A Comprehensive Examination of Severely Ill ME/CFS Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Severely Ill ME/CFS Patients Inclusion and Exclusion Criteria
- Age 18–70, inclusive;
- Must carry a diagnosis of ME/CFS as defined by the ICC criteria;
- Subjects must be homebound and spend >14 h per day sedentary and in a reclined position (measured by FitBit and patient/family report);
- SF-36 physical functioning score < 70; and
- Be able to provide informed consent.
- Patients, age < 18 years or > 70 years;
- Women who are pregnant;
- Unable to understand informed consent; or
- Patients with known HCT < 34 mg/dL.
2.1.2. Healthy Control Inclusion and Exclusion Criteria
- Age 18–70, inclusive;
- Must not carry a diagnosis of ME/CFS as defined by the ICC criteria or active illness (acute or chronic);
- Must be sedentary ≤ 14 h; and
- SF-36 physical functioning score ≥ 70.
- Patients, age < 18 years or > 70 years;
- Women who are pregnant;
- Unable to understand informed consent; or
- Patients with a known HCT < 34 mg/dL.
2.2. Data Collection from Questionnaires
2.3. Data Collection of Patient Activity, Sleep Monitoring, and Cognitive Tests
2.4. Clinical Lab Tests
2.5. Tests of Antibodies and Antigens against Pathogens
2.6. Data Analysis
3. Results
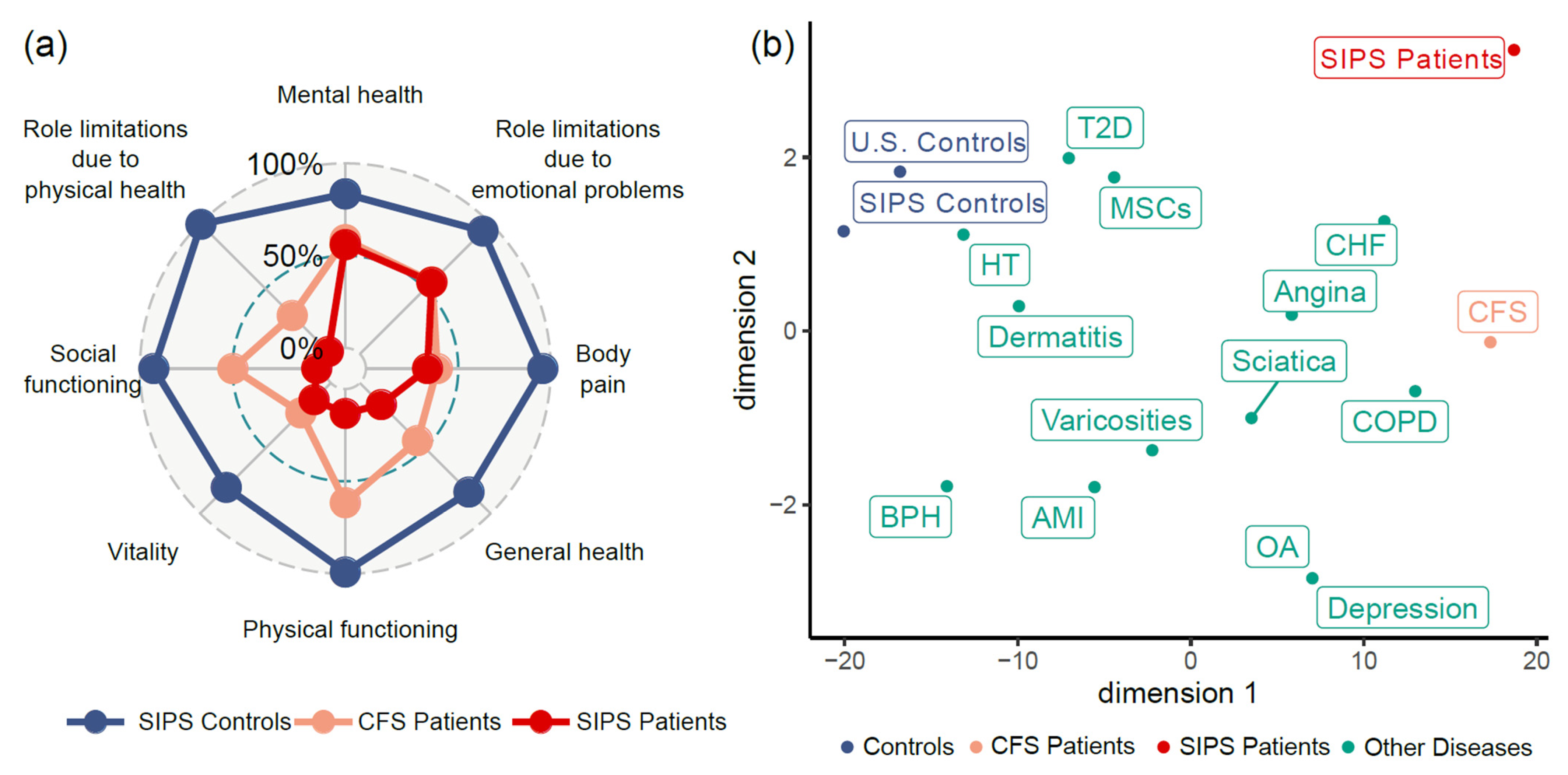
3.1. Patient-Reported Health Status and Symptoms of the Severely Ill
3.1.1. Demographics and Quality of Life of the Patients
3.1.2. Patient-Reported Health Status
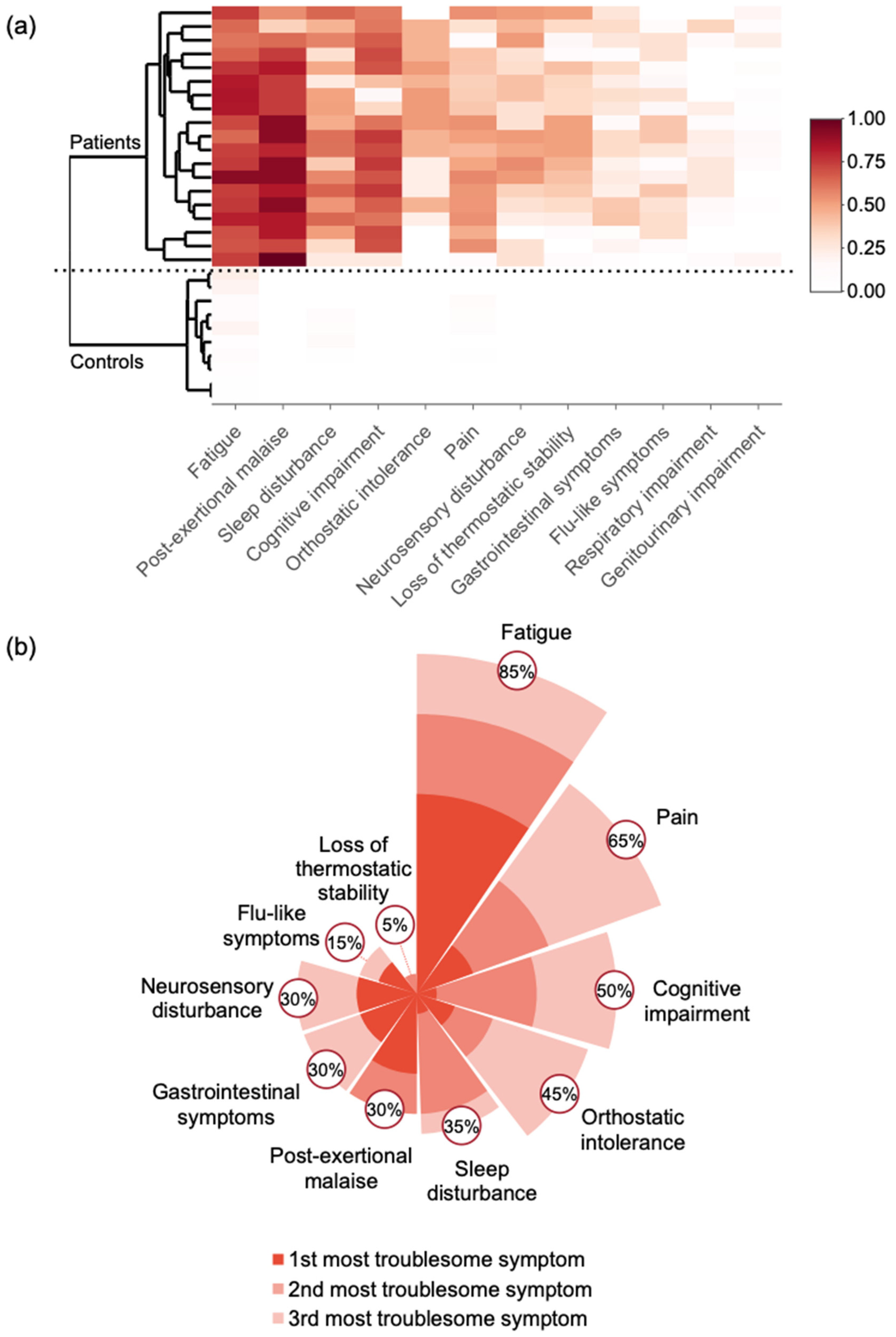
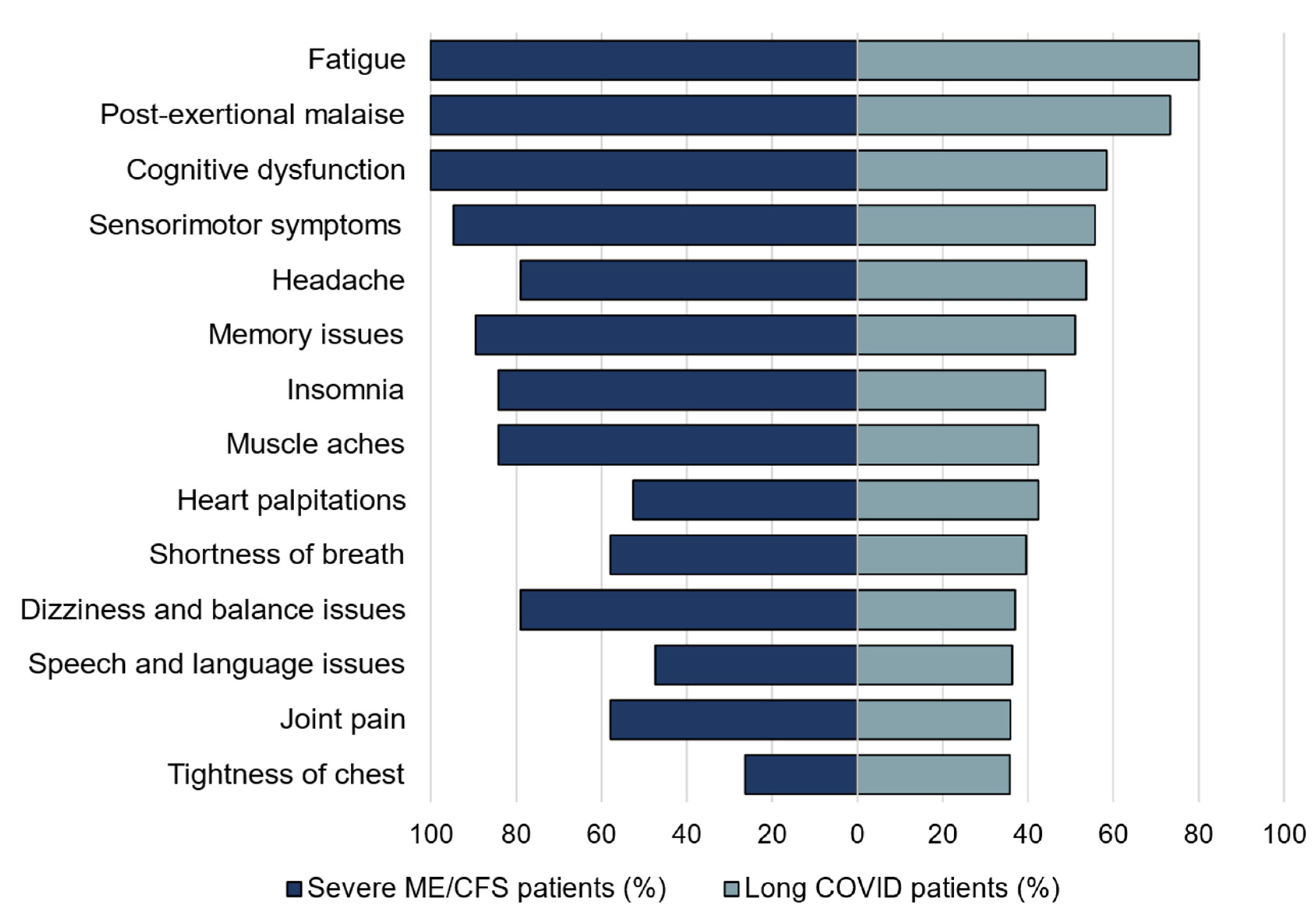
3.1.3. Evaluation of the Common Symptoms in the Patients
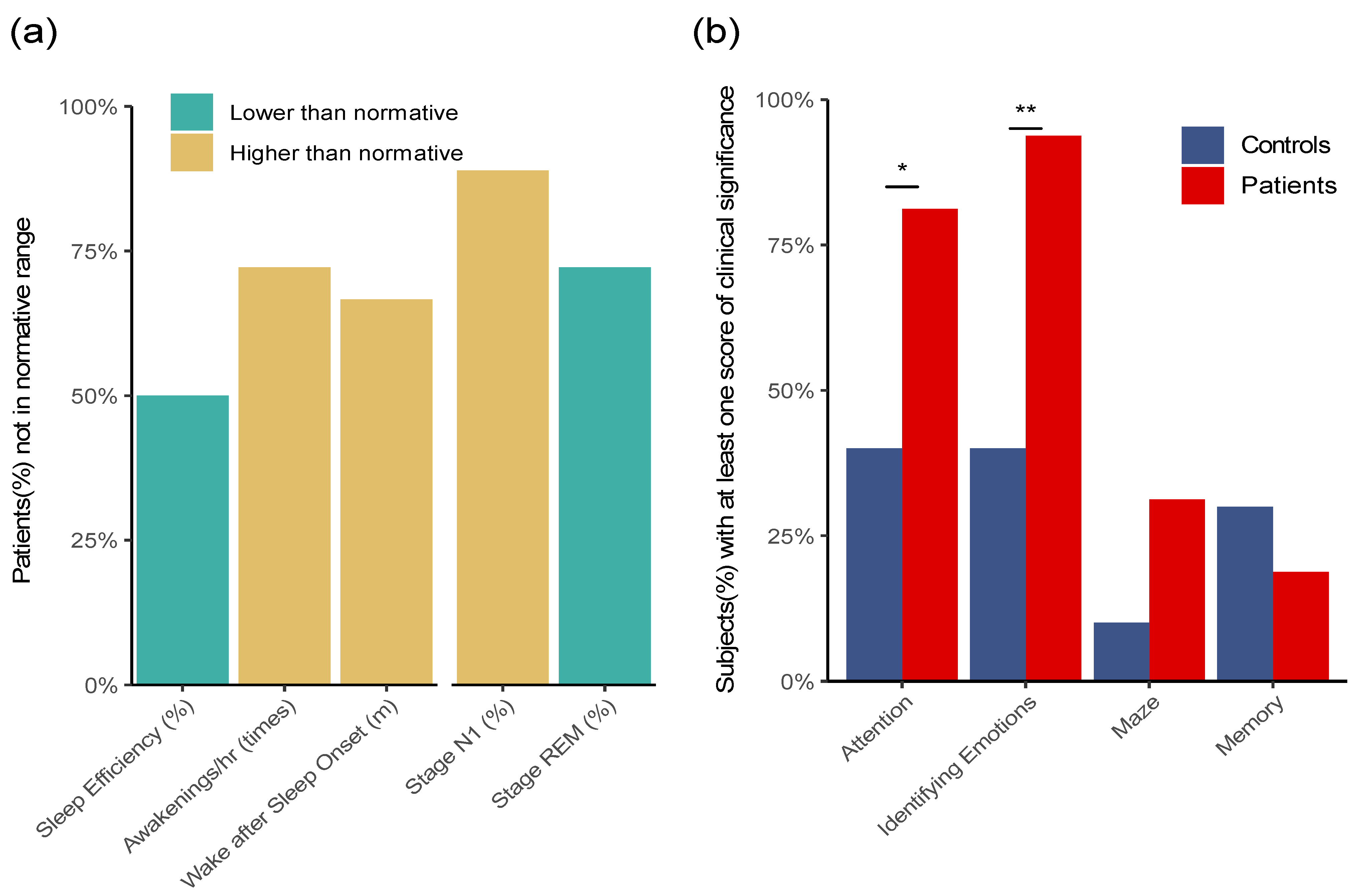
3.2. Activity, Sleep Monitoring, and Cognitive Tests of the Severe ME/CFS Patients
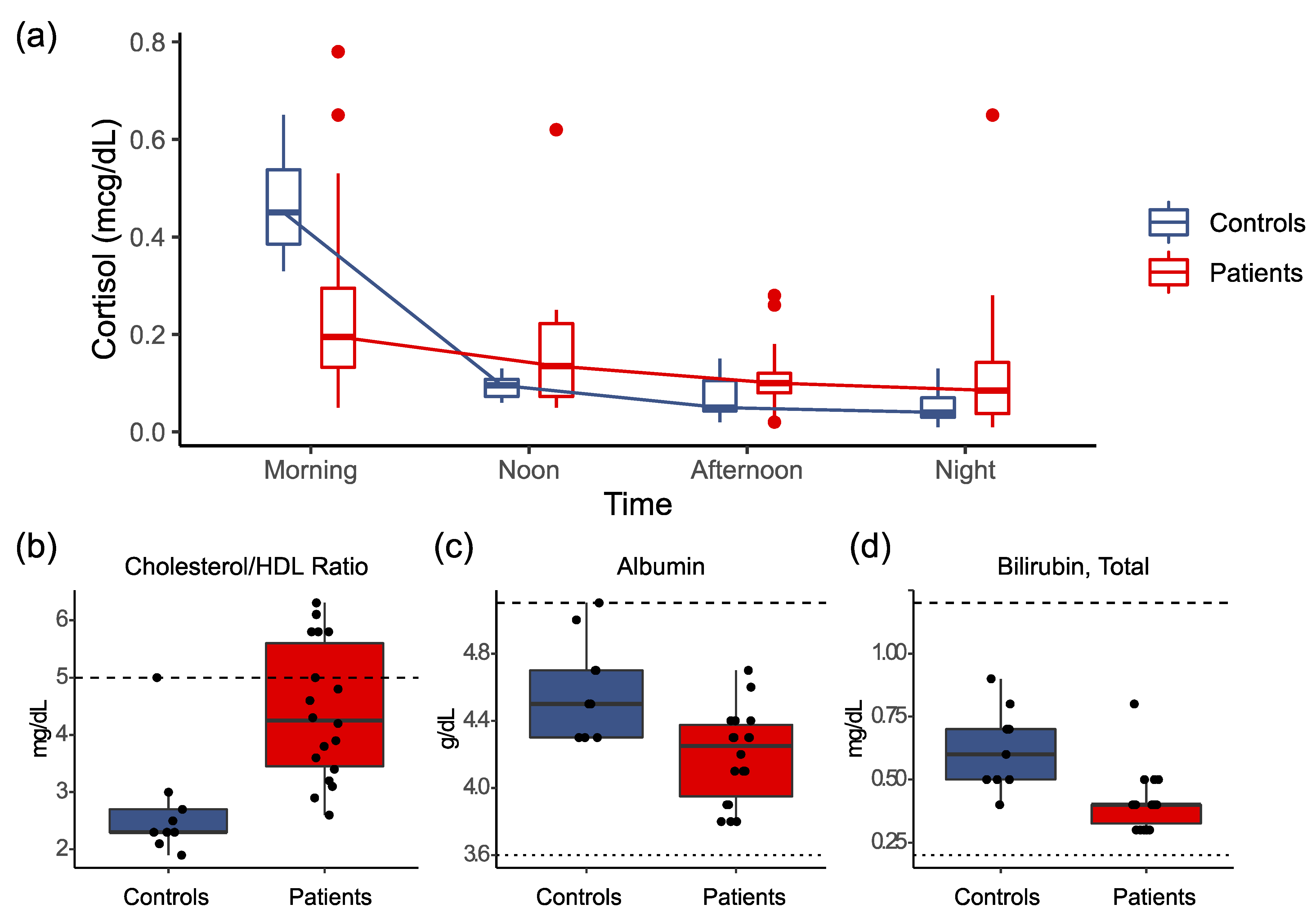
3.3. Results of Clinical Laboratory Testing
3.4. Tests on Antigens and Antibodies against Viral and Bacterial Pathogens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Committee on the Diagnostic Criteria for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome; Board on the Health of Select Populations; Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2015; ISBN 978-0-309-31689-7. [Google Scholar]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Comprehensive