Health Information Technology and Doctor Shopping: A Systematic Review
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search
2.5. Study Selection
2.6. Data Collection Process
2.7. Data Items
2.8. Risk of Bias within and across Studies
2.9. Summary Measures
2.10. Additional Analysis
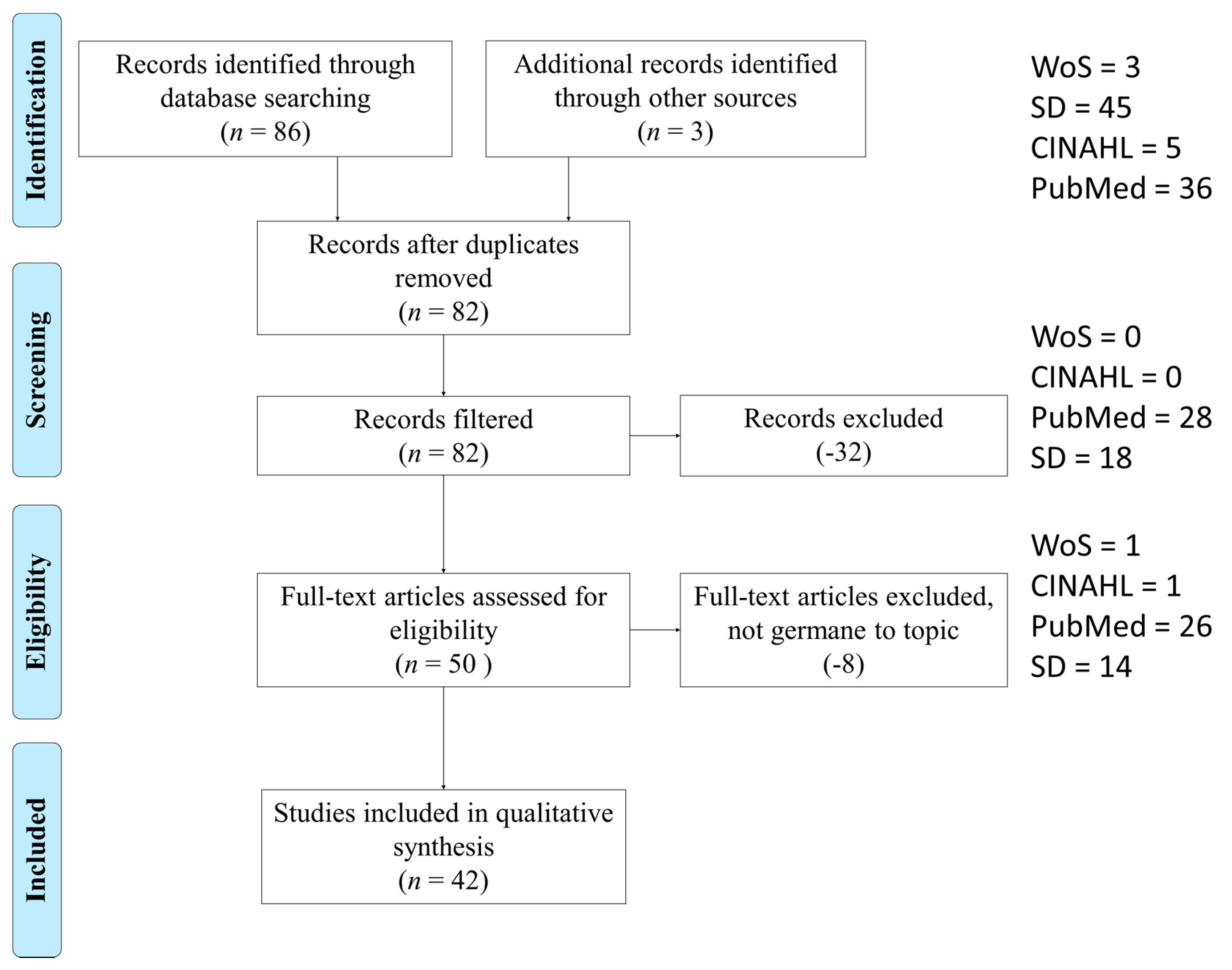
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
3.4. Results of Individual Studies
3.5. Synthesis of Results
3.6. Risk of Bias across Studies
3.7. Additional Analysis
3.7.1. Interventions of HIT
3.7.2. Facilitators of HIT
3.7.3. Barriers of HIT
3.7.4. Medical Outcomes Commensurate with HIT as Intervention
3.8. Interactions between Observations
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CEIP | French Centers for Evaluation and Information on Pharmacodependence |
| CNCP | Chronic non-cancer pain |
| DSI | Drug Shopping Indicator |
| HIE | Health Information Exchange |
| NPI | National provider identifier |
| MME | morphine milligram equivalents |
| NMPR | Nonmedical prescription use |
| PBM | Pharmacy Benefit Managers |
| PDMR | Prescription Drug Monitoring Program |
| SNIIRAM | Global health insurance reimbursement database |
Appendix A
| Authors | Intervention | Intervention Theme | Medical Outcomes Reported | Outcome Theme | Facilitators | Facilitator Theme | Barriers | Barrier Theme |
|---|---|---|---|---|---|---|---|---|
| Pett RG, et al. | PDMP | PDMP | Naloxone prescribed to shoppers | Shoppers prescribed treatment | Pharmacists support PDMP | Pharmacists support | Not reported | Not reported |
| Durand Z, et al. | PDMP and worker’s compensation claims | Combination | Not reported | Not reported | Government support for PDMP | Government support | PDMP systems do not share data across state lines | Data sharing |
| Freeman PR, et al. | Interviews about PDMP | PDMP | Not reported | Not reported | Government support for PDMP, Pharmacists support for PDMP, PCP support for PDMP | Government support | Access not mandatory | Access not mandatory |
| Pharmacists support | ||||||||
| Prescriber support | ||||||||
| Nagarajan R and Talbert J | computer model | Computer model | Not reported | Not reported | Model only requires data and a statistician | Simple to implement | Not reported | Not reported |
| Perry BL, et al. | computer model PageRank | Computer model | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Soffin EM, et al. | PDMP | PDMP | Accessing real-time information about patients’ prescription opioid status using PDMP reduces opioid quantities prescribed | Reduced opioids prescribed | State supports PDMP, Providers support PDMP | Government support | Not reported | Not reported |
| Prescriber support | ||||||||
| Stopka TJ, et al. | computer model | Computer model | Not reported | Not reported | Computer model not difficult to implement (low cost) | Simple to implement | Not reported | Not reported |
| Wang Y, et al. | Pharmacological database | Other | Not reported | Not reported | State supports prescription monitoring | Government support | Not reported | Not reported |
| Butler JM, et al. | PDMP, Lock-in programs | Combination | Broader range of drug schedules and an increases frequency of updating the PDMP results in lower opioid-related mortality | Reduced mortality | A “must use” law (ten states) significantly decreased doctor shopping | Must use law | PDMP is not mandatory, Lock-in programs do not treat substance abuse (may just drive users to black-market) Only billing information is used (not cash) | Access not mandatory |
| Does not treat addiction | ||||||||
| Lin JH, et al. | Medication record sharing program | Other | Not reported | Not reported | Government support for the medication record sharing program | Government support | Not reported | Not reported |
| Ponte C, et al. | SNIIRAM database | National health system DB | Not reported | Not reported | Government support for prescription monitoring | Government support | Not reported | Not reported |
| Torrance N, et al. | NHS and Generation Scotland databases combined to identify trends | National health system DB | Not reported | Not reported | State supports prescription monitoring | Government support | Not reported | Not reported |
| Ali MM, et al. | Survey instrument to assess effectiveness of the PDMP | PDMP | 10–20 fewer days of NMPR use. | Fewer days of use | Not reported | Not reported | Not reported | Not reported |
| Rutkow L, et al. | PDMP | PDMP | Not reported | Not reported | Government support for prescription monitoring | Government support | PDMP systems do not share data across state lines | Data sharing |
| Simeone R | PDMP, education efforts, pharmacy panels that span the country | Combination | Decline in overdose mortality | Reduced mortality | Government support for PDMP, Pharmacy groups have designed a proprietary system to track prescriptions | Government support | Enterprising dealers can use more drug collection agents | Easily thwarted |
| Pharmacists support | ||||||||
| Chenaf C, et al. | French national health system | National health system DB | Not reported | Not reported | French national health system monitors drug use | Government support | Not reported | Not reported |
