Renal Dysfunction and Tubulopathy Induced by High-Dose Tenofovir Disoproxil Fumarate in C57BL/6 Mice
Abstract
1. Introduction
2. Materials and Methods
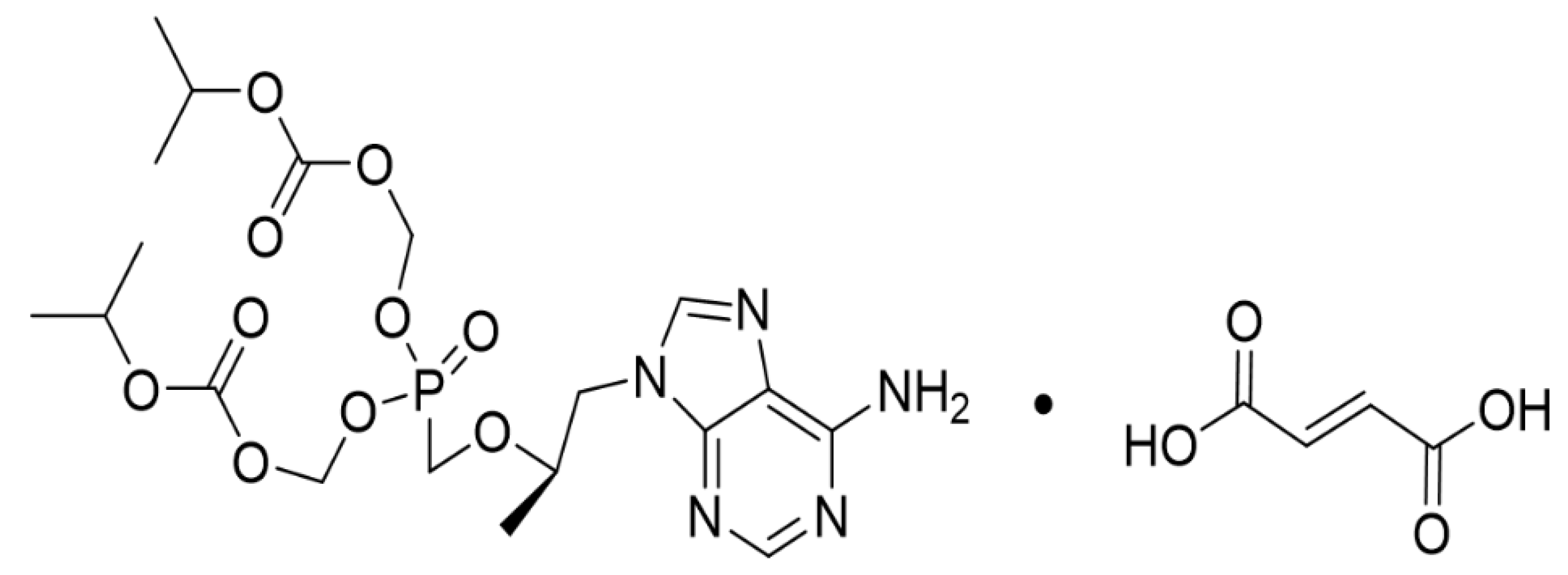
2.1. Materials
2.2. Animals and Experimental Design
2.3. Evaluation of Body Weight
2.4. Sample Collection
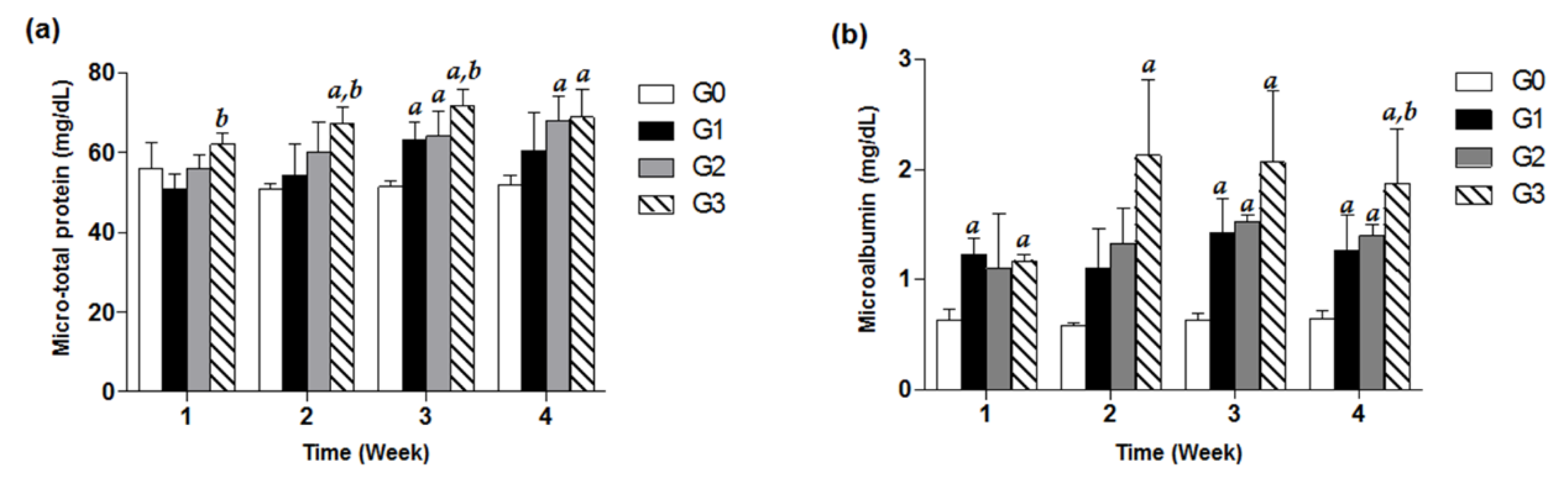
2.5. Evaluation of Micro-Total Protein and Microalbumin from Urine
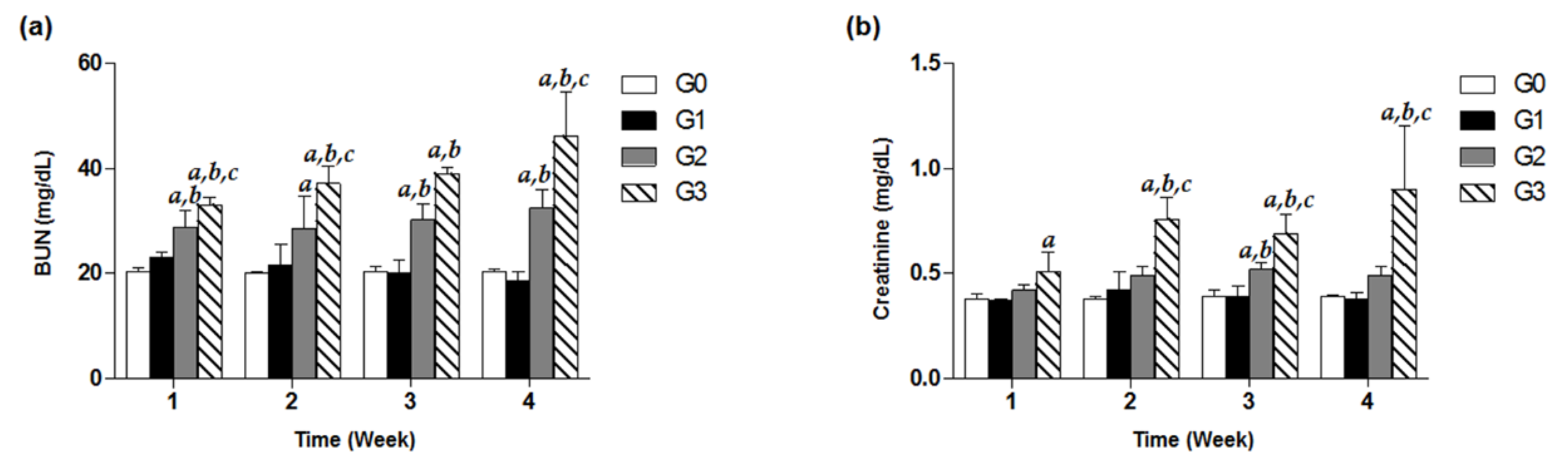
2.6. Evaluation of BUN and Creatinine from Serum
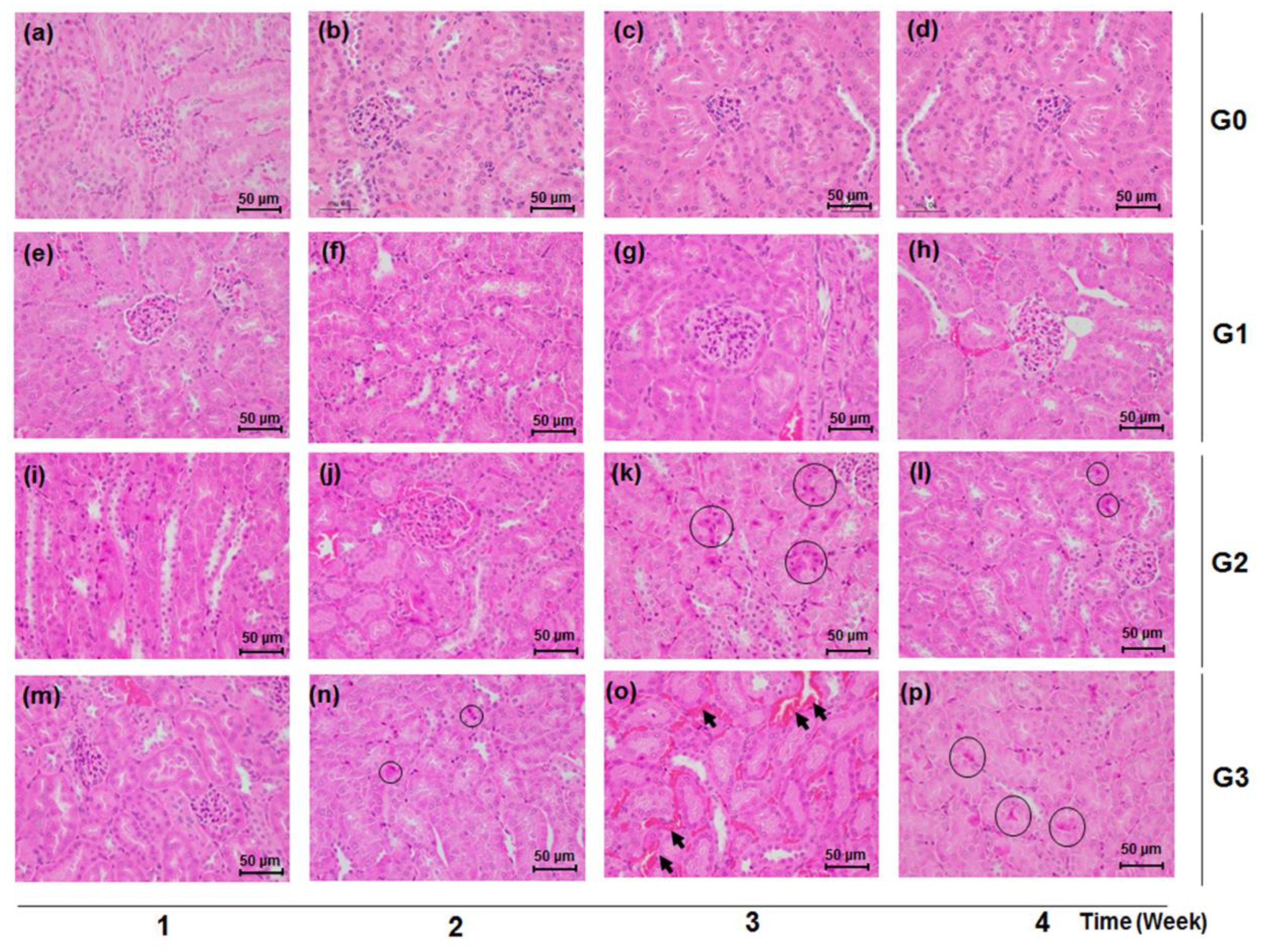
2.7. Histopathological Analysis of the Kidney
2.8. Statistical Analysis
3. Results
3.1. Effects of TDF on Body Weight Changes
3.2. Effects of TDF on Urine Micro-Total Protein and Microalbumin
3.3. Effects of TDF on Serum BUN and Creatinine
3.4. Effects of TDF on Histological Manifestations of Renal Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cho, H.; Cho, Y.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Oh, K.H.; Lee, K.; Mustika, S.; Yoon, J.H.; Kim, Y.J. Tenofovir-associated nephrotoxicity in patients with chronic hepatitis B: Two cases. Clin. Mol. Hepatol. 2016, 22, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nóvoa, S.; Alvarez, E.; Labarga, P.; Soriano, V. Renal toxicity associated with tenofovir use. Expert Opin. Drug Saf. 2010, 9, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Ristig, M.B.; Crippin, J.; Aberg, J.A.; Powderly, W.G.; Lisker-Melman, M.; Kessels, L.; Tebas, P. Tenofovir Disoproxil Fumarate Therapy for Chronic Hepatitis B in Human Immunodeficiency Virus/Hepatitis B Virus–Coinfected Individuals for Whom Interferon-α and Lamivudine Therapy Have Failed. J. Infect. Dis. 2002, 186, 1844–1847. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.R.; Katlama, C.; Montaner, J.S.; Cooper, D.A.; Gazzard, B.; Clotet, B.; Lazzarin, A.; Schewe, K.; Lange, J.; Wyatt, C. The safety of tenofovir disoproxil fumarate for the treatment of HIV infection in adults: The first 4 years. AIDS 2007, 21, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.D.; Wiebe, N.; Smith, N.; Keiser, P.; Naicker, S.; Tonelli, M. Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin. Infect. Dis. 2010, 51, 496–505. [Google Scholar] [CrossRef]
- Hall, A.M.; Hendry, B.M.; Nitsch, D.; Connolly, J.O. Tenofovir-associated kidney toxicity in HIV-infected patients: A review of the evidence. Am. J. Kidney Dis. 2011, 57, 773–780. [Google Scholar] [CrossRef]
- Fux, C.A.; Simcock, M.; Wolbers, M.; Bucher, H.C.; Hirschel, B.; Opravil, M.; Vernazza, P.; Cavassini, M.; Bernasconi, E.; Elzi, L. Tenofovir use is associated with a reduction in calculated glomerular filtration rates in the Swiss HIV Cohort Study. Antivir. Ther. 2007, 12, 1165–1173. [Google Scholar]
- Verhelst, D.; Monge, M.; Meynard, J.-L.; Fouqueray, B.; Mougenot, B.; Girard, P.-M.; Ronco, P.; Rossert, J. Fanconi syndrome and renal failure induced by tenofovir: A first case report. Am. J. Kidney Dis. 2002, 40, 1331–1333. [Google Scholar] [CrossRef]
- Van Rompay, K.K.; Brignolo, L.L.; Meyer, D.J.; Jerome, C.; Tarara, R.; Spinner, A.; Hamilton, M.; Hirst, L.L.; Bennett, D.R.; Canfield, D.R. Biological effects of short-term or prolonged administration of 9-[2-(phosphonomethoxy) propyl] adenine (tenofovir) to newborn and infant rhesus macaques. Antimicrob. Agents Chemother. 2004, 48, 1469–1487. [Google Scholar] [CrossRef]
