Drug Induced Liver Injury: Perspective of the Adverse Drug Reaction Reports to the Portuguese Pharmacovigilance System from 2010 to 2019
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Liver Adverse Drug Reactions
2.3. Source and Information Contained in Reports
2.4. Report Selection
2.5. Statistical Analysis
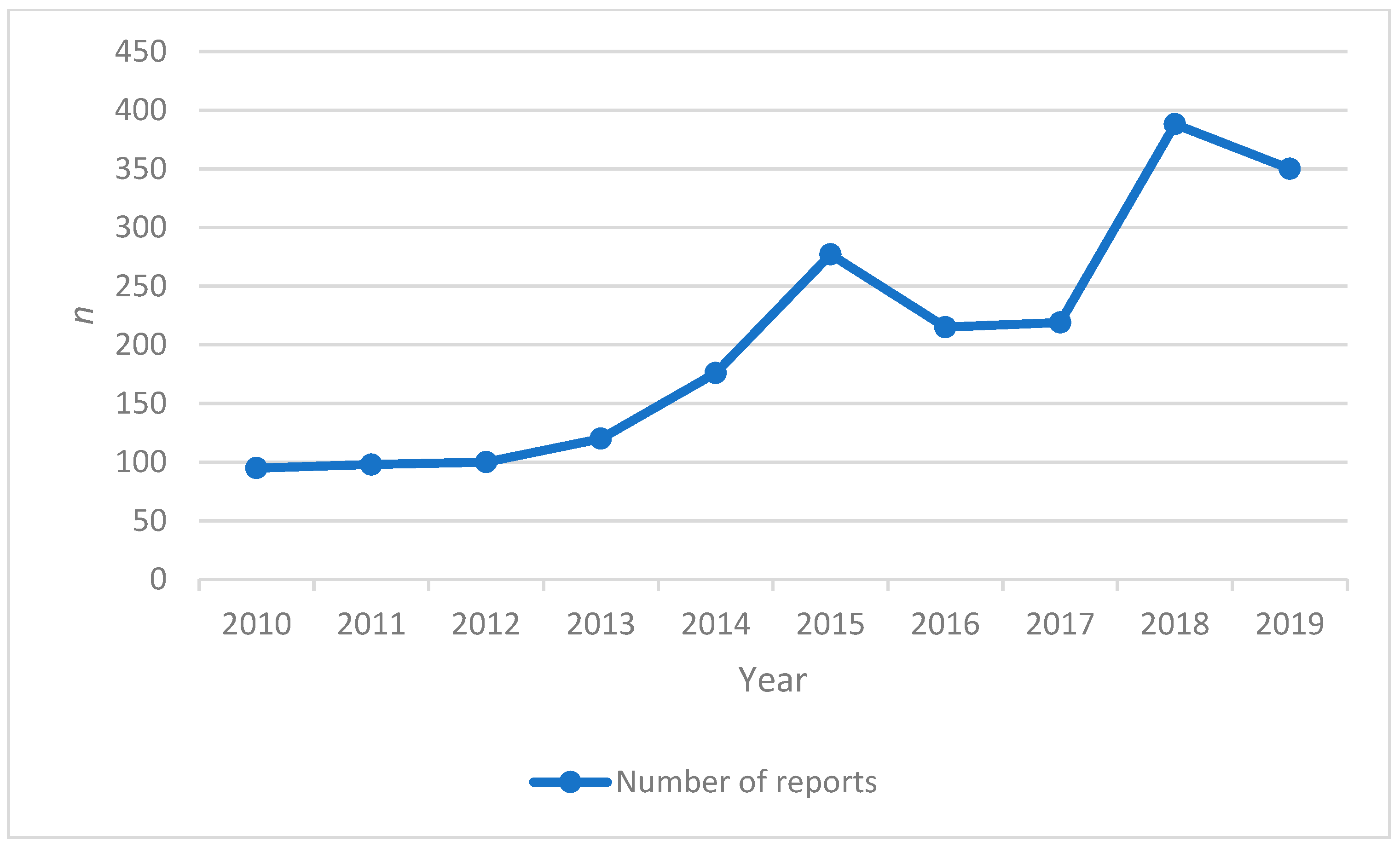
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoofnagle, J.H.; Björnsson, E.S. Drug-Induced Liver Injury-Types and Phenotypes. N. Engl. J. Med. 2019, 381, 264–273. [Google Scholar] [CrossRef]
- Katarey, D.; Verma, S. Drug-induced liver injury. Clin. Med. 2016, 16, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Geneva: Council for International Organizations of Medical Sciences (CIOMS) Drug-Induced Liver Injury (DILI): Current Status and Future Directions for Drug Development and the Post-Market Setting. A Consensus by a CIOMS Working Group. 2020. Available online: https://cioms.ch/wp-content/uploads/2020/06/CIOMS_DILI_Web_16Jun2020.pdf (accessed on 18 December 2020).
