Preoperative Aspirin Management in Redo Tetralogy of Fallot Population: Single Centre Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
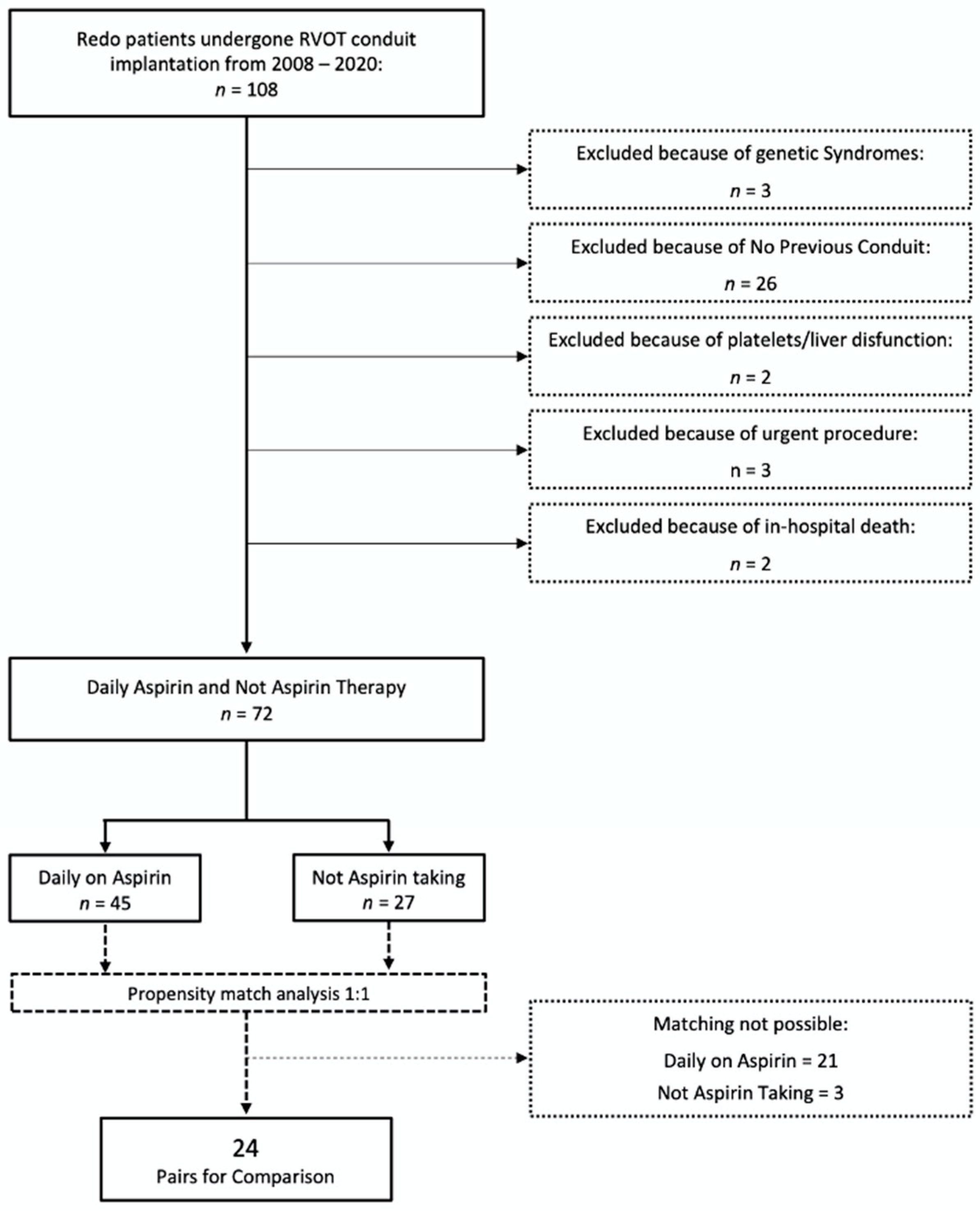
2.2. Participants
2.3. Anesthetic Protocols
2.4. Statistical Analysis
2.5. Ethical Consideration
3. Results
4. Discussion
5. Conclusions
Take-Home Message
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart disease and stroke statistics-2019 update: A report from the american heart association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.L.; Jacobs, J.P.; Hill, K.D.; Hornik, C.; O’Brien, S.M.; Pasquali, S.K.; Vener, D.; Kumar, S.R.; Habib, R.H.; Shahian, D.M.; et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2017 update on research. Ann. Thorac. Surg. 2017, 104, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Giglia, T.M.; Massicotte, M.P.; Tweddell, J.S.; Barst, R.J.; Bauman, M.; Erickson, C.C.; Feltes, T.F.; Foster, E.; Hinoki, K.; Ichord, R.N.; et al. Prevention and treatment of thrombosis in pediatric and congenital heart disease: A scientific statement from the American Heart Association. Circulation 2013, 128, 2622–2703. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S. Management of congenital heart disease: State of the art-part ii-cyanotic heart defects. Children 2019, 6, E54. [Google Scholar] [CrossRef]
- Yin, C.; Yan, J.; Li, S.; Li, D.; Wang, Q.; Wang, E. Effect analysis of repeat sternotomy in pediatric cardiac operations. J. Cardiothorac Surg. 2015, 10, 179. [Google Scholar] [CrossRef]
- Kato, H.; Chasovskyi, K.; Gandhi, S.K. Are blood products routinely required in pediatric heart surgery? Pediatr. Cardiol. 2020. [Google Scholar] [CrossRef]
