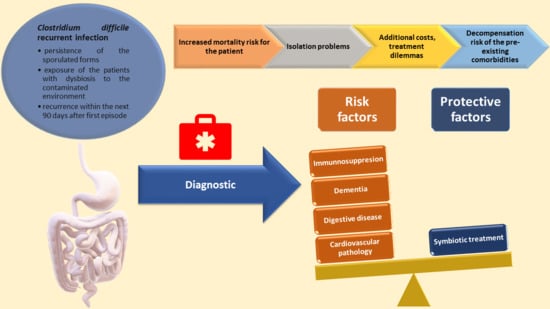
Risk Factors Associated with Recurrent Clostridioides difficile Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Diagnosis of CDI
2.3. Statistical Analysis
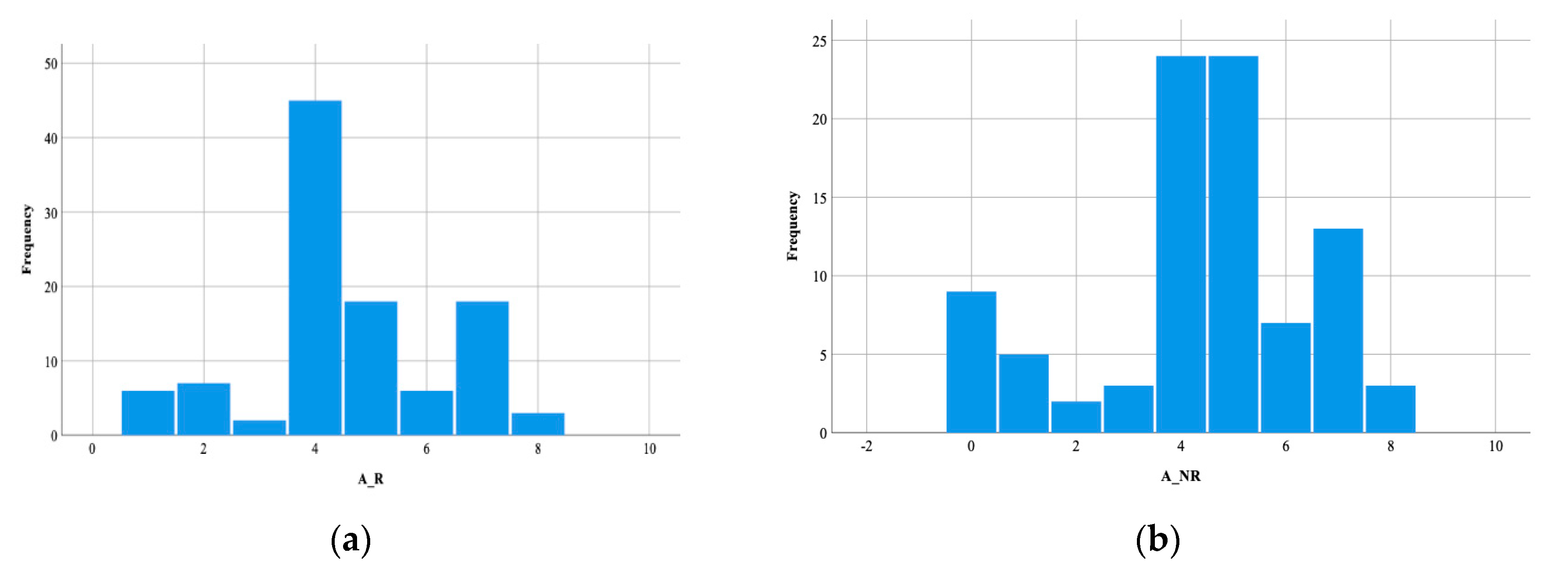
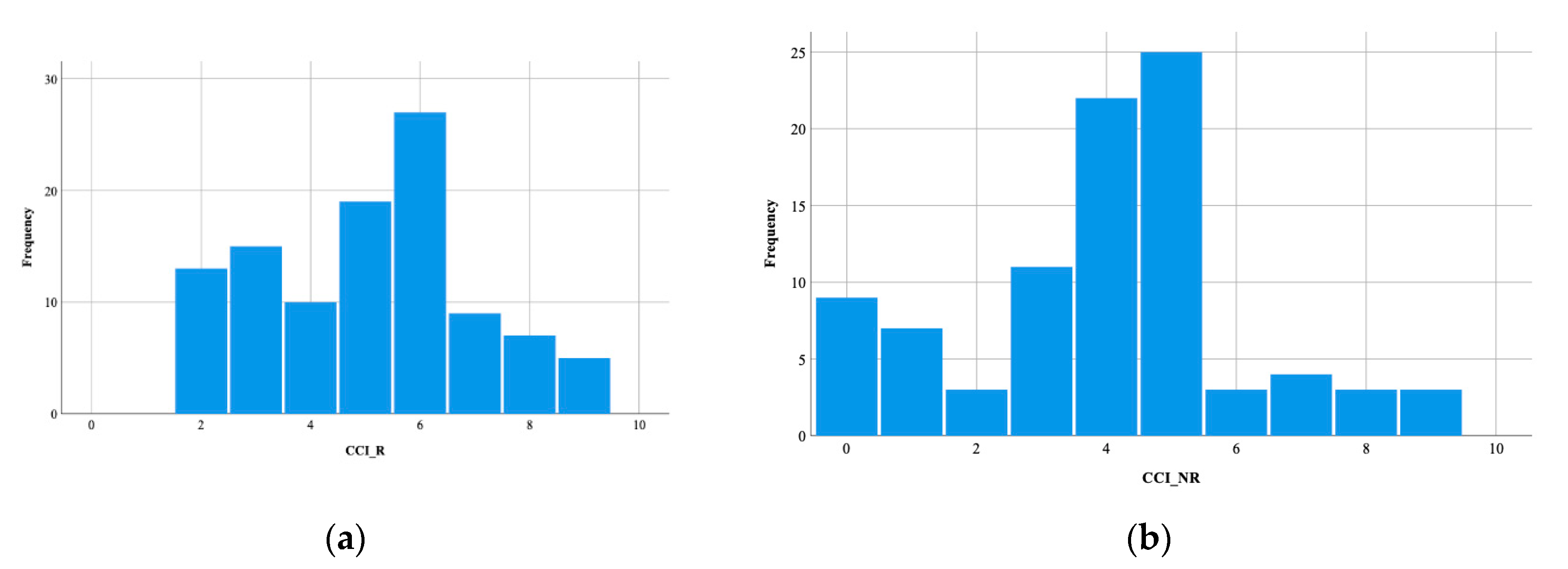
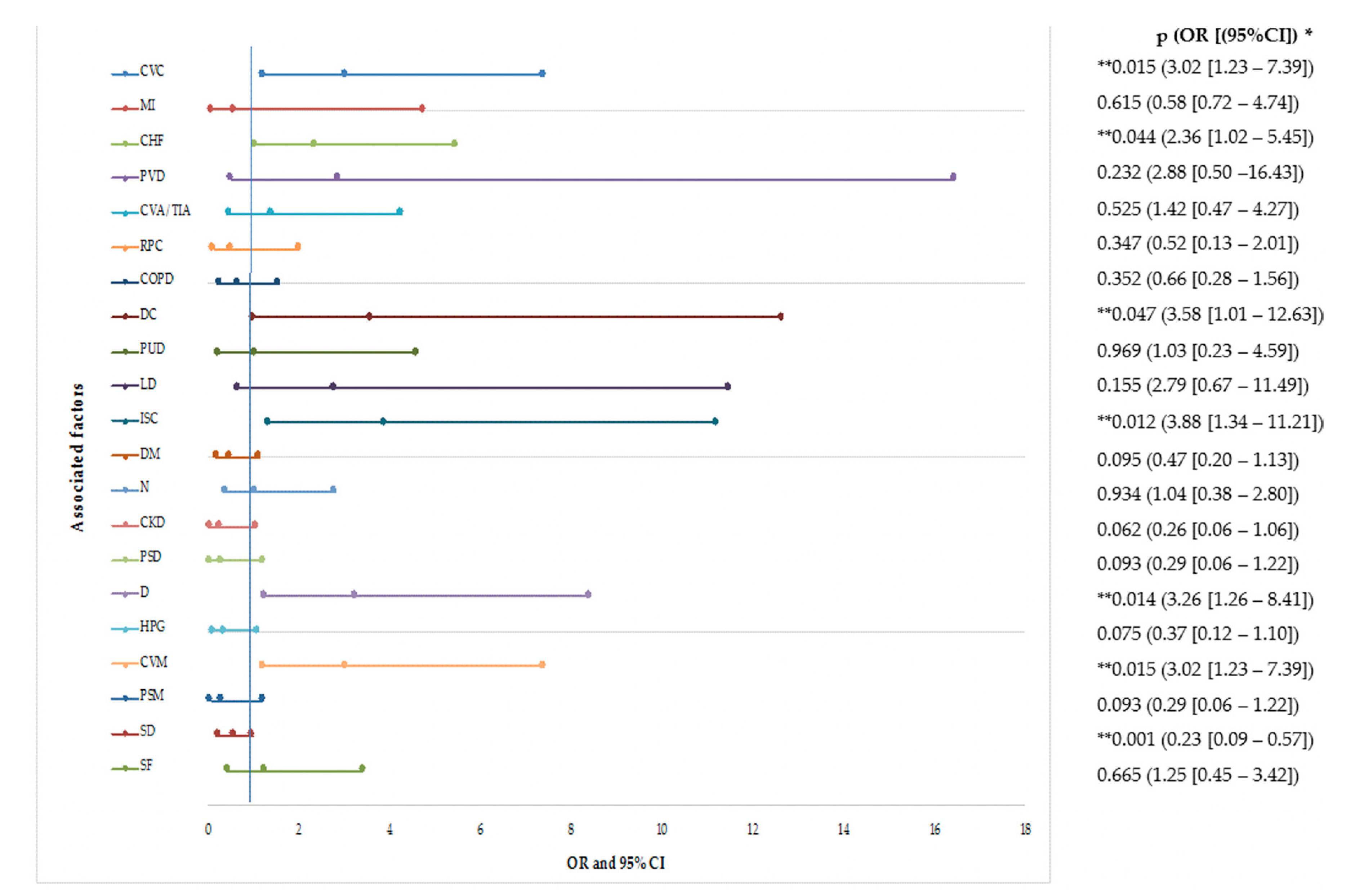
3. Results
4. Discussion
4.1. Associated Factors with Recurrent CDI
4.1.1. Demographic Factors
4.1.2. Clinical Factors
4.1.3. Environmental Conditions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kelly, C.P. Can we identify patients at high risk of recurrent clostridium difficile infection? Clin. Microbiol. Infect. 2012, 18, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, Y.S. Recurrent clostridium difficile infection: Risk factors, treatment, and prevention. Gut Liver 2019, 13, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Knafl, D.; Vossen, M.G.; Gerges, C.; Lobmeyr, E.; Karolyi, M.; Wagner, L.; Thalhammer, F. Hypoalbuminemia as predictor of recurrence of clostridium difficile infection. Wien. Klin. Wochenschr. 2019, 131, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.; Hernandez, A.V.; Donskey, C.J.; Fraser, T.G. Risk factors for recurrent clostridium difficile infection: A systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2015, 36, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Higgins, P.D.R.; Young, V.B. An observational cohort study of clostridium difficile ribotype 027 and recurrent infection. mSphere 2018, 3, e00033-18. [Google Scholar] [CrossRef] [PubMed]
- Louie, T.J.; Miller, M.A.; Mullane, K.M.; Weiss, K.; Lentnek, A.; Golan, Y.; Gorbach, S.; Sears, P.; Shue, Y.K. Fidaxomicin versus vancomycin for clostridium difficile infection. N. Engl. J. Med. 2011, 364, 422–431. [Google Scholar] [CrossRef]
- Cornely, O.A.; Miller, M.A.; Louie, T.J.; Crook, D.W.; Gorbach, S.L. Treatment of first recurrence of clostridium difficile infection: Fidaxomicin versus vancomycin. Clin. Infect. Dis. 2012, 55, S154–S161. [Google Scholar] [CrossRef]
- Johnson, S.; Gerding, D.N. Fidaxomicin “chaser” regimen following vancomycin for patients with multiple clostridium difficile recurrences. Clin. Infect. Dis. 2013, 56, 309–310. [Google Scholar] [CrossRef]
- Alonso, C.D.; Mahoney, M.V. Bezlotoxumab for the prevention of clostridium difficile infection: A review of current evidence and safety profile. Infect. Drug Resist. 2018, 12, 1–9. [Google Scholar] [CrossRef]
- Bezlotoxumab as Secondary Prophylaxis for c. Difficile in High-Risk Hospitalized Patients Exposed to Antibiotics. Available online: https://clinicaltrials.gov/ct2/show/NCT03937999 (accessed on 21 February 2020).