- Lebrecht, D.; Venhoff, A.C.; Kirschner, J.; Wiech, T.; Venhoff, N.; Walker, U.A. Mitochondrial tubulopathy in tenofovir disoproxil fumarate-treated rats. J. Acquir. Immune Defic. Syndr. 2009, 51, 258–263. [Google Scholar] [CrossRef]
- Kohler, J.J.; Hosseini, S.H.; Hoying-Brandt, A.; Green, E.; Johnson, D.M.; Russ, R.; Tran, D.; Raper, C.M.; Santoianni, R.; Lewis, W. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab. Investig. 2009, 89, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Biesecker, G.; Karimi, S.; Desjardins, J.; Meyer, D.; Abbott, B.; Bendele, R.; Richardson, F. Evaluation of mitochondrial DNA content and enzyme levels in tenofovir DF-treated rats, rhesus monkeys and woodchucks. Antivir. Res. 2003, 58, 217–225. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Adewumi, O.M.; Adesanoye, O.A.; Faokunla, O.O.; Farombi, E.O. Effect of tenofovir, an antiretroviral drug, on hepatic and renal functional indices of Wistar rats: Protective role of vitamin E. Basic Clin. Physiol. Pharmacol. 2012, 23, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Abraham, P.; Hemalatha, R.; Isaac, B. A reliable and reproducible rodent model of Tenofovir disoproxil fumarate (TDF)(anti-HIV drug) nephrotoxicity that resembles human TDF tubulopathy. Biomed. Res. 2016, 27, 974–982. [Google Scholar]
- Shin, J.-W.; Seol, I.-C.; Son, C.-G. Interpretation of animal dose and human equivalent dose for drug development. J. Korean Orient. Med. 2010, 31, 1–7. [Google Scholar]
- Johnson, R.J.; Couser, W.G. Hepatitis B infection and renal disease: Clinical, immunopathogenetic and therapeutic considerations. Kidney Int. 1990, 37, 663–676. [Google Scholar] [CrossRef]
