Sexuality of Women after Gynecological Surgeries
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Participation in the Study
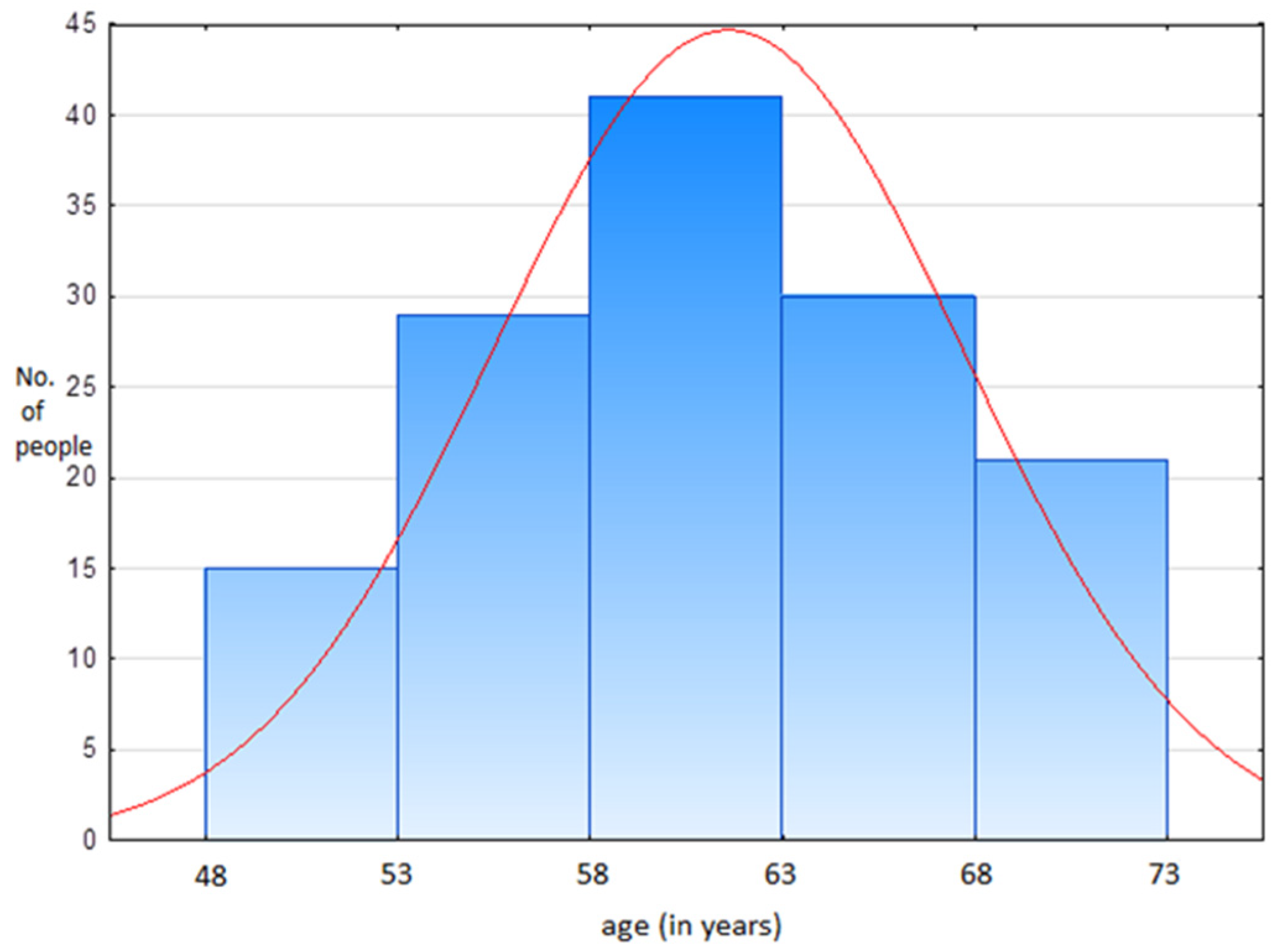
2.1.2. Characteristics of the Questioned Group
2.2. Instruments
2.3. Procedure
2.4. Data Analysis
2.5. Ethical Statement
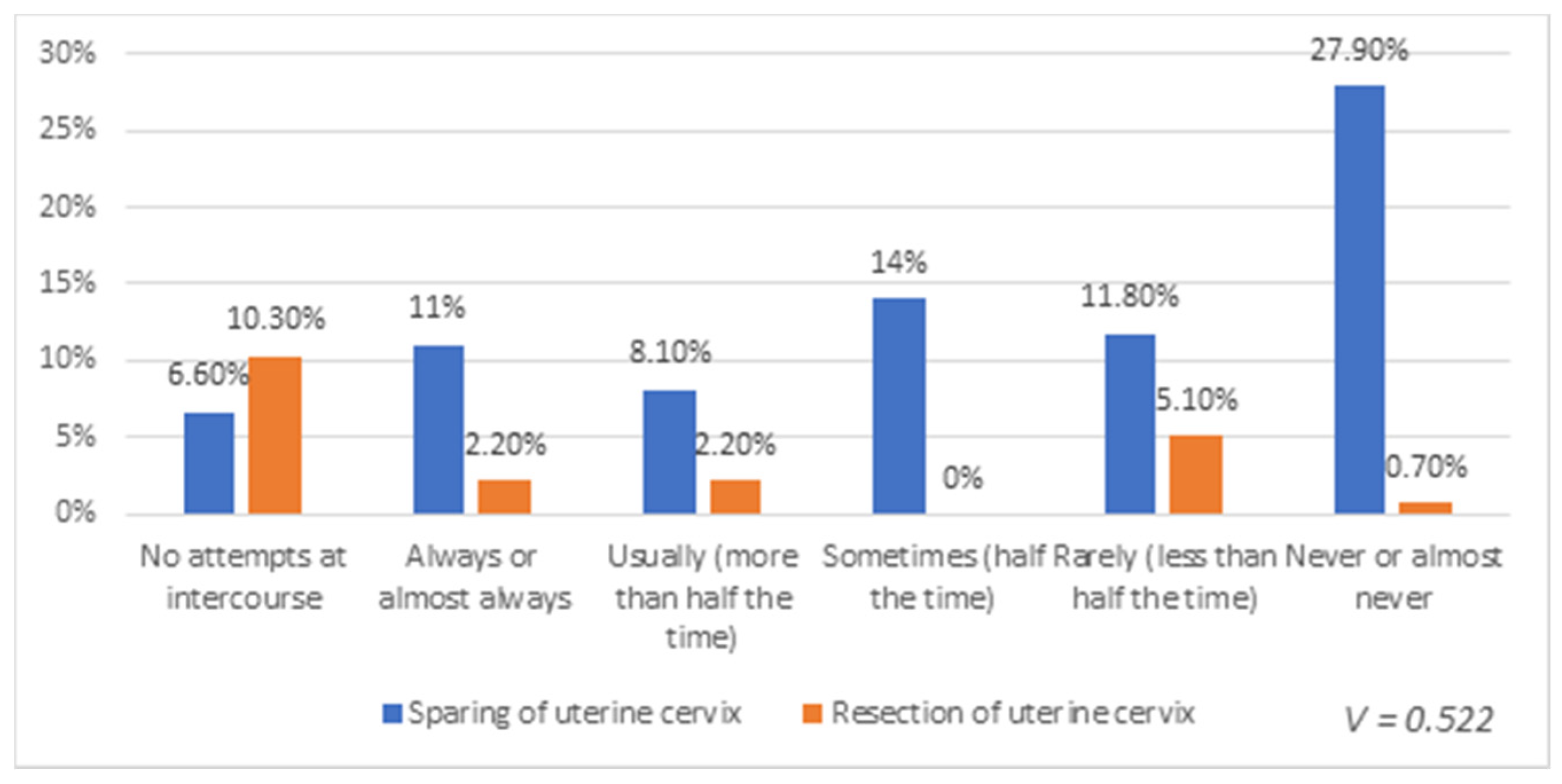
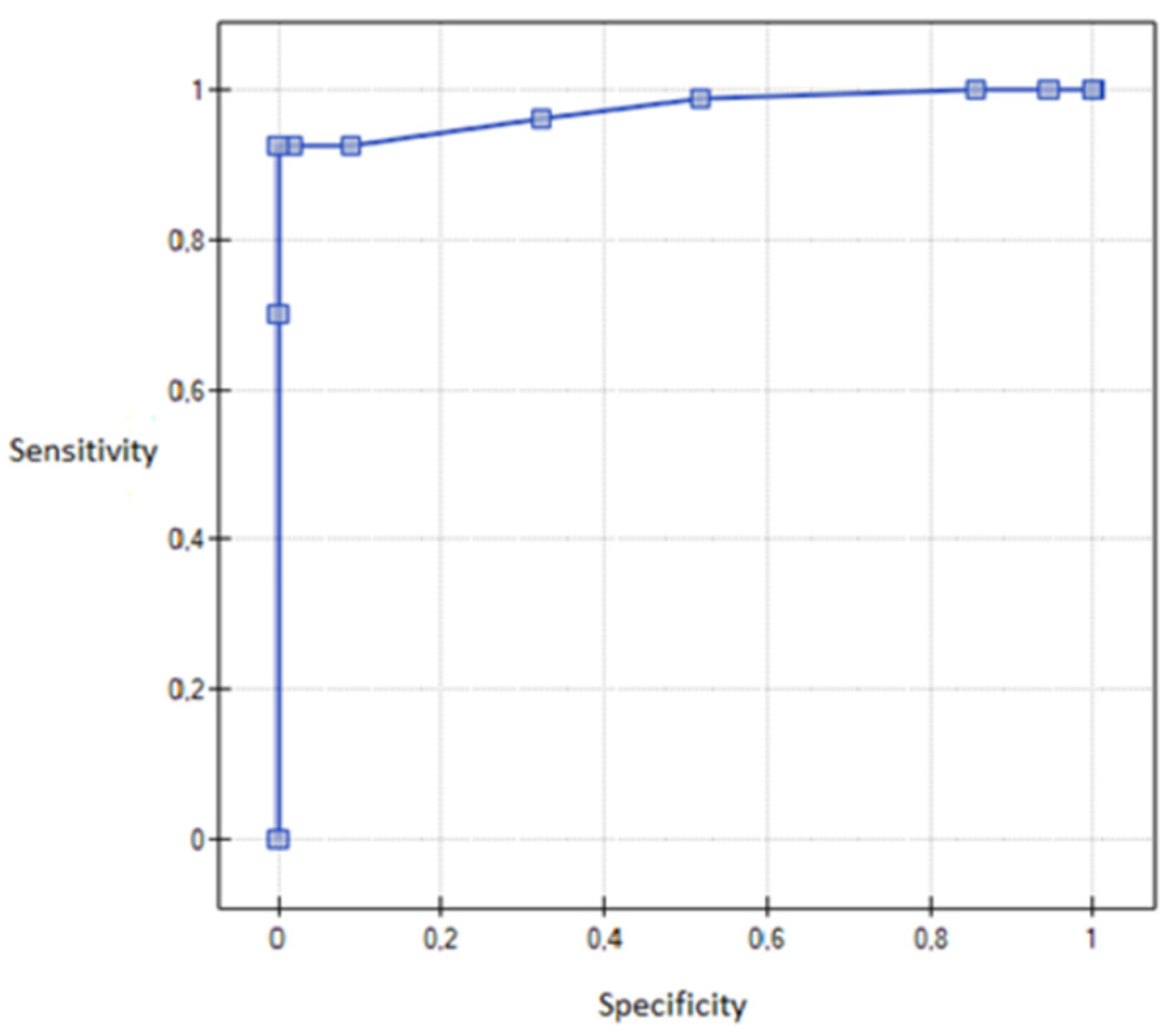
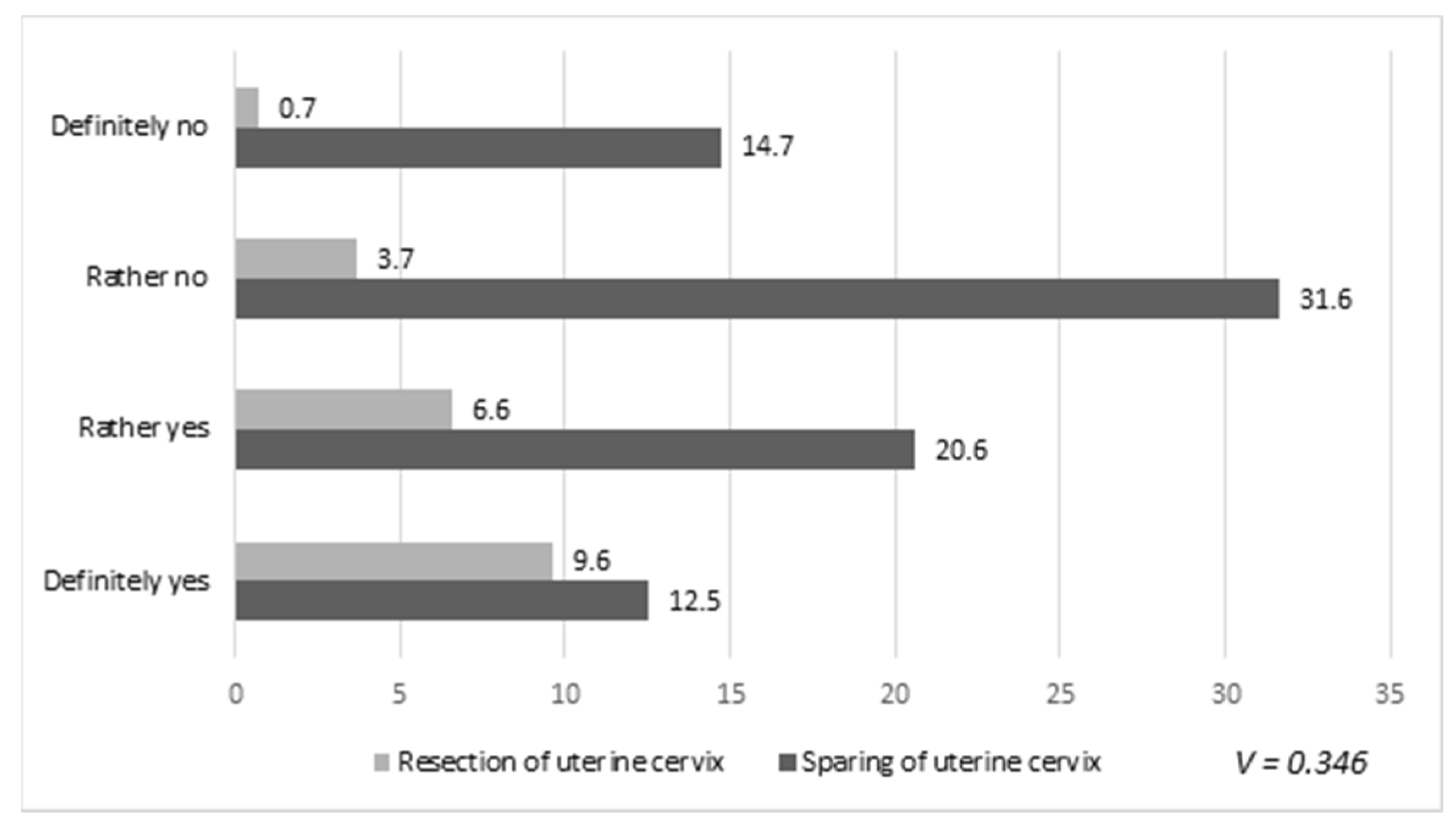
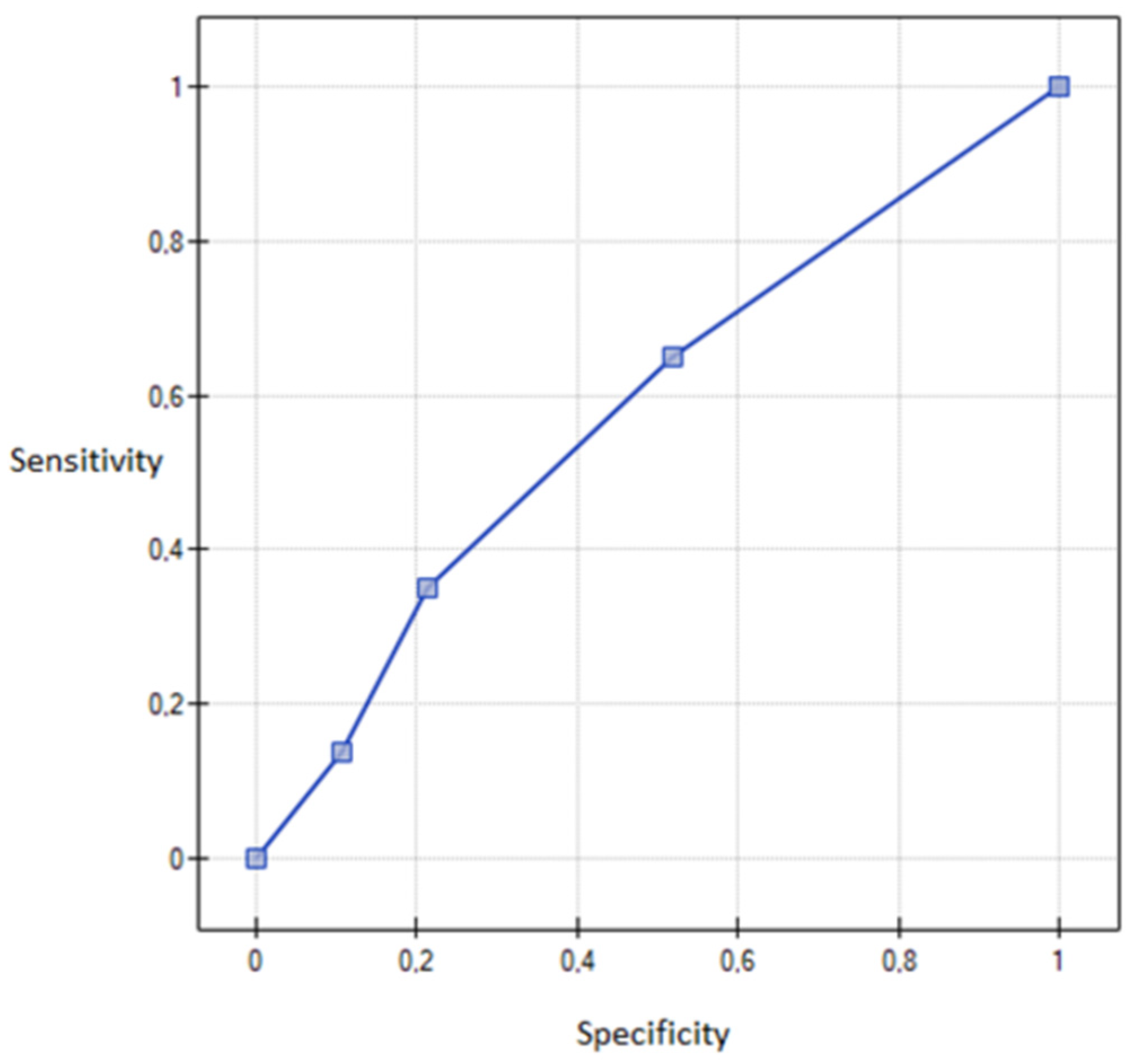
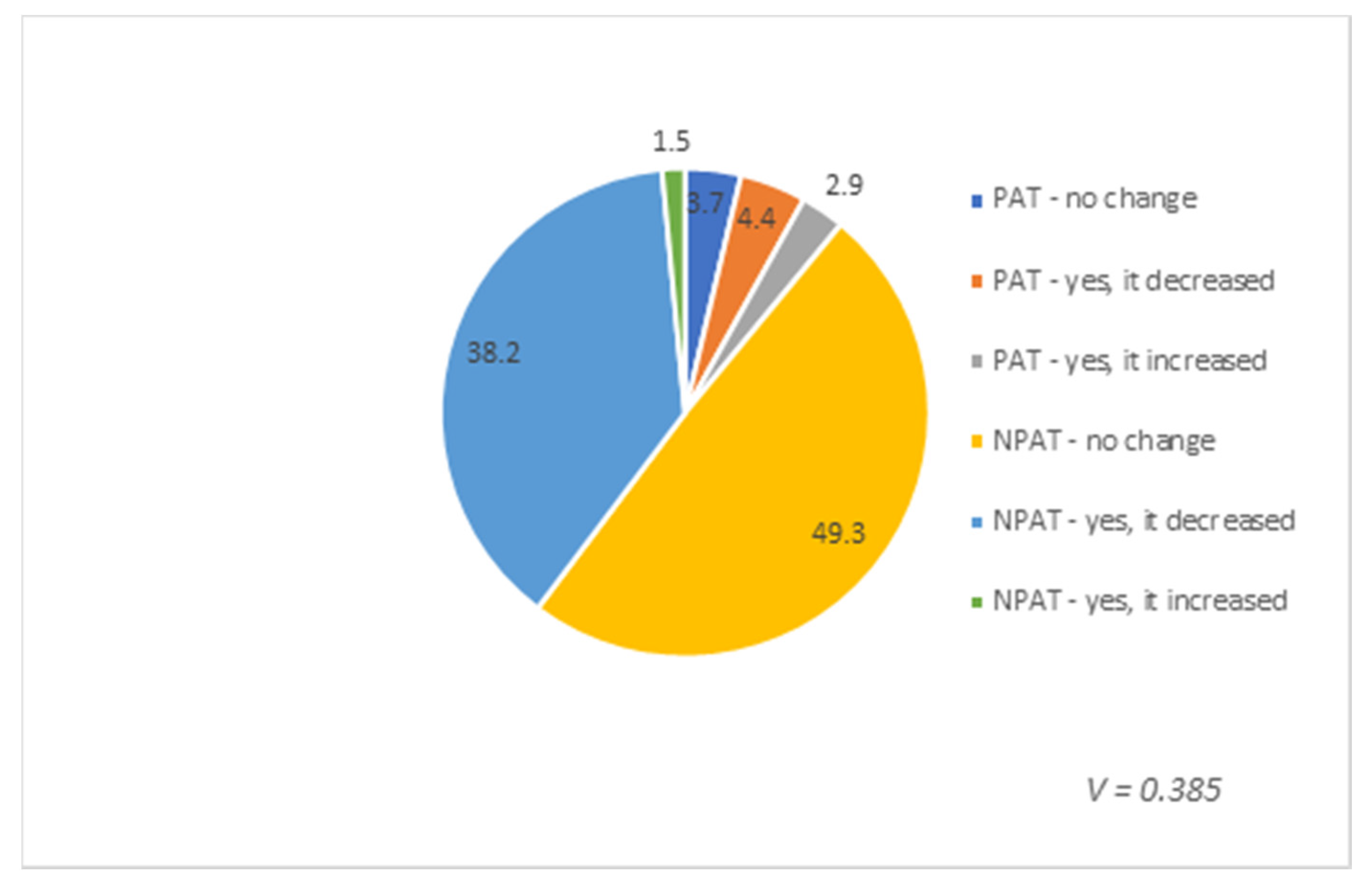
3. Results
4. Discussion
5. Conclusions
- There were no differences in the level of sexual interest between a group of patients who were operated on 1 year ago or less and a group of patients who were operated on 2 to 5 years ago.
- Minor gynecological procedures do not affect the sexual lives of patients.
