A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser
Abstract
1. Introduction
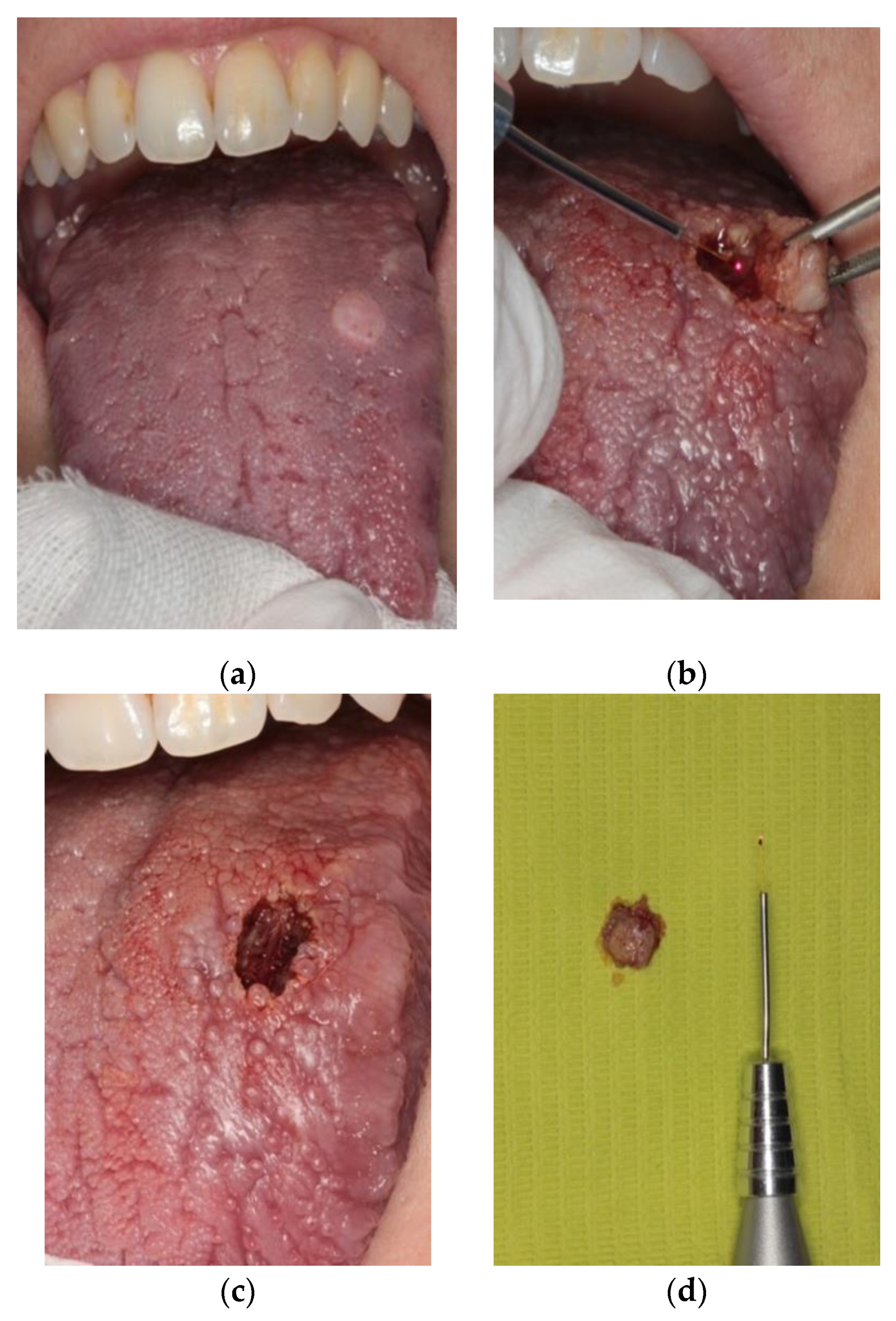
2. Materials and Methods
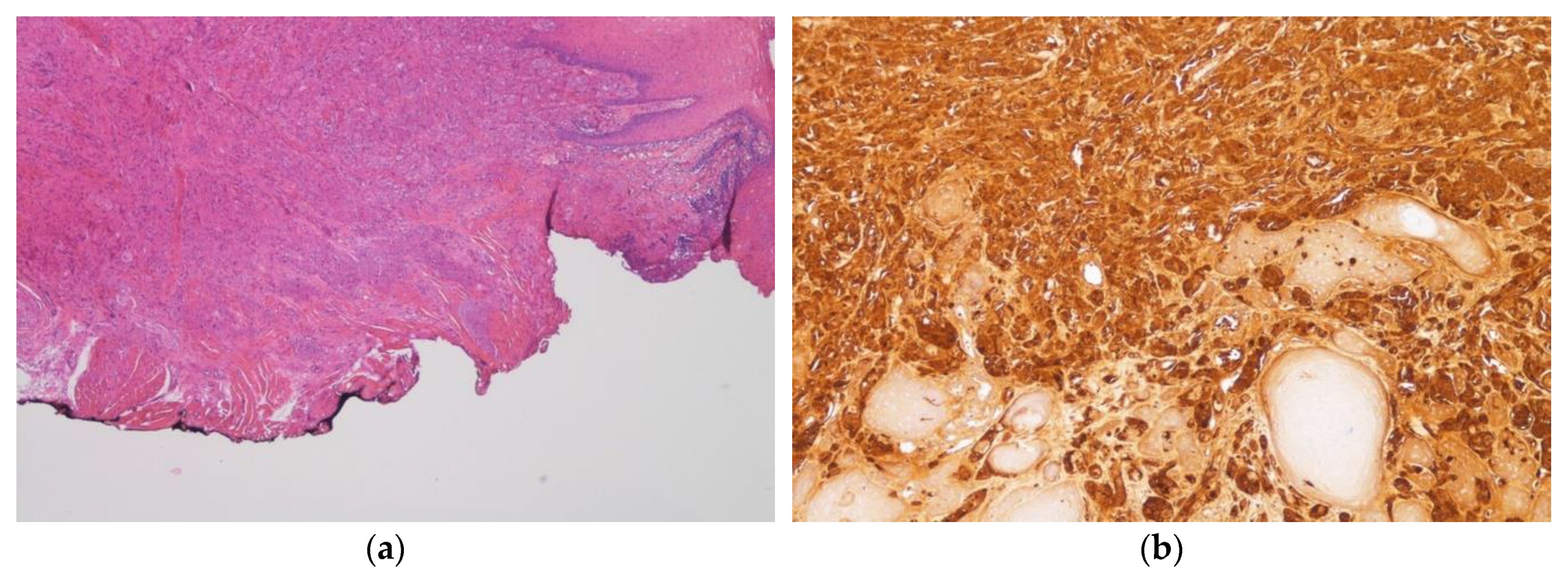
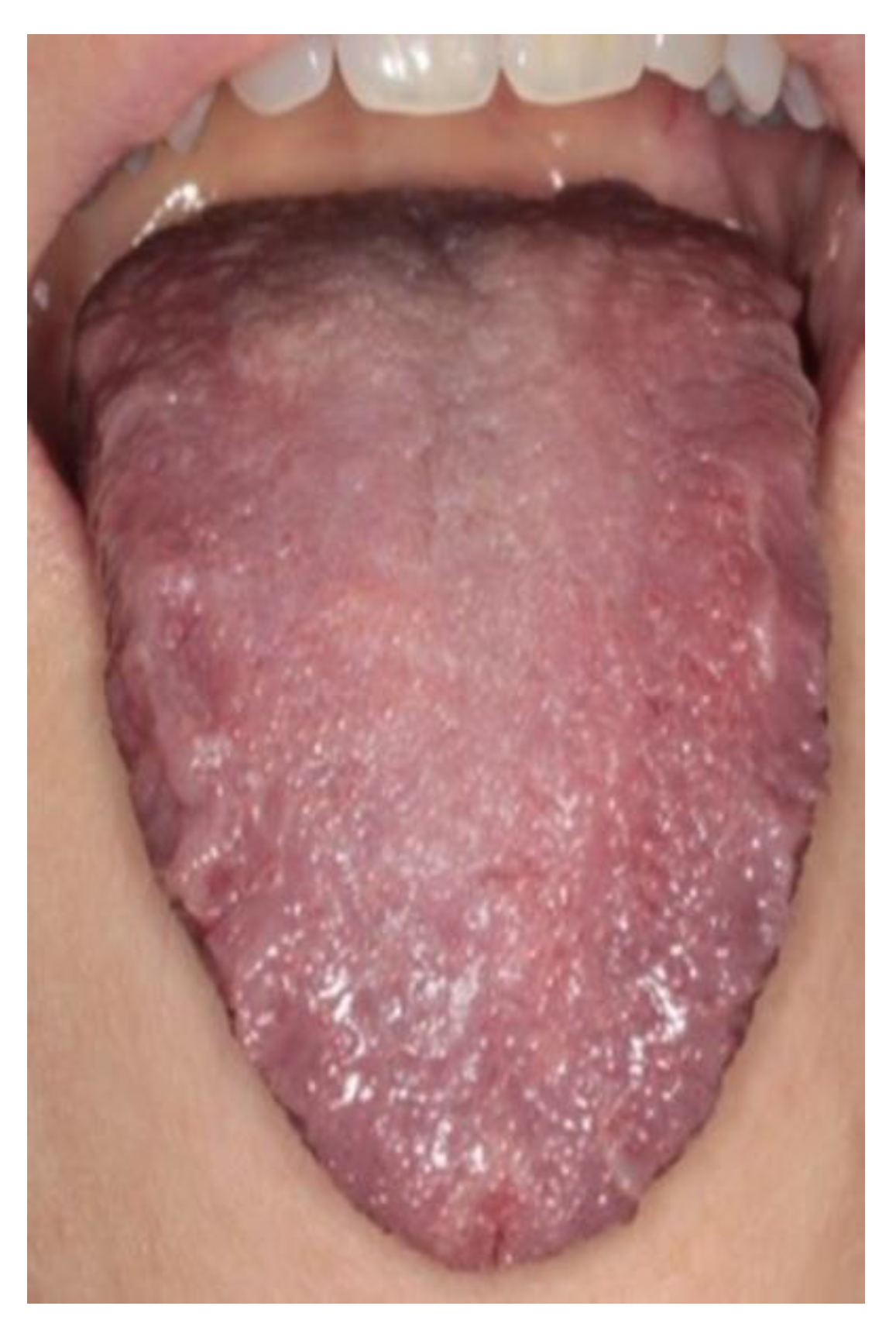
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pertile, D.; Scabini, S.; Romairone, E.; Scordamiglia, R.; Rimini, E.; Ferrando, V. Gastric Abrikosoff tumor (granular cell tumor): Case report. G. Chir. 2010, 31, 433–434. [Google Scholar] [PubMed]
- Van de Loo, S.; Thunnissen, E.; van der Waal, I. Granular cell tumor of the oral cavity; a case series including a case of metachronous occurrence in the tongue and the lung. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e30. [Google Scholar] [PubMed]
- Pushpa, G.; Karve, P.P.; Ahmad, P.B. Abrikossoff’s Tumor: An Unusual Presentation. Indian J. Dermatol. 2013, 58, 407. [Google Scholar] [PubMed]
- Tobouti, P.L.; Pigatti, F.M.; Martins-Mussi, M.C.; Sedassari, B.T.; de Sousa, S.C. Extra-tongue oral granular cell tumor: Histological and immunohistochemical aspect. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e31–e35. [Google Scholar] [PubMed]
- Ferreira, J.C.; Ferreira Oton-Leite, A.; Guidi, R.; Mendonça, E.F. Granular cell tumor mimicking a squamous cell carcinoma of the tongue: A case report. BMC Res. Notes 2017, 10, 14. [Google Scholar]
- Suchitra, G.; Tambekar, K.N.; Gopal, K.P. Abrikossoff’s tumor of tongue: Report of an uncommon lesion. J. Oral Maxillofac. Pathol. 2014, 18, 134–136. [Google Scholar]
- Azma, E.; Safavi, N. Diode Laser Application in Soft Tissue Oral Surgery. J. Lasers Med. Sci. 2013, 4, 206–211. [Google Scholar]
- Berlucchi, M. Oral Granular Cell Tumor Mimicking a Giant Sialolith in a Child. J. Pediatr. 2018, 196, 332. [Google Scholar]