| Delorme J, et al. | French national health system | National health system DB | Not reported | Not reported | French national health system monitors drug use | Government support | Not reported | Not reported |
| Kea B, et al. | PDMP | PDMP | Not reported | Not reported | Government support for PDMP | Government support | Not reported | Not reported |
| National Council of State Boards of Nursing | PDMP | PDMP | Not reported | Not reported | Government support for PDMP | Government support | Not reported | Not reported |
| Okumura Y, et al. | Japan national health database | National health system DB | Not reported | Not reported | Government support for monitoring program | Government support | Not reported | Not reported |
| Ong MS, et al. | Provider patient-sharing networks | Health insurance claims | Not reported | Not reported | State supports PDMP, Providers support PDMP | Government support | State PDMPs cannot share data across state lines | Data sharing |
| Prescriber support | ||||||||
| Takahashi Y, et al. | Social network analysis | Computer model | Not reported | Not reported | Social network analysis is not difficult to implement (low cost) | Simple to implement | Not reported | Not reported |
| Huang SK, et al. | PharmaCloud | Other | Not reported | Not reported | The drug expense per person declined (2–5%), Government support for PharmaCloud | Cost savings | Intervention is not widely adopted and not mandatory | Participation not mandatory |
| Government support | Access not mandatory | |||||||
| Lin MH, et al. | Medication record sharing program | Health insurance claims | Not reported | Not reported | Government support for the medication record sharing program | Government support | Not reported | Not reported |
| Lu TH, et al. | Medication record sharing program | Health insurance claims | Not reported | Not reported | Government support for the medication record sharing program | Government support | Not reported | Not reported |
| Webster LR and Grabois M | PDMP | PDMP | Not reported | Not reported | State supports prescription monitoring | Government support | Access to PDMP not mandatory | Access not mandatory |
| Han H, et al. | PDMP | PDMP | prior to the PDMP in California, the prevalence of schedule II opioid users in California increased by 150%–280% and prevalence of doctor shoppers increased 111%–213% over 9 years. | Reduced shopping | Not reported | Not reported | Not reported | Not reported |
| Hypponen H, et al. | HIE | Health information exchange | Not reported | Not reported | Government support for the HIE, Decrease in healthcare costs through reduced duplicate testing, Increased efficiency of clinical information gathering | Government support | Not reported | Not reported |
| Cost savings | ||||||||
| Increased efficiency | ||||||||
| Shepherd J | PDMP and PBM | Combination | Not reported | Not reported | Not reported | Not reported | Inadequate data collection, PBM do not process all painkiller prescriptions, | Inadequate data collection |
| Ineffective utilization of data, | Ineffective data use | |||||||
| Insufficient interstate data sharing, | Data sharing | |||||||
| Constraints on data sharing with law enforcement and state agencies, | Constraints on enforcement | |||||||
| Prescription drugs purchased with cash (not insurance) not processed through PBM | Cash-only not captured | |||||||
| Cepeda MS, et al. | PDMP | PDMP | Shopping decreased within the same state, | Reduced shopping | Not reported | Not reported | PDMP systems do not share data across state lines | Data sharing |
| Modarai F, et al. | Automation of Reports and Consolidation Orders System (ARCOS), Drug Abuse Reporting Network (DAWN) | Other | Not reported | Not reported | Government support for ARCOS and DAWN | Government support | Not reported | Not reported |
| Rouby F, et al. | Reimbursement database | National health system DB | Not reported | Not reported | Government support for prescription monitoring | Government support | Not reported | Not reported |
| Simoni-Wastila and Qian J | PDMP | PDMP | No decline in overdose mortality | No decline in mortality | State and federal government support for PDMP | Government support | Not reported | Not reported |
| Worley J, et al. | PDMP | PDMP | PDMPs decrease diversion and doctor shopping | Reduced shopping | State supports PDMP, | Government support | Not reported | Not reported |
| Providers support PDMP | Prescriber support | |||||||
| Worley J, et al. | PDMP | PDMP | Not reported | Not reported | State supports PDMP, | Government support | Not reported | Not reported |
| Providers support PDMP | Prescriber support | |||||||
| Fass JA and Hardigan PC | PDMP | PDMP | Not reported | Not reported | Government support for PDMP | Government support | Not reported | Not reported |
| Frauger E, et al. | CEIP | Other | Not reported | Not reported | Government support for CEIP | Government support | Not reported | Not reported |
| Hincapie AL, et al. | Interviews about HIE | Health information exchange | Not reported | Not reported | Government support for HIE | Government support | Not all participate in the HIE, therefore data cannot be universally exchanged | Participation not mandatory |
