Baricitinib: The First Jak Inhibitor Approved in Europe for the Treatment of Moderate to Severe Atopic Dermatitis in Adult Patients
Abstract
:1. Introduction
2. JAK Inhibitors: Mechanism of Action and Role in the Treatment of AD
3. Efficacy and Safety Results from Phase II and III Trials
3.1. Methods
3.2. Efficacy
3.2.1. Phase II Trials
3.2.2. Phase III Trials
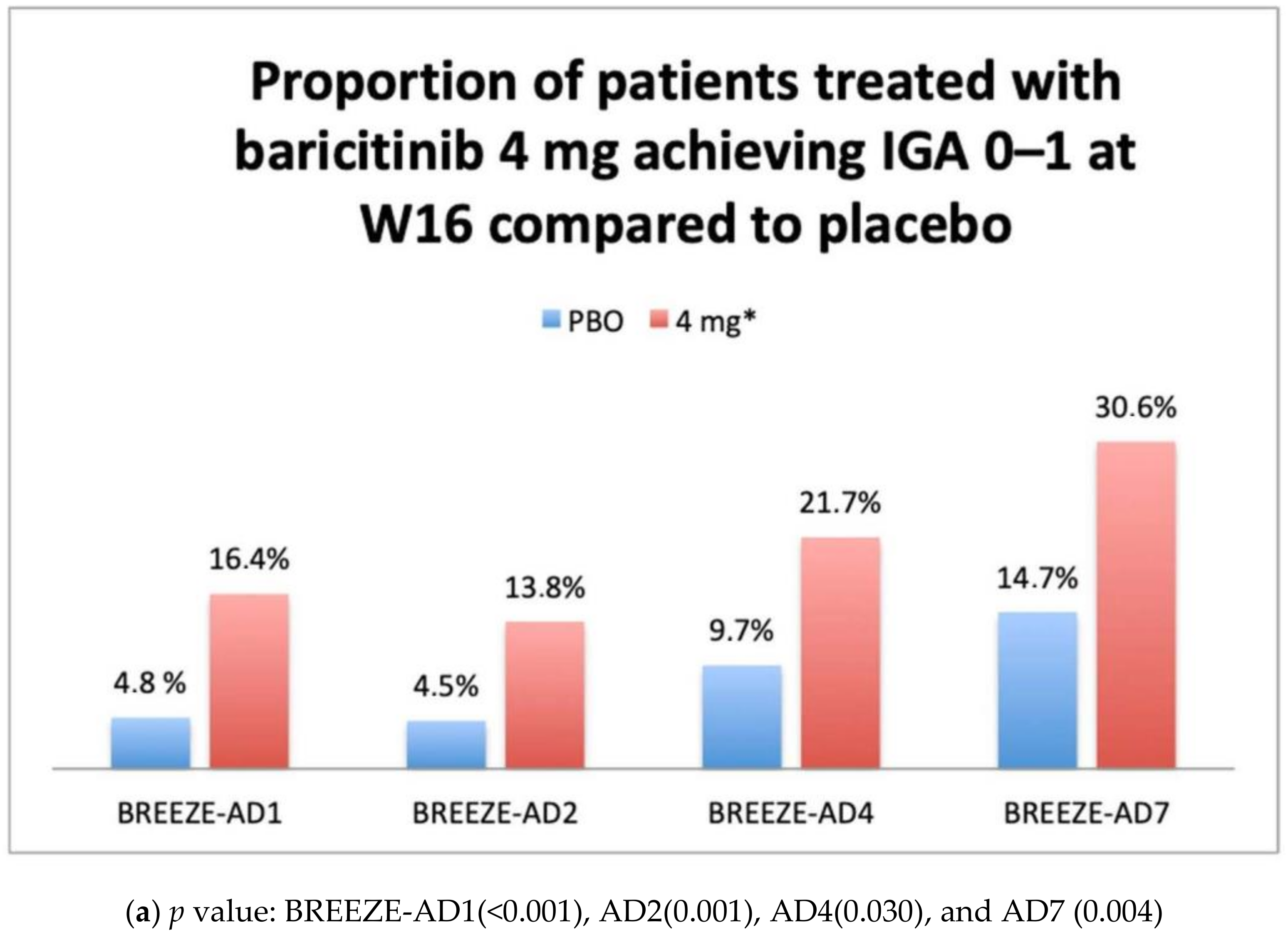
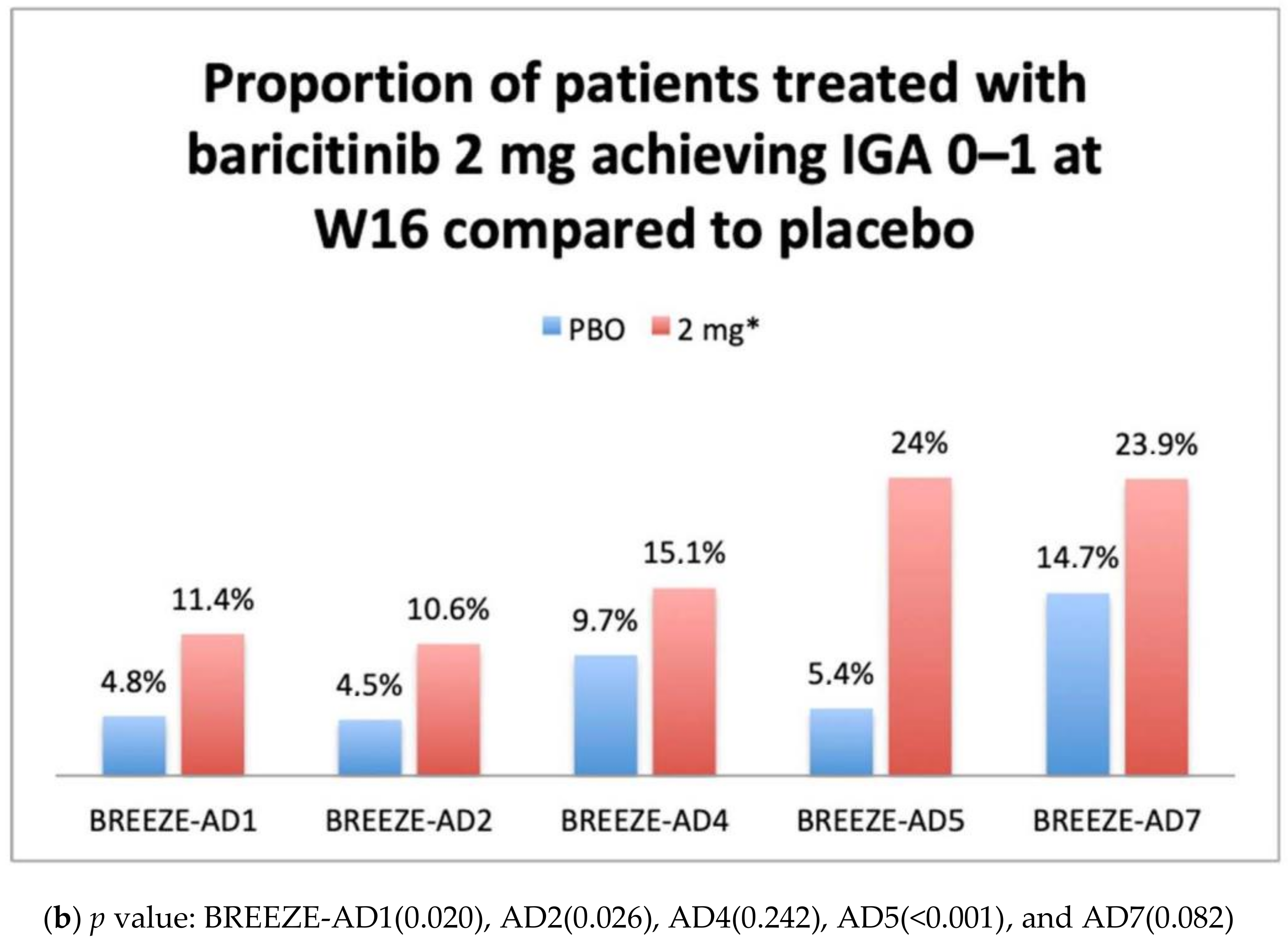
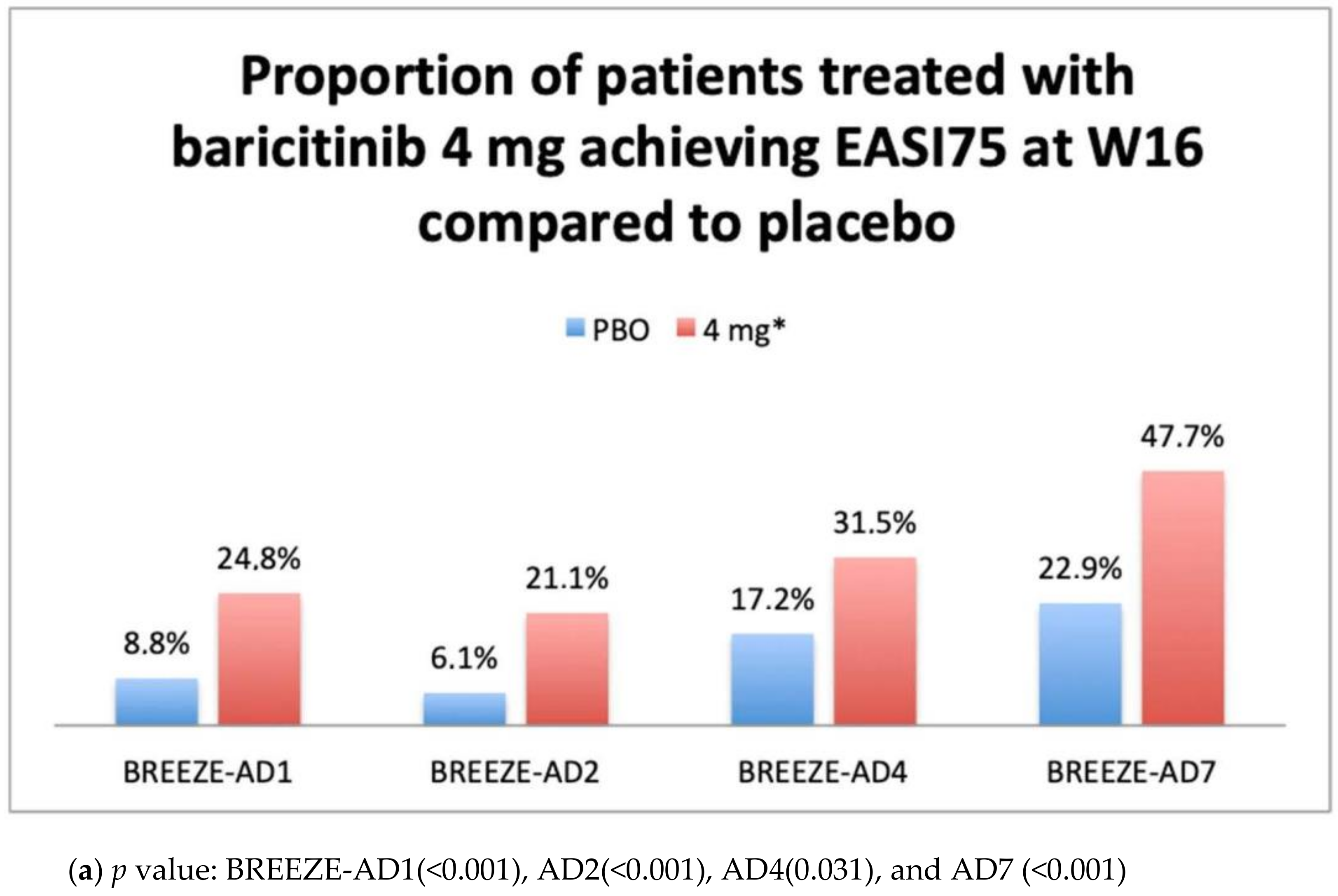
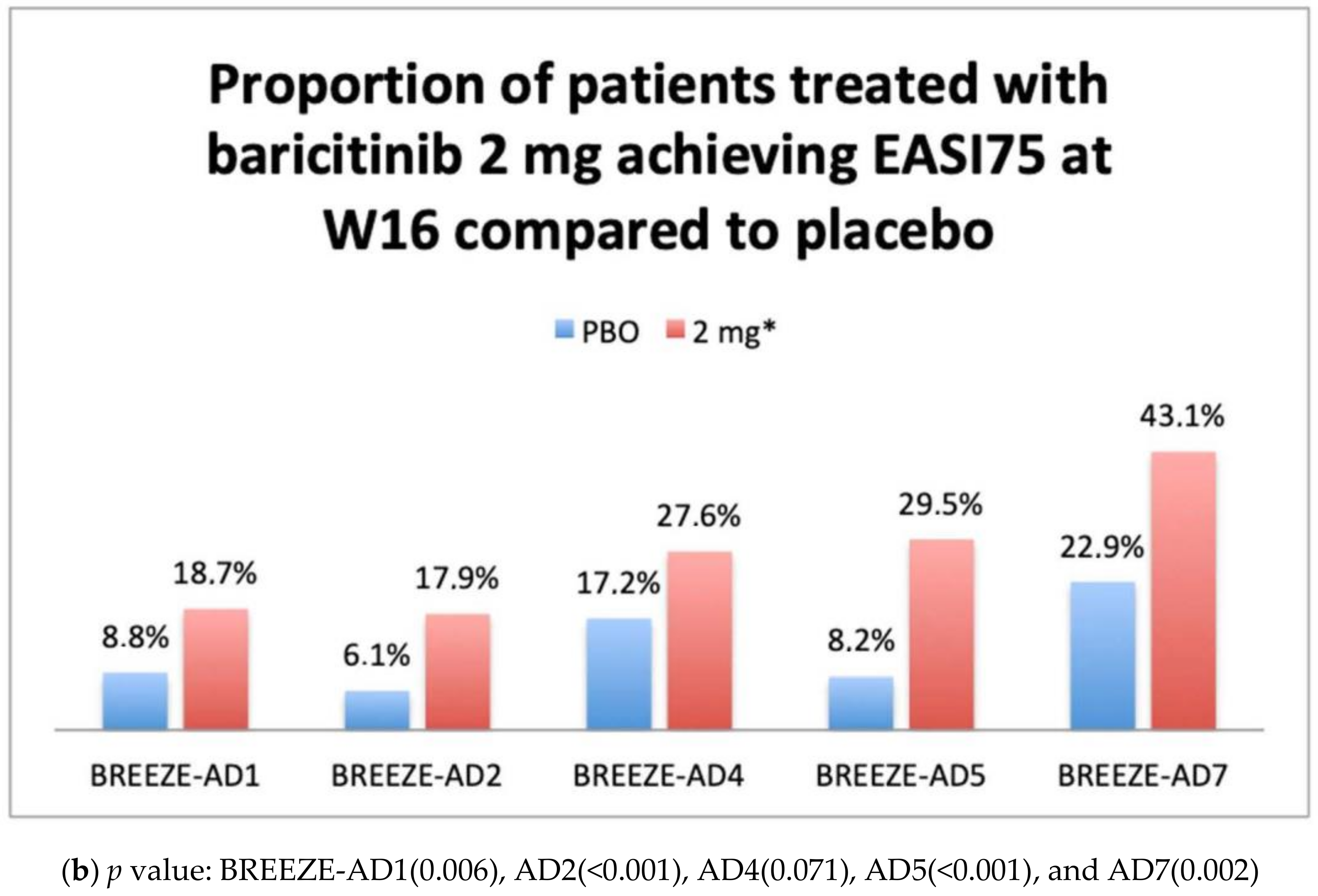
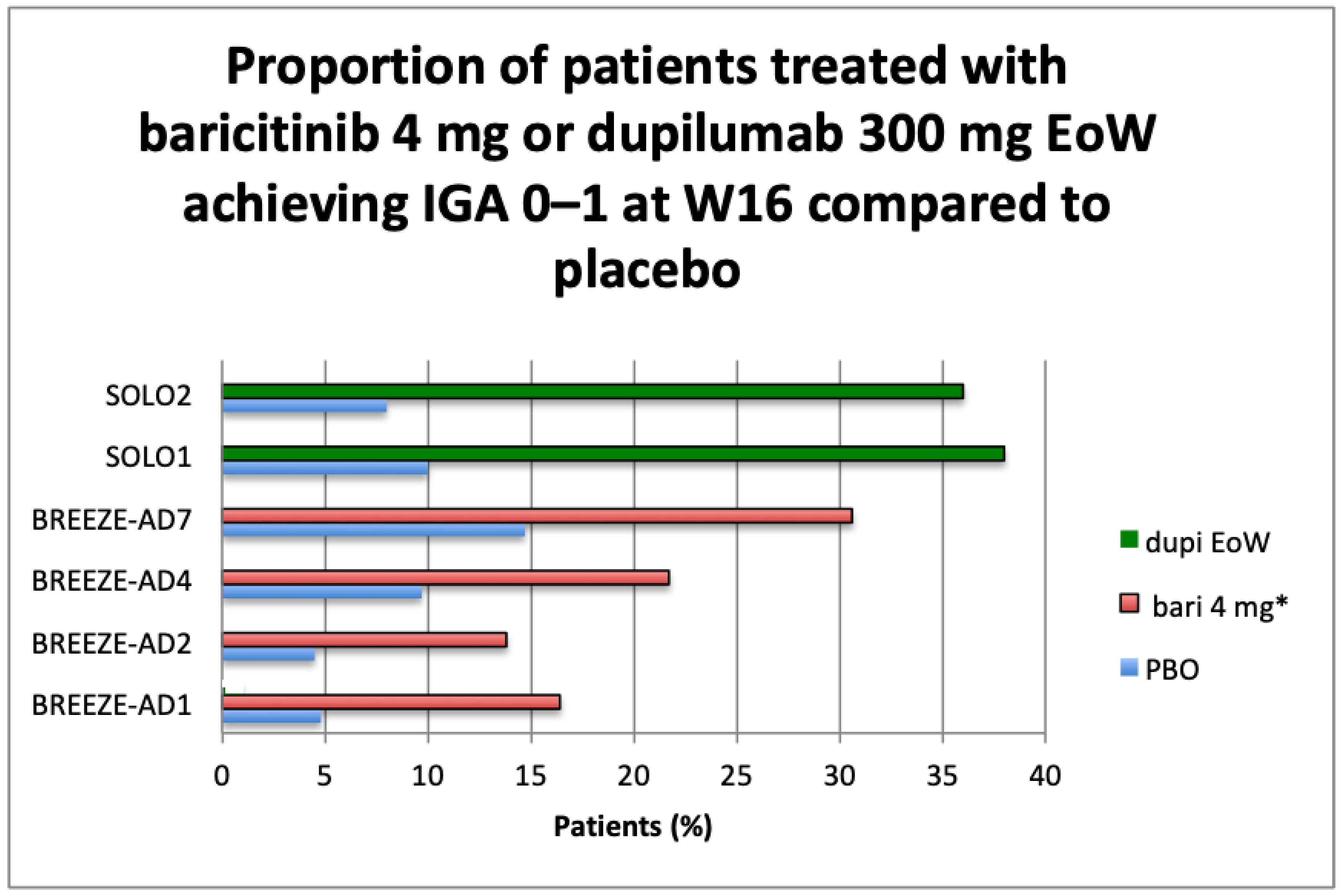
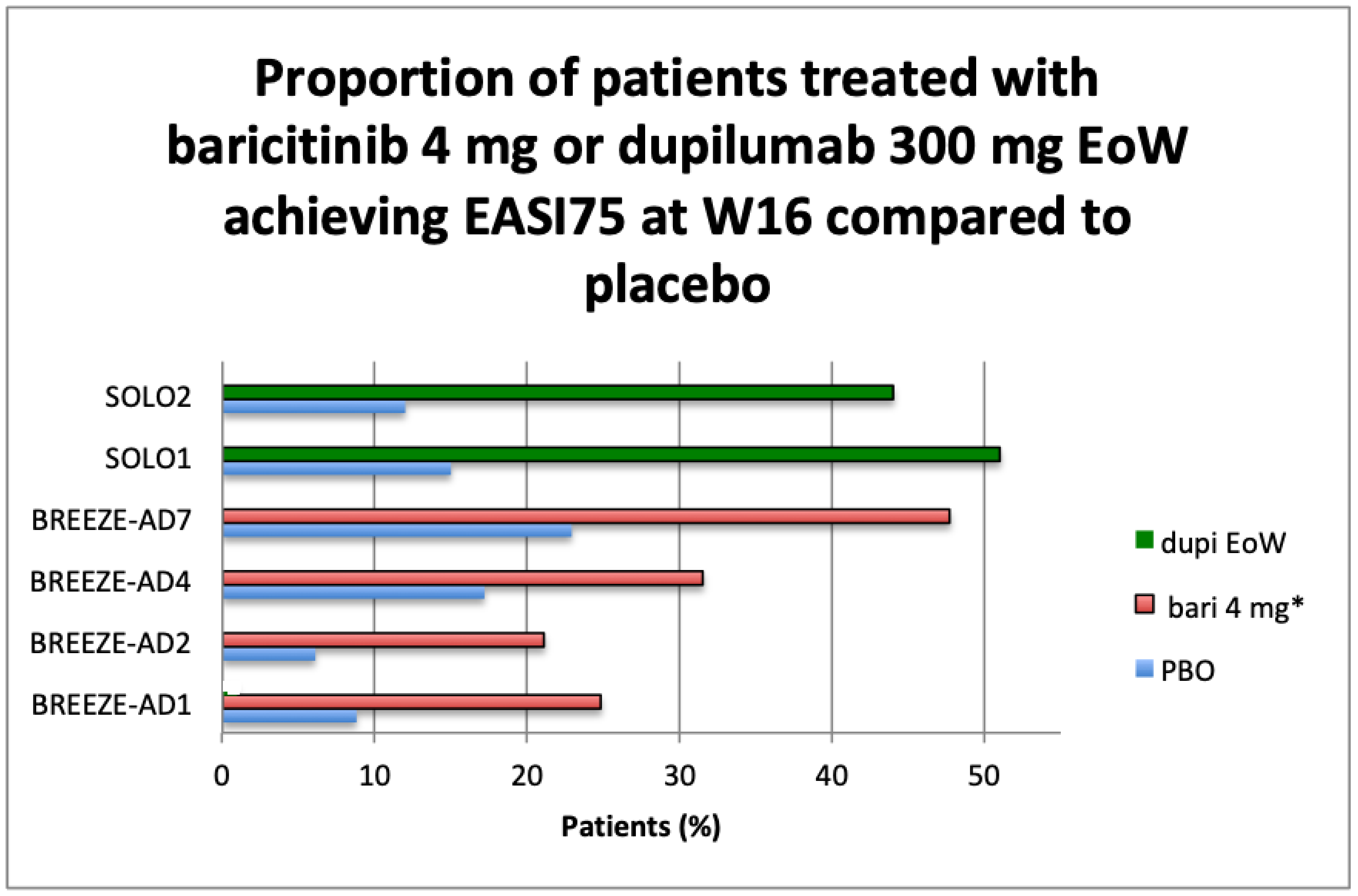
IGA 0–1 and EASI75 Results
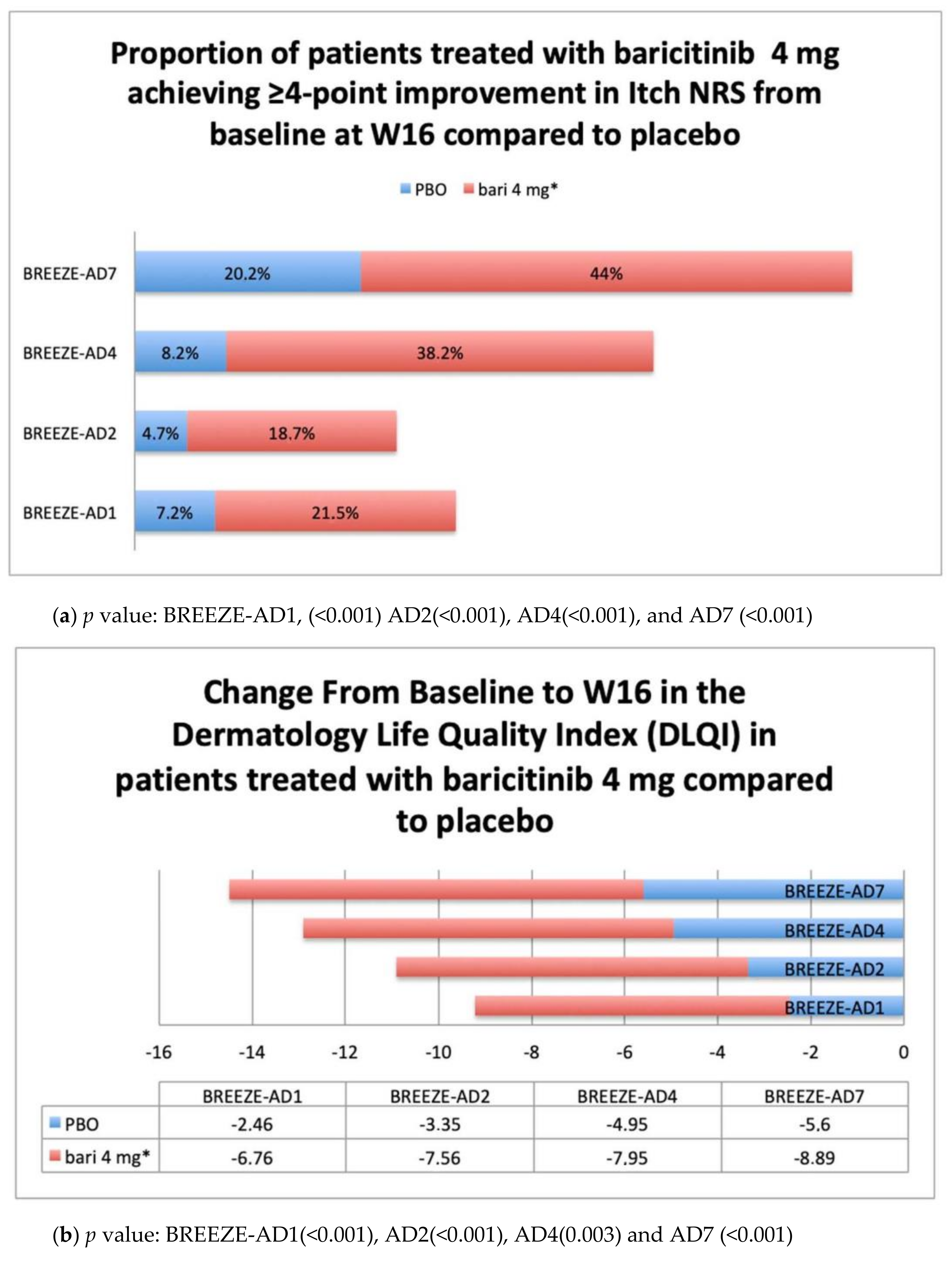
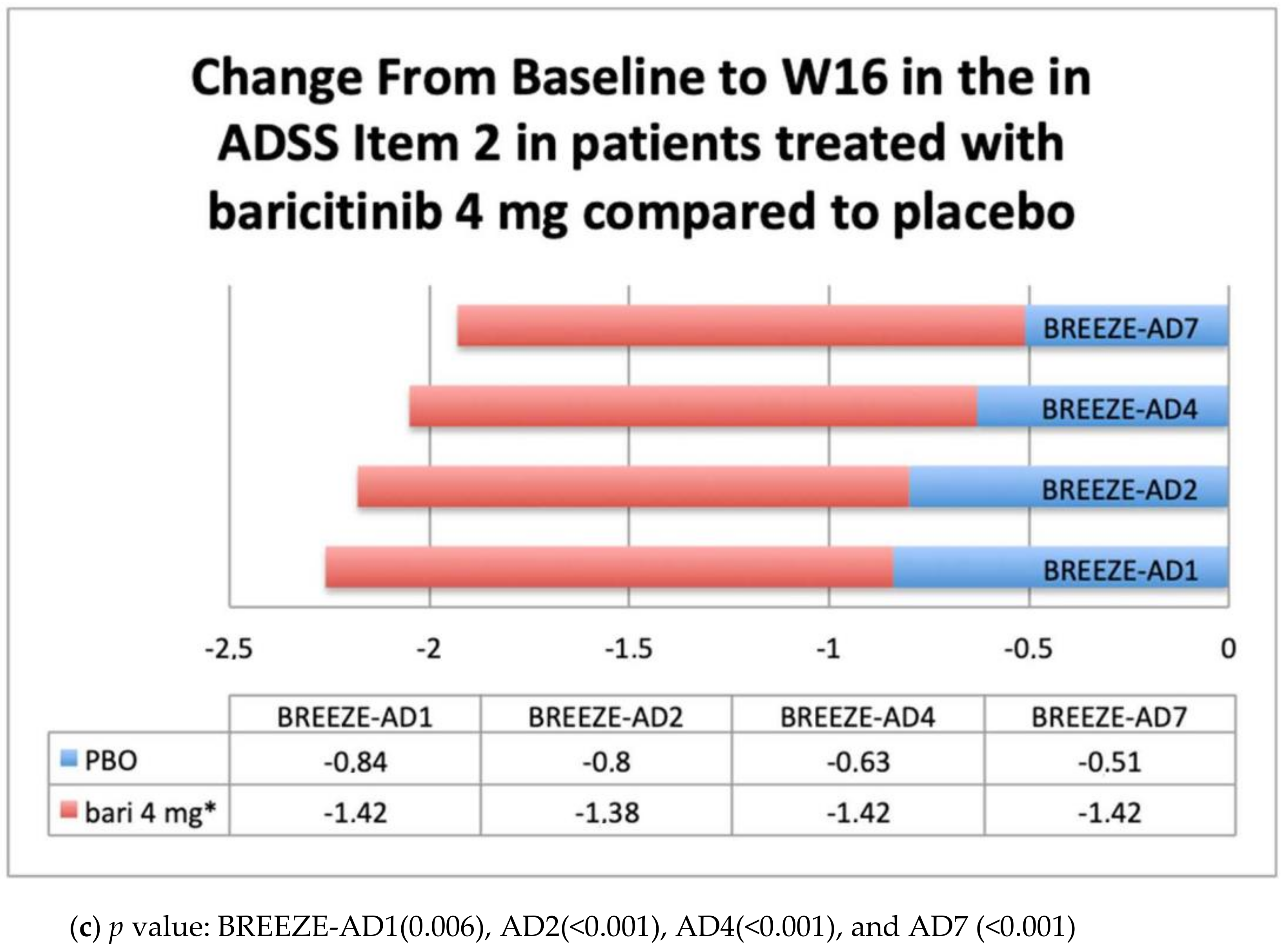
Patient-Reported Outcomes PROs
3.3. Safety
4. Discussion
4.1. Therapeutic Uses of JAK Inhibitors
4.2. Baricitinib: Evidence in Atopic Dermatitis
4.2.1. Efficacy on Eczema
4.2.2. Efficacy on Symptoms and Quality of Life
4.2.3. Safety and Tolerability
4.3. Regulatory Affair
4.4. Posology and Precautions for Use
4.5. Upcoming Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weidinger, S.; Beck, L.A.; Bieber, T.; Kabashima, K.; Irvine, A.D. Atopic dermatitis. Nat. Rev. Dis. Primers 2018, 4, 1. [Google Scholar] [CrossRef]
