Ganoderma lucidum Effects on Mood and Health-Related Quality of Life in Women with Fibromyalgia
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Instruments
2.3. Procedure
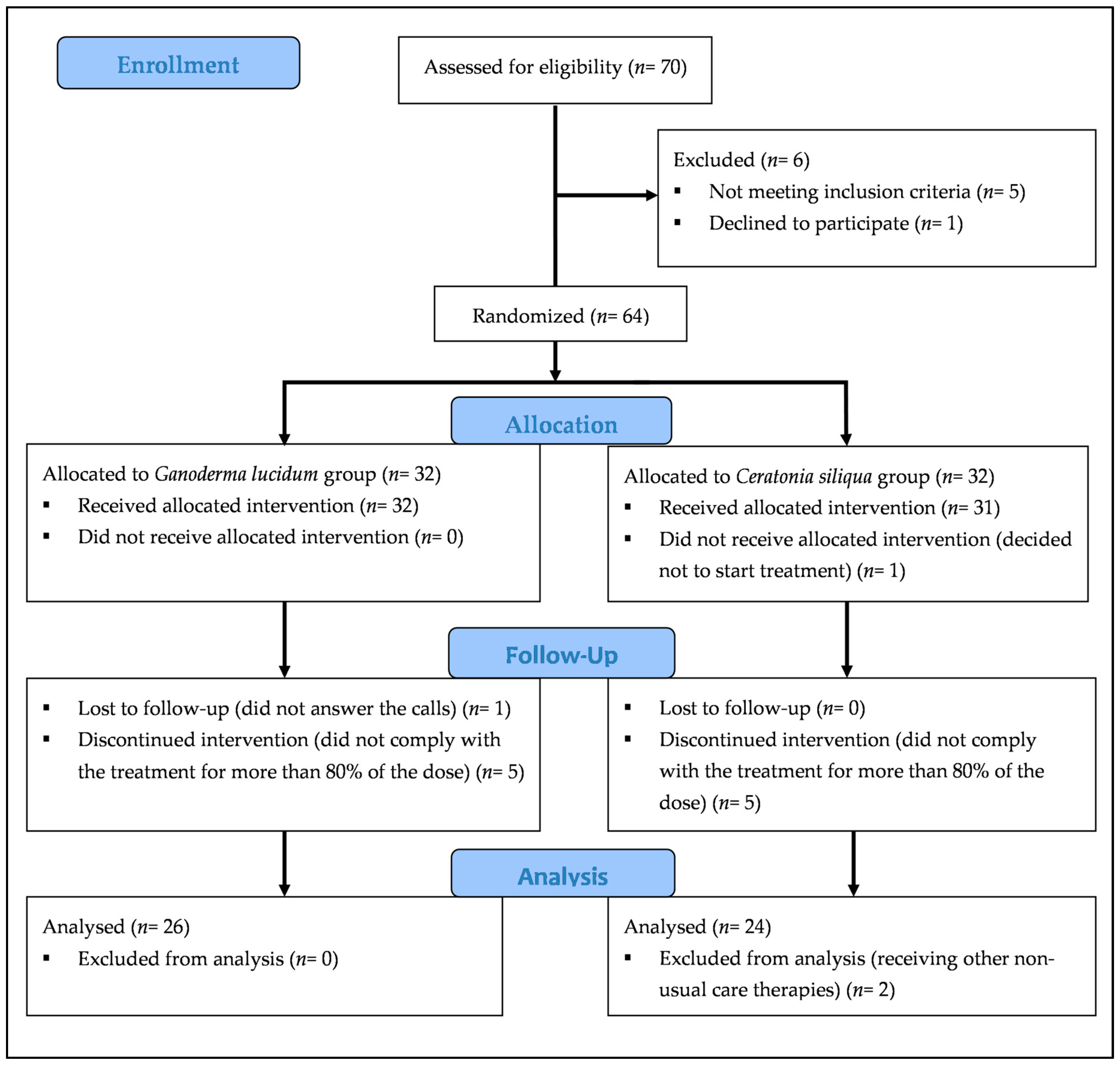
2.4. Sample and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hadlandsmyth, K.; Dailey, D.L.; Rakel, B.A.; Zimmerman, M.B.; Vance, C.G.; Merriwether, E.N.; Chimenti, R.L.; Geasland, K.M.; Crofford, L.J.; A Sluka, K.A. Somatic symptom presentations in women with fibromyalgia are differentially associated with elevated depression and anxiety. J. Health Psychol. 2020, 25, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Moukaddem, A.; Chaaya, M.; Slim, Z.F.N.; Jaffa, M.; Sibai, A.M.; Uthman, I. Fibromyalgia: Epidemiology and risk factors, a population-based case-control study in Lebanon. Int. J. Rheum. Dis. 2017, 20, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Skaer, T.L.; Kwong, W.J. Illness perceptions and burden of disease in fibromyalgia. Expert Rev. Pharm. Outcomes Res. 2017, 17, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Eröksüz, R.; Erol Forestier, F.B.; Karaaslan, F.; Forestier, R.; Işsever, H.; Erdoğan, N.; Karagülle, M.Z.; Donmez, A. Comparison of intermittent and consecutive balneological outpatient treatment (hydrotherapy and peloidotherapy) in fibromyalgia syndrome: A randomized, single-blind, pilot study. Int. J. Biometeorol. 2020, 64, 513–520. [Google Scholar] [CrossRef]
- Prabhakar, A.; Kaiser, J.M.; Novitch, M.B.; Cornett, E.M.; Urman, R.D.; Kaye, A.D. The Role of Complementary and Alternative Medicine Treatments in Fibromyalgia: A Comprehensive Review. Curr. Rheumatol. Rep. 2019, 21, 14. [Google Scholar] [CrossRef]
- Feng, X.; Wang, Y. Anti-inflammatory, anti-nociceptive and sedative-hypnotic activities of lucidone D extracted from Ganoderma lucidum. Cell. Mol. Biol. 2019, 65, 37–42. [Google Scholar] [CrossRef]
- Collado-Mateo, D.; Pazzi, F.; Dominguez-Muñoz, F.J.; Martín-Martínez, J.P.; Olivares, P.R.; Gusi, N.; Adsuar, J.C. Ganoderma Lucidum Improves Physical Fitness in Women with Fibromyalgia. Nutrición Hospitalaria 2015, 32, 2126–2135. [Google Scholar]
- Garcia-Gordillo, M.A.; Collado-Mateo, D.; Hernández-Mocholi, M.A.; Pazzi, F.; Gusi, N.; Dominguez-Muñoz, F.J.; Adsuar, J.C. Cost-Utility Analysis of a Six-Weeks Ganoderma Lucidum-Based Treatment for Women with Fibromyalgia: A Randomized Double-Blind, Active Placebo-Controlled Trial. Myopain 2015, 23, 188–194. [Google Scholar] [CrossRef]
- Ramírez-Tejero, J.A.; Martinez-Lara, E.; Peinado, M.A.; Del Moral, M.L.; Siles, E. Hydroxytyrosol as a Promising Ally in the Treatment of Fibromyalgia. Nutients 2020, 12, 2386. [Google Scholar] [CrossRef]