Review. Diagnostics 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Valdez, A.R.; Hancock, E.E.; Adebayo, S.; Kiernicki, D.J.; Proskauer, D.; Attewell, J.R.; Bateman, L.; DeMaria, A.; Lapp, C.W.; Rowe, P.C.; et al. Estimating Prevalence, Demographics, and Costs of ME/CFS Using Large Scale Medical Claims Data and Machine Learning. Front. Pediatr. 2018, 6, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bateman, L.; Bested, A.C.; Bonilla, H.F.; Chheda, B.V.; Chu, L.; Curtin, J.M.; Dempsey, T.T.; Dimmock, M.E.; Dowell, T.G.; Felsenstein, D.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Essentials of Diagnosis and Management. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Rasa, S.; Nora-Krukle, Z.; Henning, N.; Eliassen, E.; Shikova, E.; Harrer, T.; Scheibenbogen, C.; Murovska, M.; Prusty, B.K.; European Network on ME/CFS (EUROMENE). Chronic Viral Infections in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J. Transl. Med. 2018, 16, 268. [Google Scholar] [CrossRef] [Green Version]
- Falk Hvidberg, M.; Brinth, L.S.; Olesen, A.V.; Petersen, K.D.; Ehlers, L. The Health-Related Quality of Life for Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). PLoS ONE 2015, 10, e0132421. [Google Scholar] [CrossRef]
- Bombardier, C.H.; Buchwald, D. Outcome and Prognosis of Patients with Chronic Fatigue vs Chronic Fatigue Syndrome. Arch. Intern. Med. 1995, 155, 2105–2110. [Google Scholar] [CrossRef]
- Wilson, A.; Hickie, I.; Lloyd, A.; Hadzi-Pavlovic, D.; Boughton, C.; Dwyer, J.; Wakefield, D. Longitudinal Study of Outcome of Chronic Fatigue Syndrome. BMJ 1994, 308, 756–759. [Google Scholar] [CrossRef] [Green Version]
- Fennell, P.A.; Jason, L.A.; Klein, S.M. Capturing the Different Phases of the CFS Illness. CFIDS Chron. 1998, 11, 13–16. [Google Scholar]
- Pendergrast, T.; Brown, A.; Sunnquist, M.; Jantke, R.; Newton, J.L.; Strand, E.B.; Jason, L.A. Housebound versus Nonhousebound Patients with Myalgic Encephalomyelitis and Chronic Fatigue Syndrome. Chronic Illn. 2016, 12, 292–307. [Google Scholar] [CrossRef] [Green Version]