- Discomfort or pain during intercourse occurs more frequently in patients after complete uterine resection.
- Patients treated oncologically are less likely to engage in sexual activities.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ventriglio, A.; Bhugra, D. Sexuality in the 21st Century: Sexual Fluidity. East Asian Arch. Psychiatry 2019, 29, 30–34. [Google Scholar] [CrossRef]
- Traumer, L.; Jacobsen, M.H.; Laursen, B.S. Patients’ experiences of sexuality as a taboo subject in the Danish healthcare system: A qualitative interview study. Scand. J. Caring Sci. 2018, 33, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Hay, C.M.; Donovan, H.S.; Hartnett, E.G.; Carter, J.; Roberge, M.C.; Campbell, G.B.; Zuchelkowski, B.E.; Taylor, S.E. Sexual Health as Part of Gynecologic Cancer Care: What Do Patients Want? Int. J. Gynecol. Cancer 2018, 28, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Cook, O.Y.; Yeganeh, L.; Huang, C.; Ding, J.; Johnson, C. Patient-Reported Barriers and Facilitators to Seeking and Accessing Support in Gynecologic and Breast Cancer Survivors with Sexual Problems: A Systematic Review of Qualitative and Quantitative Studies. J. Sex. Med. 2020, 17, 1326–1358. [Google Scholar] [CrossRef] [PubMed]
- Halley, M.C.; May, S.G.; Rendle, K.A.; Frosch, D.L.; Kurian, A.W. Beyond Barriers: Fundamental ‘Disconnects’ Underlying The Treatment of Breast Cancer Patients’ Sexual Health. Cult. Health Sex. 2014, 16, 1169–1180. [Google Scholar] [CrossRef]
- Canzona, M.R.; Garcia, D.; Fisher, C.L.; Raleigh, M.; Kalish, V.; Ledford, C.J.W. Communication about sexual health with breast cancer survivors: Variation among patient and provider perspectives. Patient Educ. Couns. 