- Bua, A.; Usai, D.; Donadu, M.G.; Delgado Ospina, J.; Paparella, A.; Chaves-Lopez, C.; Serio, A.; Rossi, C.; Zanetti, S.; Molicotti, P. Antimicrobial activity of austroeupatorium inulaefolium (h.B.K.) against intracellular and extracellular organisms. Nat. Prod. Res. 2018, 32, 2869–2871. [Google Scholar] [CrossRef]
- Thapa, D.; Losa, R.; Zweifel, B.; Wallace, R.J. Sensitivity of pathogenic and commensal bacteria from the human colon to essential oils. Microbiology 2012, 158, 2870–2877. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition and antimicrobial activity of angelica archangelica l. (apiaceae) roots. J. Med. Food 2014, 17, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Chaves-López, C.; Usai, D.; Donadu, M.G.; Serio, A.; González-Mina, R.T.; Simeoni, M.C.; Molicotti, P.; Zanetti, S.; Pinna, A.; Paparella, A. Potential of borojoa patinoi cuatrecasas water extract to inhibit nosocomial antibiotic resistant bacteria and cancer cell proliferation in vitro. Food Funct. 2018, 9, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Louie, T.; Mullane, K.; Weiss, K.; Lentnek, A.; Golan, Y.; Kean, Y.; Sears, P. Derivation and validation of a simple clinical bedside score (atlas) for clostridium difficile infection which predicts response to therapy. BMC Infect. Dis. 2013, 13, 148. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef]
- Certest Clostridium Difficile gdh+toxin a+b. Available online: https://www.certest.es/wp-content/uploads/2020/05/IU-GX87V-v.01.pdf (accessed on 25 May 2020).
- Detection of Clostridium Difficile Infection with an Independent Call-Out of Binary Toxin and Differentiation of the 027 Strain in under an Hour. Available online: https://www.cepheid.com/en/tests/Healthcare-Associated-Infections/Xpert-C.-difficile-BT (accessed on 25 May 2020).
