Applying the Minimal Detectable Change of a Static and Dynamic Balance Test Using a Portable Stabilometric Platform to Individually Assess Patients with Balance Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Ethics Statement
2.2. Instrumentation
2.3. Protocol
2.4. Statistical Analysis: Test-Retest Study
2.4.1. Selection of Variables
2.4.2. Minimal Detectable Change Calculation
2.5. Clinical Application Foundations: Assessment of Patients’ Progression
2.5.1. Patients’ Initial Diagnosis
2.5.2. Clinician 1 Assessment: History and Physical Examination
2.5.3. Clinician 2 Assessment: Patient Progression Evaluation
2.5.4. Magnitude-Based Decision (MBD) to Monitor Patients with Balance Disorders
- Xdif: difference between the measures taken at two temporal points: pre-value and post-value (Equation (5)). In this case, the pre-value is the measure of each variable just before starting the treatment; and the post-value is the measure three months after starting the treatment.
- MBD threshold: for this method, a threshold (numerical value) must be defined from which a change is considered relevant. In our case, we selected the MDC previously calculated.
3. Results
3.1. Test-Retest Results from Balance Analysis. Minimal Detectable Changes Index
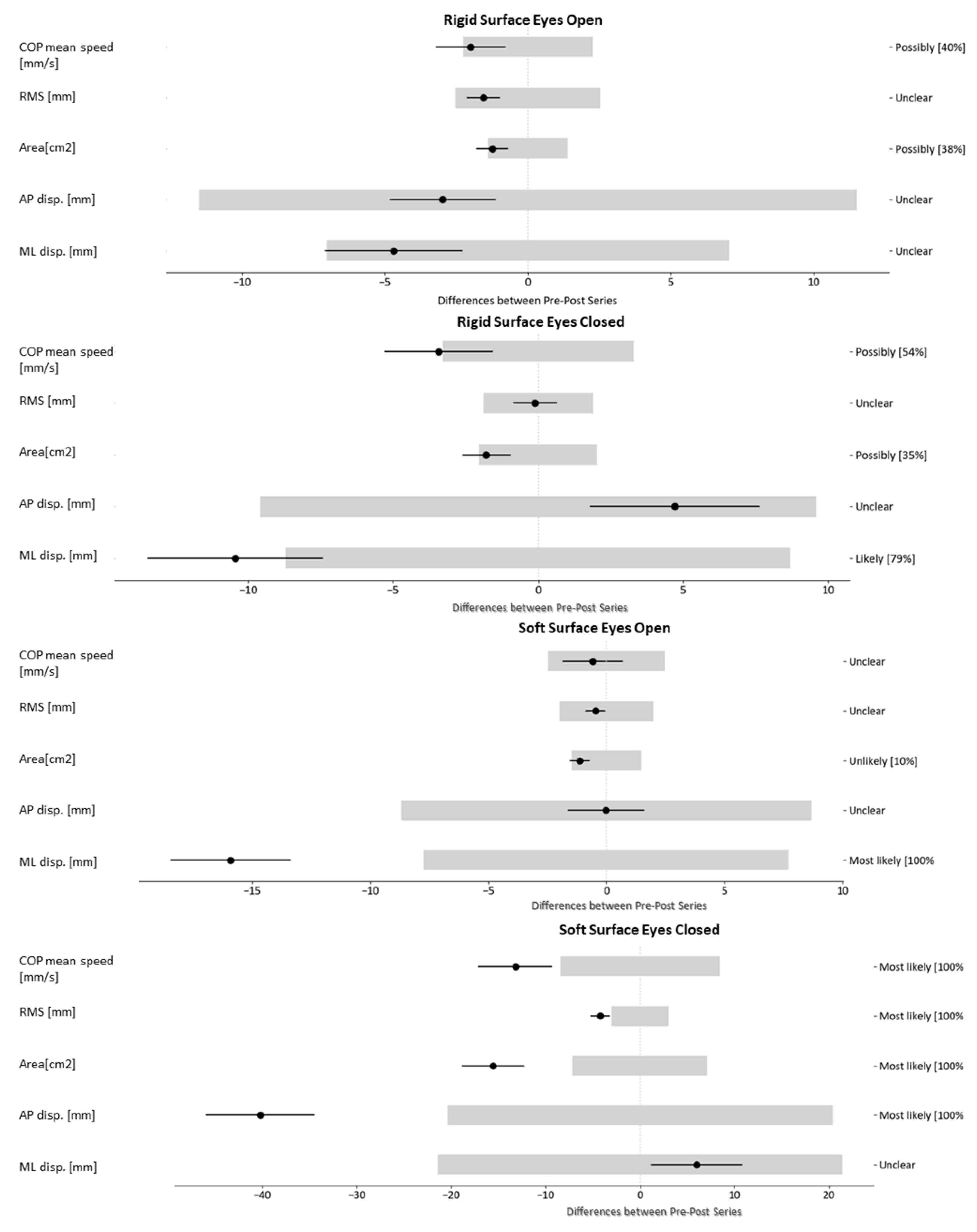
3.2. Results of the Patients-Level Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Penger, M.; Strobl, R.; Grill, E. Country-specific and individual determinants of dizziness in Europe: Results from the survey of health ageing and retirement in Europe (SHARE). Public Health 2017, 149, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, L.A.; Heuveline, P. Aging of population. Encycl. Popul. 2003, 1, 32–37. [Google Scholar]
- Vaupel, J.W.; Loichinger, E. Redistributing work in aging Europe. Science 2006, 312, 1911–1913. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Seol, H.; Nussbaum, M.A.; Madigan, M.L. Reliability of COP-based postural sway measures and age-related differences. Gait Posture 2008, 28, 337–342. [Google Scholar] [CrossRef]
- Baydal-Bertomeu, J.M.; Barberà I Guillem, R.; Soler-Gracia, C.; Peydro De Moya, M.F.; Prat, J.M.; Barona De Guzmán, R. Determinación de los patrones de comportamiento postural en población sana Española. Acta Otorrinolaringológica Española 2004, 55, 260–269. [Google Scholar] [CrossRef]
- Tinetti, M.E. Preventing falls in elderly persons. N. Engl. J. Med. 2003, 348, 42–49. [Google Scholar] [CrossRef]
- Ma, C.Z.; Wong, D.W.; Lam, W.K.; Wan, A.H.; Lee, W.C. Balance improvement effects of biofeedback systems with state-of-the-art wearable sensors: A systematic review. Sensors 2016, 16, 434. [Google Scholar] [CrossRef]
- Salehi, R.; Ebrahimi-Takamjani, I.; Esteki, A.; Maroufi, N.; Parnianpour, M. Test-retest reliability and minimal detectable change for center of pressure measures of postural stability in elderly subjects. Med. J. Islamic Repub. Iran 2010, 23, 224–232. [Google Scholar]