| Hsu MH, et al. | CPOE and NHI-IC cards | Combination | Not reported | Not reported | Physicians accept the intervention | Prescriber support | Participation is not universal or mandatory | Participation not mandatory |
| Pauly V, et al. | computer model | Computer model | Not reported | Not reported | Government supports prescription monitoring | Government support | Not reported | Not reported |
| Wilsey BL, et al. | PDMP | PDMP | Not reported | Not reported | State supports prescription monitoring | Government support | Not reported | Not reported |
| Pradel V, et al. | Prescription database | National health system DB | Not reported | Not reported | Government support for prescription monitoring | Government support | Not reported | Not reported |
Appendix B
| Authors | Sample Size | Bias within Study | Country of Origin | Statistics Used | JHNEBP | |
|---|---|---|---|---|---|---|
| Strength | Quality | |||||
| Pett RG, et al. | 967, average age not reported | United States only | United States | Logistic regression | II | A |
| Durand Z, et al. | 172,256, average age 41 | United States only | United States | t-test | III | A |
| Freeman PR, et al. | 48 PCP, 60 pharmacists, average age not reported | United States only | United States | n/a | III | B |
| Nagarajan R and Talbert J | 11,596 | United States only | United States | Tukey’s outlier detection and surrogate testing | III | B |
| Perry BL, et al. | 526,914 patients, 2,107,656 quarterly prescription entries | United States only | United States | Regression | III | A |
| Soffin EM, et al. | n/a | United States only | United States | n/a | IV | C |
| Stopka TJ, et al. | 3,143,817, average age not reported | United States only | United States | t-test | IV | C |
| Wang Y, et al. | 17,000, average age not reported | United States only | United States | t-test and descriptives | III | A |
| Butler JM, et al. | n/a | United States only | United States | n/a | IV | B |
| Lin JH, et al. | 106,508, average age not reported | Taiwan only | Taiwan | Regression | II | A |
| Ponte C, et al. | 11.7 million | France only | France | t-test | III | A |
| Torrance N, et al. | 1,036,446, average age not reported | Scotland only | Scotland | Chi squared | III | A |
| Ali MM, et al. | , average age not reported | United States only | United States | Logit regression | III | A |
| Rutkow L, et al. | 37, average age not reported | United States only | United States | language processing | III | B |
| Simeone R | 11 billion | United States Only, Selection bias because pharmacies involved in illicit activity are not going to provide data | United States | t-test | III | A |
| Chenaf C, et al. | 3505, average age not reported | France only | France | Cox proportional hazard model | III | A |
| Delorme J, et al. | 2043 | France only | France | Cox proportional hazard model | III | A |
| Kea B, et al. | 139,256, average age not reported | United States only | United States | descriptives and natural language processing | III | B |
| National Council of State Boards of Nursing | n/a | United States only | United States | n/a | IV | B |
| Okumura Y, et al. | 1,178,361, average age not reported | Japan only | Japan | t-test | III | A |
| Ong MS, et al. | 5659 patients and 1448 provider pairs | United States only | United States | Logistic regression | III | A |
| Takahashi Y, et al. | 1.24 million, average age not reported | Japan only | Japan | regression | III | A |
| Huang SK, et al. | 30,000 physicians at 1898 facilities | Taiwan only | Taiwan | natural language processing | III | A |
| Lin MH, et al. | 32,813,217 visits, average age not reported | Taiwan only | Taiwan | descriptives and natural language processing | II | A |
| Lu TH, et al. | 6947, average age not reported | Taiwan only | Taiwan | descriptives and natural language processing | II | A |
| Webster LR and Grabois M | n/a | United States only | United States | n/a | IV | C |
| Han H, et al. | 3,260,824, average age not reported | United States only | United States | n/a | III | B |
| Hypponen H, et al. | 1693, average age not reported | Finland only | Finland | natural language processing | III | A |
| Shepherd J | n/a | United States only | United States | n/a | IV | A |
| Cepeda MS, et al. | 10,910,451, average age 45 | United States only | United States | t-test | III | A |
| Modarai F, et al. | United States only | United States | Regression, spatial cluster analysis | III | B | |
| Rouby F, et al. | 4.5 million | France only | France | t-test | III | A |
| Simoni-Wastila and Qian J | 2,175,012, average age not reported | United States only | United States | multinomial regressions | III | A |
| Worley J, et al. | n/a | United States only | United States | n/a | IV | C |
| Worley J, et al. | n/a | United States only | United States | n/a | IV | C |
| Fass JA and Hardigan PC | 836, average age not reported | United States only | United States | language processing | III | A |
| Frauger E, et al. | n/a | France only | France | n/a | IV | B |
| Hincapie AL, et al. | 34, average age not reported | United States only | United States | language processing | III | B |
| Hsu MH, et al. | 8, average age 47.9, 88% male | Taiwan only | Taiwan | Chi-squared and two-sided z-tests with Bonferroni adjustments | III | B |
