Risk Factors for Dementia Incidence Based on Previous Results of the Specific Health Checkups in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alladi, S.; Hachinski, V. World dementia: One approach does not fit all. Neurology 2018, 91, 264–270. [Google Scholar] [CrossRef]
- World Health Organization; Alzheimer’s Disease International. Dementia: A Public Health Priority; WHO Press: Geneva, Switzerland, 2012. [Google Scholar]
- UK to host G8 dementia summit. Clin. Pharm. 2013. [CrossRef]
- Ninomiya, T. General Research Report to the Future Estimation of the Elderly Population of the Dementia of a Japanese. Available online: https://mhlw-grants.niph.go.jp/niph/search/NIDD00.do?resrchNum=201405037A (accessed on 14 October 2020).
- Keang, L.T.; Feng, L.; Nyunt, M.S.; Feng, L.; Gao, Q.; Lim, M.L.; Collinson, S.L.; Chong, M.S.; Lim, W.S.; Lee, T.-S.; et al. Metabolic Syndrome and the Risk of Mild Cognitive Impairment and Progression to Dementia. JAMA Neurol. 2016, 73, 456–463. [Google Scholar] [CrossRef]
- Biessels, G.J.; Staekenborg, S.; Brunner, E.; Brayne, C.; Scheltens, P. Risk of dementia in diabetes mellitus: A systematic review. Lancet Neurol. 2006, 5, 64–74. [Google Scholar] [CrossRef]
- Katon, W.; Lin, E.H.B.; Williams, L.H.; Ciechanowski, P.; Heckbert, S.R.; Ludman, E.; Rutter, C.; Crane, P.K.; Oliver, M.; Von Korff, M. Comorbid Depression is Associated with an Increased Risk of Dementia Diagnosis in Patients with Diabetes: A Prospective Cohort Study. J. Gen. Intern. Med. 2010, 25, 423–429. [Google Scholar] [CrossRef]
- Ma, F.; Wu, T.; Miao, R.; Xiao, Y.Y.; Zhang, W.; Huang, G. Conversion of Mild Cognitive Impairment to Dementia among Subjects with Diabetes: A Population-Based Study of Incidence and Risk Factors with Five Years of Follow-up. J. Alzheimer’s Dis. 2014, 43, 1441–1449. [Google Scholar] [CrossRef]
- Ninomiya, T. Epidemiological Evidence of the Relationship Between Diabetes and Dementia. Single Mol. Single Cell Seq. 2019, 1128, 13–25. [Google Scholar] [CrossRef]
- Bello-Chavolla, O.Y.; Antonio-Villa, N.E.; Vargas-Vázquez, A.; Ávila-Funes, J.A.; Aguilar-Salinas, C.A. Pathophysiological Mechanisms Linking Type 2 Diabetes and Dementia: Review of Evidence from Clinical, Translational and Epidemiological Research. Curr. Diabetes Rev. 2019, 15, 456–470. [Google Scholar] [CrossRef]
- Yokomichi, H.; Nagai, A.; Hirata, M.; Kiyohara, Y.; Muto, K.; Ninomiya, T.; Matsuda, K.; Kamatani, Y.; Tamakoshi, A.; Kubo, M.; et al. Serum glucose, cholesterol and blood pressure levels in Japanese type 1 and 2 diabetic patients: BioBank Japan. J. Epidemiol. 2017, 27, S92–S97. [Google Scholar] [CrossRef]
- Albanese, E.; Launer, L.J.; Egger, M.; Prince, M.J.; Giannakopoulos, P.; Wolters, F.J.; Egan, K. Body mass index in midlife and dementia: Systematic review and meta-regression analysis of 589,649 men and women followed in longitudinal studies. Alzheimer’s Dementia: Diagn. Assess. Dis. Monit. 2017, 8, 165–178. [Google Scholar] [CrossRef]
- Pedditizi, E.; Peters, R.; Beckett, N. The risk of overweight/obesity in mid-life and late life for the development of dementia: A systematic review and meta-analysis of longitudinal studies. Age Ageing 2016, 45, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Masaki, K.; Xue, Q.-L.; Peila, R.; Petrovitch, H.; White, L.R.; Launer, L.J. A 32-Year Prospective Study of Change in Body Weight and Incident Dementia. Arch. Neurol. 2005, 62, 55–60. [Google Scholar] [CrossRef]