- Singh, T.; Bedi, P.; Bumrah, K.; Singh, J.; Rai, M.; Seelam, S. Updates in treatment of recurrent clostridium difficile infection. J. Clin. Med. Res. 2019, 11, 465–471. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of america (idsa) and society for healthcare epidemiology of america (shea). Clin. Infect. Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Marsh, J.W.; Arora, R.; Schlackman, J.L.; Shutt, K.A.; Curry, S.R.; Harrison, L.H. Association of relapse of clostridium difficile disease with bi/nap1/027. J. Clin. Microbiol. 2012, 50, 4078–4082. [Google Scholar] [CrossRef]
- Negrut, N.; Nistor-Cseppento, D.C.; Khan, S.A.; Pantis, C.; Maghiar, T.A.; Maghiar, O.; Aleya, S.; Rus, M.; Tit, D.M.; Aleya, L.; et al. Clostridium difficile infection epidemiology over a period of 8 years—A single centre study. Sustainability 2020, 12, 4439. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; El-Tawil, O.S.; Bungau, S.G.; Atanasov, A.G. Applications of antioxidants in metabolic disorders and degenerative diseases: Mechanistic approach. Oxid. Med. Cell. Longev. 2019, 2019, 1–3. [Google Scholar] [CrossRef]
- Taneja, V. Sex hormones determine immune response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.J.; Travers, P.; Walport, M.; Shlomchik, M.J. Immunobiology, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Xu, C.; Zhu, H.; Qiu, P. Aging progression of human gut microbiota. BMC Microbiol. 2019, 19, 236. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W. (dis)trust your gut: The gut microbiome in age-related inflammation, health, and disease. Microbiome 2017, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Index. Foods and Dietary Supplements in the Prevention and Treatment of Disease in Older Adults; Watson, R.R., Ed.; Academic Press: San Diego, CA, USA, 2015; pp. 373–380. [Google Scholar]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.Z.; Abe, F.; Osawa, R. Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, F.; Wu, Q.; Gao, J.; Liu, W.; Liu, C.; Guo, X.; Suwal, S.; Kou, Y.; Zhang, B.; et al. Protective effects of bifidobacterial strains against toxigenic clostridium difficile. Front. Microbiol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Maisa, A.; Ross, G.; Verlander, N.; Fairley, D.; Bradley, D.; Patterson, L. Comparing the epidemiology of community- and hospital-associated clostridium difficile infections in northern ireland, 2012–2016: A population data linkage and case–case study. Epidemiol. Infect. 2019, 147, e141. [Google Scholar] [CrossRef]
- Negruţ, N.; Khan, S.; Bungau, S.; Zaha, D.; Aron, R.; Bratu, O.; Diaconu, C.; Ionita Radu, F. Diagnostic challenges in gastrointestinal infections. Rom. J. Mil. Med. 2020, CXXIII, 83–90. [Google Scholar]
- Redding, L.E.; Kelly, B.J.; Stefanovski, D.; Lautenbach, J.K.; Tolomeo, P.; Cressman, L.; Gruber, E.; Meily, P.; Lautenbach, E. Pet ownership protects against recurrence of clostridioides difficile infection. Open Forum Infect. Dis. 2020, 7, ofz541. [Google Scholar] [CrossRef]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in fecal microbiota in different european study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef]
- Jacobson, S.M.; Slain, D. Evaluation of a bedside scoring system for predicting clinical cure and recurrence of clostridium difficile infections. Am. J. Health Syst. Pharm. 2015, 72, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Petrella, L.A.; Sambol, S.P.; Cheknis, A.; Nagaro, K.; Kean, Y.; Sears, P.S.; Babakhani, F.; Johnson, S.; Gerding, D.N. Decreased cure and increased recurrence rates for clostridium difficile infection caused by the epidemic c. Difficile bi strain. Clin. Infect. Dis. 2012, 55, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Snijder, R.; Sugitani, T. Characterization and risk factors for recurrence of clostridioides (clostridium) difficile infection in japan: A nationwide real-world analysis using a large hospital-based administrative dataset. J. Infect. Chemother. 2019, 25, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Olivos, N.E.; Balderas-Garcés, B.; Gómez-Gutiérrez, C.; Noffal-Nuno, V.; Méndez-Sánchez, N. Risk factors associated with recurrent clostridium difficile infection in a group of inpatients in mexico. Med. Surg. 2016, 23, 97–99. [Google Scholar]
- World Health Organization. Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 (accessed on 4 June 2020).