2016, 99, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- McClelland, S.I.; Holland, K.J.; Griggs, J.J. Vaginal Dryness and Beyond: The Sexual Health Needs of Women Diagnosed with Metastatic Breast Cancer. J. Sex Res. 2014, 52, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Ussher, J.M.; Perz, J.; Gilbert, E. Information needs associated with changes to sexual well-being after breast cancer. J. Adv. Nurs. 2012, 69, 327–337. [Google Scholar] [CrossRef]
- Stead, M.L.; Brown, J.M.; Fallowfield, L.; Selby, P. Lack of Communication Between Healthcare Professionals and Women with Ovarian Cancer About Sexual Issues. Br. J. Cancer 2003, 88, 666–671. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, S.R.; Connaghan, J.; Maguire, R.; Kotronoulas, G.; Flannagan, C.; Jain, S.; Brady, N.; McCaughan, E. Healthcare Professional Perceived Barriers and Facilitators to Discussing Sexual Wellbeing With Patients after Diagnosis of Chronic Illness: A Mixed-Methods Evidence Synthesis. Patient Educ. Couns. 2019, 102, 850–863. [Google Scholar] [CrossRef] [PubMed]
- Hubbs, J.L.; Michelson, E.L.D.; Vogel, R.I.; Rivard, C.L.; Teoh, D.G.K.; Geller, M.A. Sexual Quality of Life after the Treatment of Gynecologic Cancer: What Women Want. Support. Care Cancer 2019, 27, 4649–4654. [Google Scholar] [CrossRef] [PubMed]
- Seksualność Kobieca po Operacjach Ginekologicznych—Sexuality of Women after Gynecologic Surgery. Available online: http://przeglad-seksuologiczny.pl/numery/24/PS_NR_24_ART_4_24.pdf (accessed on 10 October 2020).