- Buchman, A.S.; Schneider, J.A.; Wilson, R.S.; Bienias, J.L.; Bennett, D.A. Body mass index in older persons is associated with Alzheimer disease pathology. Neurology 2006, 67, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.K.; Wilkins, C.H.; Morris, J.C. Accelerated Weight Loss May Precede Diagnosis in Alzheimer Disease. Arch. Neurol. 2006, 63, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- van der Burg, J.M.; Pijl, H.; Campman, Y.J.; Roos, R.A.; Aziz, N.A. Does midlife obesity really lower dementia risk? Lancet Diabetes Endocrinol. 2015, 3, 499–500. [Google Scholar] [CrossRef]
- Yokomichi, H.; Kondo, K.; Nagamine, Y.; Yamagata, Z.; Kondo, N. Dementia risk by combinations of metabolic diseases and body mass index: Japan Gerontological Evaluation Study Cohort Study. J. Diabetes Investig. 2019, 11, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, Y.; Okamoto, E.; Hiratsuka, Y.; Kumakawa, T. Influence of Specific Health Guidance on the Consultation Rate of Metabolic-Related Diseases. Adv. Public Health 2019, 2019, 9735127. [Google Scholar] [CrossRef]
- Japanese Ministry of Health, Labour and Welfare. Long-Term Care, Health and Welfare Services for the Elderly. Available online: http://www.mhlw.go.jp/english/policy/care-welfare/care-welfare-elderly/ (accessed on 14 October 2020).
- Japanese Ministry of Health, Labour and Welfare. “Long-Term Care Insurance Business Situation,”. Available online: https://www.mhlw.go.jp/topics/kaigo/toukei/joukyou.html (accessed on 14 October 2020).
- Tamaki, Y.; Hiratsuka, Y.; Kumakawa, T.; Miura, H. Relationship between the Necessary Support Level for Oral Hygiene and Performance of Physical, Daily Activity, and Cognitive Functions. Int. J. Dent. 2018, 2018, 1542713. [Google Scholar] [CrossRef] [PubMed]
- Japanese Ministry of Health, Labour and Welfare. Ethical Guidelines for Epidemiological Research. Ministry of Education, Culture, Sports, Science and Technology. Available online: http://www.lifescience.mext.go.jp/files/pdf/n796_01.pdf (accessed on 14 October 2020).
- Japanese Ministry of Health, Labour and Welfare. Guideline for Provision of Database for National Health Insurance Claim and the Specific Medical Checkup and Specific Health Guidance. Available online: http://www.mhlw.go.jp/file/05-Shingikai-12401000-Hokenkyoku-Soumuka/0000064238_3.pdf (accessed on 14 October 2020).
- Japanese Ministry of Health, Labour and Welfare. Security Guidelines for Health Information Systems. Available online: http://www.mhlw.go.jp/file/05-Shingikai-12601000-Seisakutoukatsukan-Sanjikanshitsu_Shakaihoshoutantou/0000166260.pdf (accessed on 14 October 2020).
- Okamoto, E. Cost-Benefit of Health Promotion: Will it Pay Off? Japan’s Venture Against Metabolic Syndrome. In Asian Perspectives and Evidence on Health Promotion and Education; Springer: Tokyo, Japan, 2011; pp. 182–195. [Google Scholar] [CrossRef]
- Japanese Ministry of Health, Labour and Welfare. Estimated Medical Cost Database. Available online: http://www.mhlw.go.jp/bunya/iryouhoken/iryouhoken03/01.html (accessed on 14 October 2020).
