Effects of Handgrip Strength on 10-Year Cardiovascular Risk among the Korean Middle-Aged Population: The Korea National Health and Nutrition Examination Survey 2014
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
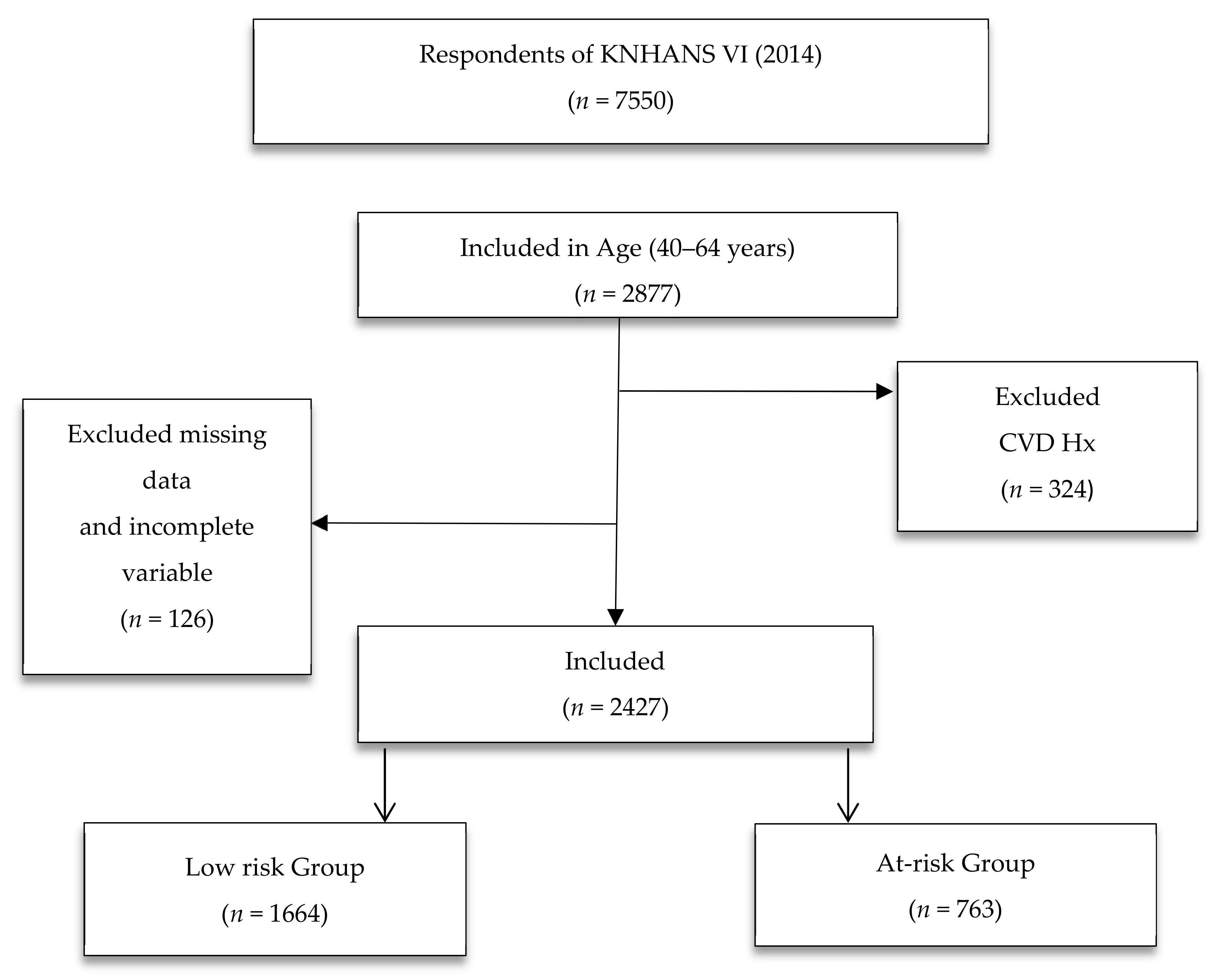
2.2. Setting and Sample
2.3. Measurements
2.3.1. The FRS
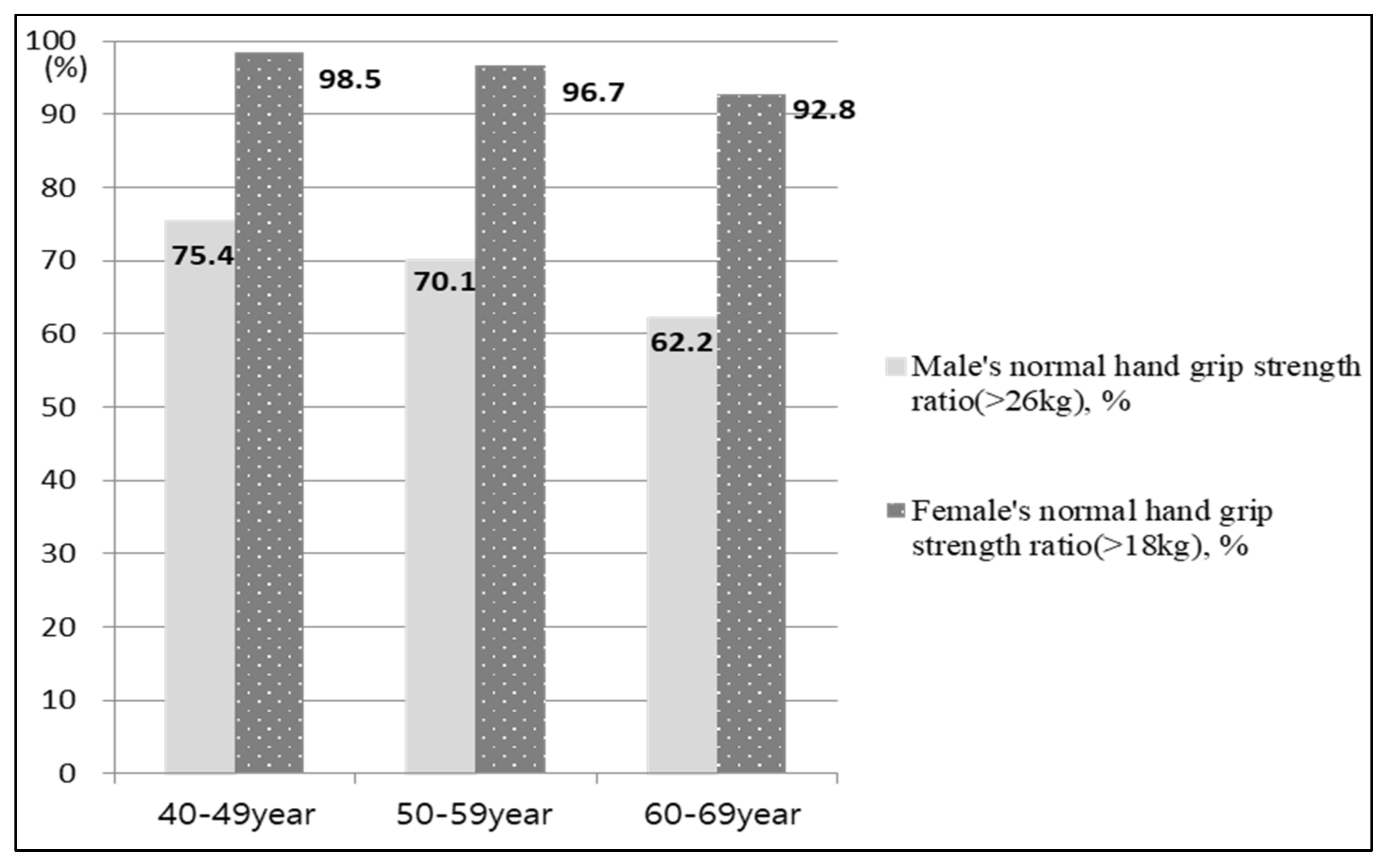
2.3.2. HGS
2.3.3. Level of Physical Activity Assessment
2.3.4. Central Obesity
2.4. Analysis
3. Results
3.1. General Characteristics of Study Participants
3.2. Differences in HGS and CVD Risk Factors between the Two Groups
3.3. Factors Influencing the 10-Year Risk of CVD
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Statistics Korea. The Statistical Result about Cause of Death at 2019. 2019. Available online: http://www.index.go.kr/potal/main/EachDtlPageDetail.do?idx_cd=1012 (accessed on 3 April 2020).
