1. Introduction
Fibromyalgia (FM) is a complex systemic pathology characterized by generalized musculoskeletal pain, sleep disturbance, stiffness, fatigue, and psychological problems [
1,
2]. FM is defined by the American College of Rheumatology (ACR) [
3] as a chronic musculoskeletal pain syndrome whose etiology is currently unknown, characterized by widespread pain for more than 3 months and soreness in at least 11 out of 18 tender points.
Diagnostic criteria were modified by the ACR in 2010 and 2011, firstly shifting the diagnostic focus away from tender points and towards a clearer and more specific variety of symptoms, then allowing for the possibility of self-reported diagnosis in a research setting, as well as adding a fibromyalgia severity score [
3].
Cognitive difficulties, while not commonly assessed at diagnosis, are also common in FM. These include so called “Fibro Fog,” or dyscognition, defined as cognitive dysfunction characterized by memory lapses, confusion, as well as impairing concentration, planning, and organization [
4]. Fibro Fog is experienced by 76.4–82.5% of patients with FM [
5], yet cognitive impairment was only added to the ACR diagnostic criteria in 2010 [
3]. Major depression has been found to be 20–60% more prevalent among patients with FM when compared to the general population [
6].
The etiology of FM is unknown, and it is likely multifactorial. First-degree relatives of patients with FM are 8.5 times more likely to have this disorder than the general population [
7]. However, genetic factors associated with FM are unknown. It has been suggested that serotonin- and dopamine-related genes may play a role in the pathogenesis of FM [
8].
The strongest evidence points to dysregulation of pain modulation, based upon the results of experimentally induced pain testing yielding heightened processing and attenuated inhibitory process of noxious stimuli [
9,
10], as well as imaging studies showing increased neural response to pain.
Patients with FM have a three-times-higher concentration of substance P in the cerebrospinal fluid [
11]. Activation of the N-methyl-d-aspartate receptor (NMDAR) is also increased in FM patients. Substance P modulates the responsiveness of the NMDAR to glutamate, which consequently leads to temporary central sensitization and temporal summation in otherwise healthy individuals [
12,
13]. It has been demonstrated that FM patients’ serotonin levels in the serum are reduced and inversely correlated with pain threshold [
14,
15]. Combined dysfunctional neurotransmitter systems, such as low serotonin and high substance P levels, can produce more pain than either abnormality on their own and be responsible for the onset of FM [
16,
17].
Research has demonstrated that FM patients have a lower level of dopamine, which plays a central role in painful conditions modulating pain perception and natural analgesia within supraspinal regions and the spine [
18,
19]. It was found that several serum pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin, are involved in the generation of symptoms in FM, including sleep disturbances, fatigue, and myalgia [
8,
20,
21,
22].
Some studies have shown a relationship between smoking habit and FM [
23].
Prevalence rates of FM have been found to vary between 0.2–4.7% [
24]. Among women, prevalence rates range between 2.4–6.8% [
24], with about a 9:1 female-to-male prevalence ratio [
25], although one study found slightly less of a distinct gender difference [
26]. Interestingly, in one recent study conducted by Wolfe and colleagues [
27] among a sample of 2445 adults, no significant gender difference in FM prevalence rates was found. One explanation concerning this disparity could be the changing diagnostic criteria. The reliance of the original ACR criteria on tender points may have resulted in higher FM rates among women, as women were found to have more tender points than men [
3]. Since Wolfe and colleagues [
27] utilized the modified ACR criteria, which rely on tender points to a lesser degree, the gender ratios may have become more proportionate.
There is currently no mass screening program for the detection of fibromyalgia; we decided to evaluate the possibility of assuming the diagnostic suspicion of pain using well-established mass screening procedures (breast cancer mammography screening programs, in our case).
In the literature, there are a series of reference scales validated for diagnosing pain [
28], including the VAS (visual analogue scale) andNRS (numeric pain rating scale) one-dimensional scales, which are certainly simple and rapid but poor in separate information regarding the extent of the pain. These scales identify two extremes consisting of the absence of pain and the maximum possible pain by identifying intermediate situations expressed by numerical values(
Figure 1 and
Figure 2).