- Boguniewicz, M.; Alexis, A.F.; Beck, L.A.; Block, J.; Eichenfield, L.F.; Fonacier, L.; Guttman-Yassky, E.; Paller, A.S.; Pariser, D.; Silverberg, J.I.; et al. Expert Perspectives on Management of Moderate-to-Severe Atopic Dermatitis: A Multidisciplinary Consensus Addressing Current and Emerging Therapies. J. Allergy Clin. Immunol. Pr. 2017, 5, 1519–1531. [Google Scholar] [CrossRef]
- Wollenberg, A.; Barbarot, S.; Bieber, T.; Christen-Zaech, S.; Deleuran, M.; Fink-Wagner, A.; Gieler, U.; Girolomoni, G.; Lau, S.; Muraro, A.; et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef] [Green Version]
- Deckers, I.A.G.; Mclean, S.; Linssen, S.; Mommers, M.; Van Schayck, C.P.; Sheikh, A. Investigating International Time Trends in the Incidence and Prevalence of Atopic Eczema 1990–2010: A Systematic Review of Epidemiological Studies. PLoS ONE 2012, 7, e39803. [Google Scholar] [CrossRef] [Green Version]
- Naldi, L.; Parazzini, F.; Gallus, S. Prevalence of atopic dermatitis in Italian schoolchildren: Factors affecting its variation. Acta Derm. Venereol. 2009, 89, 122–125. [Google Scholar] [CrossRef] [PubMed]
- El Hachem, M.; Gesualdo, F.; Ricci, G.; Diociaiuti, A.; Giraldi, L.; Ametrano, O.; Occella, C.; Fortina, A.B.; Milioto, M.; Arcangeli, F.; et al. Topical corticosteroid phobia in parents of pediatric patients with atopic dermatitis: A multicentre survey. Ital. J. Pediatr. 2017, 43, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.P.; Chao, L.X.; Simpson, E.L.; Silverberg, J.I. Persistence of atopic dermatitis(AD): A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2016, 75, 681–687.e11. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Patel, K.R.; Singam, V.; Rastogi, S.; Silverberg, J.I. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 1526–1532.e7. [Google Scholar] [CrossRef]
- Boothe, W.D.; Tarbox, J.A.; Tarbox, M.B. Atopic Dermatitis: Pathophysiology. Adv. Exp. Med. Biol. 2017, 1027, 21–37. [Google Scholar] [CrossRef]
- Gooderham, M.J.; Hong, H.C.-H.; Eshtiaghi, P.; Papp, K.A. Dupilumab: A review of its use in the treatment of atopic dermatitis. J. Am. Acad. Dermatol. 2018, 78, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Howell, M.D.; Guttman-Yassky, E.; Silverberg, J.I.; Kell, C.; Ranade, K.; Moate, R.; van der Merwe, R. Treatment of atopic dermatitis with tralokinumab, an anti–IL-13 mAb. J. Allergy Clin. Immunol. 2019, 143, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, E.L.; Flohr, C.; Eichenfield, L.F.; Bieber, T.; Sofen, H.; Taïeb, A.; Owen, R.; Putnam, W.; Castro, M.; DeBusk, K.; et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: A randomized, placebo-controlled phase II trial (TREBLE). J. Am. Acad. Dermatol. 2018, 78, 863–871.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabashima, K.; Furue, M.; Hanifin, J.M.; Pulka, G.; Wollenberg, A.; Galus, R.; Etoh, T.; Mihara, R.; Nakano, M.; Ruzicka, T. Nemolizumab in patients with moderate-to-severe atopic dermatitis: Randomized, phase II, long-term extension study. J. Allergy Clin. Immunol. 2018, 142, 1121–1130.e7. [Google Scholar] [CrossRef]