- Extremera, N.; Fernández-Berrocal, P. The Subjective Happiness Scale: Translation and Preliminary Psychometric Evaluation of a Spanish Version. Soc. Indic. Res. 2013, 119, 473–481. [Google Scholar] [CrossRef]
- Vazquez, C.; Duque, A.; Hervás, G. Satisfaction with Life Scale in a Representative Sample of Spanish Adults: Validation and Normative Data. Span. J. Psychol. 2013, 16, E82. [Google Scholar] [CrossRef] [PubMed]
- Estévez-López, F.; Gray, C.M.; Segura-Jiménez, V.; Soriano-Maldonado, A.; Álvarez-Gallardo, I.C.; Arrayás-Grajera, M.J.; Carbonell-Baeza, A.; Aparicio, V.A.; Delgado-Fernández, M.; Pulido-Martos, M. Independent and combined association of overall physical fitness and subjective well-being with fibromyalgia severity: The al-Ándalus project. Qual. Life Res. 2015, 24, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-San Martín, M.I.; Andrade-Rosa, C.; Molina, J.; Muñoz, P.E.; Carretero, B.; Rodríguez, M.; Silva, A. Validation of the Spanish version of the geriatric depression scale (GDS) in primary care. Int. J. Geriatr. Psychiatry 2002, 17, 279–287. [Google Scholar] [CrossRef]
- Park, D.C.; Glass, J.M.; Minear, M.; Crofford, L.J. Cognitive function in fibromyalgia patients. Arthritis Rheum. 2001, 44, 2125–2133. [Google Scholar] [CrossRef]
- Gandek, B.; Ware, J.E.; Aaronson, N.K.; Apolone, G.; Bjorner, J.B.; Brazier, J.; Bullinger, M.; Kaasa, S.; Leplege, A.; Prieto, L.; et al. Cross-Validation of Item Selection and Scoring for the SF-12 Health Survey in Nine Countries. J. Clin. Epidemiol. 1998, 51, 1171–1178. [Google Scholar] [CrossRef]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretación de los cuestionarios de salud SF-36 y SF-12 en España: Componentes físico y mental. Med. Clín. 2008, 130, 726–735. [Google Scholar] [CrossRef]
- Busner, J.; Targum, S.D. The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 2007, 4, 28–37. [Google Scholar]
- Hudson, J.I.; Arnold, L.M.; Bradley, L.A.; Choy, E.H.S.; Mease, P.J.; Wang, F.; Ahl, J.; Wohlreich, M.M. What Makes Patients with Fibromyalgia Feel Better? Correlations Between Patient Global Impression of Improvement and Changes in Clinical Symptoms and Function: A Pooled Analysis of 4 Randomized Placebo-controlled Trials of Duloxetine. J. Rheumatol. 2009, 36, 2517–2522. [Google Scholar] [CrossRef]
- Soo, T.S. Effective Dosage of the Extract of Ganoderma Lucidum in the Treatment of Various Ailments. In Mushroom Biology and Mushroom Products; Pennsylvania State University: State College, PA, USA, 1996; pp. 177–185. [Google Scholar]