- Lai, K.N.; Lai, F.M. Clinical features and the natural course of hepatitis B virus-related glomerulopathy in adults. Kidney Int. 1991, 35, S40–S45. [Google Scholar]
- Crum-Cianflone, N.; Ganesan, A.; Teneza-Mora, N.; Riddle, M.; Medina, S.; Barahona, I.; Brodine, S. Prevalence and factors associated with renal dysfunction among HIV-infected patients. AIDS Patient Care STDS 2010, 24, 353–360. [Google Scholar] [CrossRef]
- Jose, S.; Hamzah, L.; Campbell, L.J.; Hill, T.; Fisher, M.; Leen, C.; Gilson, R.; Walsh, J.; Nelson, M.; Hay, P. Incomplete reversibility of estimated glomerular filtration rate decline following tenofovir disoproxil fumarate exposure. J. Infect. Dis. 2014, 210, 363–373. [Google Scholar] [CrossRef]
- Wu, M.T.; Lam, K.K.; Lee, W.C.; Hsu, K.T.; Wu, C.H.; Cheng, B.C.; Ng, H.Y.; Chi, P.J.; Lee, Y.T.; Lee, C.T. Albuminuria, proteinuria, and urinary albumin to protein ratio in chronic kidney disease. J. Clin. Lab. Anal. 2012, 26, 82–92. [Google Scholar] [CrossRef]
- Finco, D.; Duncan, J. Evaluation of blood urea nitrogen and serum creatinine concentrations as indicators of renal dysfunction: A study of 111 cases and a review of related literature. J. Am. Vet. Med. Assoc. 1976, 168, 593–601. [Google Scholar] [PubMed]
- Goicoechea, M.; Liu, S.; Best, B.; Sun, S.; Jain, S.; Kemper, C.; Witt, M.; Diamond, C.; Haubrich, R.; Louie, S. Greater tenofovir-associated renal function decline with protease inhibitor-based versus nonnucleoside reverse-transcriptase inhibitor-based therapy. J. Infect. Dis. 2008, 197, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Novoa, S.; Labarga, P.; D’Avolio, A.; Barreiro, P.; Albalate, M.; Vispo, E.; Solera, C.; Siccardi, M.; Bonora, S.; Di Perri, G.; et al. Impairment in kidney tubular function in patients receiving tenofovir is associated with higher tenofovir plasma concentrations. AIDS 2010, 24, 1064–1066. [Google Scholar] [CrossRef]
- Perazella, M.A. Crystal-induced acute renal failure. Am. J. Med. 1999, 106, 459–465. [Google Scholar] [CrossRef]
- Markowitz, G.S.; Perazella, M.A. Drug-induced renal failure: A focus on tubulointerstitial disease. Clin. Chim. Acta 2005, 351, 31–47. [Google Scholar] [CrossRef]
- Waheed, S.; Attia, D.; Estrella, M.M.; Zafar, Y.; Atta, M.G.; Lucas, G.M.; Fine, D.M. Proximal tubular dysfunction and kidney injury associated with tenofovir in HIV patients: A case series. Clin. Kidney J. 2015, 8, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Karras, A.; Lafaurie, M.; Furco, A.; Bourgarit, A.; Droz, D.; Sereni, D.; Legendre, C.; Martinez, F.; Molina, J.-M. Tenofovir-Related Nephrotoxicity in Human Immunodeficiency Virus-Infected Patients: Three Cases of Renal Failure, Fanconi Syndrome, and Nephrogenic Diabetes Insipidus. Clin. Infect. Dis. 2003, 36, 1070–1073. [Google Scholar] [CrossRef]
- Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Available online: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/drug-interactions-between-nucleoside-reverse-transcriptase?view=full (accessed on 18 October 2020).
- Ng, H.H.; Stock, H.; Rausch, L.; Bunin, D.; Wang, A.; Brill, S.; Gow, J.; Mirsalis, J.C. Tenofovir Disoproxil Fumarate: Toxicity, Toxicokinetics, and Toxicogenomics Analysis After 13 weeks of Oral Administration in Mice. Int. J. Toxicol. 2015, 34, 4–10. [Google Scholar] [CrossRef]