- Nezamololama, N.; Fieldhouse, K.; Metzger, K.; Gooderham, M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: A review of abrocitinib, baricitinib, and upadacitinib. Drugs Context 2020, 9, 1–7. [Google Scholar] [CrossRef]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak–STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Sonbol, M.; Firwana, B.; Zarzour, A.; Morad, M.; Rana, V.; Tiu, R.V. Comprehensive review of JAK inhibitors in myeloproliferative neoplasms. Ther. Adv. Hematol. 2013, 4, 15–35. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.M.; Bonelli, M.; Gadina, M.; O’Shea, J.J. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat. Rev. Rheumatol. 2016, 12, 25–36. [Google Scholar] [CrossRef]
- Oetjen, L.K.; Mack, M.R.; Feng, J.; Whelan, T.M.; Niu, H.; Guo, C.J.; Chen, S.; Trier, A.M.; Xu, A.Z.; Tripathi, S.V.; et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017, 171, 217–228.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rochman, Y.; Kashyap, M.; Robinson, G.W.; Sakamoto, K.; Gomez-Rodriguez, J.; Wagner, K.-U.; Leonard, W.J. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 19455–19460. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Guttman-Yassky, E. JAK Inhibitors for Atopic Dermatitis: An Update. Am. J. Clin. Dermatol. 2019, 20, 181–192. [Google Scholar] [CrossRef]