- Wiborg, J.F.; van der Werf, S.; Prins, J.B.; Bleijenberg, G. Being Homebound with Chronic Fatigue Syndrome: A Multidimensional Comparison with Outpatients. Psychiatry Res. 2010, 177, 246–249. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; National Center for Emerging and Zoonotic Infectious Diseases (NCEZID); Division of High-Consequence Pathogens and Pathology (DHCPP). Severely Affected Patients|Clinical Care of Patients|Healthcare Providers|Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)|CDC. Available online: https://www.cdc.gov/me-cfs/healthcare-providers/clinical-care-patients-mecfs/severely-affected-patients.html (accessed on 22 August 2021).
- Dafoe, W. Extremely Severe ME/CFS-A Personal Account. Healthc. Basel Switz. 2021, 9, 504. [Google Scholar] [CrossRef]
- Komaroff, A.L. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: When Suffering Is Multiplied. Healthcare 2021, 9, 919. [Google Scholar] [CrossRef]
- Carruthers, B.M.; van de Sande, M.I.; De Meirleir, K.L.; Klimas, N.G.; Broderick, G.; Mitchell, T.; Staines, D.; Powles, A.C.P.; Speight, N.; Vallings, R.; et al. Myalgic Encephalomyelitis: International Consensus Criteria. J. Intern. Med. 2011, 270, 327–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, J.E.; New England Medical Center Hospital; Health Institute. SF-36 Physical and Mental Health Summary Scales: A User’s Manual; Health Institute, New England Medical Center: Boston, MA, USA, 1994. [Google Scholar]
- Karnofsky, D.A.; Abelmann, W.H.; Craver, L.F.; Burchenal, J.H. The Use of the Nitrogen Mustards in the Palliative Treatment of Carcinoma. With Particular Reference to Bronchogenic Carcinoma. Cancer 1948, 1, 634–656. [Google Scholar] [CrossRef]
- PROMIS: Patient-Reported Outcomes Measurement Information System—Home Page. Available online: https://commonfund.nih.gov/promis/index (accessed on 27 April 2021).
- Cella, D.; Yount, S.; Rothrock, N.; Gershon, R.; Cook, K.; Reeve, B.; Ader, D.; Fries, J.F.; Bruce, B.; Rose, M.; et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap Cooperative Group during Its First Two Years. Med. Care 2007, 45, S3–S11. [Google Scholar] [CrossRef] [Green Version]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A New Instrument for Psychiatric Practice and Research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Spencer, B.R.; Kleinman, S.; Wright, D.J.; Glynn, S.A.; Rye, D.B.; Kiss, J.E.; Mast, A.E.; Cable, R.G.; REDS-II RISE Analysis Group. Restless Legs Syndrome, Pica, and Iron Status in Blood Donors. Transfusion (Paris) 2013, 53, 1645–1652. [Google Scholar] [CrossRef] [Green Version]