Numerical rating scales have shown high correlations with other pain assessment tools in several studies [
29,
30]. The feasibility of its use and good conformity [
31,
32] has also been demonstrated. Since it is easily possible to administer NRS verbally, it can be used in telephone interviews [
33].
More complex scales (e.g., McGill Pain Questionnaire (MPQ)) help to better frame not only the presence/absence of pain but also the impact on the amount of life and daily activities; there are also dedicated scales to detect the possible presence of neuropathic pain (e.g., DN 4 douleur neuropatique 4), or the risk of addiction in patients undergoing opioid therapy (ORT, opioid risk tool, recently also available in Italian) [
28].
It has been shown that pain intensity can be reported quite easily by most patients and that several methods of pain intensity measurement have shown a high intercorrelation [
29,
34]. However, many factors such as the social situation, the work situation and environment and the history of a previous injury can influence the perception of pain and show great differences between individuals [
28].
The aim of this study is, therefore, to investigate whether a link can exist between the level of pain experienced by female patients while undergoing standard mammographic cancer screening procedures and an eventual diagnosis of FM; a subordinate aim was to assess the association between the level of pain endured by the patients and a range of other factors gathered with a questionnaire filled by the patients.
2. Materials and Methods
This prospective study was performed between June 2017 and July 2020.
We analyzed a sample of 1060 patients, all females and all Caucasian, to be subjected to standard breast cancer screening procedures, including mammography and 3D prone ecography with the Sofia system (Hitachi, Tokyo, Japan); we excluded from our study those patients who had previously received breast surgical interventions, those whose breasts were affected by benign pathologies, those who were breastfeeding and those who were affected by other rheumatologic pathologies.
Our study proceeded in two distinct phases: during the first phase (round 1) our patients, after having undergone their mammographic exam, were interviewed in order to acquire the following information: age, level of pain on a semiquantitative basis (1–4, where 1 corresponds to “little to no pain”, 2 to “moderate pain”, 3 to “significant pain” and 4 to “severe or unbearable pain”), smoking habit and number of cigarettes/day, coffee drinking habit and number of coffee cups/day, number of children, previous breastfeeding, level of perceived psychological stress, educational qualification, annual income, breast density along with ACR BIRADS classification. We did not consider breast size as a factor as there is no significant evidence of a relation between size and breast pain; in fact, looking at the medical literature, there isnot enough research on the subject in order to demonstrate the contrary [
35].
According to the American College of Rheumatology preliminary diagnostic criteria for fibromyalgia [
3], muscle pain is a frequent symptom due to its recognition in multiple body areas including the chest (
Figure 3 and
Figure 4). The recognition of this condition is therefore foreseeable in an examination such as mammography in which the skin and pectoral muscles are compressed and stretched during its execution. The further advantage of this investigation is that it is one of the cancer screening tests for breast cancer on the entire female population and could therefore be useful in identifying a subtle pathology such as fibromyalgia at no additional cost.
We applied the same level of compression (13 daN) for all the exams we performed, so as to eliminate a possible bias in the onset of pain arousing from a different level compression. After having collected the said information, we proceeded to perform a statistical analysis to investigate the association between the above-mentioned factors and the level of pain. Our patients were informed in writing about the study’s objectives and methods and all agreed to be interviewed; the data were collected prospectively as part of a data collection for subsequent ultrasound evaluation directed at patients whose mammography were negative for malignant tumors (approval by ethic committee number 187.20).Those patients who experienced the highest level of pain (level 4) were invited to receive a rheumatological examination in order to assess a possible diagnosis of fibromyalgia along with the ACR criteria. The reason why we selected only these patients for rheumatologic examination is that they were the ones who experienced a level of pain far higher than what is reasonably expected from a standard mammography. When, one year later, these patients came back for a new roundof routine breast cancer screening (round 2), we interviewed them in order to assess how many, within this selected cluster, had received an actual diagnosis of fibromyalgia after the rheumatologic exam which we suggested.