- Yamaoka, K.; Saharinen, P.; Pesu, M.; Holt, V.E.T.; Silvennoinen, O.; O’Shea, J.J. The Janus kinases (Jaks). Genome Biol. 2004, 5, 253. [Google Scholar] [CrossRef] [Green Version]
- Cornez, I.; Yajnanarayana, S.P.; Wolf, A.M.; Wolf, D. JAK/STAT disruption induces immuno-deficiency: Rationale for the development of JAK inhibitors as immunosuppressive drugs. Mol. Cell. Endocrinol. 2017, 451, 88–96. [Google Scholar] [CrossRef]
- Damsky, W.; King, B.A. JAK inhibitors in dermatology: The promise of a new drug class. J. Am. Acad. Dermatol. 2017, 76, 736–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://investor.lilly.com/news-releases/news-release-details/chmp-recommends-approval-lillys-baricitinib-treatment-adults-0 (accessed on 30 October 2021).
- A Study of Baricitinib (LY3009104) in Participants with Moderate-to-Severe Atopic Dermatitis. Available online: https://clinicaltrials.gov/ct2/show/study/NCT02576938 (accessed on 2 November 2021).
- A Study of Baricitinib (LY3009104) in Patients with Moderate to Severe Atopic Dermatitis (BREEZE-AD1) l. Available online: https://clinicaltrials.gov/ct2/show/results/NCT03334396 (accessed on 2 November 2021).
- Study of Baricitinib (LY3009104) in Patients with Moderate to Severe Atopic Dermatitis (BREEZE-AD2). Available online: https://clinicaltrials.gov/ct2/show/results/NCT03334422 (accessed on 2 November 2021).