- Musha, A.; Ogawa, M.; Yokoo, S. Granular cell tumors of the tongue: Fibroma or schwannoma. Head Face Med. 2018, 14, 1. [Google Scholar]
- Dhareula, A.; Jaiswal, M.; Goyal, A.; Gauba, K. Congenital granular cell tumor of the newborn-Spontaneous regression or early surgial intervention. J. Indian Soc. Pedod. Prev. Dent. 2018, 36, 319–323. [Google Scholar]
- Meleti, M.; Mooi, W.J.; van der Waal, I. Oral malignant melanoma associated with pseudoepitheliomatous hyperplasia. Report of a case. J. Cutan. Pathol. 2006, 33, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Sproat, R.; Wong, G.; Rubin, J. Granular Cell Tumor of the Larynx. Head Neck Pathol. 2016, 10, 538–540. [Google Scholar] [PubMed]
- Angiero, F.; Crippa, R.; Stefani, M. Granular Cells Tumour in the Oral Cavity: Report of Eleven Cases Treated with Laser Surgery. Minerva Stomatol. 2006, 55, 423–430. [Google Scholar] [PubMed]
- Romeo, U.; Palaia, G.; Galanakis, A.; Gaimari, G.; Lo Giudice, R.; Tenore, G.; del Vecchio, A. Granular cell tumour of the tongue: Two clinical cases treated with laser. Italian Oral Surg. 2012, 11, S90–S95. [Google Scholar]
- Choi, P.M.; Schneider, L. Endoscopic Nd: YAG laser treatment of granular cell tumor of the esophagus. Gastrointest. Enosc. 1990, 36, 144–146. [Google Scholar]
- Van der Maten, J.; Blaauwgeers, J.L.G.; Sutedja, T.G.; Kwa, H.B.; Postmus, P.E.; Wagenaar, S.S. Granular cell tumors of the tracheobronchial tree. J. Thorac. Cardiovasc. Surg. 2003, 126, 740–743. [Google Scholar]
- Piazza, C.; Casirati, C.; Peretti, G.; Battaglia, G.; Manfredini, C.; Nicolai, P. Granular Cell Tumor of the Hypopharynx Treated by Endoscopic CO2 Laser Excision: Report of two cases. Head Neck 2000, 22, 524–529. [Google Scholar]