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β cell axis promoting metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of trimethylamine n-oxide (tmao) in disease: Potential biomarker or new therapeutic target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut microbiota in cardiovascular health and disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Ryan, P.M.; Stanton, C.; Caplice, N.M. Bile acids at the cross-roads of gut microbiome-host cardiometabolic interactions. Diabetol. Metab. Syndr. 2017, 9, 102. [Google Scholar] [CrossRef]
- Mamic, P.; Heidenreich, P.A.; Hedlin, H.; Tennakoon, L.; Staudenmayer, K.L. Hospitalized patients with heart failure and common bacterial infections: A nationwide analysis of concomitant clostridium difficile infection rates and in-hospital mortality. J. Card. Fail. 2016, 22, 891–900. [Google Scholar] [CrossRef]
- Pero, R.; Brancaccio, M.; Laneri, S.; Biasi, M.G.; Lombardo, B.; Scudiero, O. A novel view of human helicobacter pylori infections: Interplay between microbiota and beta-defensins. Biomolecules 2019, 9, 237. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (ibd): Cause or consequence? Ibd treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [PubMed]
- Ilias, T.; Bungau, S.; Tit, D.M.; Maghiar, D.; Hocopan, C.; Brata, R.; Bratu, O.G.; Negrut, N.; Diaconu, C.; Fratila, O. Psychosocial profile of the patients with inflammatory bowel disease. Exp. Ther. Med. 2020, 20, 2493–2500. [Google Scholar] [PubMed]
- Purza, L.; Abdel-Daim, M.; Belba, A.; Iovan, C.; Bumbu, A.; Lazar, L.; Bungau, S.; Tit, D.M. Monitoring the effects of various combination of specific drug therapies at different stages of alzheimer′s dementia. Farmacia 2019, 67, 477–481. [Google Scholar] [CrossRef]
- Codrean, A.; Dumitrascu, D.L.; Codrean, V.; Tit, D.M.; Bungau, S.; Aleya, S.; Rus, M.; Fratila, O.; Nistor-Cseppento, D.C.; Aleya, L.; et al. Epidemiology of human giardiasis in romania: A 14 years survey. Sci. Total Environ. 2020, 705, 135784. [Google Scholar] [CrossRef]
- Beatty, J.K.; Akierman, S.V.; Motta, J.P.; Muise, S.; Workentine, M.L.; Harrison, J.J.; Bhargava, A.; Beck, P.L.; Rioux, K.P.; McKnight, G.W.; et al. Giardia duodenalis induces pathogenic dysbiosis of human intestinal microbiota biofilms. Int. J. Parasitol. 2017, 47, 311–326. [Google Scholar] [CrossRef]
- Trifan, A.; Stoica, O.; Stanciu, C.; Cojocariu, C.; Singeap, A.M.; Girleanu, I.; Miftode, E. Clostridium difficile infection in patients with liver disease: A review. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2313–2324. [Google Scholar] [CrossRef]
- Dharbhamulla, N.; Abdelhady, A.; Domadia, M.; Patel, S.; Gaughan, J.; Roy, S. Risk factors associated with recurrent clostridium difficile infection. J. Clin. Med. Res. 2019, 11, 1–6. [Google Scholar] [CrossRef]
- Appaneal, H.J.; Caffrey, A.R.; Beganovic, M.; Avramovic, S.; LaPlante, K.L. Predictors of clostridioides difficile recurrence across a national cohort of veterans in outpatient, acute, and long-term care settings. Am. J. Health Syst. Pharm. 2019, 76, 581–590. [Google Scholar] [CrossRef]
- Avni, T.; Babitch, T.; Ben-Zvi, H.; Hijazi, R.; Ayada, G.; Atamna, A.; Bishara, J. Clostridioides difficile infection in immunocompromised hospitalized patients is associated with a high recurrence rate. Int. J. Infect. Dis. 2020, 90, 237–242. [Google Scholar] [CrossRef]