- Finan, P.H.; Richards, J.M.; Gamaldo, C.E.; Han, D.; Leoutsakos, J.M.; Salas, R.; Irwin, M.R.; Smith, M.T. Validation of a Wireless, Self-Application, Ambulatory Electroencephalographic Sleep Monitoring Device in Healthy Volunteers. J. Clin. Sleep Med. 2016, 12, 1443–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsleben, J.A.; Kapur, V.K.; Newman, A.B.; Shahar, E.; Bootzin, R.R.; Rosenberg, C.E.; O’Connor, G.; Nieto, F.J. Sleep and Reported Daytime Sleepiness in Normal Subjects: The Sleep Heart Health Study. Sleep 2004, 27, 293–298. [Google Scholar] [CrossRef] [Green Version]
- Levendowski, D.J.; Ferini-Strambi, L.; Gamaldo, C.; Cetel, M.; Rosenberg, R.; Westbrook, P.R. The Accuracy, Night-to-Night Variability, and Stability of Frontopolar Sleep Electroencephalography Biomarkers. J. Clin. Sleep Med. 2017, 13, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, S.M.; Berten, S.; Olson, P.; Paul, R.; Willams, L.M.; Cooper, N.; Gordon, E. Development and Validation of a World-Wide-Web-Based Neurocognitive Assessment Battery: WebNeuro. Behav. Res. Methods 2007, 39, 940–949. [Google Scholar] [CrossRef]
- Gordon, E.; Cooper, N.; Rennie, C.; Hermens, D.; Williams, L.M. Integrative Neuroscience: The Role of a Standardized Database. Clin. EEG Neurosci. 2005, 36, 64–75. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Recommendations for Test Performance and Interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 1995, 44, 590–591. [Google Scholar]
- Mead, P.; Petersen, J.; Hinckley, A. Updated CDC Recommendation for Serologic Diagnosis of Lyme Disease. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magni, R.; Espina, B.H.; Shah, K.; Lepene, B.; Mayuga, C.; Douglas, T.A.; Espina, V.; Rucker, S.; Dunlap, R.; Petricoin, E.F.I.; et al. Application of Nanotrap Technology for High Sensitivity Measurement of Urinary Outer Surface Protein A Carboxyl-Terminus Domain in Early Stage Lyme Borreliosis. J. Transl. Med. 2015, 13, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theel, E.S.; Ross, T. Seasonality of Bartonella Henselae IgM and IgG Antibody Positivity Rates. J. Clin. Microbiol. 2019, 57, e01263-19. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing Long COVID in an International Cohort: 7 Months of Symptoms and Their Impact. EClinicalMedicine 2021, 101019. [Google Scholar] [CrossRef]
