. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 87, 93–106. [Google Scholar] [CrossRef]
- Son, D.E.; Ahn, Y.W.; Park, J.S.; Ko, M.Y. An Epidemiological Study of Temporomandibular Disorders Patients by Screening Questionnaire. J. Oral Med. Pain 2004, 29, 341–351. [Google Scholar]
- Manfredi, A.; Silva, A.; Vendite, L. The sensibility appreciation of the questionnaire for selection of orofacial pain and temporomandibular disorders recommended by the American Academy of Orofacial Pain. Rev. Bras. De Otorrinolaringol. 2001, 67, 763–768. [Google Scholar] [CrossRef]
- McLester, C.N.; Nickerson, B.S.; Kliszczewicz, B.M.; McLester, J.R. Reliability and Agreement of Various InBody Body Composition Analyzers as Compared to Dual-Energy X-Ray Absorptiometry in Healthy Men and Women. J. Clin. Densitom. Off. J. Int. Soc. Clin. Densitom. 2020, 23, 443–450. [Google Scholar] [CrossRef]
- Herr, K.A.; Spratt, K.; Mobily, P.R.; Richardson, G. Pain intensity assessment in older adults: Use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. Clin. J. Pain 2004, 20, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Emshoff, R.; Emshoff, I.; Rudisch, A.; Bertram, S. Reliability and temporal variation of masseter muscle thickness measurements utilizing ultrasonography. J. Oral Rehabil. 2003, 30, 1168–1172. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tohara, H.; Hara, K.; Nakane, A.; Kajisa, E.; Yoshimi, K.; Minakuchi, S. Relationship of aging, skeletal muscle mass, and tooth loss with masseter muscle thickness. BMC Geriatr. 2018, 18, 67. [Google Scholar] [CrossRef]