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef]
- Ata, A.M.; Kara, M.; Kaymak, B.; Gürçay, E.; Çakır, B.; Unlu, H.; Akıncı, A.; Özçakar, L. Regional and total muscle mass, muscle strength and physical performance: The potential use of ultrasound imaging for sarcopenia. Arch. Gerontol. Geriatr. 2019, 83, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Beyer, S.E.; Sanghvi, M.M.; Aung, N.; Hosking, A.; Cooper, J.A.; Paiva, J.M.; Lee, A.M.; Fung, K.; Lukaschuk, E.; Carapella, V.; et al. Prospective association between handgrip strength and cardiac structure and function in UK adults. PLoS ONE 2018, 13, e193124. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Patel, T.; Costa, A.; Bryce, E.; Hillier, L.M.; Slonim, K. Screening for frailty in primary care: Accuracy of gait speed and hand-grip strength. Can. Fam. Physician. 2017, 63, e51–e57. [Google Scholar]
- Van Schooten, K.S.; Pijnappels, M.; Rispens, S.M.; Elders, P.J.M.; Lips, P.; van Dieen, J.H. Ambulatory fall-risk assessment: Amount and quality of daily-life gait predict falls in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wu, Y.; Li, W.; Li, S.; Sun, Y.; Li, S. Weak grip strength and cognition predict functional limitation in older Europeans. J. Am. Geriatr. Soc. 2019, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Daphnee, D.K.; John, S.; Vaidya, A.; Khakhar, A.; Bhuvaneshwari, S.; Ramamurthy, A. Hand grip strength: A reliable, reproducible, cost-effective tool to assess the nutritional status and outcomes of cirrhotics awaiting liver transplant. Clin. Nutr. ESPEN 2017, 19, 49–53. [Google Scholar] [CrossRef]
- Cetinus, E.; Buyukbese, M.A.; Uzel, M.; Ekerbicer, H.; Karaoguz, A. Hand grip strength in patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2005, 70, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fu, L.; Jia, L.; Han, P.; Kang, L.; Yu, H.; Chen, X.; Yu, X.; Hou, L.; Wang, L.; et al. Muscle strength rather than muscle mass is associated with osteoporosis in older Chinese adults. J. Formos. Med. Assoc. 2018, 117, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, R.; Ninomiya, D.; Kasai, Y.; Kusunoki, T.; Ohtsuka, N.; Kumagi, T.; Abe, M. Handgrip strength is associated with metabolic syndrome among middle-aged and elderly community-dwelling persons. Clin. Exp. Hypertens. 2016, 38, 245–251. [Google Scholar] [CrossRef]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R.; et al. Prognostic value of grip strength: Findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef]
- Kishimoto, H.; Hata, J.; Ninomiya, T.; Nemeth, H.; Hirakawa, Y.; Yoshida, D.; Kumagai, S.; Kitazono, T.; Kiyohara, Y. Midlife and late-life handgrip strength and risk of cause-specific death in a general Japanese population: The Hisayama Study. J. Epidemiol. Community Health 2014, 68, 663–668. [Google Scholar] [CrossRef]
- Kim, M.; Won, C.W.; Kim, M. Muscular grip strength normative values for a Korean population from the Korea National Health and Nutrition Examination Survey, 2014–2015. PLoS ONE 2018, 13, e201275. [Google Scholar] [CrossRef]
- Appelman, Y.; Rijn, B.B.V.; Haaf, M.E.T.; Boersma, E.; Peters, S.A. Sex differences in cardiovascular risk factors and disease prevention. Atherosclerosis 2015, 241, 211–218. [Google Scholar] [CrossRef]
- Public Health Weekly Report. Available online: https://www.cdc.go.kr/board.es?mid=a30501000000&bid=0031&nPage=1 (accessed on 5 January 2018).
- Mulat, E.; Gebremariam, T.; Markos, Y.; Zawdie, B.; Nigatu, T.A. Cardiovascular risk in correlation with physical activity level and body mass index among adults with type 2 diabetes mellitus in Ethiopia. Endocrinol. Metab. Syndr. 2020, 9, 310. [Google Scholar] [CrossRef]
- Lam, B.C.C.; Koh, G.C.H.; Chen, C.; Wong, M.T.K.; Fallows, S.J. Comparison of body mass index (BMI), body adiposity index (BAI), waist circumference (WC), waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) as predictors of cardiovascular disease risk factors in an adult population in Singapore. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Jahangiry, L.; Farhangi, M.A.; Rezaei, F. Framingham risk score for estimation of 10 years of cardiovascular diseases risk in patients with metabolic syndrome. J. Health Popul. Nutr. 2017, 36, 36. [Google Scholar] [CrossRef]
- Framingham Heart Study. Available online: https://framinghamheartstudy.org/ (accessed on 14 June 2020).