- Rajeevan, M.S.; Dimulescu, I.; Murray, J.; Falkenberg, V.R.; Unger, E.R. Pathway-Focused Genetic Evaluation of Immune and Inflammation Related Genes with Chronic Fatigue Syndrome. Hum. Immunol. 2015, 76, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fluge, Ø.; Rekeland, I.G.; Lien, K.; Thürmer, H.; Borchgrevink, P.C.; Schäfer, C.; Sørland, K.; Aßmus, J.; Ktoridou-Valen, I.; Herder, I.; et al. B-Lymphocyte Depletion in Patients With Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Intern. Med. 2019, 170, 585–593. [Google Scholar] [CrossRef]
- Ware, J.E.; Snow, K.K.; Kosinski, M.; Gandek, B. SF-36 Health Survey: MANUAL and Interpretation Guide; Health Institute, New England Medical Center: Boston, MA, USA, 1993. [Google Scholar]
- Tudor-Locke, C.E.; Myers, A.M. Methodological Considerations for Researchers and Practitioners Using Pedometers to Measure Physical (Ambulatory) Activity. Res. Q. Exerc. Sport 2001, 72, 1–12. [Google Scholar] [CrossRef]
- Whitt, M.C.; DuBose, K.D.; Ainsworth, B.E.; Tudor-Locke, C. Walking Patterns in a Sample of African American, Native American, and Caucasian Women: The Cross-Cultural Activity Participation Study. Health Educ. Behav. Off. Publ. Soc. Public Health Educ. 2004, 31, 45S–56S. [Google Scholar] [CrossRef]
- Jackson, M.L.; Bruck, D. Sleep Abnormalities in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis: A Review. J. Clin. Sleep Med. 2012, 8, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Gotts, Z.M.; Newton, J.L.; Ellis, J.G.; Deary, V. The Experience of Sleep in Chronic Fatigue Syndrome: A Qualitative Interview Study with Patients. Br. J. Health Psychol. 2016, 21, 71–92. [Google Scholar] [CrossRef]
- Shrivastava, D.; Jung, S.; Saadat, M.; Sirohi, R.; Crewson, K. How to Interpret the Results of a Sleep Study. J. Community Hosp. Intern. Med. Perspect. 2014, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, T.; Edwards, M.J.; Isaacs, J.D. A Unifying Theory for Cognitive Abnormalities in Functional Neurological Disorders, Fibromyalgia and Chronic Fatigue Syndrome: Systematic Review. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1308–1319. [Google Scholar] [CrossRef] [Green Version]