- Korhonen, R.K.; Vain, A.; Vanninen, E.; Viir, R.; Jurvelin, J.S. Can mechanical myotonometry or electromyography be used for the prediction of intramuscular pressure? Physiol. Meas. 2005, 26, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Aird, L.; Samuel, D.; Stokes, M. Quadriceps muscle tone, elasticity and stiffness in older males: Reliability and symmetry using the MyotonPRO. Arch. Gerontol. Geriatr. 2012, 55, e31–e39. [Google Scholar] [CrossRef] [PubMed]
- Best, N.; Best, S.; Loudovici-Krug, D.; Smolenski, U.C. Measurement of mandible movements using a vernier caliper--an evaluation of the intrasession-, intersession- and interobserver reliability. Cranio J. Craniomandib. Pract. 2013, 31, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Satiroğlu, F.; Arun, T.; Işik, F. Comparative data on facial morphology and muscle thickness using ultrasonography. Eur. J. Orthod. 2005, 27, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Kiliaridis, S.; Kälebo, P. Masseter muscle thickness measured by ultrasonography and its relation to facial morphology. J. Dent. Res. 1991, 70, 1262–1265. [Google Scholar] [CrossRef]
- Jesus, F.M.; Ferreira, P.H.; Ferreira, M.L. Ultrasonographic measurement of neck muscle recruitment: A preliminary investigation. J. Man. Manip. Ther. 2008, 16, 89–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocur, P.; Grzeskowiak, M.; Wiernicka, M.; Goliwas, M.; Lewandowski, J.; Łochyński, D. Effects of aging on mechanical properties of sternocleidomastoid and trapezius muscles during transition from lying to sitting position-A cross-sectional study. Arch. Gerontol. Geriatr. 2017, 70, 14–18. [Google Scholar] [CrossRef]