- Janda, M.; Armfield, N.R.; Kerr, G.; Kurz, S.; Jackson, G.; Currie, J.; Page, K.; Weaver, E.; Yazdani, A.; Obermair, A. Patient-Reported Experiences After Hysterectomy: A Cross-Sectional Study of the Views of Over 2300 Women. J. Patient Exp. 2019, 7, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Bręborowicz, G.H.; Banaszewska, B. Położnictwo i Ginekologia. T. 2, Ginekologia—Obstetrics and Gynaecology; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2007; Volume 2. [Google Scholar]
- Neis, K.J.; Zubke, W.; Römer, T.; Schwerdtfeger, K.; Schollmeyer, T.; Rimbach, S.; Holthaus, B.; Solomayer, E.; Bojahr, B.; Neis, F.; et al. Indications and Route of Hysterectomy for Benign Diseases. Guideline of the DGGG, OEGGG and SGGG (S3 Level, AWMF Registry No. 015/070, April 2015). Geburtshilfe und Frauenheilkd. 2016, 76, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Parys, B.T.; Haylen, B.T.; Hutton, J.L.; Parsons, K.F. Effects of Simple Hysterectomy on Vesicourethral Func. BJU Int. 1989, 64, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.H.; Ballantyne, B. The neuroanatomical basis for denervation of the urinary bladder following major pelvic surgery. Br. J. Surg. 1968, 55, 929–933. [Google Scholar] [CrossRef]
- Helström, L.; Sörbom, D.; Bäckström, T. Influence of partner relationship on sexuality after subtotal hysterectomy. Acta Obstet. Gynecol. Scand. 1995, 74, 142–146. [Google Scholar] [CrossRef]
- Rhodes, J.C.; Kjerulff, K.H.; Langenberg, P.W.; Guzinski, G.M. Hysterectomy and sexual functioning. JAMA 1999, 282, 1934–1941. [Google Scholar] [CrossRef]
- Virtanen, H.; Makinen, J.; Tenho, T.; Kiilholma, P.; Pitkänen, Y.; Hirvonen, T. Effects of Abdominal Hysterectomy on Urinary and Sexual Symptoms. BJU Int. 1993, 72, 868–872. [Google Scholar] [CrossRef]
- Greimel, E.R.; Vlasic, K.K.; Waldenström, A.-C.; Duric, V.M.; Jensen, P.T.; Singer, S.; Chie, W.; Nordin, A.; Bjelic-Radisic, V.; Wydra, D.G.; et al. The European Organization for Research and Treatment of Cancer (EORTC) Quality-of-Life questionnaire cervical cancer module. Cancer 2006, 107, 1812–1822. [Google Scholar] [CrossRef]
- Mcquellon, R.P.; Thaler, H.T.; Cella, D.; Moore, D.H. Quality of life (QOL) outcomes from a randomized trial of cisplatin versus cisplatin plus paclitaxel in advanced cervical cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2006, 101, 296–304. [Google Scholar] [CrossRef]
- Sompolska-Rzechuła, A.; Machowska-Szewczyk, M.; Chudecka-Głaz, A.; Cymbaluk-Płoska, A.; Menkiszak, J. The use of logistic regression in the ovarian cancer diagnostics. Ekonometria 2014. [Google Scholar] [CrossRef]
- Sompolska-Rzechuła, A. Pomiar i Ocena Jakości Życia w Ujęciu Regionalnym. Measuring and Evaluating the Quality of Life in Regional Terms. Available online: http://cejsh.icm.edu.pl/cejsh/element/bwmeta1.element.desklight-bff39273-72b8-4e5f-92b2-251957afa0f8/c/WS_06_2017__05_Agnieszka_SOMPOLSKA-RZECHULA___Przestrzenne_zroznicowanie_poziomu_jakosci_zycia_w_Polsce.pdf (accessed on 10 October 2020).