- Liu, L.K.; Lee, W.J.; Chen, L.Y.; Hwang, A.C.; Lin, M.H.; Peng, L.N. Sarcopenia, and its association with cardiometabolic and functional characteristics in Taiwan: Results from I-Lan Longitudinal Aging Study. Geriatr. Gerontol. Int. 2014, 14, 36–45. [Google Scholar] [CrossRef]
- Gubelmann, C.; Vollenweider, P.; Marques-Vidal, P. Association of grip strength with cardiovascular risk markers. Eur. J. Prev. Cardiol. 2017, 24, 514–521. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- The Korea National Health and Nutrition Examination Survey. Available online: https://knhanes.cdc.go.kr/knhanes/main.do (accessed on 15 April 2020).
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef]
- Nakitto, M.; Asano, K.; Choi, I.; Yoon, J. Dietary intakes of adolescents from food insecure households: Analysis of data from the 6th (2013–2015) Korea National Health and Nutrition Examination Survey. Nutr. Res. Pract. 2017, 11, 507–516. [Google Scholar] [CrossRef]
- Lawman, H.G.; Troiano, R.P.; Perna, F.M.; Wang, C.Y.; Fryar, C.D.; Ogden, C.L. Associations of relative handgrip strength and cardiovascular disease biomarkers in U.S. adults, 2011–2012. Am. J. Prev. Med. 2016, 50, 677–683. [Google Scholar] [CrossRef]
- International Physical Activity Questionnaire Research Committee. Guideline for Data Processing and Analysis of the International Physical Activity Questionnaire. Available online: https://ci.nii.ac.jp/naid/1003031855 (accessed on 15 April 2020).
- Lee, H.J. WHO International Physical Activity Questionnaire: IPAQ. J. Korean Acad. Fam. Med. 2004, 11, 396–406. [Google Scholar]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S516. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S. Compendium of physical activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef]
- Brown, D.W.; Heath, G.W.; Balluz, L.; Giles, W.H.; Ford, E.S.; Mokdad, A.H.; Brown, D.R. Associations between Physical Activity Dose and Health-Related Quality of Life. Med. Sci. Sports Exerc. 2004, 36, 890–896. [Google Scholar] [CrossRef]
- Ashwell, M.; Hsieh, S.D. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Hsu, P.S.; Krairit, O.; Lee, J.S.; et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef]
- Go, A.S.; Mozaffarian, D.; Roger, V.L.; Benjamin, E.J.; Berry, J.D.; Borden, W.B.; Bravata, D.M.; Dai, S.; Ford, E.S.; Fox, C.S.; et al. Heart disease and stroke statistics—2013 update: A report from the American Heart Association. Circulation 2013, 127, e6–e245. [Google Scholar] [CrossRef] [PubMed]
- Napoli, N. Heart Attacks Increasingly Common in Young Adults. American College of Cardiology, 7 March 2019. Available online: https://www.acc.org/about-acc/press-releases/2019/03/07/08/45/heart-attacks-increasingly-common-in-young-adults (accessed on 3 November 2020).