- Wolf, S.L.; Barnhart, H.X.; Kutner, N.G.; McNeely, E.; Coogler, C.; Xu, T.; Atlanta FICSIT Group. Reducing frailty and falls in older persons: An investigation of Tai Chi and computerized balance training. J. Am. Geriatr. Soc. 1996, 44, 489–497. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, M.L.; Belda-Lois, J.; Mena-del Horno, S.; Viosca-Herrero, E.; Igual-Camacho, C.; Gisbert-Morant, B. A new methodology based on functional principal component analysis to study postural stability post-stroke. Clin. Biomech. 2018, 56, 18–26. [Google Scholar] [CrossRef]
- Patrícia Paludette, D.; Fabrício Santana da, S.; Carlos Bolli, M. Comparação do equilíbrio postural entre grupos de mulheres com diferentes faixas etárias/Comparison of postural balance among groups of women with different age ranges. Fisioterapia e Pesquisa 2015, 22, 392. [Google Scholar]
- Baker, R. Gait analysis methods in rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- López, L.F.C.; Calidonio, J.D.L. “CgMed” Design and construction of a platform to determine the position of the center of gravity in standing. Revista Ingeniería Biomédica 2009, 3, 26–36. [Google Scholar]
- Postolache, O.A.; Postolache, G.B. Development and selection of balance sensing devices. IEEE Instrum. Meas. Mag. 2017, 20, 38–48. [Google Scholar]
- Ito, T.; Sakai, Y.; Ito, Y.; Yamazaki, K.; Morita, Y. In Association between back muscle strength and proprioception or mechanoreceptor control strategy in postural balance in elderly adults with lumbar spondylosis. Healthcare 2020, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Lee, J. In immediate effect of balance taping using kinesiology tape on dynamic and static balance after ankle muscle fatigue. Healthcare 2020, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Ku, P.X.; Abu Osman, N.A.; Wan Abas, W.A.B. The limits of stability and muscle activity in middle-aged adults during static and dynamic stance. J. Biomech. 2016, 49, 3943–3948. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.; Marin, J.; Marin, J.J.; Auria, J.M.; Sanchez-Valverde, M.B. Balance study in asymptomatic subjects: Determination of significant variables and reference patterns to improve clinical application. J. Biomech. 2017, 65, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Meshkati, Z.; Namazizadeh, M.; Salavati, M.; Mazaheri, M. Reliability of force-platform measures of postural sway and expertise-related differences. J. Sport Rehab. 2011, 20, 442–456. [Google Scholar] [CrossRef]
- García, R.B.; Corresa, S.P.; Bertomeu, J.M.B.; Suárez-Varela, M.M.M. Posturografía estática con pruebas dinámicas. Utilidad de los parámetros biomecánicos en la valoración del paciente vestibular. Acta Otorrinolaringológica Española 2012, 63, 332–338. [Google Scholar] [CrossRef]
- Swanenburg, J.; de Bruin, E.D.; Favero, K.; Uebelhart, D.; Mulder, T. The reliability of postural balance measures in single and dual tasking in elderly fallers and non-fallers. BMC Musculoskelet. Disord. 2008, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.; Gröger, I.; Rupprecht, R.; Gaßmann, K.G. Intrasession Reliability of Force Platform Parameters in Community-Dwelling Older Adults. Arch. Phys. Med. Rehabil. 2008, 89, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- De Sá Ferreira, A.; Baracat, P.J.F. Test–retest reliability for assessment of postural stability using center of pressure spatial patterns of three-dimensional statokinesigrams in young health participants. J. Biomech. 2014, 47, 2919–2924. [Google Scholar] [CrossRef] [PubMed]
