Long-Term HbA1c, Physical Fitness, Nerve Conduction Velocities, and Quality of Life in Children with Type 1 Diabetes Mellitus—A Pilot Study
Abstract
1. Introduction
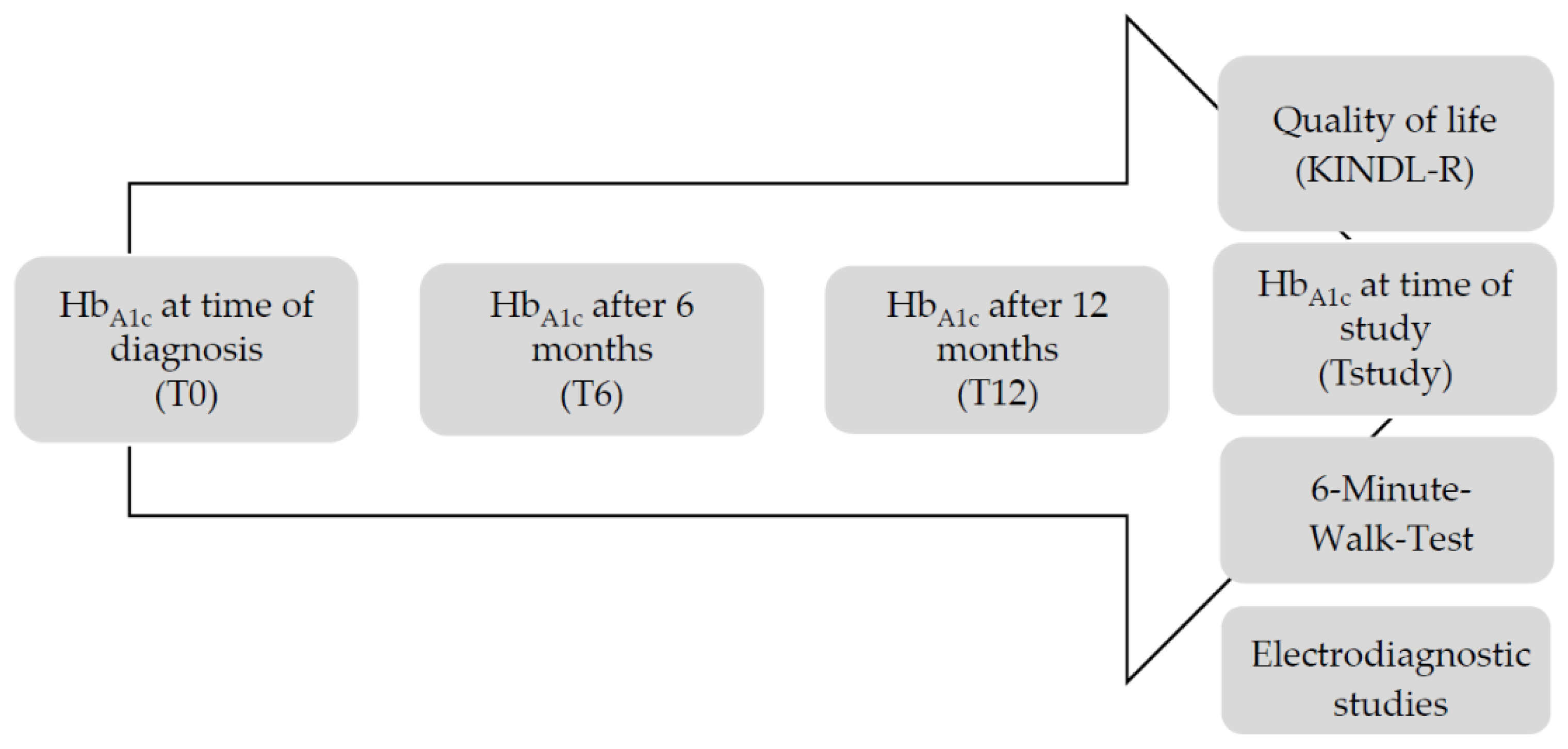
2. Materials and Methods
2.1. HbA1c Measurements
2.2. Quality of Life Questionnaire
2.3. 6-Minute Walk Test (6MWT)
2.4. Nerve Conduction Velocity (NCV)
2.5. Statistical Analysis
3. Results
3.1. Quality of Life (QoL)
3.2. Anthropometric Parameters and 6-Minute Walk Test (6MWT)
3.3. Nerve Conduction Velocity (NCV)
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bradley, C.; Gamsu, D.S. Guidelines for encouraging psychological well-being: Report of a Working Group of the World Health Organization Regional Office for Europe and International Diabetes Federation European Region St Vincent Declaration Action Programme for Diabetes. Diabet. Med. 1994, 11, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Guttmann-Bauman, I.; Flaherty, B.P.; Strugger, M.; McEvoy, R.C. Metabolic control and quality-of-life self-assessment in adolescents with IDDM. Diabet. Care 1998, 21, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Hoey, H.; Aanstoot, H.-J.; Chiarelli, F.; Daneman, D.; Danne, T.; Dorchy, H.; Fitzgerald, M.; Garandeau, P.; Greene, S.; Holl, R.; et al. Good metabolic control is associated with better quality of life in 2101 adolescents with type 1 diabetes. Diabet. Care 2001, 24, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, M.A.; Aaronson, N.K. The role of health care providers and significant others in evaluating the quality of life of patients with chronic disease: A review. J. Clin. Epidemiol. 1992, 45, 743–760. [Google Scholar] [CrossRef]
- Ziegler, D.; Mayer, P.; Gries, F.A. The natural history of somatosensory and autonomic nerve dysfunction in relation to glycaemic control during the first 5 years after diagnosis of Type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1991, 34, 822–829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maser, R.E.; Steenkiste, A.R.; Dorman, J.S.; Nielsen, V.K.; Bass, E.B.; Manjoo, Q.; Drash, A.L.; Becker, D.J.; Kuller, L.H.; Greene, D.A.; et al. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989, 38, 1456–1461. [Google Scholar] [CrossRef]
- Maser, R.E.; Nielsen, V.K.; Dorman, J.S.; Drash, A.L.; Becker, D.J.; Orchard, T.J. Measuring subclinical neuropathy: Does it relate to clinical neuropathy? Pittsburgh epidemiology of diabetes complications study-V. J. Diabet. Complicat. 1991, 5, 6–12. [Google Scholar] [CrossRef]