- Cannata, A.; Petrella, D.; Russo, C.F.; Bruschi, G.; Fratto, P.; Gambacorta, M.; Martinelli, L. Postsurgical intrapericardial adhesions: Mechanisms of formation and prevention. Ann. Thorac. Surg. 2013, 95, 1818–1826. [Google Scholar] [CrossRef]
- Kirshbom, P.M.; Myung, R.J.; Simsic, J.M.; Kramer, Z.B.; Leong, T.; Kogon, B.E.; Kanter, K.R. One thousand repeat sternotomies for congenital cardiac surgery: Risk factors for reentry injury. Ann. Thorac. Surg. 2009, 88, 158–161. [Google Scholar] [CrossRef]
- Russell, J.L.; LeBlanc, J.G.; Sett, S.S.; Potts, J.E. Risks of repeat pediatric cardiac operations. Ann. Thorac. Surg. 1998, 66, 1575–1578. [Google Scholar] [CrossRef]
- Sousa-Uva, M.; Head, S.J.; Milojevic, M.; Collet, J.-P.; Landoni, G.; Castella, M.; Dunning, J.; Gudbjartsson, T.; Linker, N.J.; Sandoval, E.; et al. 2017 EACTS Guidelines on perioperative medication in adult cardiac surgery. Eur. J. Cardio Thorac. Surg. 2018, 53, 5–33. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, K.J.; Gauvreau, K.; Newburger, J.W.; Spray, T.L. Consensus-based method for risk adjustment for surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 2020, 123, 110–118. [Google Scholar] [CrossRef]
- Ashikhmina, E.A.; Schaff, H.V.; Sinak, L.J.; Li, Z.; Dearani, J.A.; Suri, R.M.; Park, S.J.; Orszulak, T.A.; Sundt, T.M. 3rd Pericardial effusion after cardiac surgery: Risk factors, patient profiles, and contemporary management. Ann. Thorac. Surg. 2010, 89, 112–118. [Google Scholar] [CrossRef]