Statistical Analyses
Data are presented as mean and standard deviation for continuous variables and as frequency and percentages for categorical variables. Pain scores are reported as frequency (percentages) with 95% C.I. p-values to measure the association between each of the considered variables and pain scores were computed with a simple ordinal logistic regression model. Variables that resulted to significant to the univariate regression analysis were included in a multivariate ordinal logistic regression model. A p-value < 0.05 was considered statistically significant. All analyses were performed using the statistical software R, version 4.0.2.
3. Results
The demographic characteristics of the sample are reported in
Table 1.
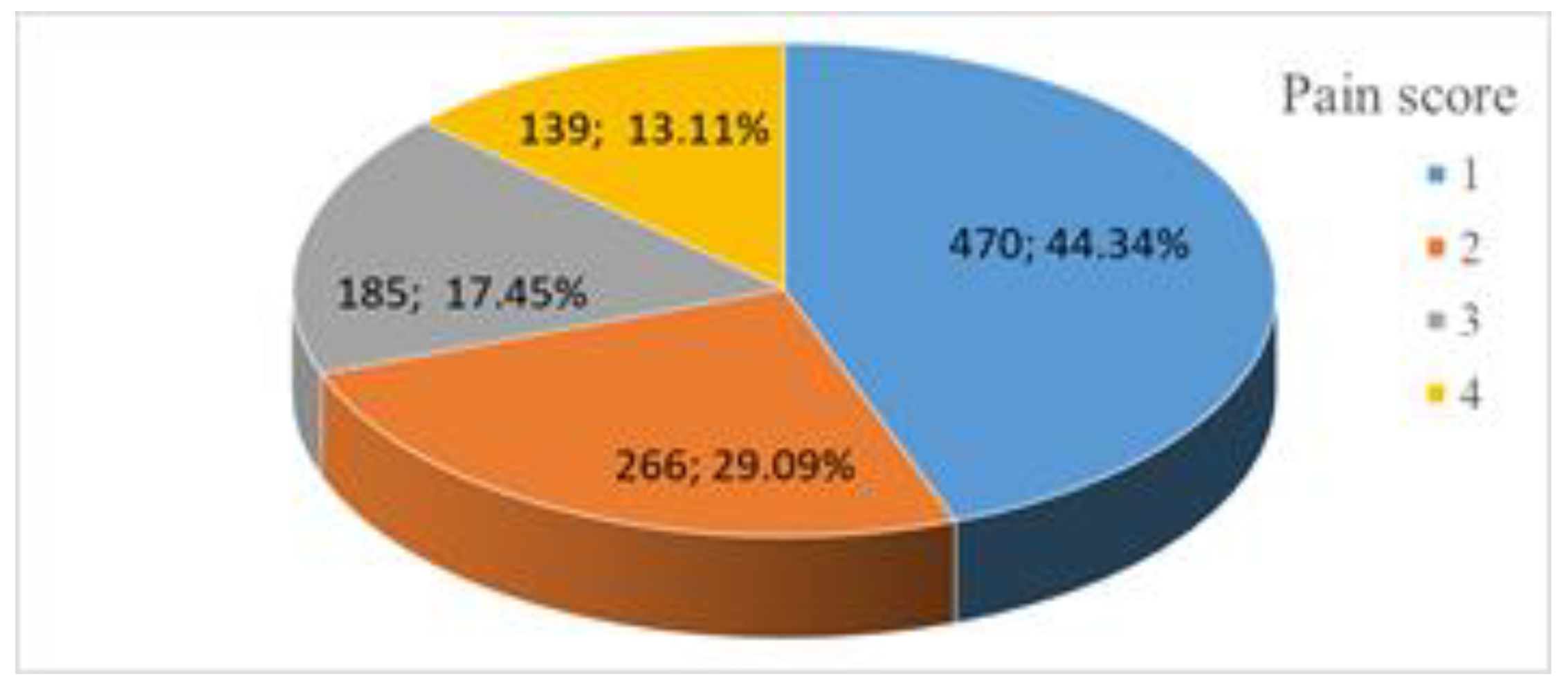
Out of the 1060 patients who participated inour study, 470 presented level 1 pain intensity (44.34%, 41.35–47.33 CI 95%), 266 presented level 2 pain intensity (25.09%, 22.48–27.70, CI 95%), 185 presented with level 3 pain intensity (17.45% 15.17–19.74 CI 95%), 139 presented level 4 pain intensity (13.11% 11.08–15.15 CI 95%) (
Figure 5); one of these patients endured such intense pain that we were unable to complete the mammography procedure; we decided not to exclude her, because for the purposes of this study, the actual completion of the screening procedure was not relevant.