- Michiels, V.; de Gucht, V.; Cluydts, R.; Fischler, B. Attention and Information Processing Efficiency in Patients with Chronic Fatigue Syndrome. J. Clin. Exp. Neuropsychol. 1999, 21, 709–729. [Google Scholar] [CrossRef]
- Schmaling, K.B.; Betterton, K.L. Neurocognitive Complaints and Functional Status among Patients with Chronic Fatigue Syndrome and Fibromyalgia. Qual. Life Res. 2016, 25, 1257–1263. [Google Scholar] [CrossRef]
- OptumHealth; Brain Resource Company. WebNeuro User Manual; Brain Resource Company (BRC): San Francisco, CA, USA, 2008. [Google Scholar]
- Nacul, L.C.; Lacerda, E.M.; Campion, P.; Pheby, D.; de Drachler, L.M.; Leite, J.C.; Poland, F.; Howe, A.; Fayyaz, S.; Molokhia, M. The Functional Status and Well Being of People with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Their Carers. BMC Public Health 2011, 11, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, W.C.; Wagner, D.; Nisenbaum, R.; Jones, J.F.; Gurbaxani, B.; Solomon, L.; Papanicolaou, D.A.; Unger, E.R.; Vernon, S.D.; Heim, C. Chronic Fatigue Syndrome—A Clinically Empirical Approach to Its Definition and Study. BMC Med. 2005, 3, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jason, L.; Brown, M.; Evans, M.; Anderson, V.; Lerch, A.; Brown, A.; Hunnell, J.; Porter, N. Measuring Substantial Reductions in Functioning in Patients with Chronic Fatigue Syndrome. Disabil. Rehabil. 2011, 33, 589–598. [Google Scholar] [CrossRef]
- Nacul, L.; Authier, F.J.; Scheibenbogen, C.; Lorusso, L.; Helland, I.B.; Martin, J.A.; Sirbu, C.A.; Mengshoel, A.M.; Polo, O.; Behrends, U.; et al. European Network on Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (EUROMENE): Expert Consensus on the Diagnosis, Service Provision, and Care of People with ME/CFS in Europe. Medicina 2021, 57, 510. [Google Scholar] [CrossRef]
- US ME/CFS Clinician Coalition. ME/CFS Treatment Recommendations, Version 1; The US ME/CFS Clinician Coalition, 2021. Available online: https://mecfscliniciancoalition.org (accessed on 22 August 2021).