- Miller, B.E.; Mochizuki, T.; Levy, J.H.; Bailey, J.; Tosone, S.R.; Tam, V.; Kanter, K. Predicting and treating coagulopathies after cardiopulmonary bypass in children. Anesth. Analg. 1997, 85, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Kirshbom, P.M.; Miller, B.E.; Spitzer, K.; Easley, K.A.; Spainhour, C.E.; Kogon, B.E.; Kanter, K.R. Failure of surface-modified bypass circuits to improve platelet function during pediatric cardiac surgery. J. Thorac. Cardiovasc. Surg. 2006, 132, 675–680. [Google Scholar] [CrossRef][Green Version]
- Hwang, T.W.; Kim, S.O.; Lee, S.Y.; Kim, S.-H.; Choi, E.-Y.; Jang, S.-I.; Park, S.-J.; Kwon, H.-W.; Lim, H.-B.; Lee, C.-H.; et al. Impact of postoperative duration of Aspirin use on longevity of bioprosthetic pulmonary valve in patients who underwent congenital heart disease repair. Korean J. Pediatr. 2016, 59, 446–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sambasivan, A.; Tibble, A.; Donahue, B.S. Low arterial saturation is associated with increased sensitivity to activated protein c in children with congenital heart disease. J. Cardiothorac. Vasc. Anesth. 2006, 20, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Hovels-Gurich, H.H.; Schumacher, K.; Vazquez-Jimenez, J.F.; Qing, M.; Hüffmeier, U.; Buding, B.; Messmer, B.J.; von Bernuth, G.; Seghaye, M.-C. Cytokine balance in infants undergoing cardiac operation. Ann. Thorac. Surg. 2002, 73, 601–609. [Google Scholar] [CrossRef]
- Le Roux, G.; Larmignat, P.; Marchal, M.; Richalet, J.P. Haemostasis at high altitude. Int. J. Sports Med. 1992, 13, S49–S51. [Google Scholar] [CrossRef]
- Zisman, E.; Erport, A.; Kohanovsky, E.; Ballagulah, M.; Cassel, A.; Quitt, M. Reuven Pizov Platelet function recovery after cessation of aspirin: Preliminary study of volunteers and surgical patients. Eur. J. Anaesthesiol. 2010, 27, 617–623. [Google Scholar] [CrossRef]