- Tolosa, L.; Jiménez, N.; Pelechá, M.; Castell, J.V.; Gómez-Lechón, M.J.; Donato, M.T. Long-term and mechanistic evaluation of drug-induced liver injury in Upcyte human hepatocytes. Arch. Toxicol. 2019, 93, 519–532. [Google Scholar] [CrossRef] [Green Version]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.P.; Hayashi, P.H.; Bonkovsky, H.L.; Navarro, V.J.; Lee, W.M.; Fontana, R.J. ACG Clinical Guideline: The Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am. J. Gastroenterol. 2014, 109, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Cho, W.C.; Upadhyay, G. Drug-Induced Liver Toxicity and Prevention by Herbal Antioxidants: An Overview. Front. Physiol. 2016, 6, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kullak-Ublick, G.A.; Andrade, R.J.; Merz, M.; End, P.; Benesic, A.; Gerbes, A.L.; Aithal, G.P. Drug-induced liver injury: Recent advances in diagnosis and risk assessment. Gut 2017, 66, 1154–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuna, L.; Bozic, I.; Kizivat, T.; Bojanic, K.; Mrso, M.; Kralj, E.; Smolic, R.; Wu, G.Y.; Smolic, M. Models of Drug Induced Liver Injury (DILI)-Current Issues and Future Perspectives. Curr. Drug Metab. 2018, 19, 830–838. [Google Scholar] [CrossRef]
- Schenker, S.; Martin, R.R.; Hoyumpa, A.M. Antecedent liver disease and drug toxicity. J. Hepatol. 1999, 31, 1088–1097. [Google Scholar] [CrossRef]
- Teschke, R. Idiosyncratic DILI: Analysis of 46,266 Cases Assessed for Causality by RUCAM and Published From 2014 to Early 2019. Front. Pharmacol. 2019, 10, 730. [Google Scholar] [CrossRef]
- Raschi, E.; Ponti, F. Drug- and herb-induced liver injury: Progress, current challenges and emerging signals of post-marketing risk. World J. Hepatol. 2015, 7, 1761–1771. [Google Scholar] [CrossRef]
- Publications of the World Health Organization Proceedings of the Name of The Importance of Pharmacovigilance Safety Monitoring of Medicinal Products, The United Kingdom. 2002. Available online: https://apps.who.int/iris/handle/10665/42493 (accessed on 20 December 2020).
- Inácio, P.; Cavaco, A.; Airaksinen, M. The value of patient reporting to the pharmacovigilance system: A systematic review. Br. J. Clin. Pharmacol. 2017, 83, 227–246. [Google Scholar] [CrossRef] [Green Version]
- Santoro, A.; Genov, G.; Spooner, A.; Raine, J.; Arlett, P. Promoting and Protecting Public Health: How the European Union Pharmacovigilance System Works. Drug Saf. 2017, 40, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Pontefract, S.K. Adverse drug reactions. Clin. Med. 2016, 16, 481–485. [Google Scholar] [CrossRef]
- Batel-Marques, F.; Mendes, D.; Alves, C.; Penedones, A.; Dias, P.; Martins, A.; Santiago, L.M.; Fontes-Ribeiro, C.; Caramona, M.; Macedo, T. Pharmacovigilance in Portugal: Activity of the Central Pharmacovigilance Unit. Acta Med. Port. 2015, 28, 222–232. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency and Heads of Medicines Agencies Guideline on Good Pharmacovigilance Practices (GVP) Module VI-Management and Reporting of Adverse Reactions to Medicinal Products (Rev 1). EMA/873138/2011 Rev 1. 2014. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf (accessed on 27 December 2020).
- WHO Collaborating Centre for Drug Statistics Methodology, Norwegian Institute of Public Health. International Language for Drug Utilization Research ATC/DDD. Available online: https://www.whocc.no/ (accessed on 16 January 2021).