- Abad, F.; Díaz-Gómez, N.M.; Rodríguez, I.; Pérez, R.; Delgado, J.A. Subclinical pain and thermal sensory dysfunction in children and adolescents with Type 1 diabetes mellitus. Diabet. Med. 2002, 19, 827–831. [Google Scholar] [CrossRef]
- Meh, D.; Denišlič, M. Subclinical neuropathy in type I diabetic children. Electroencephalogr. Clin. Neurophysiol. 1998, 109, 274–280. [Google Scholar] [CrossRef]
- Nelson, D.; Mah, J.K.; Adams, C.; Hui, S.; Crawford, S.; Darwish, H.; Stephure, D.; Pacaud, D. Comparison of conventional and non-invasive techniques for the early identification of diabetic neuropathy in children and adolescents with type 1 diabetes. Pediatr. Diabet. 2006, 7, 305–310. [Google Scholar] [CrossRef]
- Duck, S.C.; Wei, F.F.; Parke, J.; Swick, H.M. Role of Height and Glycosylated Hemoglobin in Abnormal Nerve Conduction in Pediatric Patients With Type I Diabetes Mellitus After 4–9 yr of Disease. Diabet. Care 1991, 14, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Hyllienmark, L.; Brismar, T.; Ludvigsson, J. Subclinical nerve dysfunction in children and adolescents with IDDM. Diabetologia 1995, 38, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Hyllienmark, L.; Alstrand, N.; Jonsson, B.; Ludvigsson, J.; Cooray, G.K.; Wahlberg-Topp, J. Early Electrophysiological Abnormalities and Clinical Neuropathy. Diabet. Care 2013, 36, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Louraki, M.; Karayianni, C.; Kanaka-Gantenbein, C.; Katsalouli, M.; Karavanaki, K. Peripheral neuropathy in children with type 1 diabetes. Diabet. Metab. 2012, 38, 281–289. [Google Scholar] [CrossRef]
- Jegdic, V.; Roncevic, Z.; Skrabic, V. Physical Fitness in Children with Type 1 Diabetes Measured with Six-Minute Walk Test. Int. J. Endocrinol. 2013, 2013, 1–6. [Google Scholar] [CrossRef]
- Nguyen, T.; Obeid, J.; Walker, R.G.; Krause, M.P.; Hawke, T.J.; McAssey, K.; Vandermeulen, J.; Timmons, B.W. Fitness and physical activity in youth with type 1 diabetes mellitus in good or poor glycemic control. Pediatr. Diabet. 2014, 16, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Rosario, A.S.; Schienkiewitz, A.; Neuhauser, H. German height references for children aged 0 to under 18 years compared to WHO and CDC growth charts. Ann. Hum. Biol. 2010, 38, 121–130. [Google Scholar] [CrossRef]