- Sun, J.C.; Whitlock, R.; Cheng, J.; Eikelboom, J.W.; Thabane, L.; Crowther, M.A.; Teoh, K.H.T. The effect of pre-operative aspirin on bleeding, transfusion, myocardial infarction, and mortality in coronary artery bypass surgery: A systematic review of randomized and observational studies. Eur. Heart J. 2008, 29, 1057–1071. [Google Scholar] [CrossRef]
- Smith, A.H.; Laussen, P.C. Cardiac critical care: What really makes a difference. Curr. Opin. Pediatr. 2013, 25, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Truong, D.T.; Johnson, J.T.; Bailly, D.K. Platelet inhibition in shunted infants on aspirin at short and midterm follow-up. Pediatr. Cardiol. 2017, 38, 401–409. [Google Scholar] [CrossRef]
| Variables | Daily on ASA (N = 45) | No Home ASA (N = 27) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 13.05 ± 4.3 | 15.07 ± 4.8 | 0.15 |
| Gender, male (n, %) | 27 (60) | 18 (67) | 0.57 |
| Weight, kg (mean ± SD) | 43.1 ± 14.8 | 48.2 ± 16.8 | 0.18 |
| Heart defect (n, %) | |||
| TOF | 33 (73.3) | 24 (88.9) | 0.11 |
| TOF + PAb | 1 (2.3) | 0 | 0.43 |
| TOF + PAt | 7 (15.6) | 3 (11.1) | 0.59 |
| Truncus Arteriosus | 2 (4.4) | 0 | - |
| DORV | 2 (4.4) | 0 | - |
| RACHS-1 score (n, %) | |||
| 1 Category | 0 | 0 | - |
| 2 Category | 34 (82.3) | 24 (84.6) | - |
| 3 Category | 10 (17.7) | 3 (15.4) | - |
| 4 Category | 2 | 0 | - |
| 5 Category | 0 | 0 | - |
| 6 Category | 0 | 0 | - |
| Aortic Cross Clamp (n, %) | 6 | 5 | - |
| Conduit Type (n, %) | |||
| Contegra® | 27 (78.4) | 16 (59.2) | - |
| Hancock® | 18 (21.6) | 11 (40.8) | - |
| Homograft | 0 | 0 |
| Variables | Daily on ASA (N = 24) | No Home ASA (N = 24) | p-Value |