| Pauly V, et al. | 4787 patients, average age 37.6 | France only | France | Clustering | III | A |
| Wilsey BL, et al. | 2,849,464 patients, average age of shoppers 50.7 | United States only | United States | Regression | IV | C |
| Pradel V, et al. | 128,000, average age not reported | France only | France | t-test | III | A |
References
- Kieffer, C.H. Citizen empowerment: A developmental perspective. Prev. Hum. Serv. 1984, 3, 9–36. [Google Scholar] [CrossRef]
- Castro, E.M.; Van Regenmortel, T.; Vanhaecht, K.; Sermeus, W.; Van Hecke, A. Patient empowerment, patient participation and patient-centeredness in hospital care: A concept analysis based on a literature review. Patient Educ. Couns. 2016, 99, 1923–1939. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014; ISBN 978 92 4 156485 4. [Google Scholar]
- Schneberk, T.; Raffetto, B.; Friedman, J.; Wilson, A.; Kim, D.; Schriger, D.L. Opioid prescription patterns among patients who doctor shop; Implications for providers. PLoS ONE 2020, 15. [Google Scholar] [CrossRef] [PubMed]
- Balazote, P.S.; Sicilia, M.-A. Interoperability in Healthcare Information Systems: Standards, Management, and Technology; IGI Global: Hershey, PA, USA, 2013. [Google Scholar]
- What Is Interoperability. Available online: https://www.healthit.gov/topic/interoperability (accessed on 11 July 2020).
- Biernikiewicz, M.; Taieb, V.; Toumi, M. Characteristics of doctor-shoppers: A systematic literature review. J. Mark. Access Health Policy 2019, 7. [Google Scholar] [CrossRef]
- Fink, D.; Schleimer, J.; Sarvet, A.; Grover, K.; Delcher, C.; Castillo-Carniglia, A.; Kim, J.; Rivera-Aguirre, A.; Henry, S.; Martins, S.; et al. Association between prescription drug monitoring programs and nonfatal and fatal drug overdoses: A systematic review. Ann. Intern. Med. 2018, 168, 783–790. [Google Scholar] [CrossRef]
- Casati, A.; Sedefov, R.; Pfeiffer-Gerschel, T. Misuse of medicines in the European union: A systematic review of the literature. Eur. Addict. Res. 2012, 18, 228. [Google Scholar] [CrossRef]
- Report of the International Narcotics Control Board for 2007. Available online: https://www.incb.org/incb/en/publications/annual-reports/annual-report-2007.html (accessed on 11 July 2020).
- Shepherd, J. Combating the prescription painkiller epidemic: A national prescription drug reporting program. Am. J. Law Med. 2014, 40, 85–112. [Google Scholar] [CrossRef]
- CDC. Prescription Opiod Data. Available online: https://www.cdc.gov/drugoverdose/data/prescribing.html (accessed on 11 July 2020).
- Fass, J.A.; Hardigan, P.C. Attitudes of florida pharmacists toward implementing a state prescription drug monitoring program for controlled substances. J. Manag. Care Pharm. 2011, 17, 430–438. [Google Scholar] [CrossRef]
- Hyppönen, H.; Reponen, J.; Lääveri, T.; Kaipio, J. User experiences with different regional health information exchange systems in Finland. Int. J. Med. Inform. 2014, 83, 1–18. [Google Scholar] [CrossRef]
- Huang, S.-K.; Wang, P.-J.; Tseng, W.-F.; Syu, F.-K.; Lee, M.-C.; Shih, R.-L.; Sheen, M.-T.; Chen, M.S. NHI-PharmaCloud in Taiwan—A preliminary evaluation using the RE-AIM framework and lessons learned. Int. J. Med. Inform. 2015, 84, 817–825. [Google Scholar] [CrossRef]
- Hsu, M.-H.; Yeh, Y.-T.; Chen, C.-Y.; Liu, C.-H.; Liu, C.-T. Online detection of potential duplicate medications and changes of physician behavior for outpatients visiting multiple hospitals using national health insurance smart cards in Taiwan. Int. J. Med. Inform. 2011, 80, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Chenaf, C.; Kabore, J.L.; Delorme, J.; Roche, L.; Eschalier, A.; Delage, N.; Authier, N.; Pereira, B.; Mulliez, A. Incidence of tramadol shopping behavior in a retrospective cohort of chronic non-cancer pain patients in France. Pharmacoepidemiol. Drug Saf. 2016, 25, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Popovici, I.; Hijazi, B.; Maclean, J.C.; Radakrishnan, S. The effect of state laws designed to prevent nonmedical prescription opioid use on overdose deaths and treatment. Health Econ. 2018, 27, 294–305. [Google Scholar] [CrossRef] [PubMed]
- HHS. NPI: What You Need to Know; Centers for Medicare and Medicaid Services: Baltimore, MD, USA, 2016; p. 10. [Google Scholar]
- Kruse, C.S. Writing a systematic review for publication in a health-related degree program. JMIR Res. Protoc. 2019, 8, e15490. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Alessandro, L.; Jennifer, T.; Douglas, G.A. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ Br. Med. J. 2009, 339, 332. [Google Scholar]
- Newhouse, R.; Dearbolt, S.; Poe, S.; Pugh, L.C.; White, K. JHNEBP evidence rating scales. In The Johns Hopkins Nursing Evidence-Based Practice Rating Scale; Centers for Medicare and Medicaid Services: Baltimore, MD, USA, 2005. [Google Scholar]
- Light, R.J. Measures of response agreement for qualitative data: Some generalizations and alternatives. Psychol. Bull. 1971, 76, 365. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Pett, R.G.; Mancl, L.; Revere, D.; Stergachis, A. Prescription drug monitoring program use and utility by Washington State pharmacists: A mixed-methods study. J. Am. Pharm. Assoc. 2020, 60, 57–65. [Google Scholar] [CrossRef]