- Hara, K.; Namiki, C.; Yamaguchi, K.; Kobayashi, K.; Saito, T.; Nakagawa, K.; Ishii, M.; Okumura, T.; Tohara, H. Association between myotonometric measurement of masseter muscle stiffness and maximum bite force in healthy elders. J. Oral Rehabil. 2020, 47, 750–756. [Google Scholar] [CrossRef]
- Kocur, P.; Tomczak, M.; Wiernicka, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Relationship between age, BMI, head posture and superficial neck muscle stiffness and elasticity in adult women. Sci. Rep. 2019, 9, 8515. [Google Scholar] [CrossRef] [Green Version]
- Widmer, C.G.; English, A.W.; Morris-Wiman, J. Developmental and functional considerations of masseter muscle partitioning. Arch. Oral Biol. 2007, 52, 305–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imanimoghaddam, M.; Davachi, B.; Madani, A.S.; Nemati, S. Ultrasonographic findings of masseter muscle in females with temporomandibular disorders. J. Craniofacial Surg. 2013, 24, e108–e112. [Google Scholar] [CrossRef] [PubMed]
- Castelo, P.M.; Gavião, M.B.; Pereira, L.J.; Bonjardim, L.R. Masticatory muscle thickness, bite force, and occlusal contacts in young children with unilateral posterior crossbite. Eur. J. Orthod. 2007, 29, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ries, L.G.; Alves, M.C.; Bérzin, F. Asymmetric activation of temporalis, masseter, and sternocleidomastoid muscles in temporomandibular disorder patients. Cranio J. Craniomandib. Pract. 2008, 26, 59–64. [Google Scholar] [CrossRef]