- Bullinger, M.; The BELLA Study Group; Brütt, A.L.; Erhart, M.; Ravens-Sieberer, U. Psychometric properties of the KINDL-R questionnaire: Results of the BELLA study. Eur. Child Adolesc. Psychiatr. 2008, 17 (Suppl. 1), 125–132. [Google Scholar] [CrossRef]
- Ravens-Sieberer, U.; Bullinger, M. Assessing health-related quality of life in chronically ill children with the German KINDL: First psychometric and content analytical results. Qual. Life Res. 1998, 7, 399–407. [Google Scholar] [CrossRef]
- Enright, P.L. The six-minute walk test. Respir. Care 2003, 48, 783–785. [Google Scholar]
- Lee, S.-S.; Han, H.-S.; Kim, H. A 5-yr follow-up nerve conduction study for the detection of subclinical diabetic neuropathy in children with newly diagnosed insulin-dependent diabetes mellitus. Pediatr. Diabet. 2010, 11, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, S.; Grunewald, M.; Gibbels, C.; Jaeger, S.; Matuschek, T.; Weis, S.; Klein, A.M.; Hiemisch, A.; Von Klitzing, K.; Döhnert, M. Self-Esteem of 8–14-Year-Old Children with Psychiatric Disorders: Disorder- and Gender-Specific Effects. Child Psychiatr. Hum. Dev. 2016, 48, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Shrier, L.A.; Harris, S.K.; Sternberg, M.; Beardslee, W.R. Associations of Depression, Self-Esteem, and Substance Use with Sexual Risk among Adolescents. Prev. Med. 2001, 33, 179–189. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.A.; De Freitas, R.W.J.F.; De Lima, L.S.; Dos Santos, M.A.; Zanetti, M.L.; Damasceno, M.M.C. Health-related quality of life of adolescents with type 1 diabetes mellitus. Rev. Lat. Am. Enferm. 2019, 27. [Google Scholar] [CrossRef] [PubMed]
| Patients (n = 17) | Controls (n = 17) | ||
|---|---|---|---|
| Age in years | mean (SD) | 13.3 (3.8) | 13.5 (3.8) |
| Sex, female/male | 6/11 | 6/11 | |
| Disease duration in years | mean (SD) | 4.9 (3.6) | |
| HbA1c% (T0) | mean (SD) | 8.6 (0.9) | |
| HbA1c% (T6) | mean (SD) | 7.0 (0.9) | |
| HbA1c% (T12 | mean (SD) | 7.6 (0.9) | |
| HbA1c% (Tstudy) | mean (SD) | 7.9 (1.4) | |
| Total daily dosage insulin/kg | mean (SD) | 0.8 (0.2) | |
| Insulin pump (yes/no) | 10/7 |
| Patients (n = 17) | Controls (n = 17) | |||
|---|---|---|---|---|
| Child-Rated | Parent-Rated | Child-Rated | Parent-Rated | |
| Total QoL | 80.5 (8.6) | 80.1 (9.5) | 79.9 (11.8) | 75.7 (12.0) |
| Subscale physical wellbeing | 78.6 (11.9) | 81.3 (14.1) | 67.9 (22.6) | 70.7 (25.5) |
| Subscale emotional wellbeing | 86.1 (12.4) | 82.0 (13.9) | 84.6 (15.5) | 78.5 (17.9) |