Within the income groups we have divided our patients into percentiles: group 1 goes from the 1st to the 33rd percentile; group 2 goes from the 34th to the 66th percentile; and group 3 goes from the 67th to the 100th percentile. Stress level was assessed by asking our patients about their perceived stress level, similar to our pain level assessment: we felt that a more thorough assessment exceeded the goals of our study. Regarding the pain levels, we divided them into four groups based on the perceived stress level. Breast density, on the other hand, was evaluated in four classes with increasing density according to the guidelines of the ACR.
Out of the 139 women who stated a pain score of 4, 138 (one dropped out) have consulted a rheumatologist and 50 (36%, 28–44, 95% CI) were diagnosed with fibromyalgia. At ordinal logistic regression a statistically significant association (
p < 0.01) was found between the levels pain scores and the following factors: age, coffee cups per day, number of children, stress level, breastfeeding, higher income level, breast density, benign pathology and Previous breast surgeries; there was no significant statistical association between other factors we considered and pain level (
Table 2).
In particular, a medium-high level of pain (3 or 4) appears to be associated with a lower average age, number of cigarettes greater than 15, three or more children, a medium-high stress level, three or more coffees per day, having breastfed, low income, medium-high breast density and previous benign disease.
Compared to the multivariate ordinal regression model, the variables that lose significance in the multivariate model are breastfeeding, benign pathology, and previous surgery, while the level of education becomes a significant factor read in relation to the other factors. The multivariate model shows a significant goodness of fit to the data (p-value Pearson Chi-square coefficient < 0.01).
4. Discussion
The variety of clinical aspects with which pain can occur is probably related both to the possible involvement of different anatomical structures, and to the possible presence of various underlying pathophysiological conditions. In relation to the anatomical structures involved, we can distinguish pain due to skin involvement, pain due to involvement of the musculoskeletal structures and pain due to visceral involvement. Pain due to involvement of musculoskeletal structures can be due to various pathophysiological conditions such as tendonitis, polyarthritis, rheumatoid arthritis, fibromyalgia, bursitis and other painful musculoskeletal syndromes.
In the literature, the reference scales validated to diagnose pain are many [
28]; we remember in particular the numeric pain rating scale (NRS) and the visual analogue scale (VAS) as one-dimensional scales, certainly simple and quick to administer but poor in information. In addition to the extent of the pain, more complex scales (e.g., McGill Pain Questionnaire (MPQ)) help to better frame not only the presence/absence of pain but also the impact on the amount of life and daily activities; there are also dedicated scales to detect the possible presence of neuropathic pain (e.g., douleur neuropatique 4 (DN4)), or the risk of addiction in patients undergoing opioid therapy (opioid risk tool (ORT), which is recently also available in Italian).
Based on what has been described, in our study we considered the expressiveness of pain in four categories.
What is most interesting, though, is that if we only consider the sample of patients who had experienced the highest level of pain while undergoing mammographic screening, the prevalence value rises to a 36%; this seems to show that assessing the level of pain during routine breast cancer procedures might be a valuable asset for identifying those patients who have a high chance of being affected by fibromyalgia.
As stated before, while fibromyalgia appears to be widespread amongst the population, diagnosis of this condition still remains elusive. Recent studies show how there is a significant degree of disagreement between international classification of diseases (ICD)-based clinical diagnosis and criteria-based diagnosis for fibromyalgia, which further contributes to complicate the diagnostic algorithm for this condition [
36].
Due to such difficulties, elaborating preliminary procedures in the context of widespread and consolidated diagnostic screening paradigms can help to select those clusters of patients with an increased likelihood of being affected by such conditions and which can afterwards be redirected towards dedicated professionals for diagnosis and care.
A significant association between age, stress level, and especially breast density on one side, and pain level on the other was expected, as it is consistent with most scientific literature on the subject [
37,
38,
39,
40,
41]; the correlation between these variables and the search for the disease in all pain classes will be the subject of a future study, in which the disease was only searched for within class 4.