- Qizilbash, N.; Gregson, J.; E Johnson, M.; Pearce, N.; Douglas, I.J.; Wing, K.; Evans, S.J.W.; Pocock, S.J. BMI and risk of dementia in two million people over two decades: A retrospective cohort study. Lancet Diabetes Endocrinol. 2015, 3, 431–436. [Google Scholar] [CrossRef]
- Nam, G.E.; Park, Y.G.; Han, K.; Kim, M.K.; Koh, E.S.; Kim, E.S.; Lee, M.-K.; Kim, B.; Hong, O.-K.; Kwon, H.-S. BMI, Weight Change, and Dementia Risk in Patients With New-Onset Type 2 Diabetes: A Nationwide Cohort Study. Diabetes Care 2019, 42, 1217–1224. [Google Scholar] [CrossRef]
- Kivimäki, M.; Luukkonen, R.; Batty, G.D.; Ferrie, J.E.; Pentti, J.; Nyberg, S.T.; Shipley, M.J.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; et al. Body mass index and risk of dementia: Analysis of individual-level data from 1.3 million individuals. Alzheimer’s Dement. 2018, 14, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, N.; Hata, J.; Ohara, T.; Mukai, N.; Nagata, M.; Shibata, M.; Gotoh, S.; Furuta, Y.; Yamashita, F.; Yoshihara, K.; et al. Association Between Diabetes and Hippocampal Atrophy in Elderly Japanese: The Hisayama Study. Diabetes Care 2016, 39, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Sasaki, K.; Tanizaki, Y.; Hata, J.; Fujimi, K.; Matsui, Y.; Sekita, A.; Suzuki, S.O.; Kanba, S.; Kiyohara, Y.; et al. Insulin resistance is associated with the pathology of Alzheimer disease: The Hisayama Study. Neurology 2010, 75, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Doi, Y.; Ninomiya, T.; Hirakawa, Y.; Hata, J.; Iwaki, T.; Kanba, S.; Kiyohara, Y. Glucose tolerance status and risk of dementia in the community: The Hisayama Study. Neurology 2011, 77, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
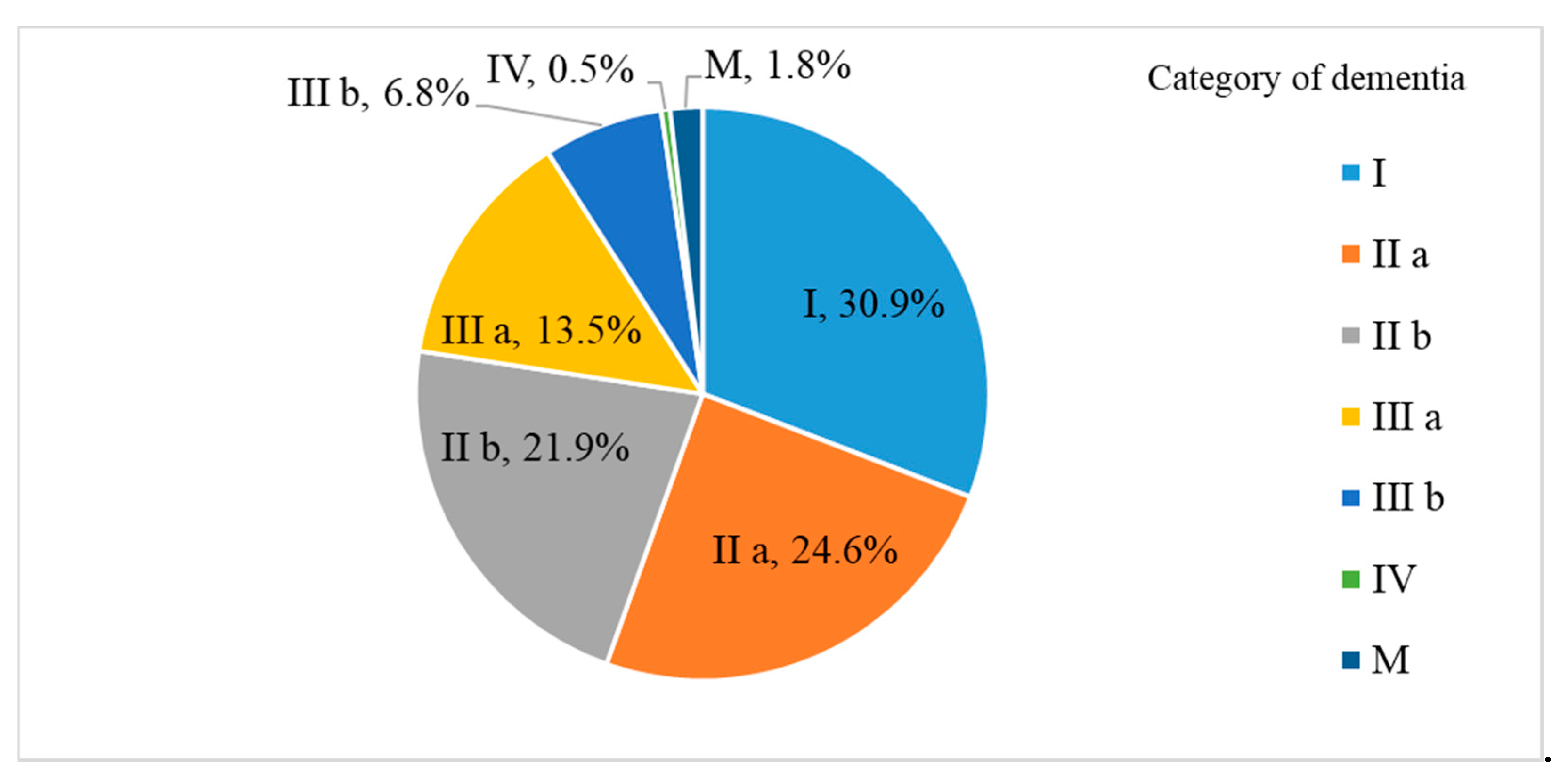
| Category | Degree of Independence in Daily Living |