- Herbalist, R.H. Reishi Mushroom. Available online: www.herbal-ahp.org (accessed on 25 November 2020).
- Littlejohn, G.; Guymer, E. Neurogenic inflammation in fibromyalgia. Semin. Immunopathol. 2018, 40, 291–300. [Google Scholar] [CrossRef]
- Chen, X.; Zou, K.; Abdullah, N.; Whiteside, N.; Sarmanova, A.; Doherty, M.; Zhang, W. The placebo effect and its determinants in fibromyalgia: Meta-analysis of randomised controlled trials. Clin. Rheumatol. 2017, 36, 1623–1630. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, Q.; Zhao, L.; Huang, X.; Wang, J.; Kang, X. Spore Powder ofGanoderma lucidumImproves Cancer-Related Fatigue in Breast Cancer Patients Undergoing Endocrine Therapy: A Pilot Clinical Trial. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Gao, Y.; Chen, G.; Gao, H.; Dai, X.; Ye, J.; Chan, E.; Huang, M.; Zhou, S. A Randomized, Double-Blind and Placebo-Controlled Study of a Ganoderma lucidum Polysaccharide Extract in Neurasthenia. J. Med. Food 2005, 8, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-H.; Wang, L.-H.; Wang, C.; Qin, L.-H. Spore powder of Ganoderma lucidum for the treatment of Alzheimer disease. Medicine 2018, 97, e0636. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | GLG (n = 26) a | PG (n = 24) a | p |
|---|---|---|---|
| Age (years) | 56.19 (7.97) | 53.74 (11.50) | 0.382 * |
| Date when fibromyalgia symptoms started | 1994 (11.86) | 1992 (12.56) | 0.601 * |
| Date of diagnosis | 2006 (6.56) | 2003 (7.02) | 0.935 * |
| Height (cm) | 157.08 (4.55) | 156.29 (6.08) | 0.541 * |
| Weight (kg) | 64.26 (9.67) | 61.30 (13.24) | 0.411 * |
| Muscle mass (%) | 61.97 (7.17) | 64.82 (8.58) | 0.245 * |
| Fat mass (%) | 34.81 (7.51) | 32.24 (7.75) | 0.285 * |
| BMI (kg/m2) | 26.05 (3.75) | 25.06 (4.75) | 0.522 * |
| Physical Activity (hours per week) | 2.96 (1.88) | 2.32 (1.43) | 0.203 * |
| Income (euros). GLG: n = 23; PG: n = 19 | 1624 (895) | 1573 (853) | 0.849 * |
| Type of treatment n (%) b | |||
| Number of participants with nonpharmacological treatment | 20 (76.9) | 18 (75) | 0.874 ** |
| Number of participants without nonpharmacological treatment | 6 (23.1) | 6 (25) | |
| Number of participants with pharmacological treatment | 13 (50) | 12 (50) | 1.000 ** |
| Number of participants without pharmacological treatment | 13 (50) | 12 (50) | |
| Educational Qualifications (%) b | |||
| No education (able to read and write) | 4 (15.4) | 2 (8.3) | 0.048 ** |
| Elementary school | 13 (50) | 7 (29.2) | |
| Secondary school | 4 (15.4) | 13 (54.2) | |
| University diploma | 3 (11.5) | 1 (4.2) | |
| University degree | 2 (7.7) | 0 (0) | |
| PhD | 0 (0) | 1 (4.2) | |
| Occupational status n (%) b | |||