- Ruhe, A.; Fejer, R.; Walker, B. The test–retest reliability of centre of pressure measures in bipedal static task conditions—A systematic review of the literature. Gait Posture 2010, 32, 436–445. [Google Scholar] [CrossRef]
- Geldhof, E.; Cardon, G.; De Bourdeaudhuij, I.; Danneels, L.; Coorevits, P.; Vanderstraeten, G.; De Clercq, D. Static and dynamic standing balance: Test-retest reliability and reference values in 9 to 10 year old children. Eur. J. Pediatr. 2006, 165, 779–786. [Google Scholar] [CrossRef]
- Lafond, D.; Corriveau, H.; Hébert, R.; Prince, F. Intrasession reliability of center of pressure measures of postural steadiness in healthy elderly people. Arch. Phys. Med. Rehabil. 2004, 85, 896–901. [Google Scholar] [CrossRef]
- Lee, P.; Liu, C.; Fan, C.; Lu, C.; Lu, W.; Hsieh, C. The test–retest reliability and the minimal detectable change of the Purdue Pegboard Test in schizophrenia. J. Formosan Med. Assoc. 2013, 112, 332–337. [Google Scholar] [CrossRef]
- Pagnacco, G.; Carrick, F.R.; Wright, C.H.G.; Oggero, E. Between-subjects differences of within-subject variability in repeated balance measures: Consequences on the minimum detectable change. Gait Posture 2015, 41, 136–140. [Google Scholar] [CrossRef]
- Pinsault, N.; Vuillerme, N. Test–retest reliability of centre of foot pressure measures to assess postural control during unperturbed stance. Med. Eng. Phys. 2009, 31, 276–286. [Google Scholar] [CrossRef]
- Steffen, T.; Seney, M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-item short-form health survey, and the unified Parkinson disease rating scale in people with parkinsonism. Phys. Ther. 2008, 88, 733–746. [Google Scholar] [CrossRef]
- Lee, H.; Granata, K.P. Process stationarity and reliability of trunk postural stability. Clin. Biomech. 2008, 23, 735–742. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Silva, D.; Briani, R.V.; Pazzinatto, M.F.; Ferrari, D.; Aragão, F.A.; de Albuquerque, C.E.; Alves, N.; de Azevedo, F.M. Reliability and differentiation capability of dynamic and static kinematic measurements of rearfoot eversion in patellofemoral pain. Clin. Biomech. 2015, 30, 144–148. [Google Scholar] [CrossRef]
- Pawar, P.K.; Dadhich, A. Study of correlation between human height and hand length in residents of Mumbai. Int. J. Biol. Med. Res. 2012, 3, 2072–2075. [Google Scholar]
- Bujang, M.A.; Baharum, N. A simplified guide to determination of sample size requirements for estimating the value of intraclass correlation coefficient: A review. Arch. Orofac. Sci. 2017, 12, 1–11. [Google Scholar]
- Charan, J.; Biswas, T. How to calculate sample size for different study designs in medical research? Indian J. Psychol. Med. 2013, 35, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Sample, R.B.; Jackson, K.; Kinney, A.L.; Diestelkamp, W.S.; Reinert, S.S.; Bigelow, K.E. Manual and cognitive dual tasks contribute to fall-risk differentiation in posturography measures. J. Appl. Biomech. 2016, 32, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Scoppa, F.; Capra, R.; Gallamini, M.; Shiffer, R. Clinical stabilometry standardization: Basic definitions–acquisition interval–sampling frequency. Gait Posture 2013, 37, 290–292. [Google Scholar] [CrossRef]