- WHO Publication The Uppsala Monitoring Centre. The Use of the WHO-UMC System for Standardized Case Causality Assessment. 2013. Available online: https://www.who.int/publications/m/item/WHO-causality-assessment (accessed on 27 December 2020).
- Available online: https://www.pordata.pt/ (accessed on 11 October 2021).
- Bell, L.; Chalasani, N. Epidemiology of Idiosyncratic Drug-Induced Liver Injury. Semin. Liver Dis. 2009, 29, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, R.; Lucena, M.I.; Fernández, M.C.; Pelaez, G.; Pachkoria, K.; García-Ruiz, E.; García-Muñoz, B.; González-Grande, R.; Pizarro, A.; Durán, J.A.; et al. Drug-Induced Liver Injury: An Analysis of 461 Incidences Submitted to the Spanish Registry Over a 10-Year Period. Gastroenterology 2005, 129, 512–521. [Google Scholar] [CrossRef]
- Teschke, R.; Wolff, A.; Frenzel, C.; Schwarzenboeck, A.; Schulze, J.; Eickhoff, A. Drug and herb induced liver injury: Council for International Organizations of Medical Sciences scale for causality assessment. World J. Hepatol. 2014, 6, 17–32. [Google Scholar] [CrossRef]
- Stine, J.G.; Sateesh, P.; Lewis, J.H. Drug-Induced Liver Injury in the Elderly. Curr. Gastroenterol. Rep. 2013, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Suzuki, A.; Borlak, J.; Andrade, R.J.; Lucena, M.I. Drug-induced liver injury: Interactions between drug properties and host factors. J. Hepatol. 2015, 63, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Chalasani, N.; Björnsson, E. Risk Factors for Idiosyncratic Drug-Induced Liver Injury. Gastroenterology 2010, 138, 2246–2259. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, M.; Crawford, J. Hepatitic inherited metabolic disorders. Semin. Diagn. Pathol. 2006, 23, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Padda, M.S.; Sanchez, M.; Akhtar, A.J.; Boyer, J.L. Drug Induced Cholestasis. Hepatology 2011, 53, 1377–1387. [Google Scholar] [CrossRef] [Green Version]
- Morales, M.; Vélez, L.; Muñoz, M. Hepatotoxicity: A Drug-Induced Cholestatic Pattern. Rev. Col. Gastroenterol. 2016, 31, 34–45. Available online: http://www.scielo.org.co/scielo.php?pid=S0120-99572016000100006&script=sci_arttext&tlng=en (accessed on 27 February 2021).
- Yang, K.; Köck, K.; Sedykh, A.; Tropsha, A.; Brouwer, K. An updated review on drug-induced cholestasis: Mechanisms and investigation of physicochemical properties and pharmacokinetic parameters. J. Pharm. Sci. 2013, 102, 3037–3057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlinger, S. Drug-induced cholestasis. J. Hepatol. 1997, 26, 1–4. [Google Scholar] [CrossRef]
- Arias, I.M.; Alter, H.J.; Boyer, J.L.; Cohen, D.E.; Shafritz, D.A.; Thorgeirsson, S.S.; Wolkoff, A.W. The Liver Biology and Pathology, 6th ed.; John Wiley Sons: Oxford, UK, 2020. [Google Scholar]
- David, S.; Hamilton, J.P. Drug-induced Liver Injury. US Gastroenterol. Hepatol. Rev. 2010, 6, 73–80. [Google Scholar]