|---|---|---|---|
| Age, years (mean ± SD) | 14.7 ± 3.9 | 14.4 ± 4.5 | 0.82 |
| Gender, male (n, %) | 18 (73.9) | 18 (73.9) | 1 |
| Weight, kg (mean ± SD) | 46.1 ± 11.8 | 46.3 ± 14.4 | 0.95 |
| Heart defect (n, %) | |||
| TOF | 19 (79.2) | 19 (79.2) | 1 |
| TOF + PAb | 0 | 0 | - |
| TOF + PAt | 5 (20.8) | 5 (20.8) | 1 |
| Truncus Arteriosus | 0 | 0 | - |
| DORV | 0 | 0 | - |
| RACHS-1 score (n, %) | |||
| 1 Category | 0 | 0 | - |
| 2 Category | 19 (79.2) | 19 (79.2) | 1 |
| 3 Category | 5 (20.8) | 5 (20.8) | 1 |
| 4 Category | 0 | 0 | - |
| 5 Category | 0 | 0 | - |
| 6 Category | 0 | 0 | - |
| Aortic Cross Clamp (n, %) | 5 (20.8) | 5 (20.8) | 1 |
| Conduit Type (n, %) | |||
| Contegra® | 22 (81.4) | 15 (62.5) | - |
| Hancock® | 2 (8.6) | 9 (37.5) | - |
| Homograft | 0 | 0 | - |
| Variables | Daily on ASA (N = 24) | No Home ASA (N = 24) | p-Value |
|---|---|---|---|
| Preoperative data | |||
| Surgery length, min (mean ± SD) | 453.1 ± 117.9 | 455.9 ± 115.1 | 0.93 |
| CPB length, min (mean ± SD) | 97.8 ± 39.6 | 103.9 ± 51.2 | 0.64 |
| Topical Hemostats, n (%) | 11 (45.8) | 10 (41,6) | 0.77 |
| Implanted Conduit (n, %) | |||
| Contegra® | 6 (25) | 4 (16.7) | - |
| Hancock® | 14 (58.4) | 17 (70.8) | - |
| Homograft | 4 (16.6) | 3 (12.5) | - |
| Postoperative data | |||
| Time to Extubation, hours (mean ± SD) | 28.8 ± 20.9 | 37.1 ± 35.6 | 0.34 |
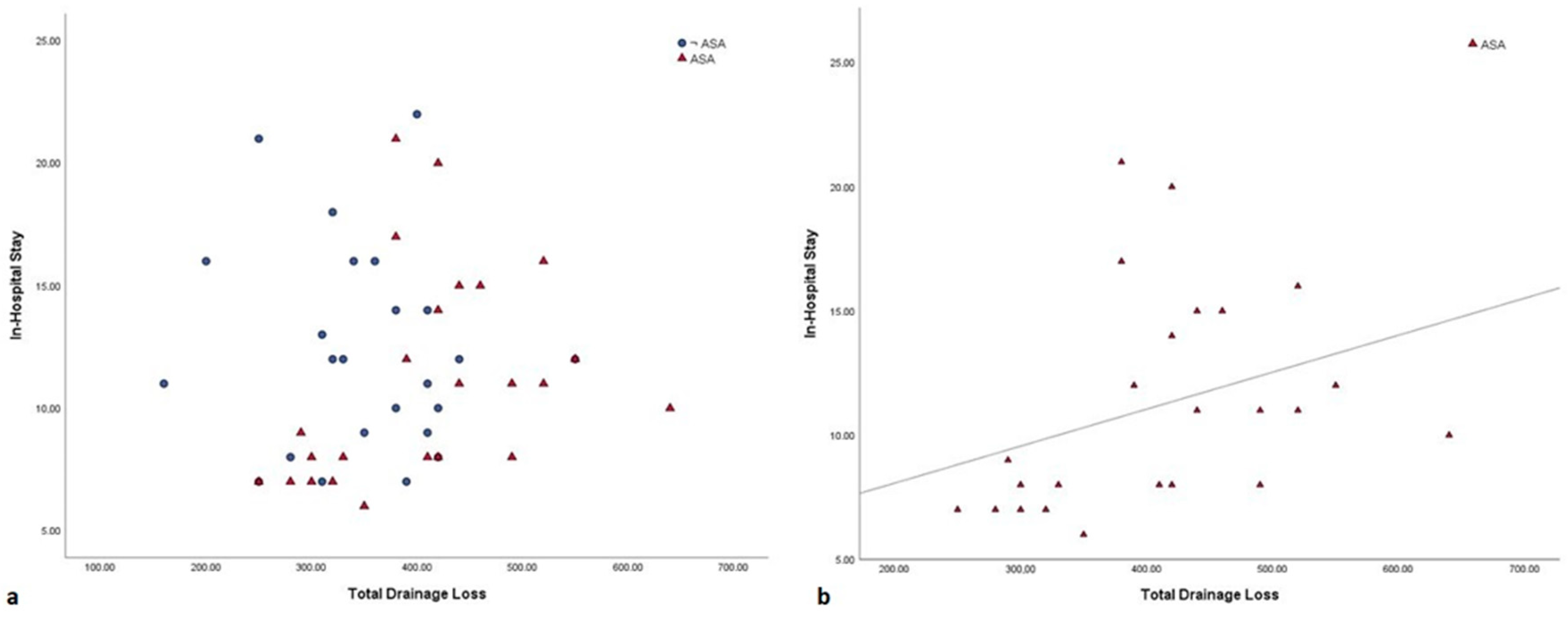
| ICU stay, days (mean ± SD) | 3.8 ± 1.5 | 4.4 ± 2.6 | 0.31 |
| Hospital stay, days (mean ± SD) | 11.1 ± 4.2 | 12.2 ± 4.2 | 0.36 |
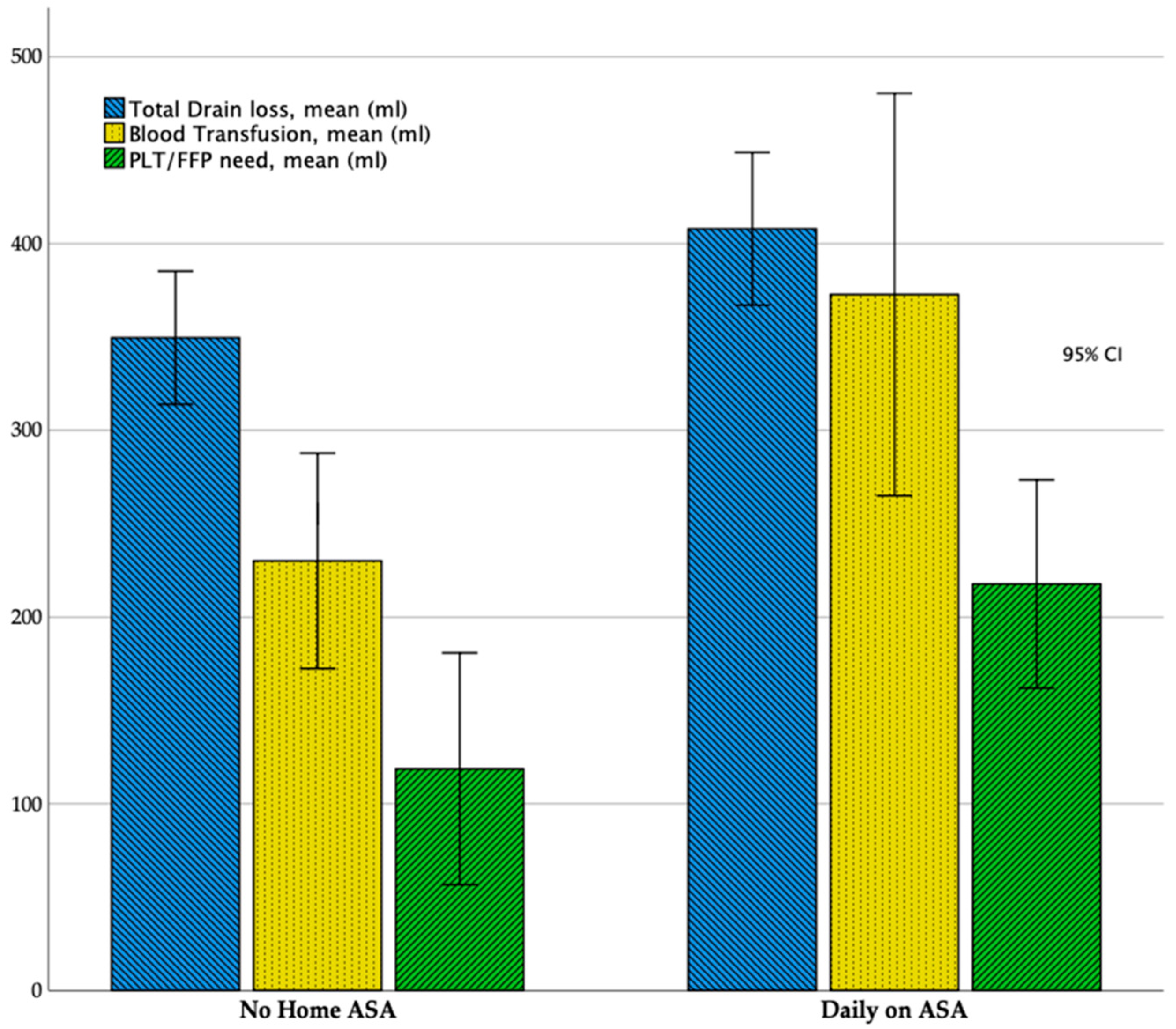
| Total Drain Loss, ml (mean ± SD) | 407.9 ± 96.7 | 349.5 ± 84.3 | 0.03 |