|---|---|
| Ⅰ | Can do on their own |
| Ⅱa | Monitoring needed only outside home |
| Ⅱb | Monitoring needed inside and outside home |
| Ⅲa | Need support during day |
| Ⅲb | Need support during night |
| IV | Need support during day and night |
| M | Need support in nursing home |
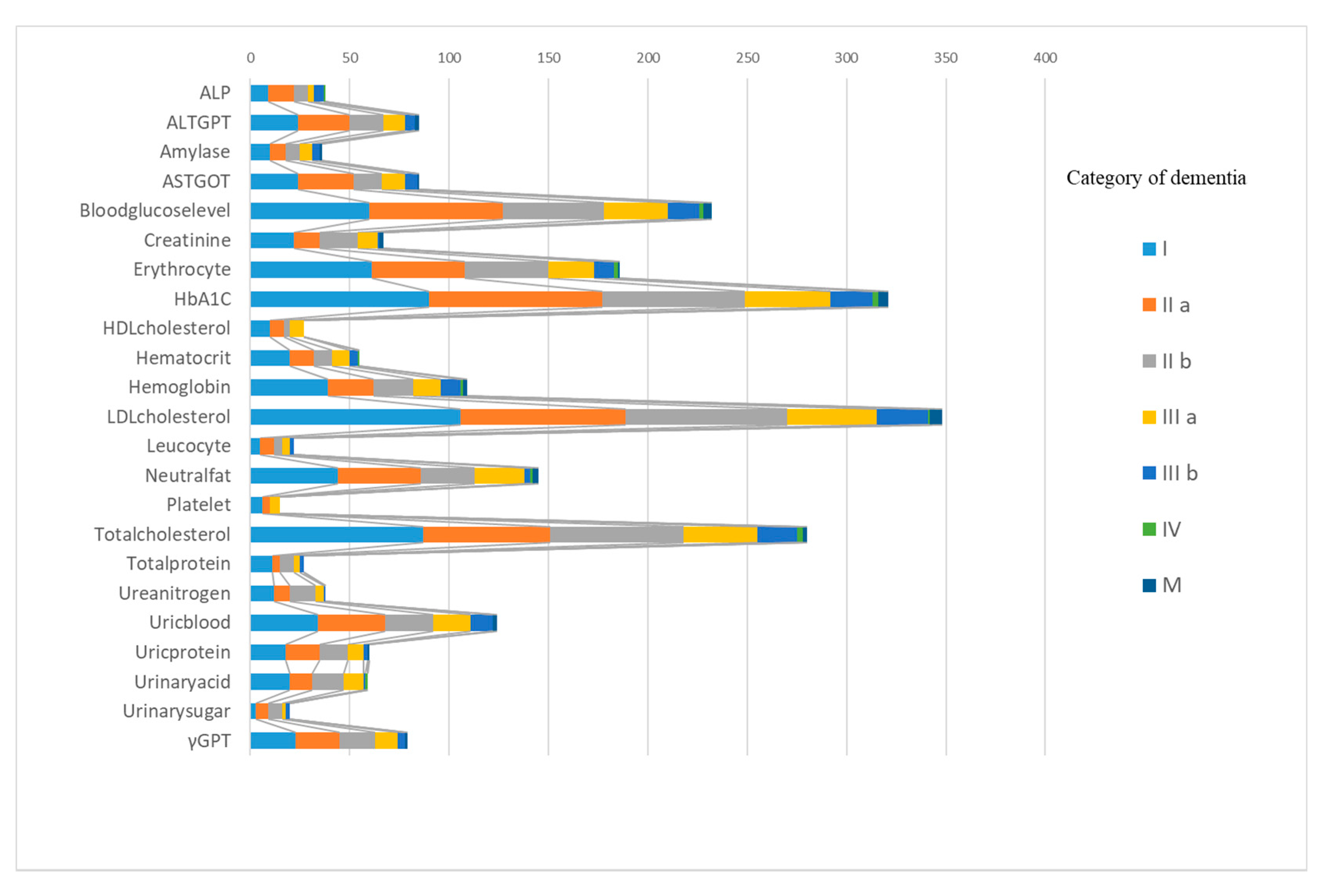
| Item | Normal | Follow-Up | Requires Further Testing | Requires Treatment | Total |
|---|---|---|---|---|---|
| ALP | 568 | 38 | 0 | 0 | 606 |
| 93.7% | 6.3% | 0.0% | 0.0% | 100.0% | |
| ALT(GPT) | 521 | 68 | 0 | 17 | 606 |
| 86.0% | 11.2% | 0.0% | 2.8% | 100.0% | |
| Amylase | 570 | 36 | 0 | 0 | 606 |
| 94.1% | 5.9% | 0.0% | 0.0% | 100.0% | |
| AST(GOT) | 521 | 71 | 0 | 14 | 606 |
| 86.0% | 11.7% | 0.0% | 2.3% | 100.0% | |
| Blood glucose level | 374 | 161 | 0 | 71 | 606 |
| 61.7% | 26.6% | 0.0% | 11.7% | 100.0% | |
| Creatinine | 539 | 67 | 0 | 0 | 606 |
| 88.9% | 11.1% | 0.0% | 0.0% | 100.0% | |
| Erythrocyte | 420 | 165 | 0 | 21 | 606 |
| 69.3% | 27.2% | 0.0% | 3.5% | 100.0% | |
| HbA1C | 285 | 237 | 0 | 84 | 606 |
| 47.0% | 39.1% | 0.0% | 13.9% | 100.0% | |
| HDL-cholesterol | 579 | 20 | 0 | 7 | 606 |
| 95.5% | 3.3% | 0.0% | 1.2% | 100.0% | |
| Hematocrit | 550 | 50 | 0 | 5 | 606 |
| 90.9% | 8.3% | 0.0% | 0.8% | 100.0% | |
| Hemoglobin | 496 | 82 | 0 | 27 | 606 |
| 82.0% | 13.5% | 0.0% | 4.5% | 100.0% | |
| LDL-cholesterol | 258 | 348 | 0 | 0 | 606 |
| 42.6% | 57.4% | 0.0% | 0.0% | 100.0% | |
| Leucocyte | 584 | 22 | 0 | 0 | 606 |
| 96.4% | 3.6% | 0.0% | 0.0% | 100.0% | |
| Neutral fat | 461 | 126 | 0 | 19 | 606 |
| 76.1% | 20.8% | 0.0% | 3.1% | 100.0% | |
| Platelet | 590 | 15 | 0 | 0 | 606 |
| 97.5% | 2.5% | 0.0% | 0.0% | 100.0% | |
| Total protein | 579 | 27 | 0 | 0 | 606 |
| 95.5% | 4.5% | 0.0% | 0.0% | 100.0% | |
| Total-cholesterol | 326 | 237 | 0 | 0 | 606 |