| self-employed | 2 (7.7) | 0 (0) | 0.167 ** |
| paid employment | 3 (11.5) | 7 (29.2) | |
| Civil servant | 6 (23.1) | 2 (8.3) | |
| Unemployed | 6 (23.1) | 2 (8.3) | |
| Retired | 3 (11.5) | 5 (20.8) | |
| Housewife | 6 (23.1) | 7 (29.2) | |
| Student | 0 (0) | 1 (4.2) | |
| Outcome Measurements | Baseline (Mean ± SD) | After 6 Weeks’ Treatment (Mean ± SD) | p * | Treatment Effect Mean (95% CI) | p ** |
|---|---|---|---|---|---|
| Analysis of the participants who completed the study | |||||
| SHS | |||||
| GLG (n = 26) | 3.83 ± 1.57 | 4.67 ± 1.44 | 0.009 | 0.66 (from 0.01 to 1.32) | 0.048 |
| PG (n = 23) | 4.55 ± 1.10 | 4.74 ± 0.93 | 0.428 | ||
| SWLS | |||||
| GLG (n = 26) | 16.58 ± 7.28 | 19.27 ± 7.17 | 0.003 | 2.69 (from −0.46 to 5.85) | 0.092 |
| PG (n = 23) | 19.13 ± 7.34 | 19.13 ± 7.31 | 0.326 | ||
| GDS | |||||
| GLG (n = 25) | 7.60 ± 3.39 | 5.36 ± 3.94 | 0.001 | −1.51 (from −3.51 to 0.48) | 0.134 |
| PG (n = 22) | 6.55 ± 3.12 | 5.81 ± 3.74 | 0.379 | ||
| GIIS | |||||
| GLG (n = 26) | NA | 2.54 ± 1.45 | NA | NA | 0.037 |
| PG (n = 24) | NA | 3.46 ± 1.59 | |||
| Intent-to-treat analysis (n = 64; GLG = 32; PG = 32) | |||||
| GHS | |||||
| GHS | 4.00 ± 1.64 | 4.69 ± 1.45 | 0.007 | 0.52 (from −0.048 to 1.09) | 0.072 |
| GLG | 4.43 ± 1.25 | 4.59 ± 1.14 | 0.305 | ||
| SLS | |||||
| SLS | 17.22 ± 7.17 | 19.60 ± 7.35 | 0.003 | 3.35 (from 0.62 to 6.09) | 0.017 |
| GLG | 19.00 ± 6.80 | 18.03 ± 6.90 | 0.326 | ||
| GDS | |||||
| GDS | 7.25 ± 3.45 | 5.60 ± 4.14 | 0.007 | −0.93 (from −3.67 to 0.23) | 0.082 |
| GLG | 6.31 ± 3.01 | 6.38 ± 4.14 | 0.927 | ||
| Outcome measurements | Baseline (Mean ± SD) | After 6 Weeks’ Treatment (Mean ± SD) | p * | Treatment Effect. Mean (95% CI) | p ** |
|---|---|---|---|---|---|
| SF12v2 (n = 50; GLG = 26; PG = 24) | |||||
| Physical Function | |||||
| GLG | 39.42 ± 37.53 | 47.12 ± 31.09 | 0.175 | 5.61 (from −10.48 to 21.70) | 0.487 |
| PG | 41.67 ± 24.08 | 43.75 ± 35.55 | 0.723 | ||
| Physical Role | |||||
| GLG | 52.88 ± 31.29 | 62.50 ± 28.06 | 0.106 | 1.8 (from −14.76 to 18.37) | 0.828 |
| PG | 46.35 ± 22.57 | 54.17 ± 27.00 | 0.200 | ||
| Bodily Pain | |||||
| GLG | 40.38 ± 39.42 | 56.73 ± 29.63 | 0.047 | 10.10 (from −9.64 to 29.84) | 0.309 |
| PG | 40.63 ± 31.98 | 46.88 ± 25.87 | 0.283 | ||
| General Health | |||||
| GLG | 17.50 ± 22.55 | 30.00 ± 25.22 | 0.015 | 4.58 (from −9.13 to 18.29) | 0.505 |
| PG | 27.08 ± 22.98 | 35.00 ± 21.37 | 0.118 | ||
| Vitality | |||||
| GLG | 30.77 ± 31.07 | 38.46 ± 32.58 | 0.319 | −7.93 (from −26.88 to 11.02) | 0.404 |
| PG | 26.04 ± 23.86 | 41.67 ± 20.41 | 0.008 | ||
| Social Functioning | |||||
| GLG | 51.92 ± 37.36 | 72.11 ± 38.94 | 0.030 | 13.94 (from −10.23 to 38.11) | 0.252 |