In some studies, drinking coffee has been shown to mitigate pain perception, owing to a direct action consisting ofcentral blocking of adenosine receptors that influence pain signaling and by interaction with peripherical adenosine receptors distributed on sensory afferent fibers [
42].Our results are in contrast with such findings;we may hypothesize that in our patients drinking significant amounts of coffeemight have heightened the pain perception by increasing the feeling of psychological stress, especially if they had drunk coffee right before undergoing mammographic examination, whereas patients physiologically feel anticipatory anxiety concerning the results of their screening. Further investigation will be needed to address the matter.
While a larger number of pregnancies appeared to be related to a higher pain level, previous breastfeeding did not show such a significant correlation; further studies will be needed to investigate the subject. We may postulate that having a larger number of children could be correlated with a higher stress level which is related to a higher pain level, or that hormonal changes happening during pregnancy may, somehow, be related to a lower pain feeling threshold.
Moreover, patients with a higher income and those who were more educatedexperienced a higher level of pain, although our statistical analyses offered results that were not particularly consistent regarding this subject;education to auniversity degree level, for instance, appeared to be correlated to a higher pain level in univariate analyses, but such result was not confirmed in multivariate analyses.
The most notable limit of our study is that our sample was an all-female one, which limits the applicability of our findings for both mixed gender and general population samples; also, our patients were all Caucasian.
Another limitation could be the great variability of the subjects subjected to screening: it has been shown that many factors such as the social situation, the situation and the work environment and the history of a previous injury can nevertheless influence the perception of pain and show great differences between individuals [
28].
Finally, as previously said, the patients we selected for rheumatologic examination were only those who experienced the highest level of pain, as we were reasonably sure that such an intense pain was not sensibly compatible with a standard mammography. This posits an inherent limit to our study which should be taken onto account when interpreting its results.
Moreover, our study is a monocentric one and further multicentric studies may provide deeper insights on the topic.
5. Conclusions
Our study showed a significant association between a high level of pain while undergoing routine mammographic exam and the diagnosis of fibromyalgia; the insidious nature of such disease, on one side, and the large and widespread use of mammographic screening, on the other, suggest that the latter might be used to at least obtain a FM’s diagnosticsuspicion, until more extensive screening routines for fibromyalgia become available such as the use of advanced imaging or artificial intelligence methods already applied or being studied in the breast sector [
43,
44,
45,
46]. With few exceptions, our study confirms most scientific findings concerning factors which relate to a heightened pain perception.
Author Contributions
Conceptualization, G.G., F.S. and A.A.H.S.; Formal analysis, G.G. and A.F.; Funding acquisition, D.L.F., A.F. and R.M.; Investigation, G.G.; Methodology, G.G., M.P.B., D.P. and S.C.; Project administration, G.G.; Software, G.G.; Supervision, G.G., D.L.F., F.S. and A.A.H.S.; Validation, G.G., F.S. and A.A.H.S.; Writing—original draft, G.G., D.L.F., A.F., R.M., F.S., M.P.B., D.P., S.C. and A.A.H.S.; Writing—review and editing, G.G., D.L.F., A.F., R.M., F.S., M.P.B., D.P., S.C. and A.A.H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Italian Ministry of Health “Ricerca Finalizzata 2018”.
Institutional Review Board Statement
The study was conducted according to the guidelines of the 409 Declaration of Helsinki, and approved by the Regional Ethic Review Board of Università della Campania “Luigi Vanvitelli” (registration number 187.20).
Informed Consent Statement
Informed consent was obtained from all subjects involved in thestudy.
Data Availability Statement
The data presented in this study are available on request from the 415 corresponding author. The data are not publicly available because are propriety of Università della Campania “Luigi Vanvitelli”, Napoli, Italy.