| Subscale self-esteem | 71.4 (13.6) | 75.8 (15.1) | 79.3 (14.8) | 70.3 (15.2) |
| Subscale family | 85.7 (9.6) | 81.3 (12.1) | 87.9 (16.1) | 79.7 (17.9) |
| Subscale friends | 85.2 (11.4) | 81.1 (9.9) | 77.5 (17.9) | 78.9 (14.8) |
| Subscale school | 68.3 (18.7) | 79.0 (19.4) | 79.0 (20.7) | 75.8 (19.7) |
| Patients (n = 17) | Controls (n = 17) | p-Value | ||
|---|---|---|---|---|
| Height SDS | 0.1 (0.8) | 0.7 (1.2) | p = 0.231 | |
| Weight SDS | 0.1 (0.7) | 0.7 (1.1) | p = 0.339 | |
| BMI SDS | 0.1 (0.8) | 0.5 (1.2) | p = 0.245 | |
| 6MWD | 639.4 (110.5) | 649.4 (60.0) | p = 0.929 | |
| Heart rate | pre-walk | 90.5 (20.2) | 85.8 (17.2) | p = 0.423 |
| post-walk | 144.1 (37.5) | 148.5 (23.5) | p = 0.323 |
| Patients (n = 17) | Controls (n = 17) | p-Value | |
|---|---|---|---|
| Motor nerves | |||
| Median | 53.1 (3.2) | 58.5 (5.1) | p = 0.006 |
| Ulnar | 52.7 (3.8) | 55.0 (6.3) | p = 0.114 |
| Peroneal | 47.3 (4.6) | 48.2 (5.1) | p = 0.316 |
| Tibial | 44.3 (7.9) | 46.5 (6.0) | p = 0.186 |
| Sensory nerves | |||
| Median | 54.6 (8.8) | 57.8 (6.0) | p = 0.178 |
| Ulnar | 55.1 (9.9) | 56.6 (8.6) | p = 0.245 |
| Sural | 48.3 (5.3) | 47.5 (3.8) | p = 0.608 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiller, K.; Kofler, M.; Frühwirth, M.; Fantur, M.; Rauchenzauner, M. Long-Term HbA1c, Physical Fitness, Nerve Conduction Velocities, and Quality of Life in Children with Type 1 Diabetes Mellitus—A Pilot Study. Healthcare 2020, 8, 384. https://doi.org/10.3390/healthcare8040384
Schiller K, Kofler M, Frühwirth M, Fantur M, Rauchenzauner M. Long-Term HbA1c, Physical Fitness, Nerve Conduction Velocities, and Quality of Life in Children with Type 1 Diabetes Mellitus—A Pilot Study. Healthcare. 2020; 8(4):384. https://doi.org/10.3390/healthcare8040384
Chicago/Turabian StyleSchiller, Katharina, Markus Kofler, Martin Frühwirth, Michaela Fantur, and Markus Rauchenzauner. 2020. "Long-Term HbA1c, Physical Fitness, Nerve Conduction Velocities, and Quality of Life in Children with Type 1 Diabetes Mellitus—A Pilot Study" Healthcare 8, no. 4: 384. https://doi.org/10.3390/healthcare8040384
APA StyleSchiller, K., Kofler, M., Frühwirth, M., Fantur, M., & Rauchenzauner, M. (2020). Long-Term HbA1c, Physical Fitness, Nerve Conduction Velocities, and Quality of Life in Children with Type 1 Diabetes Mellitus—A Pilot Study. Healthcare, 8(4), 384. https://doi.org/10.3390/healthcare8040384