- Reichelt, J.; Winter, J.; Meister, J.; Frentzen, M.; Kraus, D. A novel blue light system for surgical applications in dentistry: Evaluation of specific laser-tissue interactions in monolayer cultures. Clin. Oral Investig. 2017, 21, 985–994. [Google Scholar]
- Hess, M.M.; Fleischer, S.; Ernstberger, M. New 445 nm blue laserfor laryngeal surgery combines photoangiolytic and cutting properties. Eur. Arch. Otorhinolaryngol. 2018, 275, 1557–1567. [Google Scholar]
- Yasak, T.; Ozkaya, O.; Akçay, A.A.; Kayadibi, T. Report of two cases of granular cell tumor, a rare tumor in children. J. Ped. Surg. Case Rep. 2016, 14, 1–3. [Google Scholar]
- Ohshiro, T.; Ogata, H.; Yoshida, M.; Tanaka, Y.; Sasaki, K.; Yoshimi, K. Penetration depths of 830nm diode laser irradiation in the head and neck assessed using radiographic phantom model and wavelength-specific imaging film. Laser Ther. 1996, 8, 197–204. [Google Scholar] [CrossRef]
- Rai, S.; Kaur, M.; Bhatnagar, P. Laser: A Powerful Tool for Treatment of Pyogenic Granuloma. J. Cutan. Aesthet. Surg. 2011, 4, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, D.; Lazaridi, I.; Anagnostou, E.; Poulopoulos, A.; Panta, P.; Patil, S. Diode laser assisted excision of a gingival pyogenic granuloma: A case report. Clin. Pract. 2019, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Ishii, J.; Fujita, K.; Munemoto, S.; Komori, T. Management of oral leukoplakia by laser surgery: Relation between recurrence and malignant transformation and clinicopathological features. J. Clin. Laser Med. Surg. 2004, 22, 27–33. [Google Scholar] [CrossRef]
- Fornanini, C.; Merigo, E.; Vescovi, P.; Bonanini, M.; Antonietti, W.; Leoci, L.; Lagori, G.; Meleti, M. Different. Laser Wavelengths Comparison in the Second-Stage Implant Surgery: An ex Vivo Study. Lasers Med. Sci. 2015, 30, 1631–1639. [Google Scholar] [CrossRef]