- Huurnink, A.; Fransz, D.P.; Kingma, I.; van Dieën, J.H. Comparison of a laboratory grade force platform with a Nintendo Wii Balance Board on measurement of postural control in single-leg stance balance tasks. J. Biomech. 2013, 46, 1392–1395. [Google Scholar] [CrossRef]
- Duarte, M.; Freitas, S.M. Revision of posturography based on force plate for balance evaluation. Braz. J. Phys. Ther. 2010, 14, 183–192. [Google Scholar] [CrossRef]
- Bonnechere, B.; Jansen, B.; Jan, S.V.S. Cost-effective (gaming) motion and balance devices for functional assessment: Need or hype? J. Biomech. 2016, 49, 2561–2565. [Google Scholar] [CrossRef]
- Zhu, Y. Design and validation of a low-cost portable device to quantify postural stability. Sensors 2017, 17, 619. [Google Scholar] [CrossRef] [PubMed]
- Uebbing, T.J. User Experience in Smart Environments: Design and Prototyping. Master’s Thesis, University of Twente, Twente, The Netherlands, 2016. [Google Scholar]
- Williams, Q.I.; Gunn, A.H.; Beaulieu, J.E.; Benas, B.C.; Buley, B.; Callahan, L.F.; Cantrell, J.; Genova, A.P.; Golightly, Y.M.; Goode, A.P.; et al. Physical therapy vs. internet-based exercise training (PATH-IN) for patients with knee osteoarthritis: Study protocol of a randomized controlled trial. BMC Musculoskelet. Disord. 2015, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Peydro de Moya, M.F.; Baydal Bertomeu, J.M.; Vivas Broseta, M.J. Evaluación y rehabilitación del equilibrio mediante posturografía. Rehabilitación 2005, 39, 315–323. [Google Scholar] [CrossRef]
- Tesio, L.; Rota, V.; Longo, S.; Grzeda, M.T. Measuring standing balance in adults: Reliability and minimal real difference of 14 instrumental measures. Int. J. Rehabil. Res. 2013, 36, 362–374. [Google Scholar] [CrossRef]
- Hoving, J.L.; Pool, J.J.; van Mameren, H.; Devillé, W.J.; Assendelft, W.J.; de Vet, H.C.; de Winter, A.F.; Koes, B.W.; Bouter, L.M. Reproducibility of cervical range of motion in patients with neck pain. BMC Musculoskelet. Disord. 2005, 6, 59. [Google Scholar] [CrossRef]
- Benvenuti, F.; Mecacci, R.; Gineprari, I.; Bandinelli, S.; Benvenuti, E.; Ferrucci, L.; Baroni, A.; Rabuffetti, M.; Hallett, M.; Dambrosia, J.M. Kinematic characteristics of standing disequilibrium: Reliability and validity of a posturographic protocol. Arch. Phys. Med. Rehabil. 1999, 80, 278–287. [Google Scholar] [CrossRef]
- Doyle, T.L.; Newton, R.U.; Burnett, A.F. Reliability of traditional and fractal dimension measures of quiet stance center of pressure in young, healthy people. Arch. Phys. Med. Rehabil. 2005, 86, 2034–2040. [Google Scholar] [CrossRef]
- Schuck, P.; Zwingmann, C. The ’smallest real difference’s a measure of sensitivity to change: A critical analysis. Int. J. Rehabil. Res. 2003, 26, 85–91. [Google Scholar]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef]
- Donoghue, D.; Stokes, E.K. How much change is true change? The minimum detectable change of the Berg Balance Scale in elderly people. J. Rehabil. Med. 2009, 41, 343–346. [Google Scholar] [CrossRef]