- Shin, S.H.; Lee, T.Y. Associations of serum lipid profiles with incidence of ischemic heart diseases in Korean adults: Retrospective cohort study. J. Korea Acad. Ind. Coop. Soc. 2012, 13, 2219–2231. [Google Scholar] [CrossRef]
- Seol, S.Y.; Jeong, M.H.; Lee, S.H.; Sohn, S.J.; Cho, J.Y.; Kim, M.C.; Sim, D.S. Impact of gender differences in elderly patients with acute myocardial infarction. Korean J. Med. 2019, 94, 96–106. [Google Scholar] [CrossRef]
- Sohn, C.; Kim, J.; Bae, W. The framingham risk score, diet, and inflammatory markers in Korean men with metabolic syndrome. Nutr. Res. Pract. 2012, 6, 246–253. [Google Scholar] [CrossRef]
- Mearns, E.S.; Saulsberry, W.J.; White, C.M.; Kohn, C.G.; Lemieux, S.; Sihabout, A.; Salamucha, I.; Coleman, C.I. Efficacy and safety of antihyperglycaemic drug regimens added to metformin and sulphonylurea therapy in Type 2 diabetes: A network meta-analysis. Diabet. Med. 2015, 32, 1530–1540. [Google Scholar] [CrossRef]
- Bohannon, R.W. Grip strength: An indispensable biomarker for older adults. Clin. Interv. Aging 2019, 14, 1681–1691. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.M.; Syddall, H.E.; Cooper, R.; Kuh, D.; Cooper, C.; Sayer, A.A. Global variation in grip strength: A systematic review and meta-analysis of normative data. Age Ageing 2016, 45, 209–216. [Google Scholar] [CrossRef]
- Bae, E.J.; Park, N.J.; Sohn, H.S.; Kim, Y.H. Handgrip strength and all-cause mortality in middle-aged and older Koreans. Int. J. Environ. Res. Public Health 2019, 16, 740. [Google Scholar] [CrossRef]
- Stenholm, S.; Härkänen, T.; Sainio, P.; Heliövaara, M.; Koskinen, S. Long-term changes in handgrip strength in men and women—Accounting the effect of right censoring due to death. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1068–1074. [Google Scholar] [CrossRef]
- Musalek, C.; Kirchengast, S. Grip strength as an indicator of health-related quality of life in old age-A pilot study. Int. J. Environ. Res. Public Health 2017, 14, 1447. [Google Scholar] [CrossRef]
- Amer, M.S.; Khater, M.S.; Omar, O.H.; Mabrouk, R.A.; Mostafa, S.A. Association between Framingham risk score and subclinical atherosclerosis among elderly with both type 2 diabetes mellitus and healthy subjects. Am. J. Cardiovasc. Dis. 2014, 4, 14–19. [Google Scholar] [PubMed]
- Kim, S.; Chang, Y.; Kang, J.; Cho, A.; Cho, J.; Hong, Y.S.; Zhao, D.; Ahn, J.; Shin, H.; Guallar, E.; et al. Relationship of the Blood Pressure Categories, as Defined by the ACC/AHA 2017 Blood Pressure Guidelines, and the Risk of Development of Cardiovascular Disease in Low-Risk Young Adults: Insights From a Retrospective Cohort of Young Adults. J. Am. Hear. Assoc. 2019, 8. [Google Scholar] [CrossRef]
- Shen, S.; Lu, Y.; Qi, H.; Li, F.; Shen, Z.; Wu, L.; Yang, C.; Wang, L.; Shui, K.; Yao, W.; et al. Waist-to-height ratio is an effective indicator for comprehensive cardiovascular health. Sci. Rep. 2017, 7, 43046. [Google Scholar] [CrossRef]
- Browning, L.M.; Hsieh, S.D.; Ashwell, M.A. Systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr. Res. Rev. 2010, 23, 247–269. [Google Scholar] [CrossRef]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bulló, M.; Martínez-González, M.Á.; Corella, D.; Estruch, R.; Covas, M.I.; Arós, F.; Wärnberg, J.; Fiol, M.; Lapetra, J.; et al. Waist-to-Height Ratio and Cardiovascular Risk Factors in Elderly Individuals at High Cardiovascular Risk. PLoS ONE 2012, 7, e43275. [Google Scholar] [CrossRef]