Acknowledgments
The authors thank Luisa Fogliamanzillo for the English linguistic revision.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Clauw, D.J. Fibromyalgia: A clinical review. JAMA 2014, 311, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.K. Fibromyalgia: A review of management options. Formulary 2009, 44, 362–373. [Google Scholar]
- Wolfe, F.; Clauw, D.J.; Fitzcharles, M.-A.; Goldenberg, D.L.; Katz, R.S.; Mease, P.; Russell, A.S.; Russell, I.J.; Winfield, J.B.; Yunus, M.B. The American College of Rheumatology Preliminary Diagnostic Criteria for Fibromyalgia and Measurement of Symptom Severity. Arthritis Care Res. 2010, 62, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, K.; Gracely, R.; Glass, J. Fibromyalgia dyscognition: Concepts and issues. Reumatismo 2012, 64, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Kravitz, H.M.; Katz, R.S. Fibrofog and fibromyalgia: A narrative review and implications for clinical practice. Rheumatol. Int. 2015, 35, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Fuller-Thomson, E.; Nimigon-Young, J.; Brennenstuhl, S. Individuals with fibromyalgia and depression: Findings from a nationally representative Canadian survey. Rheumatol. Int. 2012, 32, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.M.; Hudson, J.I.; Hess, E.V.; Ware, A.E.; Fritz, D.A.; Auchenbach, M.B.; Starck, L.O.; Keck, P.E. Family study of fibromyalgia. Arthritis Rheum. 2004, 50, 944–952. [Google Scholar] [CrossRef]
- Buskila, D.; Neumann, L.; Press, J. Genetic Factors in Neuromuscular Pain. CNS Spectr. 2005, 10, 281–284. [Google Scholar] [CrossRef]
- Arroyo, J.F.; Cohen, M.L. Abnormal responses to electrocutaneous stimulation in fibromyalgia. J. Rheumatol. 1993, 20, 1925–1931. [Google Scholar]
- Petzke, F.; Clauw, D.J.; Ambrose, K.; Khine, A.; Gracely, R.H. Increased pain sensitivity in fibromyalgia: Effects of stimulus type and mode of presentation. Pain 2003, 105, 403–413. [Google Scholar] [CrossRef]
- Russell, I.J.; Orr, M.D.; Littman, B.; Vipraio, G.A.; Alboukrek, D.; Michalek, J.E.; Lopez, Y.; Mackillip, F. Elevated cerebrospinal fluid levels of substance p in patients with the fibromyalgia syndrome. Arthritis Rheum. 1994, 37, 1593–1601. [Google Scholar] [CrossRef]
- Pyke, T.L.; Osmotherly, P.G.; Baines, S. Measuring Glutamate Levels in the Brains of Fibromyalgia Patients and a Potential Role for Glutamate in the Pathophysiology of Fibromyalgia Symptoms. Clin. J. Pain 2017, 33, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M. Pathophysiology and clinical spectrum of fibromyalgia: A brief overview for medical communicators. AMWA J. 2011, 26, 50–54. [Google Scholar]
- Wolfe, F.; Russell, I.J.; Vipraio, G.; Ross, K.; Anderson, J. Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J. Rheumatol. 1997, 24, 555–559. [Google Scholar] [PubMed]
- Ernberg, M.; Voog, U.; Alstergren, P.; Lundeberg, T.; Kopp, S. Plasma and serum serotonin levels and their relationship to orofacial pain and anxiety in fibromyalgia. J. Orofac. Pain 2000, 14, 37–46. [Google Scholar]
- Cordero, M.D.; Alcocer-Gomez, E.; Cano-García, F.J.; de Miguel, M.; Sánchez-Alcázar, J.A.; Moreno Fernandez, A.M. Low levels of serotonin in serum correlates with severity of fibromyalgia. Med. Clin. 2010, 135, 644–646. [Google Scholar] [CrossRef][Green Version]