- Kovacs, F.M.; Abraira, V.; Royuela, A.; Corcoll, J.; Alegre, L.; Tomás, M.; Mir, M.A.; Cano, A.; Muriel, A.; Zamora, J. Minimum detectable and minimal clinically important changes for pain in patients with nonspecific neck pain. BMC Musculoskelet. Disord. 2008, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, G.F.; Lin, C.C.; Alghadir, A.; Whitney, S.L. Responsiveness and minimal detectable change of the dynamic gait index and functional gait index in persons with balance and vestibular disorders. J. Neurol. Phys. Ther. 2014, 38, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Batterham, A.M.; Hopkins, W.G. Making meaningful inferences about magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef]
- Cicchetti, D.V. Guidelines, criteria, and rules of thumb for evaluating normed and standardized assessment instruments in psychology. Psychol. Assess. 1994, 6, 284. [Google Scholar] [CrossRef]
- Hickey, S.; Ford, G.; Buckley, J.; O’connor, A.F. Unterberger stepping test: A useful indicator of peripheral vestibular dysfunction? J. Laryngol. Otol. 1990, 104, 599–602. [Google Scholar] [CrossRef]
- Bartual, J.; Pérez, N. El sistema vestibular y sus alteraciones. Tomo 1998, 1, 21–22. [Google Scholar]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar]
- Martínez Carrasco, Á. Análisis del riesgo de caídas en ancianos institucionalizados mediante escalas de marcha y equilibrio. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 28 January 2016. [Google Scholar]
- Vellas, B.J.; Wayne, S.J.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.Z.; Garry, P.J. One-leg balance is an important predictor of injurious falls in older persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef]
- Hansson, E.E.; Persson, L.; Malmström, E.M. Influence of vestibular rehabilitation on neck pain and cervical range of motion among patients with whiplash-associated disorder: A randomized controlled trial. J. Rehabil. Med. 2013, 45, 906–910. [Google Scholar] [CrossRef]
- Orejas, J.I.B.; Varea, J.A.; Rodrigo, J.V.; Navas, A.C. Resultados y seguimiento de la rehabilitación vestibular. Revista ORL 2020, 11, 107–114. [Google Scholar] [CrossRef]
- Hopkins, W.G. A spreadsheet for monitoring an individual’s changes and trend. Sportscience 2017, 21, 10. [Google Scholar]
- Hopkins, W.G. Rebranding MBI as magnitude-based decisions (MBD). Sportscience 2019, i-iii, 23. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Salavati, M.; Hadian, M.R.; Mazaheri, M.; Negahban, H.; Ebrahimi, I.; Talebian, S.; Jafari, A.H.; Sanjari, M.A.; Sohani, S.M.; Parnianpour, M. Test–retest reliabty of center of pressure measures of postural stability during quiet standing in a group with musculoskeletal disorders consisting of low back pain, anterior cruciate ligament injury and functional ankle instability. Gait Posture 2009, 29, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Degani, A.M.; Leonard, C.T.; Danna-dos-Santos, A. The effects of early stages of aging on postural sway: A multiple domain balance assessment using a force platform. J. Biomech. 2017, 64, 8–15. [Google Scholar] [CrossRef]
- Hanes, D.A.; McCollum, G. Cognitive-vestibular interactions: A review of patient difficulties and possible mechanisms. J. Vestib. Res. 2006, 16, 75–91. [Google Scholar]