| Group | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G0 | 19.82 | ± | 1.25 | 20.05 | ± | 1.36 | 20.21 | ± | 1.45 | 20.40 | ± | 1.49 | 20.63 | ± | 1.50 |
| G1 | 19.86 | ± | 0.91 | 20.39 | ± | 0.71 | 20.95 | ± | 0.86 | 21.15 | ± | 0.94 | 21.33 | ± | 0.97 |
| G2 | 19.82 | ± | 0.88 | 19.39 | ± | 0.87 | 19.03 | ± | 0.70 | 18.74 | ± | 0.82 | 18.41 | ± | 1.08 # |
| G3 | 19.81 | ± | 0.88 | 19.37 | ± | 0.84 | 18.76 | ± | 1.30 | 17.71 | ± | 1.77 | 16.66 | ± | 2.26 *,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, E.; Lee, J.K.; Inn, K.-S.; Chung, E.K.; Lee, K.-T.; Lee, J.-H. Renal Dysfunction and Tubulopathy Induced by High-Dose Tenofovir Disoproxil Fumarate in C57BL/6 Mice. Healthcare 2020, 8, 417. https://doi.org/10.3390/healthcare8040417
Jang E, Lee JK, Inn K-S, Chung EK, Lee K-T, Lee J-H. Renal Dysfunction and Tubulopathy Induced by High-Dose Tenofovir Disoproxil Fumarate in C57BL/6 Mice. Healthcare. 2020; 8(4):417. https://doi.org/10.3390/healthcare8040417
Chicago/Turabian StyleJang, Eungyeong, Jong Kil Lee, Kyung-Soo Inn, Eun Kyoung Chung, Kyung-Tae Lee, and Jang-Hoon Lee. 2020. "Renal Dysfunction and Tubulopathy Induced by High-Dose Tenofovir Disoproxil Fumarate in C57BL/6 Mice" Healthcare 8, no. 4: 417. https://doi.org/10.3390/healthcare8040417
APA StyleJang, E., Lee, J. K., Inn, K.-S., Chung, E. K., Lee, K.-T., & Lee, J.-H. (2020). Renal Dysfunction and Tubulopathy Induced by High-Dose Tenofovir Disoproxil Fumarate in C57BL/6 Mice. Healthcare, 8(4), 417. https://doi.org/10.3390/healthcare8040417