- Ross, S.; Fantie, B.; Straus, S.F.; Grafman, J. Divided Attention Deficits in Patients with Chronic Fatigue Syndrome. Appl. Neuropsychol. 2001, 8, 4–11. [Google Scholar] [CrossRef]
- US ME/CFS Clinician Coalition. Testing Recommendations for Suspected ME/CFS, Version 1; The US ME/CFS Clinician Coalition, 2021. Available online: https://mecfscliniciancoalition.org (accessed on 22 August 2021).
- Nater, U.M.; Maloney, E.; Boneva, R.S.; Gurbaxani, B.M.; Lin, J.-M.; Jones, J.F.; Reeves, W.C.; Heim, C. Attenuated Morning Salivary Cortisol Concentrations in a Population-Based Study of Persons with Chronic Fatigue Syndrome and Well Controls. J. Clin. Endocrinol. Metab. 2008, 93, 703–709. [Google Scholar] [CrossRef] [Green Version]
- Nater, U.M.; Youngblood, L.S.; Jones, J.F.; Unger, E.R.; Miller, A.H.; Reeves, W.C.; Heim, C. Alterations in Diurnal Salivary Cortisol Rhythm in a Population-Based Sample of Cases with Chronic Fatigue Syndrome. Psychosom. Med. 2008, 70, 298–305. [Google Scholar] [CrossRef]
- van Campen, C.L.M.C.; Rowe, P.C.; Visser, F.C. Blood Volume Status in ME/CFS Correlates With the Presence or Absence of Orthostatic Symptoms: Preliminary Results. Front. Pediatr. 2018, 6, 352. [Google Scholar] [CrossRef]
- Buchwald, D.S.; Rea, T.D.; Katon, W.J.; Russo, J.E.; Ashley, R.L. Acute Infectious Mononucleosis: Characteristics of Patients Who Report Failure to Recover. Am. J. Med. 2000, 109, 531–537. [Google Scholar] [CrossRef]
- Katz, B.Z.; Shiraishi, Y.; Mears, C.J.; Binns, H.J.; Taylor, R. Chronic Fatigue Syndrome after Infectious Mononucleosis in Adolescents. Pediatrics 2009, 124, 189–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chia, J.; Chia, A.; Voeller, M.; Lee, T.; Chang, R. Acute Enterovirus Infection Followed by Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Viral Persistence. J. Clin. Pathol. 2010, 63, 165–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, A.J.; Hanson, M.R. The Enterovirus Theory of Disease Etiology in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Critical Review. Front. Med. 2021, 8, 688486. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-Acute COVID-19 Syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; for the Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Bateman, L. Will COVID-19 Lead to Myalgic Encephalomyelitis/Chronic Fatigue Syndrome? Front. Med. 2021, 7, 1132. [Google Scholar] [CrossRef] [PubMed]
| Patients (N = 20) | Controls (N = 10) | p-Value 1 | |
|---|---|---|---|
| Age (years; mean ± s.d.) | 47.4 ± 11.6 | 46.8 ± 9.2 | 0.552 |
| Sex (% female) | 65.0% | 60.0% | 0.813 |
| BMI (kg/m2; mean ± s.d.) | 25.4 ± 6.8 | 22.0 ± 3.1 | 0.224 |
| Duration of illness (years; mean ± s.d.) | 14.5 ± 11.8 | 0.0 ± 0.0 | |
| Karnofsky Performance status index (%) | <0.001 | ||
| 30: Severely disabled; hospital admission is indicated, although death is not imminent. | 5.0% | 0.0% | |
| 40: Disabled; requires special care and assistance. | 30.0% | 0.0% | |
| 50: Require considerable assistance and frequent medical care. | 15.0% | 0.0% | |
| 60: Require occasional assistance, but is able to care for most personal needs. | 50.0% | 0.0% | |
| 100. Normal; no complaints; no evidence of disease. | 0.0% | 100.0% | |
| Quality of life (SF-36 scores; mean ± s.d.) | |||
| PF: Physical functioning | 13.3 ± 12.8 | 99.0 ± 2.1 | <0.001 |
| RP: Role limitations due to physical health | 1.9 ± 6.1 | 99.4 ± 2.0 | <0.001 |
| RE: Role limitations due to emotional problems | 55.0 ± 45.9 | 94.2 ± 9.7 | 0.037 |
| VT: Vitality/Energy/Fatigue | 12.8 ± 19.3 | 80.0 ± 14.7 | <0.001 |
| MH: Mental health/Emotional well-being | 56.0 ± 25.8 | 83.5 ± 16.0 | 0.005 |
| SF: Social functioning | 4.4 ± 12.4 | 92.5 ± 13.4 | <0.001 |
| BP: Body pain | 33.4 ± 26.2 | 95.8 ± 7.6 | <0.001 |
| GH: General health | 16.5 ± 7.3 | 83.5 ± 15.1 | <0.001 |
| Patients | Controls | p-Value 1 | |
|---|---|---|---|