| 53.8% | 39.1% | 0.0% | 0.0% | 100.0% | |
| Urea nitrogen | 568 | 38 | 0 | 0 | 606 |
| 93.7% | 6.3% | 0.0% | 0.0% | 100.0% | |
| Uric blood | 482 | 14 | 92 | 18 | 606 |
| 79.5% | 2.3% | 15.2% | 3.0% | 100.0% | |
| Uric protein | 546 | 3 | 38 | 19 | 606 |
| 90.1% | 0.5% | 6.3% | 3.1% | 100.0% | |
| Urinary acid | 547 | 59 | 0 | 0 | 606 |
| 90.3% | 9.7% | 0.0% | 0.0% | 100.0% | |
| Urinary sugar | 586 | 0 | 6 | 14 | 606 |
| 96.7% | 0.0% | 1.0% | 2.3% | 100.0% | |
| γGTP | 527 | 50 | 0 | 29 | 606 |
| 87.0% | 8.3% | 0.0% | 4.8% | 100.0% |
| Item | Multivariate Adjusted Odds Ratio | 95% CI | p-Value | |
|---|---|---|---|---|
| Lower Limit | Upper Limit | |||
| Age | 1.038 | 0.985 | 1.095 | 0.161 |
| Sex (Women/Men) | 0.975 | 0.488 | 1.948 | 0.942 |
| Height (cm) | 0.992 | 0.955 | 1.032 | 0.698 |
| Abdominal circumference (cm) | 0.961 | 0.927 | 0.997 | 0.032 |
| BMI | 1.039 | 0.934 | 1.156 | 0.484 |
| Medicine to lower blood pressure (Yes/No) | 0.907 | 0.581 | 1.416 | 0.669 |
| Insulin injections or oral hypoglycemic medications (Yes/No) | 2.635 | 1.294 | 5.365 | 0.008 |
| Medicine to lower cholesterol (Yes/No) | 1.001 | 0.630 | 1.590 | 0.998 |
| Systolic blood pressure (mmHg) | 1.007 | 0.990 | 1.025 | 0.396 |
| Diastolic blood pressure (mmHg) | 0.973 | 0.946 | 1.001 | 0.063 |
| ALP (+±/−) | 1.122 | 0.482 | 2.609 | 0.790 |
| ALT(GPT) (+±/−) | 1.082 | 0.621 | 1.884 | 0.781 |
| Amylase (+±/−) | 1.482 | 0.664 | 3.308 | 0.337 |
| AST(GOT) (+±/−) | 0.883 | 0.473 | 1.646 | 0.695 |
| Blood glucose level (+±/−) | 0.958 | 0.715 | 1.285 | 0.776 |
| Creatinine (+±/−) | 0.854 | 0.418 | 1.747 | 0.666 |
| Erythrocyte (+±/−) | 0.922 | 0.655 | 1.298 | 0.640 |
| HbA1C (+±/−) | 0.857 | 0.631 | 1.164 | 0.322 |
| HDL-cholesterol (+±/−) | 1.363 | 0.797 | 2.331 | 0.257 |