- Becker, S.; Schweinhardt, P. Dysfunctional Neurotransmitter Systems in Fibromyalgia, Their Role in Central Stress Circuitry and Pharmacological Actions on These Systems. Pain Res. Treat. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Wood, P.B.; Schweinhardt, P.; Jaeger, E.; Dagher, A.; Hakyemez, H.; Rabiner, E.A.; Bushnell, M.C.; Chizh, B.A. Fibromyalgia patients show an abnormal dopamine response to pain. Eur. J. Neurosci. 2007, 25, 3576–3582. [Google Scholar] [CrossRef]
- Wood, P.B. Role of central dopamine in pain and analgesia. Expert Rev. Neurother. 2008, 8, 781–797. [Google Scholar] [CrossRef]
- Kutu, F.C.; Özdolap, Ş.; Sarikaya, S. Pro-inflammatory cytokines and oxidized low-density-lipoprotein in patients with fibromyalgia. Arch. Rheumatol. 2018, 34, 123–129. [Google Scholar] [CrossRef]
- Ranzolin, A.; Duarte, A.L.B.P.; Bredemeier, M.; Neto, C.A.D.C.; Ascoli, B.M.; Wollenhaupt-Aguiar, B.; Kapczinski, F.; Xavier, R.M. Evaluation of cytokines, oxidative stress markers and brain-derived neurotrophic factor in patients with fibromyalgia—A controlled cross-sectional study. Cytokine 2016, 84, 25–28. [Google Scholar] [CrossRef]
- Lucas, H.; Brauch, C.; Settas, L.; Theoharides, T. Fibromyalgia—New Concepts of Pathogenesis and Treatment. Int. J. Immunopathol. Pharmacol. 2006, 19, 5–10. [Google Scholar] [CrossRef]
- Yunus, M.B.; Arslan, S.; Aldag, J.C. Relationship between fibromyalgia features and smoking. Scand. J. Rheumatol. 2002, 31, 301–305. [Google Scholar] [CrossRef]
- Marques, A.P.; Santo, A.D.S.D.E.; Berssaneti, A.A.; Matsutani, L.A.; Yuan, S.L.K. Prevalence of fibromyalgia: Literature review update. Rev. Bras. Reumatol. Engl. Ed. 2017, 57, 356–363. [Google Scholar] [CrossRef]
- Bartels, E.M.; Dreyer, L.; Jacobsen, S.; Jespersen, A.; Bliddal, H.; Danneskiold-Samsøe, B. Fibromyalgia, diagnosis and prevalence. Are gender differences explainable? Ugeskr. Laeger 2009, 171, 3588–3592. [Google Scholar]
- White, K.P.; Speechley, M.; Harth, M.; Ostbye, T. The London FM Epidemiology Study: The prevalence of fibromyalgia syndrome in London, Ontario. J. Rheumatol. 1999, 26, 1570–1576. [Google Scholar] [PubMed]
- Wolfe, F.; Brähler, E.; Hinz, A.; Häuser, W. Fibromyalgia Prevalence, Somatic Symptom Reporting, and the Dimensionality of Polysymptomatic Distress: Results From a Survey of the General Population. Arthritis Rheum. 2013, 65, 777–785. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15, S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Karoly, P.; O’Riordan, E.F.; Bland, F.; Burns, R.S. The subjective experience of acute pain. Anassessment of the utility of 10 indices. Clin. J. Pain. 1989, 5, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Kremer, E.; Atkinson, H.J.; Ignelzi, R.J. Measurement of pain: Patient preference does not confound pain measurement. Pain 1981, 10, 241–248. [Google Scholar] [CrossRef]
- Closs, S.; Barr, B.; Briggs, M.; Cash, K.; Seers, K. A comparison of five pain assessment scales for nursing home residents with varying degrees of cognitive impairment. J. Pain Symptom Manag. 2004, 27, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Farrar, J.T.; Portenoy, R.K.; Berlin, J.A.; Kinman, J.L.; Strom, B.L. Defining the clinically important difference in pain outcome measures. Pain 2000, 88, 287–294. [Google Scholar] [CrossRef]
- Hjermstad, M.J.; Fayers, P.M.; Haugen, D.F.; Caraceni, A.; Hanks, G.W.; Loge, J.H. European Palliative Care Research Collaborative (EPCRC). Studies comparing Numerical Rating Scales, Verbal Rating Scales, and Visual Analogue Scales for assessment of pain intensity in adults: A systematic literature review. J. Pain Symptom Manag. 2011, 41, 1073–1093. [Google Scholar] [CrossRef]