- Jung, S.J.; Chae, S.W. Effects of Korean diet control nutrition education on cardiovascular disease risk factors in patients who underwent cardiovascular disease surgery. J. Nutr. Health. 2018, 51, 215–227. [Google Scholar] [CrossRef]
- Carnethon, M.R. Physical activity and cardiovascular disease: How much is enough? Am. J. Lifestyle Med. 2009, 3, 44S–49S. [Google Scholar] [CrossRef]
- Luke, A.; Dugas, L.A.; Durazo-Arvizu, R.A.; Cao, G.; Cooper, R.S. Assessing physical activity and its relationship to cardiovascular risk factors: NHANES 2003–2006. BMC Public Health 2011, 11, 387. [Google Scholar] [CrossRef]
- Stamatakis, E.; Coombs, N.; Rowlands, A.; Shelton, N.; Hillsdon, M. Objectively-assessed and self-reported sedentary time in relation to multiple socioeconomic status indicators among adults in England: A cross-sectional study. BMJ Open 2014, 4, 363–373. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corra, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 7, 2315–2381. [Google Scholar] [CrossRef]
- Bankoski, A.; Harris, T.B.; McClain, J.J.; Brychta, R.J.; Caserotti, P.; Chen, K.Y.; Berrigan, D.; Troiano, R.P.; Koster, A. Sedentary activity associated with metabolic syndrome independent of physical activity. Diabetes Care 2011, 34, 497–503. [Google Scholar] [CrossRef]
- Same, R.V.; Feldman, D.I.; Shah, N.; Martin, S.S.; Al Rifai, M.; Blaha, M.J.; Graham, G.; Ahmed, H.M. Relationship between Sedentary Behavior and Cardiovascular Risk. Curr. Cardiol. Rep. 2015, 18, 1–7. [Google Scholar] [CrossRef]
| Variable | Low Risk (<FRS 9%) (n = 1664) | At-Risk * (≥FRS 10%) (n = 763) | p | ||
|---|---|---|---|---|---|
| n or Estimates % (SE) | |||||
| Age (years), mean (SE) | 49.31 (0.21) | 54.92 (0.27) | <0.001 | ||
| Age, n (%) | <0.001 | ||||
| 40–54 | 1230 | 76.9 (1.1) | 385 | 50.5 (0.8) | |
| 55–64 | 434 | 23.1 (2.0) | 378 | 49.5 (0.6) | |
| Sex | <0.001 | ||||
| Male | 379 | 31.0 (1.2) | 576 | 83.2 (1.3) | |
| Female | 1285 | 69.0 (1.2) | 187 | 16.8 (1.3) | |
| Marital status | <0.001 | ||||
| Married | 1435 | 87.8 (0.9) | 664 | 87.8 (1.6) | |
| Divorced/Unmarried | 167 | 8.2 (0.8) | 75 | 8.8 (1.2) | |
| Others | 62 | 4.0 (0.4) | 24 | 3.4 (0.8) | |
| Residential area | <0.001 | ||||
| Urban | 1397 | 84.1 (2.8) | 596 | 79.8 (3.1) | |
| Rural | 267 | 15.9 (2.8) | 167 | 20.2 (3.1) | |
| Education | <0.001 | ||||
| <Elementary school | 211 | 11.7 (1.0) | 188 | 22.6 (2.1) | |
| Middle school | 200 | 12.5 (1.0) | 121 | 17.1 (1.7) | |
| High school | 598 | 44.1 (1.8) | 213 | 31.6 (2.2) | |
| ≥College | 440 | 31.7 (2.0) | 171 | 28.8 (2.5) | |
| No response | 215 | 70 | |||
| Household income | <0.001 | ||||
| 1st quartile | 152 | 8.6 (0.9) | 123 | 14.0 (1.5) | |
| 2nd quartile | 417 | 23.7 (1.6) | 199 | 25.2 (1.8) | |
| 3rd quartile | 538 | 33.3 (1.7) | 225 | 30.2 (2.0) | |
| 4th quartile | 548 | 34.4 (2.2) | 214 | 30.6 (2.5) | |
| Occupation | <0.001 | ||||
| Managerial and professional | 329 | 20.8 (1.2) | 136 | 21.0 (2.1) | |
| Service and sales | 271 | 16.7 (1.2) | 78 | 10.2 (1.3) | |
| Routine and manual | 326 | 19.5 (1.3) | 305 | 39.4 (2.4) | |
| Unemployed | 738 | 42.9 (1.5) | 244 | 29.4 (2.2) | |
| Frequency of drinking | <0.001 | ||||
| None | 280 | 15.2 (1.1) | 100 | 11.9 (1.2) | |
| 1/week | 810 | 48.8 (1.3) | 286 | 36.2 (2.1) | |
| 2–3/week | 191 | 12.0 (0.9) | 157 | 24.2 (2.0) | |
| Daily | 383 | 24.0 (1.2) | 220 | 27.7 (1.9) | |