| PG | 56.25 ± 37.77 | 62.50 ± 36.11 | 0.450 | ||
| Emotional Role | |||||
| GLG | 61.06 ± 28.79 | 75.48 ± 26.81 | 0.033 | 5.57 (from −12.52 to 23.66) | 0.539 |
| PG | 59.90 ± 27.82 | 68.75 ± 26.58 | 0.174 | ||
| Mental Health | |||||
| GLG | 39.90 ± 25.25 | 59.61 ± 25.32 | 0.001 | 6.17 (from −7.61 to 19.95) | 0.373 |
| PG | 47.91 ± 19.39 | 61.46 ± 17.64 | 0.008 | ||
| Standardized Physical Component | |||||
| GLG | 34.31 ± 9.89 | 37.31 ± 10.70 | 0.104 | 2.24 (from −2.88 to 7.37) | 0.383 |
| PG | 34.55 ± 7.48 | 35.31 ± 9.21 | 0.681 | ||
| Standardized Mental Component | |||||
| GLG | 39.46 ± 10.57 | 46.33 ± 13.20 | 0.011 | −0.06 (from −6.66 to 6.65) | 0.987 |
| PG | 39.83 ± 9.65 | 46.76 ± 9.54 | 0.003 | ||
| Group | GLG (n = 5) | PG (n = 5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Participants | P1 | P2 | P3 | P4 | P5 | P1 | P2 | P3 | P4 | P5 |
| Ingested doses before withdrawal | 30 | 30 | 50 | 52 | 58 | 3 | 4 | 26 | 32 | 67 |
| Symptoms | ||||||||||
| Stomach problems (pain, acidity, burning, cramp and not specified discomfort) | x | x | x | x | x | x | x | x | x | |
| Nausea and vomiting | x | x | x | x | x | x | x | |||
| Diarrhea | x | x | x | |||||||
| Dyspepsia | x | |||||||||
| Meteorism | x | x | ||||||||
| Agitation | x | |||||||||
| Dehydration and swelling | x | |||||||||
| Headache | x | |||||||||
| Hypertension | x | |||||||||
| Itching and irritation | x | |||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pazzi, F.; Adsuar, J.C.; Domínguez-Muñoz, F.J.; García-Gordillo, M.A.; Gusi, N.; Collado-Mateo, D. Ganoderma lucidum Effects on Mood and Health-Related Quality of Life in Women with Fibromyalgia. Healthcare 2020, 8, 520. https://doi.org/10.3390/healthcare8040520
Pazzi F, Adsuar JC, Domínguez-Muñoz FJ, García-Gordillo MA, Gusi N, Collado-Mateo D. Ganoderma lucidum Effects on Mood and Health-Related Quality of Life in Women with Fibromyalgia. Healthcare. 2020; 8(4):520. https://doi.org/10.3390/healthcare8040520
Chicago/Turabian StylePazzi, Francesco, José Carmelo Adsuar, Francisco Javier Domínguez-Muñoz, Miguel Angel García-Gordillo, Narcis Gusi, and Daniel Collado-Mateo. 2020. "Ganoderma lucidum Effects on Mood and Health-Related Quality of Life in Women with Fibromyalgia" Healthcare 8, no. 4: 520. https://doi.org/10.3390/healthcare8040520
APA StylePazzi, F., Adsuar, J. C., Domínguez-Muñoz, F. J., García-Gordillo, M. A., Gusi, N., & Collado-Mateo, D. (2020). Ganoderma lucidum Effects on Mood and Health-Related Quality of Life in Women with Fibromyalgia. Healthcare, 8(4), 520. https://doi.org/10.3390/healthcare8040520