| PROMIS Instruments (T-score; mean ± s.d.) | |||
| Fatigue | 75.2 ± 5.9 | 41.8 ± 9.6 | <0.001 |
| Sleep disturbance | 64.5 ± 7.5 | 39.7 ± 7.4 | <0.001 |
| Sleep-related impairment | 65.4 ± 7.4 | 37.5 ± 8.4 | <0.001 |
| Pain interference | 67.0 ± 10.1 | 44.5 ± 4.8 | 0.003 |
| Pain behavior | 60.6 ± 8.9 | 42.4 ± 11.5 | 0.004 |
| Pittsburgh Sleep Quality Index (mean ± s.d.) | |||
| Sleep quality | 2.1 ± 0.7 | 0.0 ± 0.0 | <0.001 |
| Sleep latency | 2.1 ± 1.3 | 1.0 ± 0.8 | 0.093 |
| Sleep duration | 0.4 ± 0.8 | 0.3 ± 0.5 | 0.814 |
| Habitual sleep efficiency | 1.6 ± 1.3 | 0.0 ± 0.0 | 0.019 |
| Sleep disturbances | 1.9 ± 1.0 | 0.3 ± 0.5 | 0.009 |
| Use of sleeping medications | 2.2 ± 1.1 | 0.0 ± 0.0 | <0.001 |
| Daytime dysfunction | 1.9 ± 1.3 | 0.8 ± 0.5 | 0.144 |
| Global PSQI score | 11.9 ± 3.4 | 2.3 ± 1.7 | 0.003 |
| Restless Legs Syndrome (RLS; %) | |||
| Probable RLS | 23.5% (4/17) | 0.0% (0/4) |
| Viruses-Antibody Tests | Patients Positive/Total | Controls Positive/Total | p-Value 1 |
|---|---|---|---|
| Cytomegalovirus (IgG) | 9/18 | 4/9 | 1 |
| Cytomegalovirus (IgM) | 1/18 | 0/9 | 1 |
| Parvovirus B19 (IgG) | 14/18 | 6/9 | 0.653 |
| Parvovirus B19 (IgM) | 0/18 | 0/9 | 1 |
| Epstein-Barr Virus Early Antigen D (IgG) | 2/18 | 2/9 | 0.582 |
| Epstein-Barr Virus Viral Capsid Antigen (IgM) | 0/18 | 0/9 | 1 |
| Epstein-Barr Virus Viral Capsid Antigen (IgG) | 17/18 | 9/9 | 1 |
| Epstein-Barr Virus Nuclear Antigen (IgG) | 16/19 | 8/8 | 0.532 |
| Herpesvirus 6 (IgG) | 19/19 | 9/9 | 1 |
| Herpesvirus 6 (IgM) | 1/18 | 0/9 | 1 |
| Herpesvirus 7 (IgG) | 0/19 | 0/9 | 1 |
| Herpesvirus 7 (IgM) | 0/18 | 0/9 | 1 |
| Herpes Simplex Virus 1 (IgG) | 6/18 | 2/9 | 0.676 |
| Herpes Simplex Virus 2 (IgG) | 6/18 | 1/9 | 0.363 |
| Herpes Simplex Virus 1/2 (IgM) | 2/18 | 1/9 | 1 |
| Bacteria-Antigen and Antibody Tests | Positive/Total | Positive/Total | p-Value 1 |
| Borrelia-Ceres Nanotrap Lyme Antigen Test | 2/18 | 1/10 | 1 |
| Lyme Disease Ab with Reflex to Blot (IgG) | 0/18 | 0/9 | 1 |
| Lyme Disease Ab with Reflex to Blot (IgM) | 0/18 | 0/9 | 1 |
| Borrelia burgdorferi (IgG) | 0/18 | 0/9 | 1 |
| Borrelia burgdorferi (IgM) | 0/18 | 0/9 | 1 |
| Mycoplasma pneumoniae (IgG) | 13/18 | 6/7 | 0.637 |
| Mycoplasma pneumoniae (IgM) | 1/19 | 0/9 | 1 |
| Bartonella DNA-(Blood, Serum, and Culture) | 0/20 | 0/9 | 1 |
| Bartonella henselae (IgG) | 18/20 | 8/9 | 1 |
| Bartonella quintana (IgG) | 17/20 | 7/9 | 0.633 |
| Immunoglobulin G Subclasses Panel | Low/Total | Low/Total | p-Value 1 |
| Immunoglobulin G, subclass 1 | 1/19 | 0/9 | 1 |
| Immunoglobulin G, subclass 2 | 0/19 | 2/9 | 0.095 |
| Immunoglobulin G, subclass 3 | 3/19 | 0/9 | 0.530 |
| Immunoglobulin G, subclass 4 | 1/19 | 2/9 | 0.234 |
| Immunoglobulin G, serum | 0/19 | 0/9 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-J.; Hung, L.-Y.; Kogelnik, A.M.; Kaufman, D.; Aiyar, R.S.; Chu, A.M.; Wilhelmy, J.; Li, P.; Tannenbaum, L.; Xiao, W.; et al. A Comprehensive Examination of Severely Ill ME/CFS Patients. Healthcare 2021, 9, 1290. https://doi.org/10.3390/healthcare9101290
Chang C-J, Hung L-Y, Kogelnik AM, Kaufman D, Aiyar RS, Chu AM, Wilhelmy J, Li P, Tannenbaum L, Xiao W, et al. A Comprehensive Examination of Severely Ill ME/CFS Patients. Healthcare. 2021; 9(10):1290. https://doi.org/10.3390/healthcare9101290
Chicago/Turabian StyleChang, Chia-Jung, Li-Yuan Hung, Andreas M. Kogelnik, David Kaufman, Raeka S. Aiyar, Angela M. Chu, Julie Wilhelmy, Peng Li, Linda Tannenbaum, Wenzhong Xiao, and et al. 2021. "A Comprehensive Examination of Severely Ill ME/CFS Patients" Healthcare 9, no. 10: 1290. https://doi.org/10.3390/healthcare9101290
APA StyleChang, C.-J., Hung, L.-Y., Kogelnik, A. M., Kaufman, D., Aiyar, R. S., Chu, A. M., Wilhelmy, J., Li, P., Tannenbaum, L., Xiao, W., & Davis, R. W. (2021). A Comprehensive Examination of Severely Ill ME/CFS Patients. Healthcare, 9(10), 1290. https://doi.org/10.3390/healthcare9101290