| Smoking | <0.001 | ||||
| Current smoker | 1559 | 7.5 (0.7) | 345 | 51.9 (2.1) | |
| Ex-smoker/Never | 105 | 92.5 (0.7) | 418 | 48.1 (2.1) | |
| Perceived health status | <0.001 | ||||
| Poor | 447 | 28.0 (1.4) | 208 | 27.1 (2.0) | |
| Moderate | 788 | 45.9 (1.5) | 377 | 49.2 (2.3) | |
| Good | 429 | 26.0 (1.2) | 178 | 23.7 (1.7) | |
| Variable | Low Risk (<FRS 10%) | At-Risk (≥FRS 10%) | p |
|---|---|---|---|
| Estimates % (SE) | Estimates % (SE) | ||
| Handgrip strength (kg), mean (SD) | 36.79 (0.36) | 29.94 (0.28) | <0.001 |
| Sedentary time (hr/d), mean (SD) | 6.39 (0.14) | 6.83 (0.20) | 0.054 |
| Physical activity (min/w), mean (SD) | 671.96 (0.32) | 632.40 (0.50) | <0.001 |
| Light PA | 59.8 (0.3) | 63.5 (0.3) | |
| Moderate PA | 36.9 (0.2) | 33.8 (0.4) | |
| Vigorous PA | 3.3 (0.3) | 2.7 (0.3) | |
| Body mass index (kg/m2), mean (SD) | 23.55 (0.92) | 24.86 (0.12) | <0.001 |
| <25 | 71.8 (0.4) | 52.7 (0.3) | |
| ≥25 | 28.2 (0.3) | 47.3 (0.3) | |
| Waist to height ratio, mean (SD) | 0.49 (0.00) | 0.52 (0.00) | <0.001 |
| <0.50 | 50.8 (0.3) | 32.4 (0.3) | |
| ≥0.50 | 49.2 (0.2) | 67.6 (0.2) | |
| Blood pressure (mmHg) | |||
| Systolic | 112.63 (0.36) | 127.88 (0.83) | <0.001 |
| Diastolic | 75.04 (9.21) | 81.67 (10.16) | <0.001 |
| Fasting glucose (mg/dL), mean (SD) | 98.24 (0.58) | 110.52 (1.17) | <0.001 |
| Cholesterol (mg/dL), mean (SD) | 192.24(0.01) | 199.51 (0.02) | <0.001 |
| Total cholesterol, mean (SD) | 192.24 (0.96) | 198.86 (1.77) | <0.001 |
| Triglyceride | 122.42 (2.84) | 200.04 (5.34) | <0.001 |
| HDL cholesterol | 53.51 (0.28) | 45.90 (0.42) | <0.001 |
| LDL cholesterol | 121.18 (3.71) | 118.87 (2.61) | 0.598 |
| HbA1c (%) | 5.68 (0.02) | 6.12 (0.04) | <0.001 |
| Variables | Categories | Low Risk vs At-Risk for CVD | |
|---|---|---|---|
| OR (95% CI) | p | ||
| Age | ≥50 | 1.26 (1.19–1.33) | <0.001 |
| 40–49 | 1 | ||
| Sex | Male | 38.05 (15.80–91.58) | <0.001 |
| Female | 1 | ||
| Handgrip strength | −1.76 (1.18–3.71) | <0.001 | |
| Physical activity (min/w) | Light | 1.48 (0.25–8.58) | 0.669 |
| Moderate to Vigorous | 1 | ||
| Waist to height ratio | <0.50 | 0.30 (0.16–0.56) | <0.001 |
| ≥0.50 | 1 | ||
| Sedentary time (hr/d) | M ± SD | 0.96 (0.88–1.04) | 0.265 |
| χ2 = 41.47, p < 0.001, Negelkerke R2 = 13.8 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, J.; Yoo, H.J. Effects of Handgrip Strength on 10-Year Cardiovascular Risk among the Korean Middle-Aged Population: The Korea National Health and Nutrition Examination Survey 2014. Healthcare 2020, 8, 458. https://doi.org/10.3390/healthcare8040458
Shim J, Yoo HJ. Effects of Handgrip Strength on 10-Year Cardiovascular Risk among the Korean Middle-Aged Population: The Korea National Health and Nutrition Examination Survey 2014. Healthcare. 2020; 8(4):458. https://doi.org/10.3390/healthcare8040458
Chicago/Turabian StyleShim, JaeLan, and Hye Jin Yoo. 2020. "Effects of Handgrip Strength on 10-Year Cardiovascular Risk among the Korean Middle-Aged Population: The Korea National Health and Nutrition Examination Survey 2014" Healthcare 8, no. 4: 458. https://doi.org/10.3390/healthcare8040458
APA StyleShim, J., & Yoo, H. J. (2020). Effects of Handgrip Strength on 10-Year Cardiovascular Risk among the Korean Middle-Aged Population: The Korea National Health and Nutrition Examination Survey 2014. Healthcare, 8(4), 458. https://doi.org/10.3390/healthcare8040458