- A Study of Long-term Baricitinib (LY3009104) Therapy in Atopic Dermatitis (BREEZE-AD3). Available online: https://clinicaltrials.gov/ct2/show/results/NCT03334435 (accessed on 2 November 2021).
- A Long-term Study of Baricitinib (LY3009104) with Topical Corticosteroids in Adults with Moderate to Severe Atopic Dermatitis That Are Not Controlled with Cyclosporine or for Those Who Cannot Take Oral Cyclosporine Because It Is Not Medically Advisable (BREEZE-AD4). Available online: https://clinicaltrials.gov/ct2/show/results/NCT03428100 (accessed on 2 November 2021).
- A Study of Baricitinib (LY3009104) in Adult Participants with Moderate to Severe Atopic Dermatitis (BREEZE-AD5). Available online: https://clinicaltrials.gov/ct2/show/results/NCT03435081 (accessed on 2 November 2021).
- A Study of Baricitinib (LY3009104) in Participants with Moderate to Severe Atopic Dermatitis (BREEZE-AD6). Available online: https://clinicaltrials.gov/ct2/show/study/NCT03559270 (accessed on 2 November 2021).
- A Study of Baricitinib (LY3009104) in Combination with Topical Corticosteroids in Adults with Moderate to Severe Atopic Dermatitis (BREEZE-AD7). Available online: https://clinicaltrials.gov/ct2/show/results/NCT03733301 (accessed on 2 November 2021).
- A Study of Baricitinib (LY3009104) in Children and Adolescents with Atopic Dermatitis (BREEZE-AD-PEDS). Available online: https://clinicaltrials.gov/ct2/show/NCT03952559 (accessed on 2 November 2021).
- Guttman-Yassky, E.; Silverberg, J.I.; Nemoto, O.; Forman, S.B.; Wilke, A.; Prescilla, R.; de la Peña, A.; Nunes, F.P.; Janes, J.; Gamalo, M.; et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: A phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J. Am. Acad. Dermatol. 2019, 80, 913–921.e9. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.; Lacour, J.; Spelman, L.; Galimberti, R.; Eichenfield, L.; Bissonnette, R.; King, B.; Thyssen, J.; Silverberg, J.; Bieber, T.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: Results from two randomized monotherapy phaseIIItrials. Br. J. Dermatol. 2020, 183, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Kabashima, K.; Peris, K.; Silverberg, J.I.; Eichenfield, L.F.; Bieber, T.; Kaszuba, A.; Kolodsick, J.; Yang, F.E.; Gamalo, M.; et al. Efficacy and Safety of Baricitinib Combined with Topical Corticosteroids for Treatment of Moderate to Severe Atopic Dermatitis. JAMA Dermatol. 2020, 156, 1333. [Google Scholar] [CrossRef]
- Simpson, E.L.; Forman, S.; Silverberg, J.I.; Zirwas, M.; Maverakis, E.; Han, G.; Guttman-Yassky, E.; Marnell, D.; Bissonnette, R.; Waibel, J.; et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: Results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J. Am. Acad. Dermatol. 2021, 85, 62–70. [Google Scholar] [CrossRef]
- Reich, K.; DeLozier, A.M.; Nunes, F.P.; Thyssen, J.P.; Eichenfield, L.F.; Wollenberg, A.; Terres, J.A.R.; Watts, S.D.; Chen, Y.-F.; Simpson, E.L.; et al. Baricitinib improves symptoms in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: Patient-reported outcomes from two randomized monotherapy phase III trials. J. Dermatol. Treat. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Wollenberg, A.; Nakahara, T.; Maari, C.; Peris, K.; Lio, P.; Augustin, M.; Silverberg, J.; Rueda, M.; DeLozier, A.; Pierce, E.; et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 Phase 3 randomized trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1543–1552. [Google Scholar] [CrossRef]
- Thyssen, J.P.; Buhl, T.; Fernández-Peñas, P.; Kabashima, K.; Chen, S.; Lu, N.; DeLozier, A.M.; Casillas, M.; Ständer, S. Baricitinib Rapidly Improves Skin Pain Resulting in Improved Quality of Life for Patients with Atopic Dermatitis: Analyses from BREEZE-AD1, 2, and 7. Dermatol. Ther. 2021, 11, 1599–1611. [Google Scholar] [CrossRef]
- Buhl, T.; Rosmarin, D.; Serra-Baldrich, E.; Fernandez-Peñas, P.; Igarashi, A.; Konstantinou, M.P.; Chen, S.; Lu, N.; Pierce, E.; Casillas, M. Itch and Sleep Improvements with Baricitinib in Patients with Atopic Dermatitis: A Post Hoc Analysis of 3 Phase 3 Studies. Dermatol. Ther. 2021, 11, 971–982. [Google Scholar] [CrossRef]
- King, B.; Maari, C.; Lain, E.; Silverberg, J.I.; Issa, M.; Holzwarth, K.; Brinker, D.; Cardillo, T.; Nunes, F.P.; Simpson, E.L. Extended Safety Analysis of Baricitinib 2 mg in Adult Patients with Atopic Dermatitis: An Integrated Analysis from Eight Randomized Clinical Trials. Am. J. Clin. Dermatol. 2021, 22, 395–405. [Google Scholar] [CrossRef]
- Bieber, T.; Thyssen, J.P.; Reich, K.; Simpson, E.L.; Katoh, N.; Torrelo, A.; De Bruin-Weller, M.; Thaci, D.; Bissonnette, R.; Gooderham, M.; et al. Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Vyas, J.; Finlay, A. Counting the Burden: Atopic Dermatitis and Health-related Quality of Life. Acta Derm. Venereol. 2020, 100, adv00161. [Google Scholar] [CrossRef]
- Koszorú, K.; Borza, J.; Gulácsi, L.; Sárdy, M. Quality of life in patients with atopic dermatitis. Cutis 2019, 104, 174–177. [Google Scholar] [PubMed]
- Lilly, E. Company, Incyte Corporation. European Commission Approves Once-Daily Olumiant Tablets for Treatment of Adults with Moderate-to-Severe Active Rheumatoid Arthritis. 13 February 2017. Available online: https://investor.lilly.com/news-releases/news-release-details/european-commission-approves-once-daily-olumiant-tablets (accessed on 17 November 2021).
- Deisseroth, A.; Kaminskas, E.; Grillo, J.; Chen, W.; Saber, H.; Lu, H.L.; Rothmann, M.D.; Brar, S.; Wang, J.; Garnett, C.; et al. U.S. Food and Drug Administration Approval: Ruxolitinib for the Treatment of Patients with Intermediate and High-Risk Myelofibrosis. Clin. Cancer Res. 2012, 18, 3212–3217. [Google Scholar] [CrossRef] [Green Version]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. A Study of Baricitinib (LY3009104) in Participants with Severe or Very Severe Alopecia Areata (BRAVE-AA1). 2018. Identifier (NCT03570749). Available online: https://clinicaltrials.gov/ct2/show/NCT03570749 (accessed on 30 October 2021).
- ClinicalTrials.gov. A Study of Baricitinib (LY3009104) in Adults with Severe or Very Severe Alopecia Areata (BRAVE-AA2). 2019. Identifier (NCT03899259). Available online: https://clinicaltrials.gov/ct2/show/NCT03899259. (accessed on 31 October 2021).
- Lilly, E. Poster Presented at Virtual Ispor 2021 D. Christian Fenske, Katherine Rosettie, Cheryl Ferrufino, Mark Borns, Bilal Atiya, Nate Johnson, Elizabeth Wehler. Available online: https://www.ispor.org/docs/default-source/intl2021/bimbaricitinib-for-mod-sev-adispormay-2021-pdf (accessed on 2 November 2021).