- Swanenburg, J.; de Bruin, E.D.; Stauffacher, M.; Mulder, T.; Uebelhart, D. Effects of exercise and nutrition on postural balance and risk of falling in elderly people with decreased bone mineral density: Randomized controlled trial pilot study. Clin. Rehabil. 2007, 21, 523–534. [Google Scholar] [CrossRef]
- Tsukamoto, H.F.; Costa, V.d.S.P.; Silva Junior, R.A.d.; Pelosi, G.; Marchiori, L.L.d.M.; Vaz, C.R.S.; Fernandes, K.B.P. Effectiveness of a vestibular rehabilitation protocol to improve the health-related quality of life and postural balance in patients with vertigo. Int. Arch. Otorhinolaryngol. 2015, 19, 238–247. [Google Scholar] [CrossRef][Green Version]
- Baloh, R.W.; Jacobson, K.M.; Enrietto, J.A.; Corona, S.; Honrubia, V. Balance disorders in older persons: Quantification with posturography. Otolaryngol. Head Neck Surg. 1998, 119, 89–92. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.B.; Park, I.S.; Hong, S.J.; Kim, H.; Hong, S.M. Clinical analysis of dizzy patients with high levels of depression and anxiety. J. Audiol. Otol. 2016, 20, 174–178. [Google Scholar] [CrossRef][Green Version]
- Yuan, Q.; Yu, L.; Shi, D.; Ke, X.; Zhang, H. Anxiety and depression among patients with different types of vestibular peripheral vertigo. Medicine 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Yardley, L.; Redfern, M.S. Psychological factors influencing recovery from balance disorders. J. Anxiety Disord. 2001, 15, 107–119. [Google Scholar] [CrossRef]
- De Vet, H.C.; Terwee, C.B. The minimal detectable change should not replace the minimal important difference. J. Clin. Epidemiol. 2010, 63, 804. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, M.A.; Collins, F.S. The path to personalized medicine. N. Engl. J. Med. 2010, 363, 301–304. [Google Scholar] [CrossRef] [PubMed]
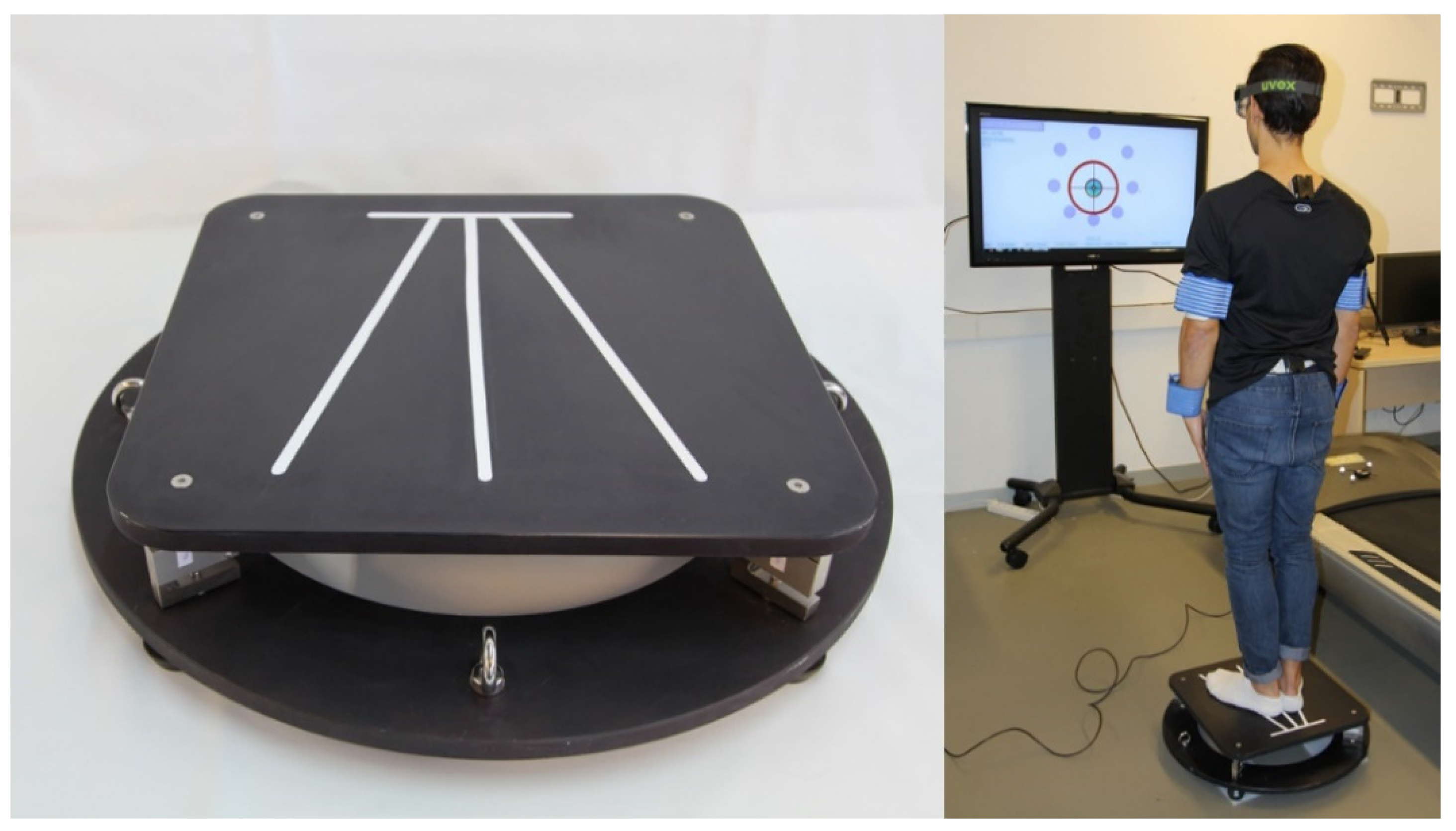

| Patient Code | Age | Gender | Anamnesis and Examination |
|---|---|---|---|
| 01 | 72 | Male | BPPV with associated signs of bilateral hearing loss (detected by audiometry) and nystagmus. |
| 02 | 73 | Female | Ménière syndrome. |
| 03 | 70 | Male | BPPV with associated signs of bilateral hearing loss (detected by audiometry). |
| 04 | 73 | Female | Osteosclerosis. Bilateral hearing loss. |
| 05 | 68 | Male | Ménière syndrome. Unilateral tinnitus and pathological nystagmus. |
| 06 | 74 | Male | Ménière syndrome. Unilateral tinnitus. |
| 07 | 68 | Female | Vestibular hypofunction. Pathological nystagmus. |
| 08 | 75 | Male | BPPV with associated signs of bilateral hearing loss (detected by audiometry). |
| Balance Tasks | Variables | Test µ (SD) | Retest µ (SD) | ICC | MDC95_es | MDC95 |
|---|---|---|---|---|---|---|
| RSEO | COP mean speed [mm/s] * | 8.1 (2.3) | 8.4 (2.2) | 0.87 | 0.9 | 2.3 |
| RMS [mm] | 5.1 (1.2) | 5.3 (1.7) | 0.61 | 2.1 | 2.6 | |
| Area [cm2] | 1.7 (0.9) | 1.7 (0.9) | 0.71 | 1.5 | 1.4 | |
| AP disp. [mm] | 19 (4.7) | 19.5 (6.1) | 0.42 | 2.4 | 11.6 | |
| ML disp. [mm] | 15.8 (4.4) | 15.8 (4.8) | 0.70 | 1.5 | 7.1 | |
| RSEC | COP mean speed [mm/s] * | 13.9 (4.2) | 13.6 (3.9) | 0.92 | 0.7 | 3.3 |
| RMS [mm] * | 6.2 (1.9) | 6.5 (1.8) | 0.87 | 0.9 | 1.9 | |