- Vescovi, P.; Corcione, L.; Meleti, M.; Merigo, E.; Fornaini, C.; Manfredi, M.; Bonanini, M.; Govoni, P.; Rocca, J.P.; Nammour, S. Nd: YAG Laser Versus Traditional Scalpel. A Preliminary Histological Analysis of Specimens from the Human Oral Mucosa. Lasers Med. Sci. 2010, 25, 685–691. [Google Scholar] [CrossRef]
- Palaia, G.; Impellizzeri, A.; Tenore, G.; Caporali, F.; Visca, P.; del Vecchio, A.; Galluccio, G.; Polimeni, A.; Romeo, U. Ex vivo histological analysis of the thermal effects created by a 445-nm diode laser in oral soft tissue biopsy. Clin. Oral Investig. 2020, 24, 2645–2652. [Google Scholar] [CrossRef]
- Monteiro, L.; Delgado, M.L.; Garces, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pachesco, J.J. A histological evaluation of the surgical margins from human oral fibrous-epithelial lesions excised with CO2 laser, Diode laser, Er: YAG laser, Nd: YAG laser, electrosurgical scalpel and cold scalpel. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e271–e280. [Google Scholar]
- Akbulut, N.; Kursun, E.S.; Tumer, M.K.; Kamburoglu, K.; Gulsen, U. Is the 810-nm diode laser the best choice in oral soft tissue therapy? Eur. J. Dent. 2013, 7, 207–211. [Google Scholar] [CrossRef]
- Cayan, T.; Hasangoglu, G.N.; Akca, G.; Kahraman, S. Comparative evaluation of diode laser and scalpel surgery in the treatment of inflammatory fibrous hyperplasia: A split-mouth study. Photobiomodul. Photomed. Laser Surg. 2019, 37, 91–98. [Google Scholar] [CrossRef]