| Hematocrit (+±/−) | 1.467 | 0.763 | 2.821 | 0.250 |
| Hemoglobin (+±/−) | 0.893 | 0.618 | 1.289 | 0.545 |
| LDL-cholesterol (+±/−) | 1.086 | 0.669 | 1.761 | 0.739 |
| Leucocyte (+±/−) | 1.222 | 0.438 | 3.405 | 0.702 |
| Neutral fat (+±/−) | 1.150 | 0.795 | 1.664 | 0.458 |
| Platelet (+±/−) | 1.554 | 0.499 | 4.837 | 0.446 |
| Total protein (+±/−) | 0.787 | 0.277 | 2.236 | 0.653 |
| Total-cholesterol (+±/−) | 0.964 | 0.716 | 1.298 | 0.807 |
| Urea nitrogen (+±/−) | 0.549 | 0.193 | 1.561 | 0.261 |
| Uric blood (+±/−) | 1.130 | 0.897 | 1.423 | 0.301 |
| Uric protein (+±/−) | 0.940 | 0.684 | 1.290 | 0.701 |
| Urinary acid (+±/−) | 1.089 | 0.503 | 2.362 | 0.828 |
| Urinary sugar (+±/−) | 0.973 | 0.600 | 1.579 | 0.913 |
| γGPT (+±/−) | 1.048 | 0.725 | 1.515 | 0.804 |
| Outpatient Medical Expenditures in 2008 | 1.000 | 1.000 | 1.000 | 0.609 |
| _cons | 1.087 | 0.985 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamaki, Y.; Hiratsuka, Y.; Kumakawa, T. Risk Factors for Dementia Incidence Based on Previous Results of the Specific Health Checkups in Japan. Healthcare 2020, 8, 491. https://doi.org/10.3390/healthcare8040491
Tamaki Y, Hiratsuka Y, Kumakawa T. Risk Factors for Dementia Incidence Based on Previous Results of the Specific Health Checkups in Japan. Healthcare. 2020; 8(4):491. https://doi.org/10.3390/healthcare8040491
Chicago/Turabian StyleTamaki, Yoh, Yoshimune Hiratsuka, and Toshiro Kumakawa. 2020. "Risk Factors for Dementia Incidence Based on Previous Results of the Specific Health Checkups in Japan" Healthcare 8, no. 4: 491. https://doi.org/10.3390/healthcare8040491
APA StyleTamaki, Y., Hiratsuka, Y., & Kumakawa, T. (2020). Risk Factors for Dementia Incidence Based on Previous Results of the Specific Health Checkups in Japan. Healthcare, 8(4), 491. https://doi.org/10.3390/healthcare8040491