- Durand, Z.; Nechuta, S.; Krishnaswami, S.; Hurwitz, E.L.; McPheeters, M. Prescription opioid use by injured workers in Tennessee: A descriptive study using linked statewide databases. Ann. Epidemiol. 2019, 32, 7–13. [Google Scholar] [CrossRef]
- Freeman, P.R.; Curran, G.M.; Drummond, K.L.; Martin, B.C.; Teeter, B.S.; Bradley, K.; Schoenberg, N.; Edlund, M.J. Utilization of prescription drug monitoring programs for prescribing and dispensing decisions: Results from a multi-site qualitative study. Res. Soc. Adm. Pharm. 2019, 15, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, R.; Talbert, J. Network abstractions of prescription patterns in a medicaid population. Amia Jt. Summits Transl. Sci. Proc. 2019, 2019, 524–532. [Google Scholar]
- Perry, B.L.; Yang, K.C.; Kaminski, P.; Odabas, M.; Park, J.; Martel, M.; Oser, C.B.; Freeman, P.R.; Ahn, Y.Y.; Talbert, J. Co-prescription network reveals social dynamics of opioid doctor shopping. PLoS ONE 2019, 14, e0223849. [Google Scholar] [CrossRef] [PubMed]
- Soffin, E.M.; Lee, B.H.; Kumar, K.K.; Wu, C.L. The prescription opioid crisis: Role of the anaesthesiologist in reducing opioid use and misuse. Br. J. Anaesth. 2019, 122, e198–e208. [Google Scholar] [CrossRef] [PubMed]
- Stopka, T.J.; Amaravadi, H.; Kaplan, A.R.; Hoh, R.; Bernson, D.; Chui, K.K.H.; Land, T.; Walley, A.Y.; LaRochelle, M.R.; Rose, A.J. Opioid overdose deaths and potentially inappropriate opioid prescribing practices (PIP): A spatial epidemiological study. Int. J. Drug Policy 2019, 68, 37–45. [Google Scholar] [CrossRef]
- Wang, Y.; Delcher, C.; Li, Y.; Goldberger, B.A.; Reisfield, G.M. Overlapping prescriptions of opioids, benzodiazepines, and carisoprodol: “Holy Trinity” prescribing in the state of Florida. Drug Alcohol Depend. 2019, 205, 107693. [Google Scholar] [CrossRef]
- Butler, J.M.; Becker, W.C.; Humphreys, K. Big data and the opioid crisis: Balancing patient privacy with public health. J. Law Med. Ethics 2018, 46, 440–453. [Google Scholar] [CrossRef]
- Lin, J.-H.; Cheng, S.-H. The impact of a medication record sharing program among diabetes patients under a single-payer system: The role of inquiry rate. Int. J. Med. Inform. 2018, 116, 18–23. [Google Scholar] [CrossRef]
- Ponté, C.; Lepelley, M.; Boucherie, Q.; Mallaret, M.; Lapeyre Mestre, M.; Pradel, V.; Micallef, J. Doctor shopping of opioid analgesics relative to benzodiazepines: A pharmacoepidemiological study among 11.7 million inhabitants in the French countries. Drug Alcohol Depend. 2018, 187, 88–94. [Google Scholar] [CrossRef]
- Torrance, N.; Mansoor, R.; Wang, H.; Gilbert, S.; Macfarlane, G.J.; Serpell, M.; Baldacchino, A.; Hales, T.G.; Donnan, P.; Wyper, G.; et al. Association of opioid prescribing practices with chronic pain and benzodiazepine co-prescription: A primary care data linkage study. Br. J. Anaesth. 2018, 120, 1345–1355. [Google Scholar] [CrossRef]
- Ali, M.M.; Dowd, W.N.; Classen, T.; Mutter, R.; Novak, S.P. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: Evidence from the National Survey of Drug Use and Health. Addict. Behav. 2017, 69, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Rutkow, L.; Smith, K.C.; Lai, A.Y.; Vernick, J.S.; Davis, C.S.; Alexander, G.C. Prescription drug monitoring program design and function: A qualitative analysis. Drug Alcohol Depend. 2017, 180, 395–400. [Google Scholar] [CrossRef]
- Simeone, R. Doctor shopping behavior and the diversion of prescription opioids. Subst. Abus. Res. Treat. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- National Council of State Boards of Nursing. A changing environment: 2016 NCSBN environmental scan. J. Nurs. Regul. 2016, 6, 4–37. [Google Scholar] [CrossRef]
- Delorme, J.; Chenaf, C.; Kabore, J.L.; Pereira, B.; Mulliez, A.; Tremey, A.; Brousse, G.; Zenut, M.; Laporte, C.; Authier, N. Incidence of high dosage buprenorphine and methadone shopping behavior in a retrospective cohort of opioid-maintained patients in France. Drug Alcohol Depend. 2016, 162, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kea, B.; Fu, R.; Lowe, R.A.; Sun, B.C. Interpreting the national hospital ambulatory medical care survey: United States emergency department opioid prescribing, 2006–2010. Acad. Emerg. Med. 2016, 23, 159–165. [Google Scholar] [CrossRef]
- Okumura, Y.; Shimizu, S.; Matsumoto, T. Prevalence, prescribed quantities, and trajectory of multiple prescriber episodes for benzodiazepines: A 2-year cohort study. Drug Alcohol Depend. 2016, 158, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.S.; Olson, K.L.; Cami, A.; Liu, C.; Tian, F.; Selvam, N.; Mandl, K.D. Provider patient-sharing networks and multiple-provider prescribing of benzodiazepines. J. Gen. Intern. Med. 2016, 31, 164–171. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ishizaki, T.; Nakayama, T.; Kawachi, I. Social network analysis of duplicative prescriptions: One-month analysis of medical facilities in Japan. Health Policy 2016, 120, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.H.; Chang, H.T.; Tu, C.Y.; Chen, T.J.; Hwang, S.J. Doctor-shopping behaviors among traditional chinese medicine users in Taiwan. Int. J. Environ. Res. Public Health 2015, 12, 9237–9247. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.H.; Lee, Y.Y.; Lee, H.C.; Lin, Y.M. Doctor shopping behavior for zolpidem among insomnia patients in taiwan: A nationwide population-based study. Sleep 2015, 38, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Webster, L.R.; Grabois, M. Current regulations related to opioid prescribing. PM&R 2015, 7, S236–S247. [Google Scholar] [CrossRef]