- Jensen, M.P.; Turner, J.A.; Romano, J.M.; Strom, S.E. The chronic pain coping inventory: Development and preliminary validation. Pain 1995, 60, 203–216. [Google Scholar] [CrossRef]
- Wood, K.; Cameron, M.; Fitzgerald, K. Breast size, bra fit and thoracic pain in young women: A correlational study. Chiropractic Osteopat. 2008, 16, 1. [Google Scholar] [CrossRef]
- Wolfe, F.; Schmukler, J.; Jamal, S.; Castrejon, I.; Gibson, K.A.; Srinivasan, S.; Häuser, W.; Pincus, T. Diagnosis of Fibromyalgia: Disagreement Between Fibromyalgia Criteria and Clinician-Based Fibromyalgia Diagnosis in a University Clinic. Arthritis Rheum. 2019, 71, 343–351. [Google Scholar] [CrossRef]
- Siqveland, J.; Hauff, E.; Ruud, T.; Brennen, T.J. Posttraumatic stress and autobiographical memory in chronic pain patients. Scand. J. Pain 2019, 19, 337–343. [Google Scholar] [CrossRef]
- Taylor, A.G.; Fischer-White, T.G.; Anderson, J.G.; Adelstein, K.E.; Murugesan, M.; Lewis, J.E.; Scott, M.M.; Gaykema, R.P.A.; Goehler, L.E. Stress, Inflammation and Pain: A Potential Role for Monocytes in Fibromyalgia-related Symptom Severity. Stress Health 2016, 32, 503–513. [Google Scholar] [CrossRef]
- McNicholas, M.M.; Heneghan, J.P.; Milner, M.H.; Tunney, T.; Hourihane, J.B.; MacErlaine, D.P. Pain and increased mammographic density in women receiving hormone replacement therapy: A prospective study. Am. J. Roentgenol. 1994, 163, 311–315. [Google Scholar] [CrossRef][Green Version]
- El Tumi, H.; Johnson, M.; Dantas, P.; Maynard, M.; Tashani, O. Age-related changes in pain sensitivity in healthy humans: A systematic review with meta-analysis. Eur. J. Pain 2017, 21, 955–964. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Peters, J.H.; Heesen, M.; Scheel, J.; Kunz, M. Age changes in pain perception: A systematic-review and meta-analysis of age effects on pain and tolerance thresholds. Neurosci. Biobehav. Rev. 2017, 75, 104–113. [Google Scholar] [CrossRef]
- Baratloo, A.; Rouhipour, A.; Forouzanfar, M.M.; Safari, S.; Amiri, M.; Negida, A. The Role of Caffeine in Pain Management: A Brief Literature Review. Anesthesiol. Pain Med. 2016, 6, e33193. [Google Scholar] [CrossRef] [PubMed]
- Fausto, A.; Bernini, M.; La Forgia, D.; Fanizzi, A.; Marcasciano, M.; Volterrani, L.; Casella, D.; Mazzei, M.A. Six-year prospective evaluation of second-look US with volume navigation for MRI-detected additional breast lesions. Eur. Radiol. 2018, 29, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Basile, T.M.A.; Fanizzi, A.; Losurdo, L.; Bellotti, R.; Tangaro, S.; La Forgia, D.; Didonna, V.; Massafra, R.; Tamborra, P.; Moschetta, M.; et al. Hough transform for clustered microcalcifications detection in full-field digital mammograms. Appl. Digit. Image Process. XL 2017, 10396, 41. [Google Scholar] [CrossRef]
- Fanizzi, A.; Pomarico, D.; Paradiso, A.; Bove, S.; Diotiaiuti, S.; Didonna, V.; Giotta, F.; La Forgia, D.; Latorre, A.; Pastena, M.; et al. Predicting of Sentinel Lymph Node Status in Breast Cancer Patients with Clinically Negative Nodes: A Validation Study. Cancers 2021, 13, 352. [Google Scholar] [CrossRef]
- Fanizzi, A.; Basile, T.M.; Losurdo, L.; Bellotti, R.; Bottigli, U.; Campobasso, F.; Didonna, V.; Fausto, A.; Massafra, R.; Tagliafico, A.; et al. Ensemble Discrete Wavelet Transform and Gray-Level Co-Occurrence Matrix for Microcalcification Cluster Classification in Digital Mammography. Appl. Sci. 2019, 9, 5388. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).