- Bataller, R.; Brenner, D. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Ferenci, P. Hepatic Encephalopathy. Gastroenterol. Rep. 2017, 5, 138–147. [Google Scholar] [CrossRef] [Green Version]
- Molleston, J.P.; Fontana, R.J.; Lopez, M.J.; Kleiner, D.E.; Gu, J.; Chalasani, N. Characteristics of Idiosyncratic Drug-induced Liver Injury in Children: Results From the DILIN Prospective Study. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, A.; Costa, J. Off-label prescription: Practice and problems. Port. J. Cardiol. 2013, 32, 681–686. [Google Scholar] [CrossRef]
- Tivoli, Y.; Rubenstein, R. Pruritus An Updated Look at an Old Problem. J. Clin. Aesthetic Dermatol. 2009, 2, 30–36. [Google Scholar]
- Huang, A.; Kaffenberger, B.H.; Reich, A.; Szepietowski, J.C.; Ständer, S.; Kwatra, S.G. Pruritus Associated with Commonly Prescribed Medications in a Tertiary Care Center. Medicines 2019, 6, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Association for the Study of the Liver EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [CrossRef]
- Ylä-Rautio, H.; Siissalo, S.; Leikola, S. Drug-related problems and pharmacy interventions in non-prescription medication, with a focus on high-risk over-the-counter medications. Int. J. Clin. Pharm. 2020, 42, 786–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masnoon, N.; Shakib, S.; Kalisch-Ellett, L.; Caughey, G.E. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017, 17, 1–10. [Google Scholar] [CrossRef] [Green Version]
| Age Group | Sex | Χ2 | p | |||
|---|---|---|---|---|---|---|
| Female n = 968 n (%) | Male n = 980 n (%) | NI 2 n = 90 n (%) | Total n = 2038 n (%) | |||
| 1–3 years | 8 (0.4) | 12 (0.6) | 1 (0.0) | 21 (1.0) | 0.75 | 0.383 |
| 4–12 years | 25 (1.2) | 24 (1.2) | 2 (0.1) | 51 (2.5) | 0.02 | 0.850 |
| 13–18 years | 18 (0.9) | 13 (0.6) | 5 (0.2) | 36 (1.8) | 0.83 | 0.347 |
| 19–64 years | 563 (27.6) | 542 (26.6) | 15 (0.7) | 1120 (55.0) | 0.37 | 0.203 |
| >64 years | 202 (9.9) | 203 (10.0) | 11 (0.5) | 416 (20.4) | 0.01 | 0.933 |
| NI 1 | 152 (7.4) | 186 (9.7) | 56 (2.7) | 394 (19.3) |
| Adverse Reaction | Age Group n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1–3 Years (n = 21) | 4–12 Years (n = 51) | 13–18 Years (n = 36) | 19–64 Years (n = 1120) | >64 Years (n = 416) | NI 1 (n = 394) | Total (n = 2038) | Χ2 | p | |
| Hepatitis | 8 (0.3) | 16 (0.7) | 14 (0.6) | 333 (14.2) | 164 (7.0) | 91 (3.9) | 626 (26.7) | 13.98 | 0.007 |
| Hepatotoxicity | 5 (0.2) | 10 (0.4) | 5 (0.2) | 362 (15.5) | 114 (4.9) | 104 (4.4) | 600 (25.6) | 11.60 | 0.020 |
| Jaundice | 6 (0.3) | 7 (0.3) | 4 (0.2) | 142 (6.1) | 67 (2.9) | 37 (1.6) | 263 (11.2) | 7.13 | 0.129 |
| Cholestasis | 3 (0.1) | 6 (0.3) | 8 (0.3) | 101 (4.3) | 80 (3.4) | 32 (1.4) | 230 (9.8) | 34.07 | <0.001 |
| Rash | 1 (0.0) | 4 (0.2) | 2 (0.1) | 62 (2.6) | 21 (0.9) | 13 (0.6) | 103 (4.4) | 0.72 | 0.947 |
| Hepatic fibrosis | 97 (4.1) | 1 (0.0) | 1 (0.0) | 99 (4.2) | 45.69 | <0.001 | |||