- Han, H.; Kass, P.H.; Wilsey, B.L.; Li, C.S. Increasing trends in Schedule II opioid use and doctor shopping during 1999–2007 in California. Pharmacoepidemiol. Drug Saf. 2014, 23, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, M.S.; Fife, D.; Yuan, Y.; Mastrogiovanni, G. Distance traveled and frequency of interstate opioid dispensing in opioid shoppers and nonshoppers. J. Pain 2013, 14, 1158–1161. [Google Scholar] [CrossRef] [PubMed]
- Modarai, F.; Mack, K.; Hicks, P.; Benoit, S.; Park, S.; Jones, C.; Proescholdbell, S.; Ising, A.; Paulozzi, L. Relationship of opioid prescription sales and overdoses, North Carolina. Drug Alcohol Depend. 2013, 132, 81–86. [Google Scholar] [CrossRef]
- Rouby, F.; Pradel, V.; Frauger, E.; Pauly, V.; Natali, F.; Reggio, P.; Thirion, X.; Micallef, J. Assessment of abuse of tianeptine from a reimbursement database using ‘doctor-shopping’ as an indicator. Fundam. Clin. Pharm. 2012, 26, 286–294. [Google Scholar] [CrossRef]
- Simoni-Wastila, L.; Qian, J. Influence of prescription monitoring programs on analgesic utilization by an insured retiree population. Pharmacoepidemiol. Drug Saf. 2012, 21, 1261–1268. [Google Scholar] [CrossRef]
- Worley, J. Prescription drug monitoring programs, a response to doctor shopping: Purpose, effectiveness, and directions for future research. Issues Ment. Health Nurs. 2012, 33, 319–328. [Google Scholar] [CrossRef]
- Worley, J. Psychiatric nursing’s role in preventing doctor shopping. J. Psychosoc. Nurs. Ment. Health Serv. 2012, 50, 4–5. [Google Scholar] [CrossRef]
- Frauger, E.; Pauly, V.; Pradel, V.; Rouby, F.; Arditti, J.; Thirion, X.; Lapeyre Mestre, M.; Micallef, J. Evidence of clonazepam abuse liability: Results of the tools developed by the French Centers for Evaluation and Information on Pharmacodependence (CEIP) network. Fundam. Clin. Pharm. 2011, 25, 633–641. [Google Scholar] [CrossRef]
- Hincapie, A.L.; Warholak, T.L.; Murcko, A.C.; Slack, M.; Malone, D.C. Physicians’ opinions of a health information exchange. J. Am. Med. Inf. Assoc. 2011, 18, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pauly, V.; Frauger, E.; Pradel, V.; Rouby, F.; Berbis, J.; Natali, F.; Reggio, P.; Coudert, H.; Micallef, J.; Thirion, X. Which indicators can public health authorities use to monitor prescription drug abuse and evaluate the impact of regulatory measures? Controlling high dosage Buprenorphine abuse. Drug Alcohol Depend. 2011, 113, 29–36. [Google Scholar] [CrossRef]
- Wilsey, B.L.; Fishman, S.M.; Gilson, A.M.; Casamalhuapa, C.; Baxi, H.; Lin, T.C.; Li, C.S. An analysis of the number of multiple prescribers for opioids utilizing data from the California Prescription Monitoring Program. Pharmacoepidemiol. Drug Saf. 2011, 20, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Pradel, V.; Delga, C.; Rouby, F.; Micallef, J.; Lapeyre-Mestre, M. Assessment of abuse potential of benzodiazepines from a prescription database using ‘doctor shopping’ as an indicator. CNS Drugs 2010, 24, 611. [Google Scholar] [CrossRef] [PubMed]
| Authors | Participants | Intervention | Results (Compared to Control Group) | Medical Outcomes Reported | Study Design |
|---|---|---|---|---|---|
| Pett RG et al. [26] | Pharmacists | PDMP | Frequent PDMP users were more likely to recommend naloxone. | Naloxone prescribed to shoppers | Explanatory, sequential 2-phase mixed-methods |
| Durand et al. [27] | Injured workers identified in worker’s compensation records | PDMP and worker’s compensation claims | No control group. Injured workers have a high prevalence of opioid use after injury, but prescribing patterns generally follow state guidelines. | Not reported | Retrospective cohort |
| Freeman et al. [28] | Pharmacists and providers | Interviews about PDMP | No control group. Both PCPs and pharmacists reported PDMPs are key tools to aid prescribing and dispensing. | Not reported | Qualitative |
| Nagarajan and Talbert [29] | Prescriber-prescriber networks | Computer model | Outliers were clearly identified in the model, which can help identify those prescribers contributing to the opioid epidemic. | Not reported | Computer model to detect prescriber outliers |
| Perry et al. [30] | Patients who submitted claims to commercial database in the Appalachian region of the U.S., 58.7% female | Computer model PageRank | Model clearly differentiates aberrant behavior identifying drug shoppers for both opioids and morphine milligram equivalents (MME). | Not reported | Computer model to detect prescriber outliers |
| Soffin et al. [31] | n/a | PDMP | n/a | Accessing real-time information about patients’ prescription opioid status using PDMP reduces opioid quantities prescribed | Opinion |
| Stopka et al. [32] | adults in Massachusetts prescribed opioids | Computer model | Hotspots were identified. | Not reported | Spatial epidemiological study |