- Takashima, M.; Arai, Y.; Kawamura, A.; Hayashi, T.; Takagi, R. Quantitative evaluation of masseter muscle stiffness in patients with temporomandibular disorders using shear wave elastography. J. Prosthodont. Res. 2017, 61, 432–438. [Google Scholar] [CrossRef]
- Schroeder, H.; Siegmund, H.; Santibáñez, G.; Kluge, A. Causes and signs of temporomandibular joint pain and dysfunction: An electromyographical investigation. J. Oral Rehabil. 1991, 18, 301–310. [Google Scholar] [CrossRef]
- Vain, A. Role of skeletal muscle tone and elasticity in the workability restoration of male crosscountry skiers. Acta Acad. Olymp. Est. 2005, 13, 95–108. [Google Scholar]
- Wozniak, E.; Loster, J.E.; Wieczorek, A. Relation between Headache and Mastication Muscle Tone in Adolescents. Pain Res. Manag. 2018, 2018, 7381973. [Google Scholar] [CrossRef]
- Rapidis, A.D.; Dijkstra, P.U.; Roodenburg, J.L.; Rodrigo, J.P.; Rinaldo, A.; Strojan, P.; Takes, R.P.; Ferlito, A. Trismus in patients with head and neck cancer: Etiopathogenesis, diagnosis and management. Clin. Otolaryngol. Off. J. ENT-UK Off. J. Neth. Soc. Oto-Rhino-Laryngol. Cervico-Facial Surg. 2015, 40, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Evcik, D.; Aksoy, O. Relationship Between Head Posture and Temporomandibular Dysfunction Syndrome. J. Musculoskelet. Pain 2004, 12, 19–24. [Google Scholar] [CrossRef]
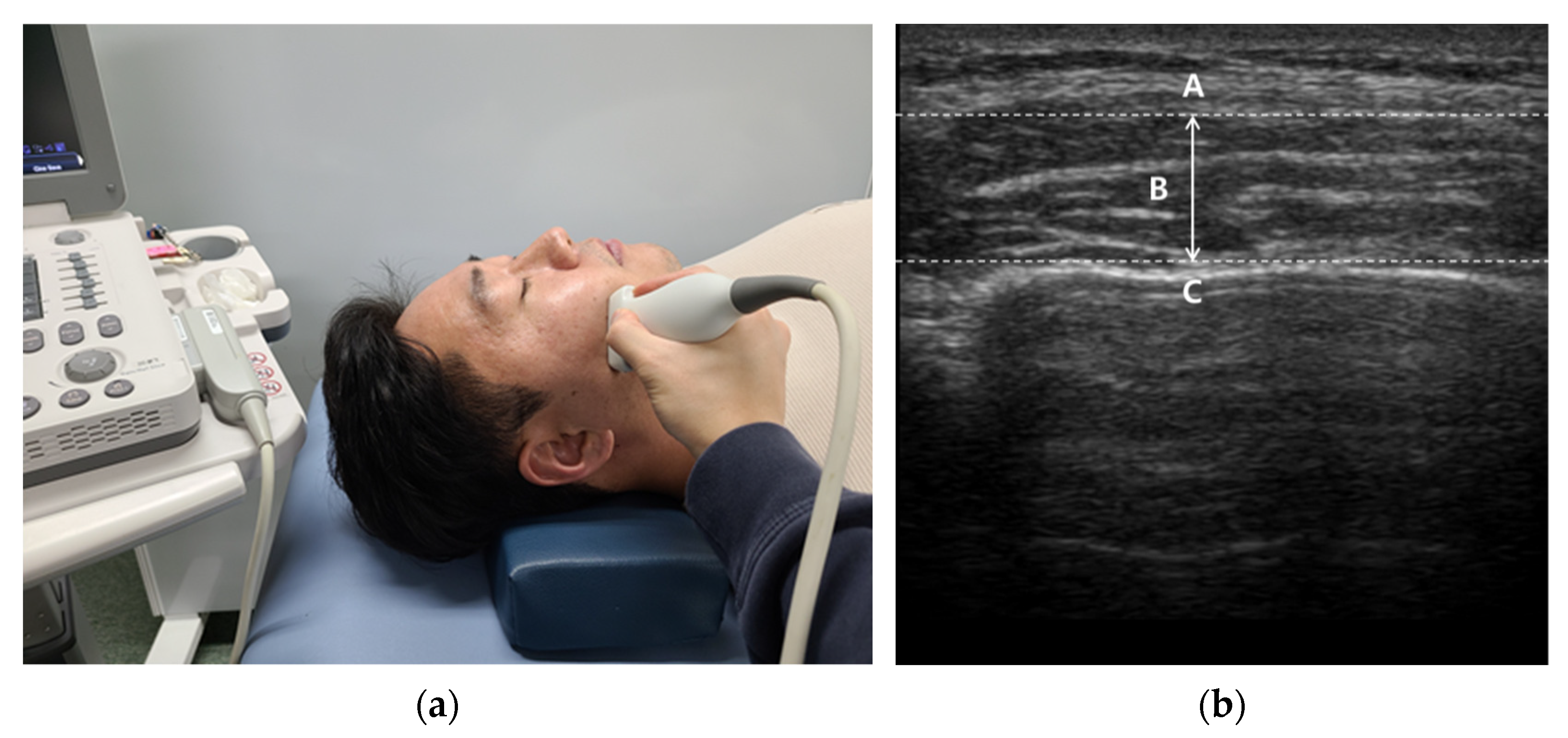
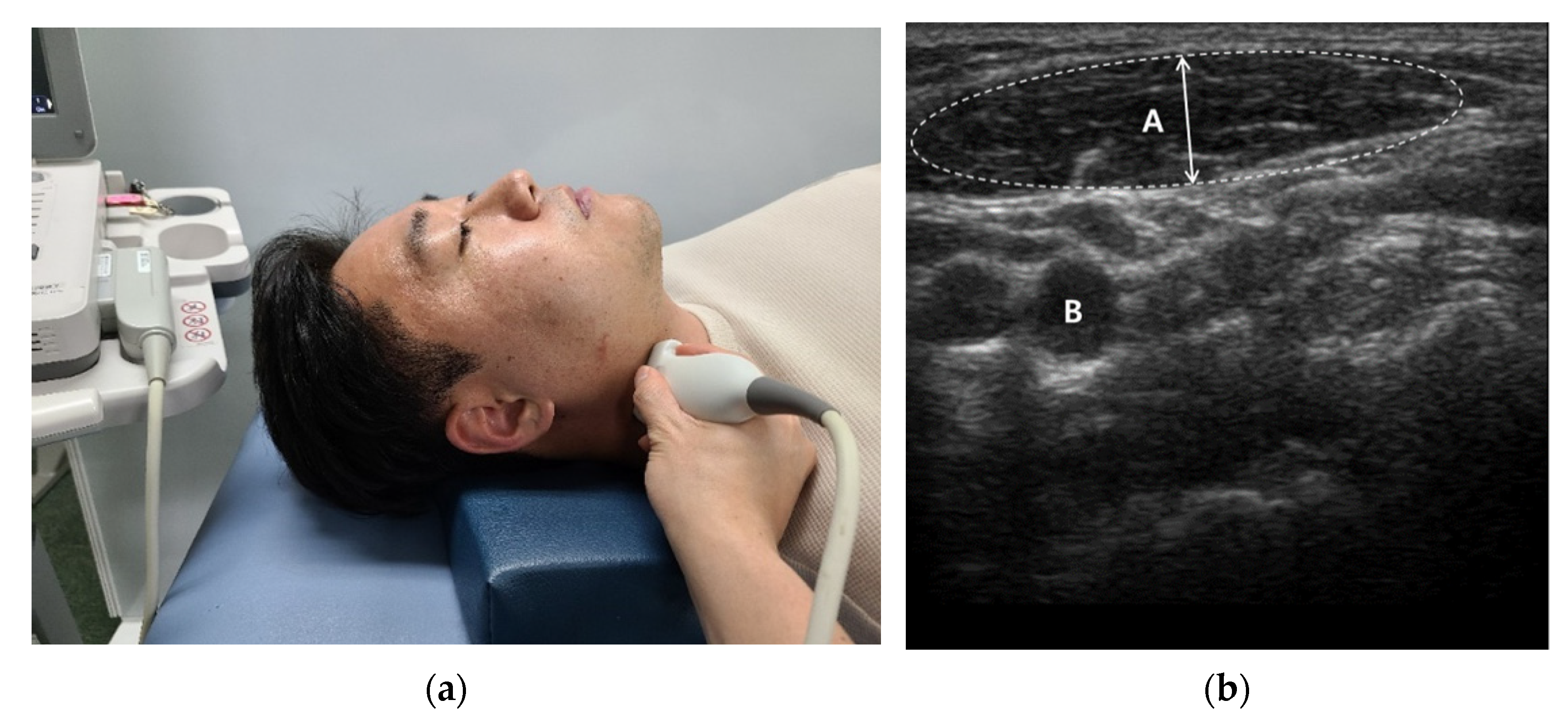
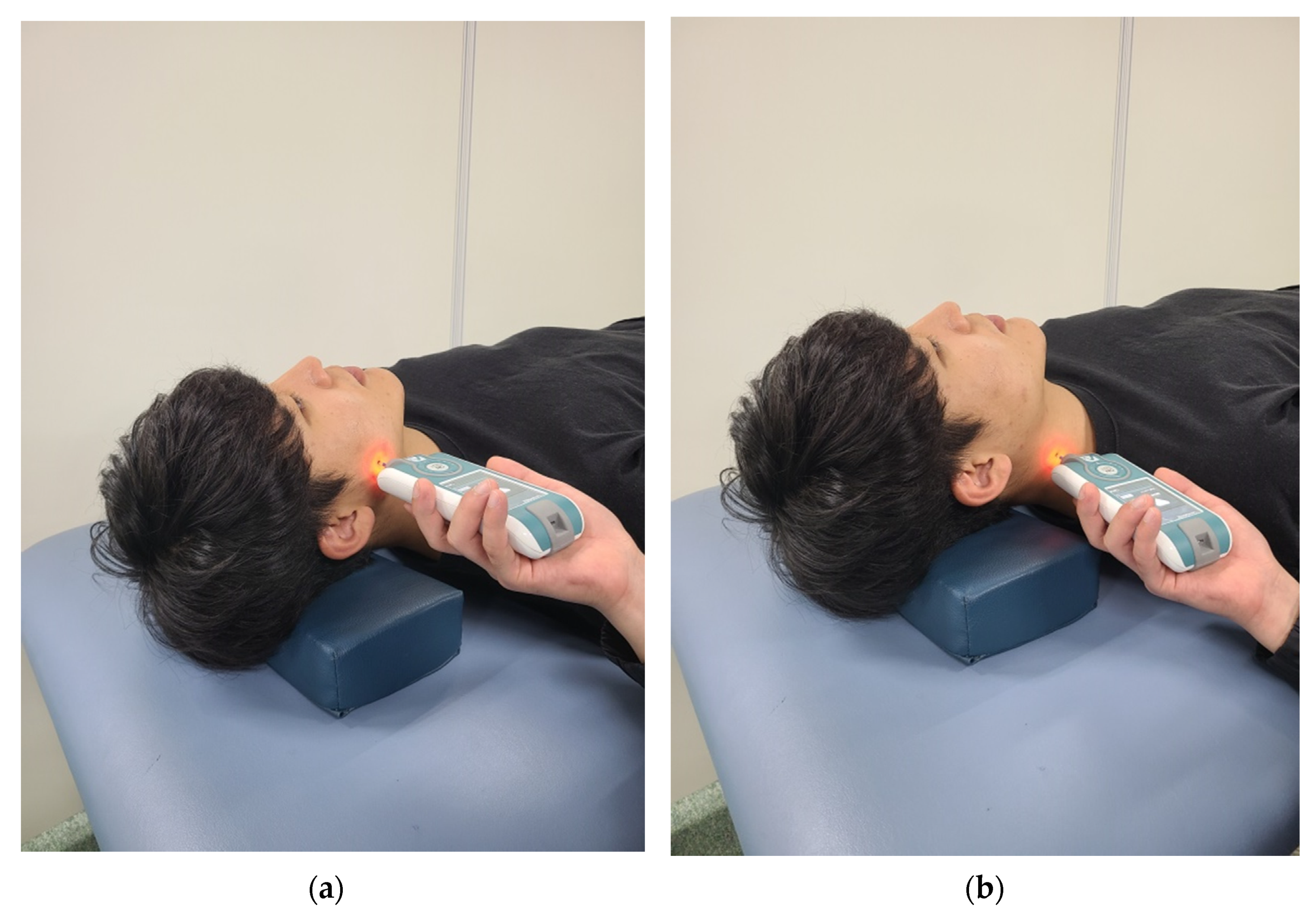
| Variable | TMD Group (n1 = 30) | Non-TMD Group (n2 = 30) | p-Value |
|---|---|---|---|