| Blood Transfusion, ml (mean ± SD) | 372.7 ± 255.1 | 220.1 ± 130.3 | 0.02 |
| PLT or FFP transfusion, ml (mean ± SD) | 217.7 ± 132.1 | 118.7 ± 147.1 | 0.01 |
| Inotropic Support length, days (mean ± SD) | 3.1 ± 2.7 | 4 ± 2.8 | 0.29 |
| Complications (n, %) | 8 (33.3) | 5 (20.8) | 0.33 |
| UTI | 4 (16.6) | 2 (8.4) | - |
| Respiratory | 3 (12.5) | 1 (4.2) | - |
| Renal | 0 | 1(4.2) | - |
| Wound Infection | 1 (4.2) | 1 (4.2) | - |
| Re-exploration for bleeding (n) | 0 | 1 | 0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comentale, G.; Palma, G.; Parisi, V.; Simeone, S.; Pucciarelli, G.; Manzo, R.; Pilato, E.; Giordano, R. Preoperative Aspirin Management in Redo Tetralogy of Fallot Population: Single Centre Experience. Healthcare 2020, 8, 455. https://doi.org/10.3390/healthcare8040455
Comentale G, Palma G, Parisi V, Simeone S, Pucciarelli G, Manzo R, Pilato E, Giordano R. Preoperative Aspirin Management in Redo Tetralogy of Fallot Population: Single Centre Experience. Healthcare. 2020; 8(4):455. https://doi.org/10.3390/healthcare8040455
Chicago/Turabian StyleComentale, Giuseppe, Gaetano Palma, Valentina Parisi, Silvio Simeone, Gianluca Pucciarelli, Rachele Manzo, Emanuele Pilato, and Raffaele Giordano. 2020. "Preoperative Aspirin Management in Redo Tetralogy of Fallot Population: Single Centre Experience" Healthcare 8, no. 4: 455. https://doi.org/10.3390/healthcare8040455
APA StyleComentale, G., Palma, G., Parisi, V., Simeone, S., Pucciarelli, G., Manzo, R., Pilato, E., & Giordano, R. (2020). Preoperative Aspirin Management in Redo Tetralogy of Fallot Population: Single Centre Experience. Healthcare, 8(4), 455. https://doi.org/10.3390/healthcare8040455