| Wang et al. [33] | Patients in pharmacological database in Florida | Pharmacological database | Recipients of opioids, benzodiazepines, and carisoprodol in 2017 compared with 2012 were younger, more likely to be female, and geographically-localized. | Not reported | Retrospective, observational |
| Butler et al. [34] | n/a | PDMP, Lock-in programs | No control group. The lock-in program decreased doctor shopping. | Broader range of drug schedules and an increases frequency of updating the PDMP results in lower opioid-related mortality | Opinion |
| Lin et al. [35] | Patients from health insurance claim data | Medication record sharing program | The medication duplication rate was reduced 7.76 percentile, average medication overlap periods shortened 4.36 days. | Not reported | Retrospective pre-post test design |
| Ponte et al. [36] | Beneficiaries of the France public health system | SNIIRAM database | The strong opioid analgesics have the highest DSI (2.79%) versus 2.06% for BZD hypnotics. Flunitrazepam ranked first according to its DSI (13.2%), followed by morphine (4%), and zolpidem (2.2%). | Not reported | Retrospective, observational |
| Torrance et al. [37] | Beneficiaries of the Scotland National Health System (NHS) | NHS and Generation Scotland databases combined to identify trends | The number of strong opioid prescriptions more than doubled between 2003–2012. Patients in the most deprived areas were more likely to receive a strong opioid. | Not reported | Descriptive analysis |
| Ali et al. [38] | Respondents to the national survey of drug use and health, aged 12 and older, 48% male | Survey instrument to assess effectiveness of the PDMP | No control group. PDMP was effective at controlling for doctor shopping for opiate pain killers. | 10–20 fewer days of NMPR use | Qualitative |
| Rutkow et al. [39] | Prescribers in four states | PDMP | Prescribers need to work with law enforcement, law-enforcement need to share data with each other, data sharing between states needs to occur. | Not reported | Qualitative |
| Simeone [40] | Prescriptions | PDMP, education efforts, pharmacy panels that span the country | No control group. The number of prescriptions diverted fell from 4.3 million in 2008 to 3.37 million in 2012. | Decline in overdose mortality | Retrospective, observational |
| Chenaf et al. [17] | Adult patients with chronic non-cancer pain (CNCP) | French national health system | No control group. Shopping very low in drugs for these conditions. | Not reported | Retrospective cohort |
| Delorme et al. [42] | Patients treated by opioid substitution treatment over 8 years | French national health system | No control group. Shopping behavior was only found in high dosage buprenorphine patients, but still very low. | Not reported | Retrospective cohort |
| Kea et al. [43] | Emergency Department (ED) discharges | PDMP | Doctor shopping was not detected in ED survey. | Not reported | Qualitative |
| Authors | Intervention Theme | Outcome Theme | Facilitator Theme | Barrier Theme |
|---|---|---|---|---|
| Pett et al. | PDMP | Shoppers prescribed treatment | Pharmacists support | Not reported |
| Durand et al. | Combination | Not reported | Government support | Data sharing |
| Freeman et al. | PDMP | Not reported | Government support | Access not mandatory |
| Pharmacists support | ||||
| Prescriber support | ||||
| Nagarajan and Talbert | Computer model | Not reported | Simple to implement | Not reported |
| Perry et al. | Computer model | Not reported | Not reported | Not reported |
| Soffin et al. | PDMP | Reduced opioids prescribed | Government support | Not reported |
| Prescriber support | ||||
| Stopka et al. | Computer model | Not reported | Simple to implement | Not reported |
| Wang et al. | Other | Not reported | Government support | Not reported |
| Butler et al. | Combination | Reduced mortality | Must use law | Access not mandatory |
| Does not treat addiction | ||||
| Lin et al. | Other | Not reported | Government support | Not reported |
| Ponte et al. | National health system database (DB) | Not reported | Government support | Not reported |
| Torrance et al. | National health system DB | Not reported | Government support | Not reported |
| Ali et al. | PDMP | Fewer days of use | Not reported | Not reported |
| Rutkow et al. | PDMP | Not reported | Government support | Data sharing |
| Simeone | Combination | Reduced mortality | Government support | Easily thwarted |
| Pharmacists support | ||||
| Chenaf et al. | National health system DB | Not reported | Government support | Not reported |
| Delorme et al. | National health system DB | Not reported | Government support | Not reported |
| Kea et al. | PDMP | Not reported | Government support | Not reported |
| National Council of State Boards of Nursing | PDMP | Not reported | Government support | Not reported |
| Okumura et al. | National health system DB | Not reported | Government support | Not reported |
| Ong et al. | Health insurance claims | Not reported | Government support | Data sharing |
| Prescriber support | ||||
| Takahashi et al. | Computer model | Not reported | Simple to implement | Not reported |
| Huang et al. | Other | Not reported | Cost savings | Participation not mandatory |
| Government support | Access not mandatory | |||