- Uivarosan, D.; Tit, D.M.; Iovan, C.; Nistor-Cseppento, D.C.; Endres, L.; Lazar, L.; Sava, C.; Sabau, A.M.; Buhas, C.; Moleriu, L.C.; et al. Effects of combining modern recovery techniques with neurotrophic medication and standard treatment in stroke patients. Sci. Total Environ. 2019, 679, 80–87. [Google Scholar] [CrossRef]
- Bretler, T.; Weisberg, H.; Koren, O.; Neuman, H. The effects of antipsychotic medications on microbiome and weight gain in children and adolescents. BMC Med. 2019, 17, 112. [Google Scholar] [CrossRef] [PubMed]
- Hort, J.; Valis, M.; Angelucci, F. Administration of pre/probiotics with conventional drug treatment in alzheimer’s disease. Neural Regen. Res. 2020, 15, 448–449. [Google Scholar] [PubMed]
- Na, X.; Kelly, C. Probiotics in clostridium difficile infection. J. Clin. Gastroenterol. 2011, 45, S154–S158. [Google Scholar] [CrossRef] [PubMed]
- Zaha, D.C.; Bungau, S.; Aleya, S.; Tit, D.M.; Vesa, C.M.; Popa, A.R.; Pantis, C.; Maghiar, O.A.; Bratu, O.G.; Furau, C.; et al. What antibiotics for what pathogens? The sensitivity spectrum of isolated strains in an intensive care unit. Sci. Total Environ. 2019, 687, 118–127. [Google Scholar] [CrossRef]
- Zaha, D.C.; Bungau, S.; Uivarosan, D.; Tit, D.M.; Maghiar, T.A.; Maghiar, O.; Pantis, C.; Fratila, O.; Rus, M.; Vesa, C.M. Antibiotic consumption and microbiological epidemiology in surgery departments: Results from a single study center. Antibiotics 2020, 9, 81. [Google Scholar] [CrossRef]
- Deshpande, A.; Pasupuleti, V.; Mossad, S.B.; Budev, M.; Schmitt, S.K.; Corey, R.; Eghtesad, B.; Mawhorter, S.D.; Hernandez, A.V.; Jain, A.; et al. Use of lactobacillus in prevention of recurrences of clostridium difficile infection in solid organ transplant recipients. Infect. Dis. Clin. Pract. 2013, 21, 292–298. [Google Scholar] [CrossRef]

| Age group (Years) | R | NR | p |
|---|---|---|---|
| <50, M, SD (N) | 42.50 ± 6.73 (14) | 38.15 ± 10.23 (13) | 0.200 |
| 50–59, M, SD (N) | 56.00 ± 2.27 (23) | 57.00 ± 0.0001(6) | 0.297 |
| 60–69, M, SD (N) | 66.92 ± 1.44 (13) | 64.14 ± 1.06 (7) | <0.001 |
| 70–79, M, SD (N) | 75.70 ± 3.49 (30) | 76.41 ± 2.96 (54) | 0.326 |
| >80, M, SD (N) | 84.16 ± 2.15 (25) | 85.10 ± 2.18 (10) | 0.253 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrut, N.; Bungau, S.; Behl, T.; Khan, S.A.; Vesa, C.M.; Bustea, C.; Nistor-Cseppento, D.C.; Rus, M.; Pavel, F.-M.; Tit, D.M. Risk Factors Associated with Recurrent Clostridioides difficile Infection. Healthcare 2020, 8, 352. https://doi.org/10.3390/healthcare8030352
Negrut N, Bungau S, Behl T, Khan SA, Vesa CM, Bustea C, Nistor-Cseppento DC, Rus M, Pavel F-M, Tit DM. Risk Factors Associated with Recurrent Clostridioides difficile Infection. Healthcare. 2020; 8(3):352. https://doi.org/10.3390/healthcare8030352
Chicago/Turabian StyleNegrut, Nicoleta, Simona Bungau, Tapan Behl, Shamim Ahmad Khan, Cosmin Mihai Vesa, Cristiana Bustea, Delia Carmen Nistor-Cseppento, Marius Rus, Flavia-Maria Pavel, and Delia Mirela Tit. 2020. "Risk Factors Associated with Recurrent Clostridioides difficile Infection" Healthcare 8, no. 3: 352. https://doi.org/10.3390/healthcare8030352
APA StyleNegrut, N., Bungau, S., Behl, T., Khan, S. A., Vesa, C. M., Bustea, C., Nistor-Cseppento, D. C., Rus, M., Pavel, F.-M., & Tit, D. M. (2020). Risk Factors Associated with Recurrent Clostridioides difficile Infection. Healthcare, 8(3), 352. https://doi.org/10.3390/healthcare8030352