| Gender (Male/Female) | 12/18 | 14/16 | 0.60 |
| Age (years) | 24.0 ± 1.7 | 24.3 ± 1.6 | 0.59 |
| Height (cm) | 167.7 ± 5.6 | 168.3 ± 4.8 | 0.69 |
| Body mass (kg) | 59.9 ± 5.0 | 60.3 ± 6.9 | 0.83 |
| BMI (kg/m2) | 20.9 ± 2.6 | 21.3 ± 2.6 | 0.49 |
| NRS | 5.3 ± 1.0 | ||
| AAOP questionnaire | 2.3 ± 1.9 |
| Variable | TMD Group (n1 = 30) | Non-TMD Group (n2 = 30) | p-Value | |
|---|---|---|---|---|
| Masseter | Relaxed (mm) | 10.7 ± 2.0 | 12.7 ± 1.9 | <0.001 |
| Clenching (mm) | 12.9 ± 2.0 | 15.0 ± 2.2 | <0.001 | |
| SCM | Relaxed (mm) | 10.0 ± 2.0 | 11.9 ± 1.6 | <0.001 |
| Clenching (mm) | 12.2 ± 2.2 | 14.4 ± 1.8 | <0.001 | |
| Variable | TMD Group (n1 = 30) | Non-TMD Group (n2 = 30) | p-Value | |
|---|---|---|---|---|
| Masseter | Frequency (Hz) | 24.1 ± 2.8 | 21.4 ± 2.4 | <0.001 |
| Decrement (log) | 1.5 ± 0.3 | 1.6 ± 0.3 | 0.35 | |
| Stiffness (N/m) | 507.2 ± 63.4 | 435.2 ± 71.2 | <0.001 | |
| SCM | Frequency (Hz) | 16.5 ± 1.3 | 14.8 ± 1.5 | <0.001 |
| Decrement (log) | 1.4 ± 0.2 | 1.4 ± 0.2 | 0.76 | |
| Stiffness (N/m) | 281.6 ± 52.8 | 246.0 ± 41.2 | 0.005 | |
| Variable | TMD Group (n1 = 30) | Non-TMD Group (n2 = 30) | p-Value |
|---|---|---|---|
| MMO (cm) | 3.4 ± 0.3 | 5.4 ± 0.5 | <0.001 |
| Variable | Masseter Muscle Tone | SCM Muscle Tone | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Decrement | Stiffness | Frequency | Decrement | Stiffness | ||||||||
| r | (p) | r | (p) | r | (p) | r | (p) | r | (p) | r | (p) | ||
| Masseter | Relaxed | −0.15 | (0.25) | −0.03 | (0.81) | −0.16 | (0.19) | −0.38 | (0.003) | −0.04 | (0.75) | −0.40 | (0.002) |