| Ascites | 2 (0.1) | 45 (1.9) | 24 (1.0) | 26 (1.1) | 97 (4.1) | 5.74 | 0.218 | ||
| Pruritus | 6 (0.3) | 2 (0.1) | 60 (2.6) | 21 (0.9) | 8 (0.3) | 97 (4.1) | 5.33 | 0.254 | |
| Autoimmune hepatitis | 2 (0.1) | 43 (1.8) | 11 (0.5) | 8 (0.3) | 64 (2.7) | 3.42 | 0.489 | ||
| Choluria | 1 (0.0) | 2 (0.1) | 2 (0.1) | 26 (1.1) | 15 (0.6) | 2 (0.1) | 48 (2.1) | 3.47 | 0.481 |
| Encephalopathy | 1 (0.0) | 2 (0.1) | 2 (0.1) | 12 (0.5) | 30 (1.3) | 47 (2.0) | 29.54 | <0.001 | |
| Cirrhosis | 28 (1.2) | 5 (0.2) | 8 (0.3) | 41 (1.8) | 4.96 | 0.290 | |||
| Acholic stool | 1 (0.0) | 13 (0.6) | 13 (0.6) | 27 (1.1) | 8.25 | 0.082 | |||
| Laboratory tests | |||||||||
| Aminotransferase | 2 (0.1) | 18 (0.9) | 8 (0.3) | 264 (13.0) | 101 (5.0) | 101 (5.0) | 494 (24.2) | 6.18 | 0.186 |
| Bilirubin | 5 (0.2) | 5 (0.2) | 2 (0.1) | 153 (7.5) | 73 (3.6) | 55 (2.7) | 293 (14.4) | 8.46 | 0.076 |
| ALT | 4 (0.2) | 4 (0.2) | 5 (0.2) | 130 (6.4) | 70 (3.4) | 31 (1.5) | 244 (12.0) | 9.23 | 0.055 |
| AST | 5 (0.2) | 4 (0.2) | 3 (0.1) | 112 (5.5) | 71 (3.5) | 26 (1.3) | 221 (10.8) | 18.57 | <0.001 |
| GGT | 4 (0.2) | 3 (0.1) | 1 (0.0) | 117 (5.7) | 67 (3.3) | 35 (1.7) | 227 (11.1) | 15.08 | 0.004 |
| Alkaline phosphatase | 2 (0.2) | 3 (0.1) | 65 (3.2) | 50 (2.5) | 16 (0.8) | 136 (6.7) | 20.53 | <0.001 | |
| Lactate dehydrogenase | 1 (0.0) | 49 (2.4) | 21 (1.0) | 6 (0.3) | 77 (3.8) | 4.48 | 0.344 | ||
| Prothrombin time | 34 (1.7) | 6 (0.3) | 1 (0.3) | 41(2.0) | 6.12 | 0.189 | |||
| Procedural complications | |||||||||
| Off label use | 2 (0.2) | 3 (0.1) | 4 (0.2) | 49 (2.4) | 2 (0.2) | 18 (0.9) | 78 (3.8) | 22.03 | <0.001 |
| Drug exposure Pregnancy | 13 (0.6) | 10 (0.4) | 23 (1.1) | 6.13 | 0.189 | ||||
| Overdose | 1 (0.0) | 1 (0.0) | 12 (0.6) | 2 (0.2) | 16 (0.8) | 6.71 | 0.151 | ||
| Medical error | 4 (0.2) | 2 (0.2) | 2 (0.2) | 8 (0.4) | 0.55 | 0.968 | |||
| Number of Suspected Drugs | 1–4 | 5–9 | ≤10 | Total | Χ2 | p |
|---|---|---|---|---|---|---|
| Adverse Reactions n (%) | ||||||
| Hepatitis | 610 (25.9) | 12 (0.5) | 4 (0.2) | 626 (26.6) | 68.48 | <0.001 |
| Hepatotoxicity | 489 (20.8) | 74 (3.1) | 37 (1.6) | 600 (25.5) | 41.59 | <0.001 |
| Jaundice | 260 (11.0) | 3 (0.1) | 0 (0.0) | 263 (11.2) | 31.87 | <0.001 |
| Cholestasis | 204 (8.7) | 22 (0.9) | 4 (0.2) | 230 (9.8) | 1.94 | 0.378 |
| Rash | 97 (4.1) | 6 (0.3) | 0 (0.0) | 103 (4.4) | 4.70 | 0.095 |
| Hepatic fibrosis | 21 (0.9) | 55 (2.3) | 23 (1.0) | 99 (4.2) | 454.55 | <0.001 |
| Pruritus | 93 (4.0) | 0 (0.0) | 4 (0.2) | 97 (4.1) | 9.55 | 0.008 |
| Ascites | 85 (3.6) | 12 (0.5) | 0 (0.0) | 97 (4.1) | 4.97 | 0.083 |
| Encephalopathy | 75 (3.2) | 10 (0.4) | 0 (0.0) | 85 (3.6) | 3.87 | 0.143 |
| Autoimmune hepatitis | 64 (2.7) | 0 (0.0) | 0 (0.0) | 64 (2.7) | 8.67 | 0.001 |
| Choluria | 48 (2.0) | 0 (0.0) | 0 (0.0) | 48 (2.0) | 6.45 | 0.039 |
| Cirrhosis | 33 (1.4) | 6 (0.3) | 2 (0.1) | 41 (1.7) | 2.53 | 0.281 |