- NICE Technology Appraisal Guidance, Published 3 March 2021. Available online: https://www.nice.org.uk/guidance/ta681/resources/baricitinib-for-treating-moderate-to-severeatopic-dermatitis-pdf-82609375014853 (accessed on 31 October 2021).
- Eli Lilly and Company. Olumiant: European Union Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/olumiant-epar-product-information_en.pdf (accessed on 28 October 2021).
- Mendes, J.T.; Balogh, E.A.; Strowd, L.C.; Feldman, S.R. An evaluation of baricitinib as a therapeutic option for adult patients with moderate to severe atopic dermatitis. Expert Opin. Pharmacother. 2020, 21, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, L.; Malvaso, D.; Chiricozzi, A.; Tambone, S.; D’Urso, D.F.; Guerriero, C.; Peris, K. Baricitinib: Therapeutic potential for moderate to severe atopic dermatitis. Expert Opin. Investig. Drugs 2020, 29, 1089–1098. [Google Scholar] [CrossRef]
- Konrad, R.J.; Higgs, R.E.; Rodgers, G.H.; Ming, W.; Qian, Y.-W.; Bivi, N.; Mack, J.K.; Siegel, R.W.; Nickoloff, B.J. Assessment and Clinical Relevance of Serum IL-19 Levels in Psoriasis and Atopic Dermatitis Using a Sensitive and Specific Novel Immunoassay. Sci. Rep. 2019, 9, 5211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| BREEZE-AD1 NCT0334396 I4V-MC-JAHL | GLOBAL n = 660 monotherapy | A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of Baricitinib in Adult Patients With Moderate to Severe Atopic Dermatitis | Study Duration W16 oral 4 mg:2 mg:1 mg:PBO | PRIMARY ENDPOINT IGA 0–1 W16 | Completed |
| BREEZE-AD2 NCT03334422 I4V-MC-JAHM | GLOBAL n = 615 monotherapy | A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of Baricitinib in Patients With Moderate to Severe Atopic Dermatitis | Study Duration W16 oral 4 mg:2 mg:1 mg:PBO | PRIMARY ENDPOINT IGA 0–1 W16 | Completed |
| BREEZE-AD3 NCT03334435; I4V-MC-JAHN | GLOBAL n = 1760 (allowed TCS) | A Phase 3, Multicenter, Double-Blind Study to Evaluate the Long-Term Safety and Efficacy of Baricitinib in Adult Patients With Atopic Dermatitis | Study Duration W104 oral High:mild:low:PBO | PRIMARY ENDPOINT IGA 0–1 W16,36,52 | Active, not recruiting |
| BREEZE-AD4 NCT03428100 I4V-MC-JAIN | GLOBAL n = 463 (TCS combo) | A Phase 3, Multicenter, Double-Blind, Randomized, Placebo-Controlled Study Evaluating the Safety and Efficacy of Baricitinib in Combination With Topical Corticosteroids in Adult Patients With Moderate to Severe Atopic Dermatitis Who Have Experienced Failure to Cyclosporine or Are Intolerant to or Have Contraindication to Cyclosporine | Study Duration W104 oral 4 mg:2 mg:1 mg:PBO | PRIMARY ENDPOINT EASI 75 W16 | Active, not recruiting |
| BREEZE-AD5 NCT03435081; I4V-MC-JAIW | US/CANADA n = 440 monotherapy | A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of Baricitinib in Adult Patients With Moderate to Severe Atopic Dermatitis | Study Duration W104 oral 2 mg:1 mg:PBO | PRIMARY ENDPOINT EASI 75 W16 | Completed |
| BREEZE-AD6 NCT03559270; I4V-MC-JAIX | US/CANADA n = 380 (allowed TCS) | A Multicenter, Open-Label, Phase 3 Study to Evaluate the Efficacy and Safety of Baricitinib in Adult Patients With Moderate to Severe Atopic Dermatitis | Study Duration W104 oral 2 mg | PRIMARY ENDPOINT EASI 75 W16 | Active, not recruiting |
| BREEZE-AD7 NCT0373301; I4V-MC-JAIY | GLOBAL n = 329 (TCS combo) | A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of Baricitinib in Combination With Topical Corticosteroids in Adult Patients With Moderate to Severe Atopic Dermatitis BREEZE-AD7 | Study Duration W16 oral 4 mg:2 mg:PBO | PRIMARY ENDPOINT IGA 0–1 W16 | Completed |
| Authors [Ref] | Title | Type of Study | Source of Data | Information about Publication |
|---|---|---|---|---|
| Promoted by Eli Lilly and Company [25] | A Study of Baricitinib (LY3009104) in Participants With Moderate to Severe Atopic Dermatitis | Phase II RCT | clinicaltrials.gov NCT02576938 (accessed on 2 November 2021) | Last update posted 17 June 2020 |
| Promoted by Eli Lilly and Company [26] | A Study of Baricitinib (LY3009104) in Patients With Moderate to Severe Atopic Dermatitis (BREEZE-AD1) | Phase III RCT | clinicaltrials.gov NCT03334396 (accessed on 2 November 2021) | Last update posted 18 August 2020 |
| Promoted by Eli Lilly and Company [27] | Study of Baricitinib (LY3009104) in Patients With Moderate to Severe Atopic Dermatitis (BREEZE-AD2) | Phase III RCT | clinicaltrials.gov NCT03334422 (accessed on 2 November 2021) | Last update posted 22 January 2020 |
| Promoted by Eli Lilly and Company [28] | A Study of Long-term Baricitinib (LY3009104) Therapy in Atopic Dermatitis (BREEZE-AD3) | Phase III RCT | clinicaltrials.gov NCT03334435 (accessed on 2 November 2021) | No results posted |
| Promoted by Eli Lilly and Company [29] | A Long-term Study of Baricitinib (LY3009104) With Topical Corticosteroids in Adults With Moderate to Severe Atopic Dermatitis That Are Not Controlled With Cyclosporine or for Those Who Cannot Take Oral Cyclosporine Because it is Not Medically Advisable (BREEZE-AD4) | Phase III RCT | clinicaltrials.gov NCT03428100 (accessed on 2 November 2021) | Last update posted 11 May 2021 (pre-print) |
| Promoted by Eli Lilly and Company [30] | A Study of Baricitinib (LY3009104) in Adult Participants With Moderate to Severe Atopic Dermatitis (BREEZE-AD5) | Phase III RCT | clinicaltrials.gov NCT03435081 (accessed on 2 November 2021) | Last update posted 19 August 2021 |
| Promoted by Eli Lilly and Company [31] | A Study of Baricitinib (LY3009104) in Participants With Moderate to Severe Atopic Dermatitis (BREEZE-AD6) | Phase III RCT | clinicaltrials.gov NCT03559270 (accessed on 2 November 2021) | No results posted |
| Promoted by Eli Lilly and Company [32] | A Study of Baricitinib (LY3009104) in Combination With Topical Corticosteroids in Adults With Moderate to Severe Atopic Dermatitis (BREEZE-AD7) | Phase III RCT | clinicaltrials.gov NCT03733301 (accessed on 2 November 2021) | Last update posted 11 August 2020 |
| Promoted by Eli Lilly and Company [33] | A Study of Baricitinib (LY3009104) in Children and Adolescents With Atopic Dermatitis (BREEZE-AD-PEDS) | Phase III RCT | clinicaltrials.gov NCT03952559 (accessed on 2 November 2021) | No results posted |