- Wydra, D.G.; Stefańska, K.; Sawicki, S.; Wilhelm, J.; Emerich, J. Comparison of sexual behavior after total or subtotal hysterectomy. Ginekol. Polska 2004, 75, 274–280. [Google Scholar]
- Kuppermann, M.; Summitt, R.L.; Varner, R.; McNeeley, S.G.; Goodman-Gruen, D.; Learman, L.A.; Ireland, C.C.; Vittinghoff, E.; Lin, F.; Richter, H.E.; et al. Sexual Functioning After Total Compared With Supracervical Hysterectomy: A Randomized Trial. Obstet. Gynecol. 2005, 105, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Lermann, J.; Häberle, L.; Merk, S.; Henglein, K.; Beckmann, M.W.; Mueller, A.; Mehlhorn, G. Comparison of prevalence of hypoactive sexual desire disorder (HSDD) in women after five different hysterectomy procedures. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Flory, N.; Bissonnette, F.; Amsel, R.T.; Binik, Y.M. The Psychosocial Outcomes of Total and Subtotal Hysterectomy: A Randomized Controlled Trial. J. Sex. Med. 2006, 3, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Roovers, J.; Van Der Bom, J.G.; Van Der Vaart, C.H.; Heintz, A.P.M. Hysterectomy and sexual wellbeing: Prospective observational study of vaginal hysterectomy, subtotal abdominal hysterectomy, and total abdominal hysterectomy. BMJ 2003, 327, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Engh, M.E.; Jerhamre, K.; Junskog, K. A randomized trial comparing changes in sexual health and psychological well-being after subtotal and total hysterectomies. Acta Obstet. Gynecol. Scand. 2010, 89. [Google Scholar] [CrossRef]
- El-Toukhy, T.; Hefni, M.A.; Davies, A.E.; Mahadevan, S. The effect of different types of hysterectomy on urinary and sexual functions: A prospective study. J. Obstet. Gynaecol. 2004, 24, 420–425. [Google Scholar] [CrossRef]
- Gütl, P.; Greimel, E.; Roth, R.; Winter, R. Women’s sexual behavior, body image and satisfaction with surgical outcomes after hysterectomy: A comparison of vaginal and abdominal surgery. J. Psychosom. Obstet. Gynecol. 2002, 23, 51–59. [Google Scholar] [CrossRef]
- Ellström, M.A.; Åström, M.; Möller, A.; Olsson, J.-H.; Hahlin, M. A randomized trial comparing changes in psychological well-being and sexuality after laparoscopic and abdominal hysterectomy. Acta Obstet. Gynecol. Scand. 2003, 82, 871–875. [Google Scholar] [CrossRef]
- Farrell, S.A. Sexuality after hysterectomy. Obstet. Gynecol. 2000, 95, 1045–1051. [Google Scholar] [CrossRef] [PubMed]
- Maas, C.P.; Weijenborg, P.T.M.; Ter Kuile, M.M. The effect of hysterectomy on sexual functioning. Annu. Rev. Sex Res. 2003, 14, 83–113. [Google Scholar] [PubMed]
- Bellerose, S.B.; Binik, Y.M. Body image and sexuality in oophorectomized women. Arch. Sex. Behav. 1993, 22, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Teplin, V.; Vittinghoff, E.; Lin, F.; Learman, L.A.; Richter, H.E.; Kuppermann, M. Oophorectomy in Premenopausal Women. Obstet. Gynecol. 2007, 109, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Doğanay, M.; Kokanalı, D.; Kokanalı, M.K.; Cavkaytar, S.; Aksakal, O.S. Comparison of female sexual function in women who underwent abdominal or vaginal hysterectomy with or without bilateral salpingo-oophorectomy. J. Gynecol. Obstet. Hum. Reprod. 2019, 48, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Tucker, P.E.; Cohen, P.A. Review Article: Sexuality and Risk-Reducing Salpingo-oophorectomy. Int. J. Gynecol. Cancer 2017, 27, 847–852. [Google Scholar] [CrossRef]
- Pieterse, Q.D.; Maas, C.; Ter Kuile, M.; Löwik, M.; Van Eijkeren, M.; Trimbos, J.; Kenter, G.G. An observational longitudinal study to evaluate miction, defecation, and sexual function after radical hysterectomy with pelvic lymphadenectomy for early-stage cervical cancer. Int. J. Gynecol. Cancer 2006, 16, 1119–1129. [Google Scholar] [CrossRef]
- Tangjitgamol, S.; Manusirivithaya, S.; Hanprasertpong, J.; Kasemsarn, P.; Soonthornthum, T.; Leelahakorn, S.; Thawaramara, T.; Lapcharoen, O. Sexual dysfunction in Thai women with early-stage cervical cancer after radical hysterectomy. Int. J. Gynecol. Cancer 2007, 17, 1104–1112. [Google Scholar] [CrossRef]
- Jensen, P.T.; Groenvold, M.; Klee, M.C.; Thranov, I.; Petersen, M.A.; Machin, D. Early-stage cervical carcinoma, radical hysterectomy, and sexual function. Cancer 2003, 100, 97–106. [Google Scholar] [CrossRef]
- Greenwald, H.P.; McCorkle, R. Sexuality and Sexual Function in Long-Term Survivors of Cervical Cancer. J. Women’s Health 2008, 17, 955–963. [Google Scholar] [CrossRef]