| Lin et al. | Health insurance claims | Not reported | Government support | Not reported |
| Lu et al. | Health insurance claims | Not reported | Government support | Not reported |
| Webster and Grabois | PDMP | Not reported | Government support | Access not mandatory |
| Han et al. | PDMP | Reduced shopping | Not reported | Not reported |
| Hypponen et al. | Health information exchange | Not reported | Government support | Not reported |
| Cost savings | ||||
| Increased efficiency | ||||
| Shepherd | Combination | Not reported | Not reported | Inadequate data collection |
| Ineffective data use | ||||
| Data sharing | ||||
| Constraints on enforcement | ||||
| Cash-only not captured | ||||
| Cepeda et al. | PDMP | Reduced shopping | Not reported | Data sharing |
| Modarai et al. | Other | Not reported | Government support | Not reported |
| Rouby et al. | National health system DB | Not reported | Government support | Not reported |
| Simoni-Wastila and Qian | PDMP | No decline in mortality | Government support | Not reported |
| Worley et al. | PDMP | Reduced shopping | Government support | Not reported |
| Prescriber support | ||||
| Worley et al. | PDMP | Not reported | Government support | Not reported |
| Prescriber support | ||||
| Fass and Hardigan | PDMP | Not reported | Government support | Not reported |
| Frauger et al. | Other | Not reported | Government support | Not reported |
| Hincapie et al. | Health information exchange | Not reported | Government support | Participation not mandatory |
| Hsu et al. | Combination | Not reported | Prescriber support | Participation not mandatory |
| Pauly et al. | Computer model | Not reported | Government support | Not reported |
| Wilsey et al. | PDMP | Not reported | Government support | Not reported |
| Pradel et al. | National health system DB | Not reported | Government support | Not reported |
| Strength of Evidence | Frequency | Quality of Evidence | Frequency |
|---|---|---|---|
| III (Non-experimental, qualitative) | 28 (67%) | A (High quality) | 25 (60%) |
| IV (Opinion) | 10 (24%) | B (Good quality) | 11 (26%) |
| II (Quasi-experimental) | 4 (10%) | C (Low quality or major flaws) | 6 (14%) |
| I (Experimental study or RCT) | 0 (0%) | ||
| (a) | (b) | ||
| Interventions | References | Occurrences (n = 42) | Frequency |
|---|---|---|---|
| PDMP | [13,26,28,31,38,39,41,43,49,50,51,54,55,56,60] | 15 | 36% |
| National health system DB | [17,36,37,42,44,53,61] | 7 | 17% |
| Computer model | [29,30,32,46,59] | 5 | 12% |
| Combination | [11,16,27,34,54] | 5 | 12% |
| Other | [15,33,35,52,57] | 5 | 12% |
| Health insurance claims | [45,47,48] | 3 | 7% |
| Health information exchange | [14,58] | 2 | 5% |
| Facilitators | References | Occurrences (n = 52) | Frequency |
|---|---|---|---|
| Government support | [13,14,17,27,28,31,33,35,36,37,39,40,41,42,43,44,45,47,48,49,52,53,54,55,56,57,58,59,60,61] | 31 | 60% |
| Prescriber support | [28,31,45,55,56] | 6 | 12% |
| Not reported | [11,30,38,50,51] | 5 | 10% |
| Simple to implement | [29,30,32,46] | 4 | 6% |
| Pharmacists support | [26,28,40] | 3 | 6% |
| Cost savings | [14,15] | 2 | 4% |
| Must use law | [34] | 1 | 2% |
| Increased efficiency | [14] | 1 | 2% |
| Barriers | References | Occurrences (n = 48) | Frequency |
|---|---|---|---|
| Not reported | [13,14,17,26,29,30,31,32,33,35,36,37,38,41,42,43,44,46,47,48,50,52,54,55,56,57,59,60,61] | 30 | 63% |
| Data sharing | [11,27,39,45,51] | 5 | 10% |
| Access not mandatory | [15,28,34,49] | 4 | 8% |
| Participation not mandatory | [15,16,58] | 3 | 6% |
| Easily thwarted | [40] | 1 | 2% |
| Inadequate data collection | [11] | 1 | 2% |
| Ineffective data use | [11] | 1 | 2% |
| Constraints on enforcement | [11] | 1 | 2% |
| Cash-only not captured | [11] | 1 | 2% |
| Does not treat addiction | [34] | 1 | 2% |
| Medical Outcomes | References | Occurrences (n = 42) | Frequency |
|---|---|---|---|
| Not reported | [11,13,14,15,16,17,27,28,29,30,32,33,35,36,37,39,41,42,43,44,45,47,48,49,52,53,56,57,58,59,60,61] | 33 | 79% |
| Reduced shopping | [50,51,55] | 3 | 7% |
| Reduced mortality | [34,40] | 2 | 5% |
| Shoppers prescribed treatment | [26] | 1 | 2% |
| Fewer days of use | [38] | 1 | 2% |
| No decline in mortality | [54] | 1 | 2% |
| Reduced opioids prescribed | [31] | 1 | 2% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruse, C.S.; Kindred, B.; Brar, S.; Gutierrez, G.; Cormier, K. Health Information Technology and Doctor Shopping: A Systematic Review. Healthcare 2020, 8, 306. https://doi.org/10.3390/healthcare8030306
Kruse CS, Kindred B, Brar S, Gutierrez G, Cormier K. Health Information Technology and Doctor Shopping: A Systematic Review. Healthcare. 2020; 8(3):306. https://doi.org/10.3390/healthcare8030306
Chicago/Turabian StyleKruse, Clemens Scott, Brady Kindred, Shaneel Brar, Guillermo Gutierrez, and Kaleigh Cormier. 2020. "Health Information Technology and Doctor Shopping: A Systematic Review" Healthcare 8, no. 3: 306. https://doi.org/10.3390/healthcare8030306
APA StyleKruse, C. S., Kindred, B., Brar, S., Gutierrez, G., & Cormier, K. (2020). Health Information Technology and Doctor Shopping: A Systematic Review. Healthcare, 8(3), 306. https://doi.org/10.3390/healthcare8030306





.jpg)