| Guttman-Yassky et al. [34] | Baricitinib in adult patients with moderate to severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. | PHASE II RCT | NCT02576938 | Published on 2019 J Am Acad Dermatol. |
| Simpson EL et al. [35] | Baricitinib in patients with moderate to severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. | Phase III RCT | BREEZE-AD1 NCT03334396 BREEZE-AD2 NCT03334422 | Published on August 2020 Br J Dermatol. |
| Reich K. et al. [36] | Efficacy and Safety of Baricitinib Combined With Topical Corticosteroids for Treatment of Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial. | Phase III RCT | BREEZE-AD7 NCT03733301 | Published on December 2020 JAMA Dermatol |
| Simpson EL et al. [37] | Baricitinib in patients with moderate-to-severe atopic dermatitis: Results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5) | Phase III RCT | BREEZE-AD5 NCT03435081 | Published on July 2021 J Am Acad Dermatol. |
| Reich K. et al. [38] | Baricitinib improves symptoms in patients with moderate to severe atopic dermatitis and inadequate response to topical corticosteroids: patient-reported outcomes from two randomized monotherapy phase III trials | Phase III RCT | BREEZE-AD1 NCT03334396 BREEZE-AD2 NCT03334422 | Published on November 2020 J Dermatolog Treat Epub ahead of print |
| Wollemberg A. et al. [39] | Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life, and functioning in adult patients with moderate to severe atopic dermatitis from the BREEZE-AD7 Phase 3 randomised trial | Phase III RCT | BREEZE-AD7 NCT03733301 | Published on July 2021 J Eur Acad Dermatol Venereol. |
| Thyssen JP et al. [40] | Baricitinib Rapidly Improves Skin Pain Resulting in Improved Quality of Life for Patients with Atopic Dermatitis: Analyses from BREEZE-AD1, 2, and 7 | Phase III RCTs | BREEZE-AD1 NCT03334396 BREEZE-AD2 NCT03334422 BREEZE-AD7 NCT03733301 | Published on October 2021 Dermatol Ther |
| Buhl T. [41] | Itch and Sleep Improvements with Baricitinib in Patients with Atopic Dermatitis: A Post-Hoc Analysis of 3 Phase 3 Studies. | Phase III RCTs | BREEZE-AD1 NCT03334396 BREEZE-AD2 NCT03334422 BREEZE-AD7 NCT03733301 | Published on June 2021 Dermatol Ther |
| King B. et al. [42] | Extended Safety Analysis of Baricitinib 2 mg in Adult Patients with Atopic Dermatitis: An Integrated Analysis from Eight Randomized Clinical Trials | Phase II–III RCTs | NCT02576938 BREEZE-AD1 NCT03334396 BREEZE-AD 2 NCT03334422 BREEZE-AD3 NCT03334435 BREEZE-AD4 NCT03428100 BREEZE-AD5 NCT03435081 BREEZE-AD6 NCT03559270 BREEZE-AD7 NCT03733301 | Published on May 2021 Am J Clin Dermatol |
| Bieber T. et al. [43] | Pooled safety analysis of baricitinib in adult patients with atopic dermatitis from 8 randomized clinical trials. | Phase III RCTs | BREEZE-AD1 NCT03334396 BREEZE-AD2 NCT03334422 BREEZE-AD3 NCT03334435 BREEZE-AD4 NCT03428100 BREEZE-AD5 NCT03435081 BREEZE-AD6 NCT03559270 BREEZE-AD7 NCT03733301 | Published on February 2021 J Eur Acad Dermatol Venereol. |
| BREEZE-AD1 | BREEZE-AD2 | BREEZE-AD4 | BREEZE-AD5 | BREEZE-AD7 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Patients | 125 | 123 | 249 | 123 | 123 | 244 | 92 | 184 | 93 | 145 | 146 | 111 | 109 | 108 |
| Baricitinib dosage | 4 mg | 2 mg | PBO | 4 mg | 2 mg | PBO | 4 mg + TCS | 2 mg + TCS | PBO + TCS | 2 mg | PBO | 4 mg + TCS | 2 mg + TCS | PBO + TCS |
| TEAES | 73 (58.4%) | 71 (57.7%) | 135 (54.2%) | 66 (53.7%) | 71 (57.7%) | 137 (56%) | 50 (54.3%) | 69 (37.5%) | 29 (31.2%) | 18 (12.4%) | 20 (13.7%) | 64 (57.7%) | 61 (56%) | 41 (38%) |
| Nasopharingitis |
12 (9.6%) | 12 (9.8%) | 26 (10.4%) | 10 (8.1%) | 16 (13%) | 30 (12%) | 27 (29.3%) | 34 (18.5%) | 13 (14%) | 9 (6.1%) | 11 (7.5%) | 17 (15%) | 12(11%) | 13 (12%) |
| URIs | 4 (3.2%) | 3 (2.4%) | 6 (2.4%) | 4 (3.3%) | 5 (4.1%) | 5 (2%) | 10 (11%) | 10 (5.4%) | 2 (2.1%) | 11 (7.6%) | 9 (6.2%) | 3 (3%) | 8 (7%) | 2 (2%) |
| Herpes simplex | 9 (7.2%) | 4 (3.3%) | 3 (1.2%) | 5 (4.1%) | 7 (5.7%) | 11 (4.5%) | 5 (5.4%) | 5 (2.7%) | 4 (4.3%) | nr | nr | 3 (3%) | 1 (1%) | 3 (3%) |
| Headache | 10 (8%) | 14 (11.4%) | 16 (6.4%) | 11 (8.9%) | 9 (7.3%) | 5 (2%) | 9 (9.8%) | 11 (6%) | 6 (6.5%) | nr | nr | nr | nr | nr |
| CPK elevations | 4 (3.2%) | 1 (0.8%) | 2 (0.8%) | 7 (5.7%) | 1 (0.8%) | 1 (0.4%) | nr | nr | nr | nr | nr | 0 | 3 (3%) | 0 |
| SAE | 2 (1.6%) | 0 | 6 (2.4%) | 1 (0.8%) | 3 (2.4%) | 9 (3.7%) | 6 (6.5%) | 4 (2.2%) | 2 (2.2%) | 2 (1.4%) | 3 (2%) | 4 (4%) | 2 (2%) | 4 (4%) |
| MACE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Malignant | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Baricitinib Reimbursed EU Contries for Atopic Dermatitis | |
|---|---|
| Austria | Norway |
| Denmark | Sweden |
| Finland | Switzerland |
| France | UK |
| Germany | Scotland |
| Netherlands | Slovenia |
| Portugal | Spain (end 2021) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radi, G.; Simonetti, O.; Rizzetto, G.; Diotallevi, F.; Molinelli, E.; Offidani, A. Baricitinib: The First Jak Inhibitor Approved in Europe for the Treatment of Moderate to Severe Atopic Dermatitis in Adult Patients. Healthcare 2021, 9, 1575. https://doi.org/10.3390/healthcare9111575
Radi G, Simonetti O, Rizzetto G, Diotallevi F, Molinelli E, Offidani A. Baricitinib: The First Jak Inhibitor Approved in Europe for the Treatment of Moderate to Severe Atopic Dermatitis in Adult Patients. Healthcare. 2021; 9(11):1575. https://doi.org/10.3390/healthcare9111575
Chicago/Turabian StyleRadi, Giulia, Oriana Simonetti, Giulio Rizzetto, Federico Diotallevi, Elisa Molinelli, and Annamaria Offidani. 2021. "Baricitinib: The First Jak Inhibitor Approved in Europe for the Treatment of Moderate to Severe Atopic Dermatitis in Adult Patients" Healthcare 9, no. 11: 1575. https://doi.org/10.3390/healthcare9111575
APA StyleRadi, G., Simonetti, O., Rizzetto, G., Diotallevi, F., Molinelli, E., & Offidani, A. (2021). Baricitinib: The First Jak Inhibitor Approved in Europe for the Treatment of Moderate to Severe Atopic Dermatitis in Adult Patients. Healthcare, 9(11), 1575. https://doi.org/10.3390/healthcare9111575