- Yen, J.-Y.; Chen, Y.-H.; Long, C.-Y.; Chang, Y.; Yen, C.-F.; Chen, C.-C.; Ko, C.-H. Risk Factors for Major Depressive Disorder and the Psychological Impact of Hysterectomy: A Prospective Investigation. Psychosomatics 2008, 49, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; Van Der Does, J. Surviving gynecologic cancer and coping with sexual morbidity: An international problem. Int. J. Gynecol. Cancer 1994, 4, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Cull, A.; Cowie, V.; Farquharson, D.; Livingstone, J.; Smart, G.; Elton, R. Early stage cervical cancer: Psychosocial and sexual outcomes of treatment. Br. J. Cancer 1993, 68, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Flay, L.D.; Matthews, J.H. The effects of radiotherapy and surgery on the sexual function of women treated for cervical cancer. Int. J. Radiat. Oncol. 1995, 31, 399–404. [Google Scholar] [CrossRef]
- Frumovitz, M.M.; Sun, C.C.; Schover, L.R.; Munsell, M.F.; Jhingran, A.; Wharton, J.T.; Eifel, P.; Bevers, T.B.; Levenback, C.F.; Gershenson, D.M.; et al. Quality of Life and Sexual Functioning in Cervical Cancer Survivors. J. Clin. Oncol. 2005, 23, 7428–7436. [Google Scholar] [CrossRef] [PubMed]
- Ditto, A.; Martinelli, F.; Borreani, C.; Kusamura, S.; Hanozet, F.; Brunelli, C.; Rossi, G.; Solima, E.; Fontanelli, R.; Zanaboni, F.; et al. Quality of Life and Sexual, Bladder, and Intestinal Dysfunctions after Class III Nerve-Sparing and Class II Radical Hysterectomies. Int. J. Gynecol. Cancer 2009, 19, 953–957. [Google Scholar] [CrossRef]
- Jensen, P.T.; Groenvold, M.; Klee, M.C.; Thranov, I.; Petersen, M.A.; Machin, D. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int. J. Radiat. Oncol. 2003, 56, 937–949. [Google Scholar] [CrossRef]
- Robinson, J.W.; Faris, P.D.; Scott, C.B. Psychoeducational group increases vaginal dilation for younger women and reduces sexual fears for women of all ages with gynecological carcinoma treated with radiotherapy. Int. J. Radiat. Oncol. 1999, 44, 497–506. [Google Scholar] [CrossRef]
- Miles, T.; Johnson, N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Coch. Datab. Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Shankar, A.; Patil, J.; Luther, A.; Mandrelle, K.; Chakraborty, A.; Dubey, A.; Saini, D.; Bharat, R.P.; Abrol, D.; Bharati, S.J.; et al. Sexual Dysfunction in Carcinoma Cervix: Assessment in Post Treated Cases by LENTSOMA Scale. Asian Pac. J. Cancer Prev. 2020, 21, 349–354. [Google Scholar] [CrossRef]
- Vermeer, W.M.; Bakker, R.M.; Stiggelbout, A.M.; Creutzberg, C.L.; Kenter, G.G.; Ter Kuile, M.M. Psychosexual support for gynecological cancer survivors: Professionals’ current practices and need for assistance. Support. Care Cancer 2014, 23, 831–839. [Google Scholar] [CrossRef] [PubMed]
| Number of Labors | Spontaneous Labor | Cesarean Section | Both | None |
|---|---|---|---|---|
| 0 labor | 0 | 0 | 0 | 21 |
| 1–2 labor/s | 44 | 22 | 5 | 0 |
| ≥3 labors | 36 | 0 | 8 | 0 |
| Answer of the Respondent (Yes/Not) | Uterine Body Removed | Cervix Intact | Postoperative Chemotherapy and/or Radiotherapy |
|---|---|---|---|
| Yes | 44 | 108 | 15 |
| Not | 92 | 28 | 121 |
| Presence of Postoperative Treatment | No Attempt at Intercourse Was Made | Always or Almost Always | Usually (More than Half the Time) | Sometimes (about Half the Time) | Rarely (Less than Half the Time) | Never or Almost Never |
|---|---|---|---|---|---|---|
| Postoperative chemo- and/or radiotherapy | 5.1 | 0.7 | 2.2 | 0.7 | 0 | 2.2 |
| No postoperative chemo- and/or radiotherapy | 11.8 | 12.5 | 8.1 | 13.2 | 16.9 | 26.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowski, M.; Gargulińska, P.; Ustianowski, Ł.; Lewandowska, R.; Kwiatkowski, S.; Cymbaluk-Płoska, A. Sexuality of Women after Gynecological Surgeries. Healthcare 2020, 8, 393. https://doi.org/10.3390/healthcare8040393
Kozłowski M, Gargulińska P, Ustianowski Ł, Lewandowska R, Kwiatkowski S, Cymbaluk-Płoska A. Sexuality of Women after Gynecological Surgeries. Healthcare. 2020; 8(4):393. https://doi.org/10.3390/healthcare8040393
Chicago/Turabian StyleKozłowski, Mateusz, Paula Gargulińska, Łukasz Ustianowski, Roksana Lewandowska, Sebastian Kwiatkowski, and Aneta Cymbaluk-Płoska. 2020. "Sexuality of Women after Gynecological Surgeries" Healthcare 8, no. 4: 393. https://doi.org/10.3390/healthcare8040393
APA StyleKozłowski, M., Gargulińska, P., Ustianowski, Ł., Lewandowska, R., Kwiatkowski, S., & Cymbaluk-Płoska, A. (2020). Sexuality of Women after Gynecological Surgeries. Healthcare, 8(4), 393. https://doi.org/10.3390/healthcare8040393