| Clenching | −0.17 | (0.17) | −0.01 | (0.89) | −0.16 | (0.19) | −0.34 | (0.008) | −0.02 | (0.86) | −0.32 | (0.011) | |
| SCM | Relaxed | −0.21 | (0.10) | 0.02 | (0.85) | −0.16 | (0.21) | −0.42 | (0.001) | 0.09 | (0.47) | −0.35 | (0.005) |
| Clenching | −0.21 | (0.10) | 0.07 | (0.56) | −0.27 | (0.35) | −0.47 | (<0.001) | 0.08 | (0.53) | −0.31 | (0.015) | |
| MMO | −0.39 | (0.002) | 0.03 | (0.77) | −0.51 | (<0.001) | −0.44 | (<0.001) | −0.07 | (0.57) | −0.34 | (0.007) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Chon, S. Assessments of Muscle Thickness and Tonicity of the Masseter and Sternocleidomastoid Muscles and Maximum Mouth Opening in Patients with Temporomandibular Disorder. Healthcare 2021, 9, 1640. https://doi.org/10.3390/healthcare9121640
Lee K, Chon S. Assessments of Muscle Thickness and Tonicity of the Masseter and Sternocleidomastoid Muscles and Maximum Mouth Opening in Patients with Temporomandibular Disorder. Healthcare. 2021; 9(12):1640. https://doi.org/10.3390/healthcare9121640
Chicago/Turabian StyleLee, Keunhyo, and Seungchul Chon. 2021. "Assessments of Muscle Thickness and Tonicity of the Masseter and Sternocleidomastoid Muscles and Maximum Mouth Opening in Patients with Temporomandibular Disorder" Healthcare 9, no. 12: 1640. https://doi.org/10.3390/healthcare9121640
APA StyleLee, K., & Chon, S. (2021). Assessments of Muscle Thickness and Tonicity of the Masseter and Sternocleidomastoid Muscles and Maximum Mouth Opening in Patients with Temporomandibular Disorder. Healthcare, 9(12), 1640. https://doi.org/10.3390/healthcare9121640