- Al-Mohaya, M.A.; Al-Malik, A.M. Excision of oral pyogenic granuloma in a diabetic patient with 940 nm diode laser. Saudi Med. J. 2016, 37, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Olan, L.B.I.; Ventura, M.A.R.; Najera, C.A.B.; Castro, A.N. Laser therapy on dental emergencies of patients with hemofilia as an alterantive for hemostasis and reducing the use of factor VIII concentrate. J. Dent. Health Oral Disord. Ther. 2019, 10, 88–89. [Google Scholar] [CrossRef]
- Monteiro, L.; Barbieri, C.; Warnakulasuriya, S.; Martins, M.; Salazar, F.; Pacheco, J.J.; Vescovi, P.; Meleti, M. Type of surgical treatment and recurrence of oral leukoplakia: A retrospective clinical study. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e520–e526. [Google Scholar] [CrossRef] [PubMed]
- Elemek, E. Gingival melanin depigmentation by 810 nm diode laser. Eur. J. Dent. 2018, 12, 149–152. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viani, M.V.; Corcione, L.; Di Blasio, C.; Bologna-Molina, R.; Vescovi, P.; Meleti, M. A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser. Healthcare 2020, 8, 267. https://doi.org/10.3390/healthcare8030267
Viani MV, Corcione L, Di Blasio C, Bologna-Molina R, Vescovi P, Meleti M. A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser. Healthcare. 2020; 8(3):267. https://doi.org/10.3390/healthcare8030267
Chicago/Turabian StyleViani, Maria Vittoria, Luigi Corcione, Chiara Di Blasio, Ronell Bologna-Molina, Paolo Vescovi, and Marco Meleti. 2020. "A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser" Healthcare 8, no. 3: 267. https://doi.org/10.3390/healthcare8030267
APA StyleViani, M. V., Corcione, L., Di Blasio, C., Bologna-Molina, R., Vescovi, P., & Meleti, M. (2020). A Single Case Report of Granular Cell Tumor of the Tongue Successfully Treated through 445 nm Diode Laser. Healthcare, 8(3), 267. https://doi.org/10.3390/healthcare8030267