| Area [cm2] * | 3.5 (1.9) | 3.4 (1.8) | 0.85 | 1.0 | 2.0 | |
| AP disp. [mm] | 24.3 (6.1) | 25.9 (7.7) | 0.75 | 1.5 | 9.7 | |
| ML disp. [mm] * | 22.4 (8.3) | 22.7 (7.6) | 0.85 | 1.0 | 8.7 | |
| SSEO | COP mean speed [mm/s] * | 13.7 (2.7) | 13.5 (2.3) | 0.88 | 0.9 | 2.5 |
| RMS [mm] | 6.9 (1.3) | 6.9 (1.5) | 0.74 | 1.4 | 2.0 | |
| Area [cm2] | 3.7 (1.0) | 3.4 (1.1) | 0.76 | 0.4 | 1.5 | |
| AP disp. [mm] | 27.5 (5.1) | 27.2 (6.7) | 0.72 | 1.7 | 8.8 | |
| ML disp. [mm] | 23.4 (4.3) | 22.2 (4) | 0.56 | 1.7 | 7.7 | |
| SSEC | COP mean speed [mm/s] * | 36.2 (7.9) | 34 (6.6) | 0.83 | 1.0 | 8.5 |
| RMS [mm] | 13.6 (2.2) | 13.3 (2.3) | 0.77 | 1.3 | 3.0 | |
| Area [cm2] | 17.2 (5.3) | 15.7 (4.7) | 0.74 | 1.3 | 7.2 | |
| AP disp. mm] | 54.8 (12.4) | 52.7 (10.9) | 0.61 | 1.6 | 20.4 | |
| ML disp. [mm] | 51.6 (10.6) | 50.4 (9.5) | 0.42 | 2.0 | 21.4 | |
| LOS | COP mean speed [mm/s] * | 15.9 (3.1) | 15.4 (3.0) | 0.93 | 0.7 | 2.3 |
| RMS [mm] * | 5.8 (0.3) | 5.9 (0.3) | 0.96 | 0.5 | 0.2 | |
| Area [cm2] * | 179.5 (43.7) | 183.2 (45.2) | 0.97 | 0.4 | 21.7 | |
| AP disp. [mm] | 153.8 (20.4) | 157 (19.7) | 0.94 | 0.7 | 14.4 | |
| ML disp. [mm] | 157.1 (20) | 158 (23.1) | 0.94 | 0.7 | 14.8 | |
| Lim.COP.Forward [mm] * | 84.6 (17.5) | 87.5 (15.3) | 0.9 | 0.8 | 15.0 | |
| Lim.COP.Forward-rightward [mm] * | 90.4 (14.4) | 91.8 (11.8) | 0.93 | 0.7 | 10.2 | |
| Lim.COP.Rightward [mm] | 77.3 (12.2) | 75.5 (12.2) | 0.83 | 1.1 | 14.0 | |
| Lim.COP.Backward-rightward [mm] | 68.2 (12.7) | 68 (10.6) | 0.88 | 0.8 | 11.4 | |
| Lim.COP.Backward [mm] | 68.1 (14.3) | 68.5 (13.6) | 0.84 | 1.0 | 15.6 | |
| Lim.COP.Backward-leftward [mm] | 71.2 (12.2) | 71 (14.1) | 0.85 | 1.1 | 14.4 | |
| Lim.COP.Leftward [mm] | 77.6 (10.5) | 81.4 (13.8) | 0.86 | 1.2 | 13.1 | |
| Lim.COP.Forward-leftward [mm] * | 91 (14.8) | 92.6 (12.4) | 0.89 | 0.8 | 12.5 | |
| Success.Forward [%] | 75.7 (14.4) | 76.8 (11.7) | 0.5 | 1.7 | 25.8 | |
| Success.Forward-rightward [%] | 79.4 (12.2) | 79.1 (13.9) | 0.87 | 1.0 | 13.2 | |
| Success.Rightward [%] | 78.3 (12.3) | 80.4 (10.7) | 0.68 | 1.4 | 18.1 | |
| Success.Backward-rightward [%] | 79.1 (11.6) | 78 (9.9) | 0.42 | 1.9 | 23.0 | |
| Success.Backward [%] | 83.5 (11.) | 85.6 (8.2) | 0.57 | 1.5 | 18.5 | |
| Success.Backward-leftward [%] | 80.4 (10.5) | 82.8 (11.7) | 0.2 | 2.6 | 27.7 | |
| Success.Leftward [%] | 80.8 (11.7) | 81.6 (11.1) | 0.8 | 1.2 | 14.4 | |
| Success.Forward-leftward [%] | 78.8 (13.3) | 80.5 (11.4) | 0.77 | 1.2 | 16.5 |
| Balance Tasks | Variables | Value Pre | Value Post | Difference | MDC | % (− / 0 / +) |
|---|---|---|---|---|---|---|
| RSEO | COP mean speed [mm/s] | 13.3 | 11.2 | −2.0 | 2.3 | 41/59/0 |
| RMS [mm] | 6.5 | 5.0 | −1.5 | 2.6 | 22/78/0 | |
| Area [cm2] | 3.4 | 2.1 | −1.2 | 1.4 | 43/57/0 | |
| AP disp. [mm] | 20.0 | 17.0 | −3.0 | 11.6 | 8/91/1 | |
| ML disp. [mm] | 22.4 | 17.7 | −4.7 | 7.1 | 26/74/0 | |
| RSEC | COP mean speed [mm/s] | 20.3 | 16.9 | −3.4 | 3.3 | 53/47/0 |
| RMS [mm] | 7.6 | 7.5 | −0.1 | 1.9 | 4/94/2 | |
| Area [cm2] | 5.1 | 3.3 | −1.8 | 2.0 | 40/60/0 | |
| AP disp. [mm] | 26.9 | 31.6 | 4.7 | 9.7 | 0/84/16 | |
| ML disp. [mm] | 30.4 | 20.0 | −10.4 | 8.7 | 65/35/0 | |
| SSEO | COP mean speed [mm/s] | 18.5 | 18.0 | −0.6 | 2.5 | 7/92/1 |