| Total | 2079 (88.4) | 200 (8.5) | 74 (3.1) | 2353 (100.0) |
| Number of Suspected Drugs | 1–4 (n = 1867) | 5–9 (n = 131) | ≥10 (n = 40) | Χ2 | p |
|---|---|---|---|---|---|
| Age (Mean ± SD) | 52 ± 20 | 38 ± 16 | 35 ± 18 | ||
| Case Evolution n (%) | |||||
| Cure | 746 (36.6) | 36 (1.8) | 14 (0.7) | 8.28 | 0.001 |
| In recovery | 285 (14.0) | 10 (0.5) | 0 (0.0) | 12.66 | 0.001 |
| Cure with sequels | 25 (1.2) | 0 (0.0) | 0 (0.0) | 2.31 | 0.313 |
| No recovery | 102 (5.0) | 2 (0.1) | 1 (0.0) | 4.46 | 0.107 |
| Death | 115 (5.6) | 11 (0.5) | 0 (0.0) | 3.74 | 0.153 |
| Unknown 1 | 594 (29.1) | 72 (3.5) | 25 (1.2) | ||
| Adverse Reaction | Total n (%) | Female n (%) | Male n (%) | NI 1 n (%) | Χ2 | p |
|---|---|---|---|---|---|---|
| Hepatotoxicity | 46 (27.5) | 23 (13.8) | 21 (12.6) | 2 (1.2) | 7.51 | 0.006 |
| Hepatitis | 37 (22.2) | 21 (12.6) | 15 (9) | 1 (0.6) | 10.64 | 0.001 |
| Encephalopathy | 25 (15) | 8 (4.8) | 17 (10.2) | 0 (0) | 0.14 | 0.702 |
| Jaundice | 17 (10.2) | 4 (2.4) | 13 (7.8) | 0 (0) | 1.16 | 0.280 |
| Ascites | 14 (8.4) | 2 (1.2) | 12 (7.2) | 0 (0) | 2.97 | 0.084 |
| Choluria | 8 (4.8) | 0 (0) | 8 (4.8) | 0 (0) | 4.60 | 0.031 |
| Splenomegaly | 8 (4.8) | 0 (0) | 8 (4.8) | 0 (0) | 4.60 | 0.031 |
| Cholestasis | 4 (2.4) | 0 (0) | 4 (2.4) | 0 (0) | 2.24 | 0.134 |
| Cirrhosis | 4 (2.4) | 0 (0) | 4 (2.4) | 0 (0) | 2.24 | 0.134 |
| Hepatomegaly | 4 (2.4) | 0 (0) | 4 (2.4) | 0 (0) | 2.24 | 0.134 |
| Number adverse reactions | 167 (100.0) | 58 (24.7) | 106 (63.5) | 3 (1.8) | ||
| Number of cases | 126 | 51 (40.5) | 70 (55.6) | 5 (4.) | ||
| Age (mean ± SD) | 57 ± 20 | 57 ± 20 | 57 ± 21 | 56 ± 00 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, D.R.d.C.M.A.; Breton, M.C.; Monteiro, C.S.d.J.; dos Santos, J.L. Drug Induced Liver Injury: Perspective of the Adverse Drug Reaction Reports to the Portuguese Pharmacovigilance System from 2010 to 2019. Healthcare 2021, 9, 1630. https://doi.org/10.3390/healthcare9121630
Nunes DRdCMA, Breton MC, Monteiro CSdJ, dos Santos JL. Drug Induced Liver Injury: Perspective of the Adverse Drug Reaction Reports to the Portuguese Pharmacovigilance System from 2010 to 2019. Healthcare. 2021; 9(12):1630. https://doi.org/10.3390/healthcare9121630
Chicago/Turabian StyleNunes, David Ricardo da Conceição Marçal Alves, Michèle Claire Breton, Cristina Sofia de Jesus Monteiro, and Jorge Luiz dos Santos. 2021. "Drug Induced Liver Injury: Perspective of the Adverse Drug Reaction Reports to the Portuguese Pharmacovigilance System from 2010 to 2019" Healthcare 9, no. 12: 1630. https://doi.org/10.3390/healthcare9121630
APA StyleNunes, D. R. d. C. M. A., Breton, M. C., Monteiro, C. S. d. J., & dos Santos, J. L. (2021). Drug Induced Liver Injury: Perspective of the Adverse Drug Reaction Reports to the Portuguese Pharmacovigilance System from 2010 to 2019. Healthcare, 9(12), 1630. https://doi.org/10.3390/healthcare9121630