| RMS [mm] | 7.3 | 6.8 | −0.5 | 2.0 | 7/92/1 | |
| Area [cm2] | 4.9 | 3.7 | −1.1 | 1.5 | 33/67/0 | |
| AP disp. [mm] | 23.3 | 23.3 | 0.0 | 8.8 | 3/94/3 | |
| ML disp. [mm] | 44.3 | 28.4 | −15.9 | 7.7 | 98/2/0 | |
| SSEC | COP mean speed [mm/s] | 62.6 | 49.4 | −13.2 | 8.5 | 86/14/0 |
| RMS [mm] | 19.2 | 15.0 | −4.2 | 3.0 | 78/22/0 | |
| Area [cm2] | 41.0 | 25.4 | −15.6 | 7.2 | 99/1/0 | |
| AP disp. [mm] | 102.4 | 62.2 | −40.2 | 20.4 | 97/3/0 | |
| ML disp. [mm] | 66.5 | 72.5 | 6.0 | 21.4 | 1/91/8 | |
| LOS | COP mean speed [mm/s] | 19.0 | 21.2 | 2.2 | 2.3 | 0/52/48 |
| RMS [mm] | 5.5 | 6.2 | 0.7 | 0.2 | 0/0/100 | |
| Area [cm2] | 170.0 | 234.0 | 64.0 | 21.7 | 0/0/100 | |
| AP disp. [mm] | 154.9 | 176.8 | 21.9 | 14.4 | 0/16/84 | |
| ML disp. [mm] | 150.3 | 179.5 | 29.2 | 14.8 | 0/3/97 | |
| Lim.COP.Forward [mm] | 86.8 | 108.2 | 21.4 | 15.0 | 0/21/79 | |
| Lim.COP.Forward-rightward [mm] | 9.5 | 108.3 | 15.8 | 10.2 | 0/14/86 | |
| Lim.COP.Rightward [mm] | 0.0 | 92.8 | 92.8 | 14.0 | 0/0/100 | |
| Lim.COP.Backward-rightward [mm] | 60.4 | 72.5 | 12.1 | 11.4 | 0/45/55 | |
| Lim.COP.Backward [mm] | 32.7 | 69.0 | 36.4 | 15.6 | 0/1/99 | |
| Lim.COP.Backward-leftward [mm] | 60.0 | 82.1 | 22.1 | 14.4 | 0/15/85 | |
| Lim.COP.Leftward [mm] | 72.7 | 87.0 | 14.3 | 13.1 | 0/43/57 | |
| Lim.COP.Forward-leftward [mm] | 106.6 | 104.7 | −1.9 | 12.5 | 5/93/2 | |
| Success.Forward [%] | 50.7 | 68.8 | 18.2 | 25.8 | 0/72/28 | |
| Success.Forward-rightward [%] | 69.0 | 73.6 | 4.6 | 13.2 | 1/89/11 | |
| Success.Rightward [%] | 43.9 | 88.2 | 44.2 | 18.1 | 0/0/100 | |
| Success.Backward-rightward [%] | 45.3 | 81.5 | 36.2 | 23.0 | 0/13/87 | |
| Success.Backward [%] | 53.9 | 91.9 | 37.9 | 18.5 | 0/2/98 | |
| Success.Backward-leftward [%] | 43.6 | 70.2 | 26.6 | 27.7 | 0/53/47 | |
| Success.Leftward [%] | 55.9 | 75.1 | 19.1 | 14.4 | 0/26/74 | |
| Success.Forward-leftward [%] | 58.5 | 79.2 | 20.8 | 16.5 | 0/31/69 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Torre, J.; Marin, J.; Polo, M.; Marín, J.J. Applying the Minimal Detectable Change of a Static and Dynamic Balance Test Using a Portable Stabilometric Platform to Individually Assess Patients with Balance Disorders. Healthcare 2020, 8, 402. https://doi.org/10.3390/healthcare8040402
De la Torre J, Marin J, Polo M, Marín JJ. Applying the Minimal Detectable Change of a Static and Dynamic Balance Test Using a Portable Stabilometric Platform to Individually Assess Patients with Balance Disorders. Healthcare. 2020; 8(4):402. https://doi.org/10.3390/healthcare8040402
Chicago/Turabian StyleDe la Torre, Juan, Javier Marin, Marco Polo, and José J. Marín. 2020. "Applying the Minimal Detectable Change of a Static and Dynamic Balance Test Using a Portable Stabilometric Platform to Individually Assess Patients with Balance Disorders" Healthcare 8, no. 4: 402. https://doi.org/10.3390/healthcare8040402
APA StyleDe la Torre, J., Marin, J., Polo, M., & Marín, J. J. (2020). Applying the Minimal Detectable Change of a Static and Dynamic Balance Test Using a Portable Stabilometric Platform to Individually Assess Patients with Balance Disorders. Healthcare, 8(4), 402. https://doi.org/10.3390/healthcare8040402





